Identification of Clubroot-Resistant Germplasm in a Radish (Raphanus sativus L.) Core Collection
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant and Pathogen Materials
2.2. Plant Cultivation
2.3. Clubroot Pathogen Inoculation
2.4. Clubroot Resistance Assessment
2.5. DNA Extraction
2.6. SSR Analysis
2.7. Plant Hybridization
2.8. Cultivation of HR and Hybrid Radishes
3. Results and Analysis
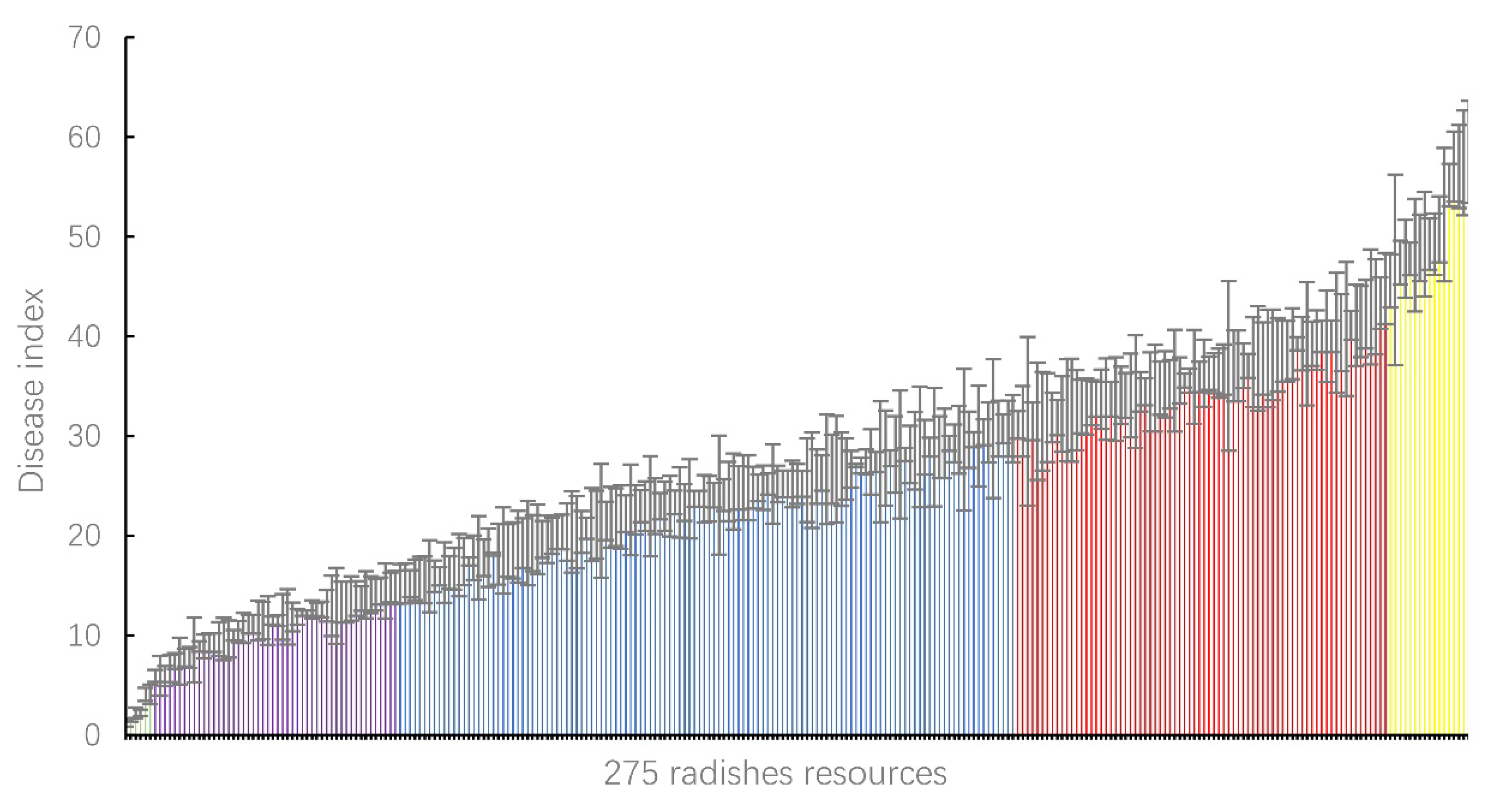
3.1. Clubroot Resistance Evaluation among Radish Resources
3.2. Geographical Distribution of Clubroot Resistance Resources
3.3. Horticultural Characters of HR Radishes
3.4. Polymorphism at Known Clubroot-Resistance QTL Not Associated with Resistance
3.5. Hybridization of HR Accessions with Main Breeding Radishes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, Z.; Jiang, Y.; Gong, J.; Li, Q.; Dun, B.; Liu, D.; Yin, F.; Yuan, L.; Zhou, X.; Wang, H.; et al. R gene triplication confers European fodder turnip with improved clubroot resistance. Plant Biotechnol. J. 2022, 20, 1502–1517. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Cui, W.; Munir, S.; He, P.; Li, X.; Wu, Y.; Yang, X.; Tang, P.; He, Y. Plasmodiophora brassicae root hair interaction and control by Bacillus subtilis XF-1 in Chinese cabbage. Biol. Control 2019, 128, 56–63. [Google Scholar] [CrossRef]
- Hirani, A.H.; Gao, F.; Liu, J.; Fu, G.; Wu, C.; McVetty, P.B.; Li, G. Combinations of independent dominant loci conferring clubroot resistance in all four turnip accessions (Brassica rapa) from the European clubroot differential set. Front. Plant Sci. 2018, 9, 1628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, G.; Li, X.; Piao, Z. Effects of exogenous ergothioneine on Brassica rapa clubroot development revealed by transcriptomic analysis. Int. J. Mol. Sci. 2023, 24, 6380. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.; Deng, X.; Cui, L.; Yu, X.; Yuan, W.; Dai, Z.; Yao, M.; Pang, W.; Ma, Y.; Yu, X.; et al. Construction of a high-density genetic linkage map and identification of quantitative trait loci associated with clubroot resistance in radish (Raphanus sativus L.). Mol. Breed. 2019, 39, 116. [Google Scholar] [CrossRef]
- Gahatraj, S.; Shrestha, S.M.; Devkota, T.R.; Rai, H.H. A review on clubroot of crucifers: Symptoms, life-cycle of pathogen, factors affecting severity, and management strategies. Arch. Agric. Environ. Sci. 2019, 4, 342–349. [Google Scholar] [CrossRef]
- Vañó, M.S.; Nourimand, M.; MacLean, A.; Pérez-López, E. Getting to the root of a club—Understanding developmental manipulation by the clubroot pathogen. Semin. Cell Dev. Biol. 2023, 148, 22–32. [Google Scholar] [CrossRef]
- Schwelm, A.; Fogelqvist, J.; Knaust, A.; Jülke, S.; Lilja, T.; Bonilla-Rosso, G.; Karlsson, M.; Shevchenko, A.; Dhandapani, V.; Choi, S.R.; et al. The Plasmodiophora brassicae genome reveals insights in its life cycle and ancestry of chitin synthases. Sci. Rep. 2015, 5, 11153. [Google Scholar] [CrossRef]
- Wagner, G.; Laperche, A.; Lariagon, C.; Marnet, N.; Renault, D.; Guitton, Y.; Bouchereau, A.; Delourme, R.; Manzanares-Dauleux, M.J.; Gravot, A.; et al. Resolution of quantitative resistance to clubroot into QTL-specific metabolic modules. J. Exp. Bot. 2019, 70, 5375–5390. [Google Scholar] [CrossRef]
- Gan, C.; Yan, C.; Pang, W.; Cui, L.; Fu, P.; Yu, X.; Qiu, Z.; Zhu, M.; Piao, Z.; Deng, X. Identification of novel locus RsCr6 related to clubroot resistance in radish (Raphanus sativus L.). Front. Plant Sci. 2022, 13, 866211. [Google Scholar] [CrossRef]
- Wang, Y.; Xiang, X.; Huang, F.; Yu, W.; Zhou, X.; Li, B.; Zhang, Y.; Chen, P.; Zhang, C. Fine mapping of clubroot resistance loci CRA8. 1 and candidate gene analysis in Chinese cabbage (Brassica rapa L.). Front. Plant Sci. 2022, 13, 898108. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, T.; Lu, J.; Xu, X.; Zhang, W. Metabonomic profiling of clubroot-susceptible and clubroot-resistant radish and the assessment of disease-resistant metabolites. Front. Plant Sci. 2022, 13, 1037633. [Google Scholar] [CrossRef]
- Zahr, K.; Sarkes, A.; Yang, Y.; Ahmed, H.; Zhou, Q.; Feindel, D.; Harding, M.W.; Feng, J. Plasmodiophora brassicae in its environment: Effects of temperature and light on resting spore survival in soil. Phytopathology 2021, 111, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Daval, S.; Gazengel, K.; Belcour, A.; Linglin, J.; Guillerm-Erckelboudt, A.Y.; Sarniguet, A.; Manzanares-Dauleux, M.J.; Lebreton, L.; Mougel, C. Soil microbiota influences clubroot disease by modulating Plasmodiophora brassicae and Brassica napus transcriptomes. Microb. Biotechnol. 2020, 13, 1648–1672. [Google Scholar] [CrossRef] [PubMed]
- Al-Daoud, F.; Gossen, B.D.; Robson, J.; Mcdonald, M.R. Propidium monoazide improves quantification of resting spores of Plasmodiophora brassicae with qPCR. Plant Dis. 2017, 101, 442–447. [Google Scholar] [CrossRef][Green Version]
- Al-Daoud, F.; Gossen, B.; Mcdonald, M. Maturation of resting spores of Plasmodiophora brassicae continues after host cell death. Plant Pathol. 2020, 69, 310–319. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, J.W.; Dong, L.H.; Li, X.; Asif, M.; Guo, Q.G.; Jiang, W.J.; Ma, P.; Zhang, L.Q. Fengycin produced by Bacillus subtilis NCD-2 is involved in suppression of clubroot on Chinese cabbage. Biol. Control 2019, 136, 104001. [Google Scholar] [CrossRef]
- Yang, X.X.; Zhang, L.; Huang, X.Q.; Wu, W.X.; Zhou, X.Q.; Du, L.; Li, H.Z.; Liu, Y. Difference of the microbial community structure in the rhizosphere of soybean and oilseed rape based on high-throughput pyrosequencing analysis. J. Appl. Ecol. 2019, 30, 2345–2351. [Google Scholar] [CrossRef]
- Ahmed, A.; Munir, S.; He, P.; Li, Y.; He, P.; Yixin, W.; He, Y. Biocontrol arsenals of bacterial endophyte: An imminent triumph against clubroot disease. Microbiol. Res. 2020, 241, 126565. [Google Scholar] [CrossRef]
- Zhu, M.; He, Y.; Li, Y.; Ren, T.; Liu, H.; Huang, J.; Jiang, D.; Hsiang, T.; Zheng, L. Two new biocontrol agents against clubroot caused by Plasmodiophora brassicae. Front. Microbiol. 2020, 10, 3099. [Google Scholar] [CrossRef]
- Zhang, J.; Ahmed, W.; Zhou, X.; Yao, B.; He, Z.; Qiu, Y.; Wei, F.; He, Y.; Wei, L.; Ji, G. Crop rotation with marigold promotes soil bacterial structure to assist in mitigating clubroot Incidence in Chinese Cabbage. Plants 2022, 11, 2295. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, X.; Cheng, J.; Xie, J.; Lin, Y.; Jiang, D.; Fu, Y.; Chen, T. Application of Trichoderma Hz36 and Hk37 as biocontrol agents against clubroot caused by Plasmodiophora brassicae. J. Fungi 2022, 8, 777. [Google Scholar] [CrossRef] [PubMed]
- Struck, C.; Rüsch, S.; Strehlow, B. Control strategies of clubroot disease caused by Plasmodiophora brassicae. Microorganisms 2022, 10, 620. [Google Scholar] [CrossRef] [PubMed]
- Ludwig-Müller, J. What can we learn from-omics approaches to understand clubroot disease. Int. J. Mol. Sci. 2022, 23, 6293. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.; Zhang, X.; Ma, Y.; Wang, Y.; Zhan, Z.; Piao, Z. Fine mapping and candidate gene analysis of CRA3. 7 conferring clubroot resistance in Brassica rapa. Theor. Appl. Genet. 2022, 135, 4541–4548. [Google Scholar] [CrossRef] [PubMed]
- Liégard, B.; Baillet, V.; Etcheverry, M.; Joseph, E.; Lariagon, C.; Lemoine, J.; Evrard, A.; Colot, V.; Gravot, A.; Manzanares-Dauleux, M.J.; et al. Quantitative resistance to clubroot infection mediated by transgenerational epigenetic variation in Arabidopsis. New Phytol. 2019, 222, 468–479. [Google Scholar] [CrossRef]
- Ce, F.; Mei, J.; He, H.; Zhao, Y.; Hu, W.; Yu, F.; Li, Q.; Ren, X.; Si, J.; Song, H.; et al. Identification of candidate genes for clubroot-resistance in Brassica oleracea using quantitative trait loci-sequencing. Front. Plant Sci. 2021, 12, 703520. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wang, X.; Xiao, Y.; Wei, S.; Wang, D.; Huang, Y.; Wang, W.; Yang, H. Specific genes identified in Pathotype 4 of the Clubroot pathogen Plasmodiophora brassicae. Plant Dis. 2019, 103, 495–503. [Google Scholar] [CrossRef]
- Askarian, H.; Akhavan, A.; González, L.G.; Hwang, S.F.; Strelkov, S.E. Genetic structure of Plasmodiophora brassicae populations virulent on clubroot resistant canola (Brassica napus). Plant Dis. 2021, 105, 3694–3704. [Google Scholar] [CrossRef]
- Pang, W.; Liang, Y.; Zhan, Z.; Li, X.; Piao, Z. Development of a sinitic clubroot differential set for the pathotype classification of Plasmodiophora brassicae. Front. Plant Sci. 2020, 11, 568771. [Google Scholar] [CrossRef]
- Kamei, A.; Tsuro, M.; Kubo, N.; Hayashi, T.; Wang, N.; Fujimura, T.; Hirai, M. QTL mapping of clubroot resistance in radish (Raphanus sativus L.). Theor. Appl. Genet. 2010, 120, 1021–1027. [Google Scholar] [CrossRef]
- Strelkov, S.; Tewari, J.; Smith-Degenhardt, E. Characterization of Plasmodiophora brassicae populations from Alberta, Canada. Can. J. Plant Pathol. 2006, 28, 467–474. [Google Scholar] [CrossRef]
- Murray, M.; Thompson, W. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4326. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.X.; Cui, L.; Zhang, X.Z.; Pang, W.X.; Yu, X.Q.; Deng, X.H.; Wang, F.; Dai, Z.Y.; Yao, M.H.; Pu, Z.Y.; et al. Development of Linkage Markers to Clubroot Resistance in Radish. Mol. Plant Breed. 2020, 20, 3692–3697. (In Chinese) [Google Scholar] [CrossRef]
- Yang, H.; Yuan, Y.; Wei, X.; Zhang, X.; Wang, H.; Song, J.; Li, X. A new identification method reveals the resistance of an extensive-source radish collection to Plasmodiophora brassicae Race 4. Agronomy 2021, 11, 792. [Google Scholar] [CrossRef]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Kopec, P.M.; Mikolajczyk, K.; Jajor, E.; Perek, A.; Nowakowska, J.; Obermeier, C.; Chawla, H.S.; Korbas, M.; Bartkowiak-Broda, I.; Karlowski, W.M.; et al. Local duplication of TIR-NBS-LRR gene marks clubroot resistance in Brassica napus cv. Tosca. Front. Plant Sci. 2021, 12, 639631. [Google Scholar] [CrossRef]
- Gururani, M.A.; Venkatesh, J.; Upadhyaya, C.P.; Nookaraju, A.; Pandey, S.K.; Park, S.W. Plant disease resistance genes: Current status and future directions. Physiol. Mol. Plant Pathol. 2012, 78, 51–65. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.; Wang, J.; Wang, P.; Qiu, Y.; Zhao, W.; Pang, S.; Li, X.; Wang, H.; Song, J.; et al. Pan-genome of Raphanus highlights genetic variation and introgression among domesticated, wild, and weedy radishes. Mol. Plant 2021, 14, 2032–2055. [Google Scholar] [CrossRef]
- Zhan, Z.; Jiang, Y.; Shah, N.; Hou, Z.; Zhou, Y.; Dun, B.; Li, S.; Zhu, L.; Li, Z.; Piao, Z.; et al. Association of clubroot resistance locus PbBa8.1 with a linkage drag of high erucic acid content in the seed of the European turnip. Front. Plant Sci. 2020, 11, 810. [Google Scholar] [CrossRef]
- Zhu, M.; Yang, L.; Zhang, Y.; Zhuang, M.; Ji, J.; Hou, X.; Li, Z.; Han, F.; Fang, Z.; Lv, H.; et al. Introgression of clubroot resistant gene into Brassica oleracea L. from Brassica rapa based on homoeologous exchange. Hortic. Res. 2022, 9, uhac195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S.; Su, W.; Liu, Y.; Wang, H.P.; Song, J.P.; Yang, W.L.; Jia, H.X.; Zhang, X.H.; Li, X.X. Germplasm Innovation and Black Rot Resistance Transferring in Brassica rapa ssp. chinensis Through Interspecific Hybridization with Brassica carinata. Acta Horticult. Sin. 2021, 48, 1304–1316. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Liu, Z.; Zhao, W.; Zhang, X.; Song, J.; Jia, H.; Yang, W.; Ma, Y.; Wang, Y.; et al. Characterization and acceleration of genome shuffling and ploidy reduction in synthetic allopolyploids by genome sequencing and editing. Nucleic Acids Res. 2023, 51, 198–217. [Google Scholar] [CrossRef] [PubMed]






| Source | Total Resources | R | HR | Source | Total Resources | R | HR |
|---|---|---|---|---|---|---|---|
| Shandong | 28 | 1 | Guangdong | 4 | 4 | ||
| Henan | 23 | 3 | Guizhou | 4 | 0 | ||
| Gansu | 22 | 2 | Taiwan | 4 | 0 | ||
| Sichuan | 22 | 3 | 3 | Tianjin | 4 | 0 | |
| Jiangsu | 15 | 5 | Liaoning | 3 | 0 | ||
| Shanxi | 15 | 1 | Shanghai | 3 | 1 | ||
| Hubei | 14 | 4 | Guangxi | 2 | 0 | ||
| Hebei | 12 | 1 | Heilongjiang | 2 | 0 | ||
| Shaanxi | 11 | 2 | Jiangxi | 2 | 2 | ||
| Anhui | 11 | 0 | Beijing | 1 | 0 | ||
| Xinjiang | 11 | 1 | Hainan | 1 | 1 | ||
| Zhejiang | 11 | 5 | Xizang | 1 | 0 | ||
| Yunnan | 8 | 3 | Russia | 3 | 1 | 2 | |
| Hunan | 7 | 3 | North Korea | 1 | 0 | ||
| Neinenggu | 7 | 1 | Germany | 1 | 0 | 1 | |
| Ningxia | 8 | 1 | France | 1 | 0 | ||
| Fujian | 6 | 4 | South Korea | 1 | 0 | ||
| Qinghai | 6 | 1 | Total | 275 | 50 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Wang, H.; Song, J.; Yang, W.; Jia, H.; Agerbirk, N.; Chen, Y.; Li, C.; Piao, Y.; Li, S.; et al. Identification of Clubroot-Resistant Germplasm in a Radish (Raphanus sativus L.) Core Collection. Agronomy 2024, 14, 157. https://doi.org/10.3390/agronomy14010157
Ma Y, Wang H, Song J, Yang W, Jia H, Agerbirk N, Chen Y, Li C, Piao Y, Li S, et al. Identification of Clubroot-Resistant Germplasm in a Radish (Raphanus sativus L.) Core Collection. Agronomy. 2024; 14(1):157. https://doi.org/10.3390/agronomy14010157
Chicago/Turabian StyleMa, Yang, Haiping Wang, Jiangping Song, Wenlong Yang, Huixia Jia, Niels Agerbirk, Yinan Chen, Chen Li, Yinglan Piao, Sen Li, and et al. 2024. "Identification of Clubroot-Resistant Germplasm in a Radish (Raphanus sativus L.) Core Collection" Agronomy 14, no. 1: 157. https://doi.org/10.3390/agronomy14010157
APA StyleMa, Y., Wang, H., Song, J., Yang, W., Jia, H., Agerbirk, N., Chen, Y., Li, C., Piao, Y., Li, S., & Zhang, X. (2024). Identification of Clubroot-Resistant Germplasm in a Radish (Raphanus sativus L.) Core Collection. Agronomy, 14(1), 157. https://doi.org/10.3390/agronomy14010157








