Identification and Functional Analysis of PR Genes in Leaves from Variegated Tea Plant (Camellia sinensis)
Abstract
1. Introduction
2. Materials and Methods
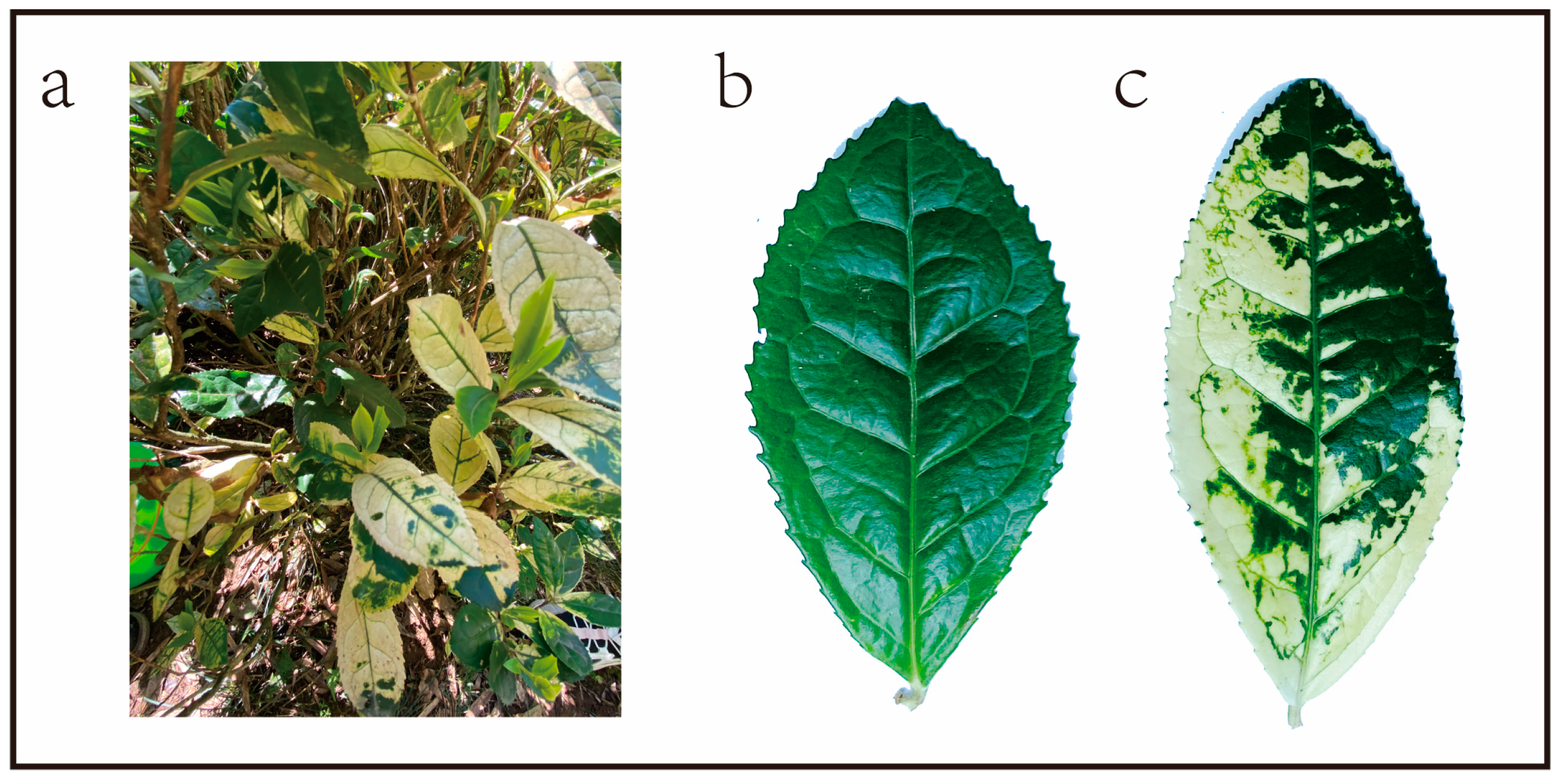
2.1. Plant Material
2.2. Identification of CsPR1, CsPR4, and CsPR5 Genes
2.3. Physicochemical Characterization of CsPRs
2.4. Promoter Analysis
2.5. Phylogenetic Analysis of CsPR1, CsPR4, and CsPR5
2.6. RNA Isolation and Real-Time Quantitative Polymerase Chain Reaction
2.7. Expression and Purification of Recombinant CsPRs
2.8. Statistical Data Analysis
3. Results
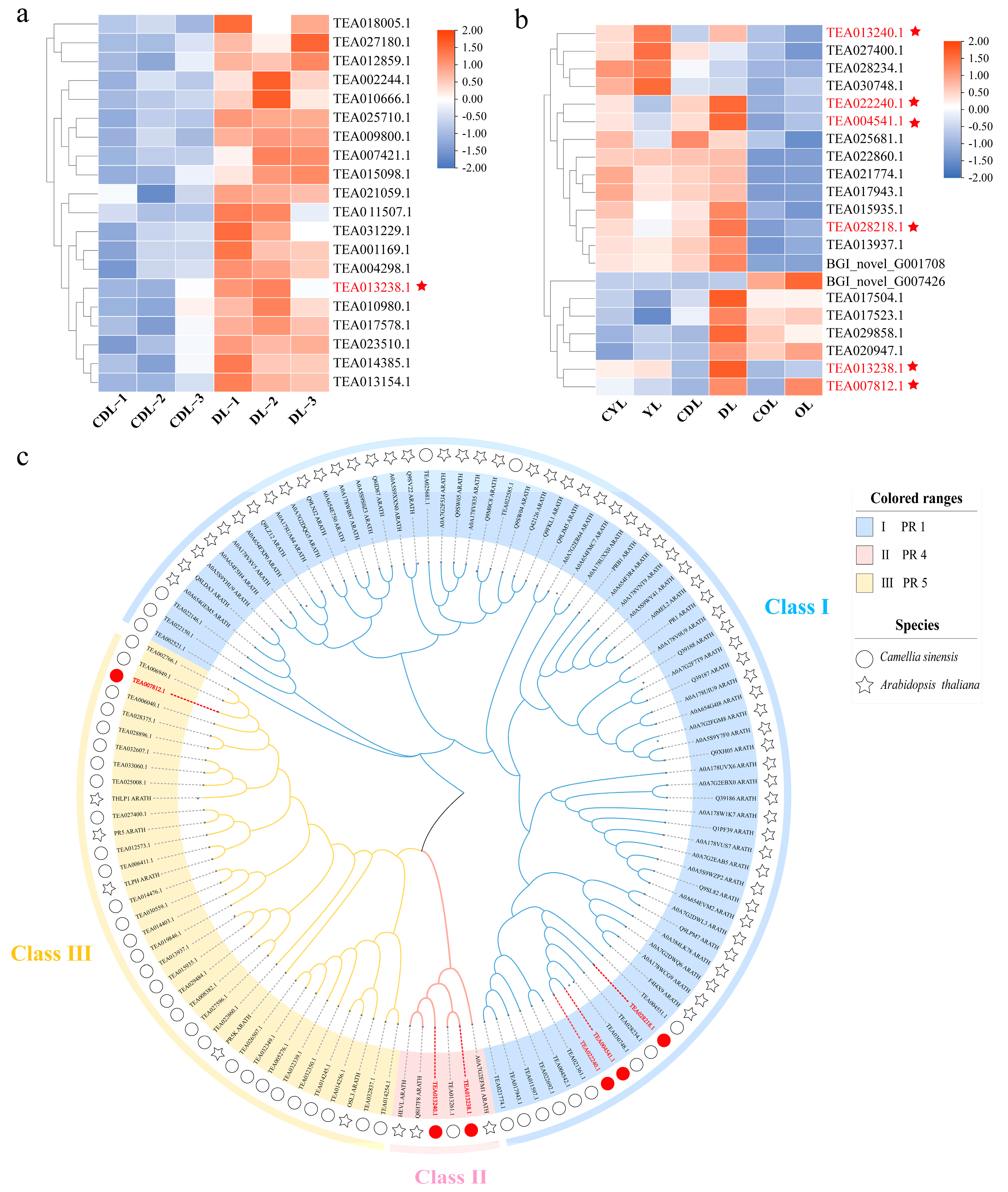
3.1. Proteomic and Transcriptomic Analyses
3.2. Identification of the PR1, PR4, and PR5 Gene Family Members in C. sinensis
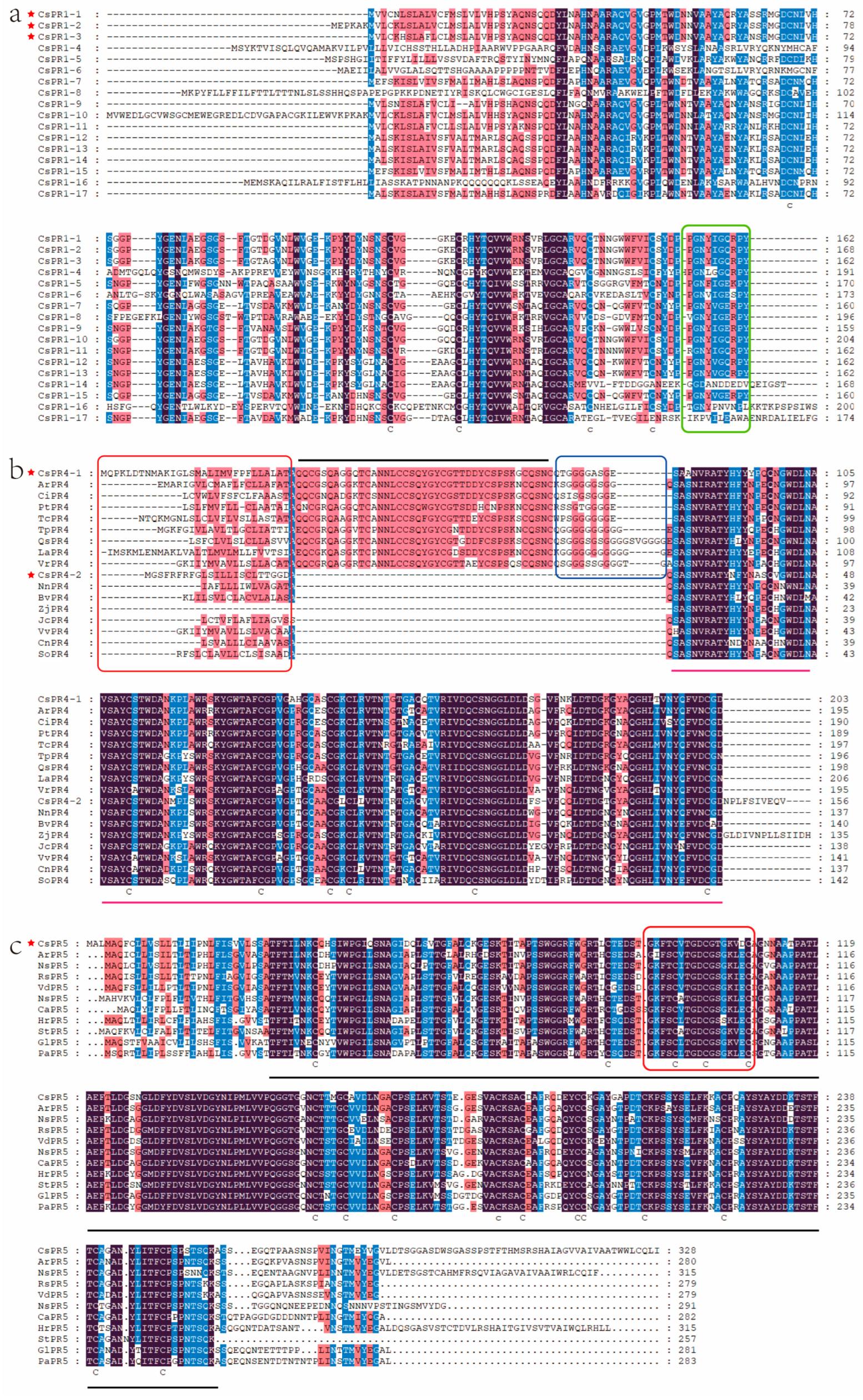
3.3. Evolutionary Relationships and Sequence Alignment of PR1, PR4, and PR5 Gene Family Members in C. sinensis
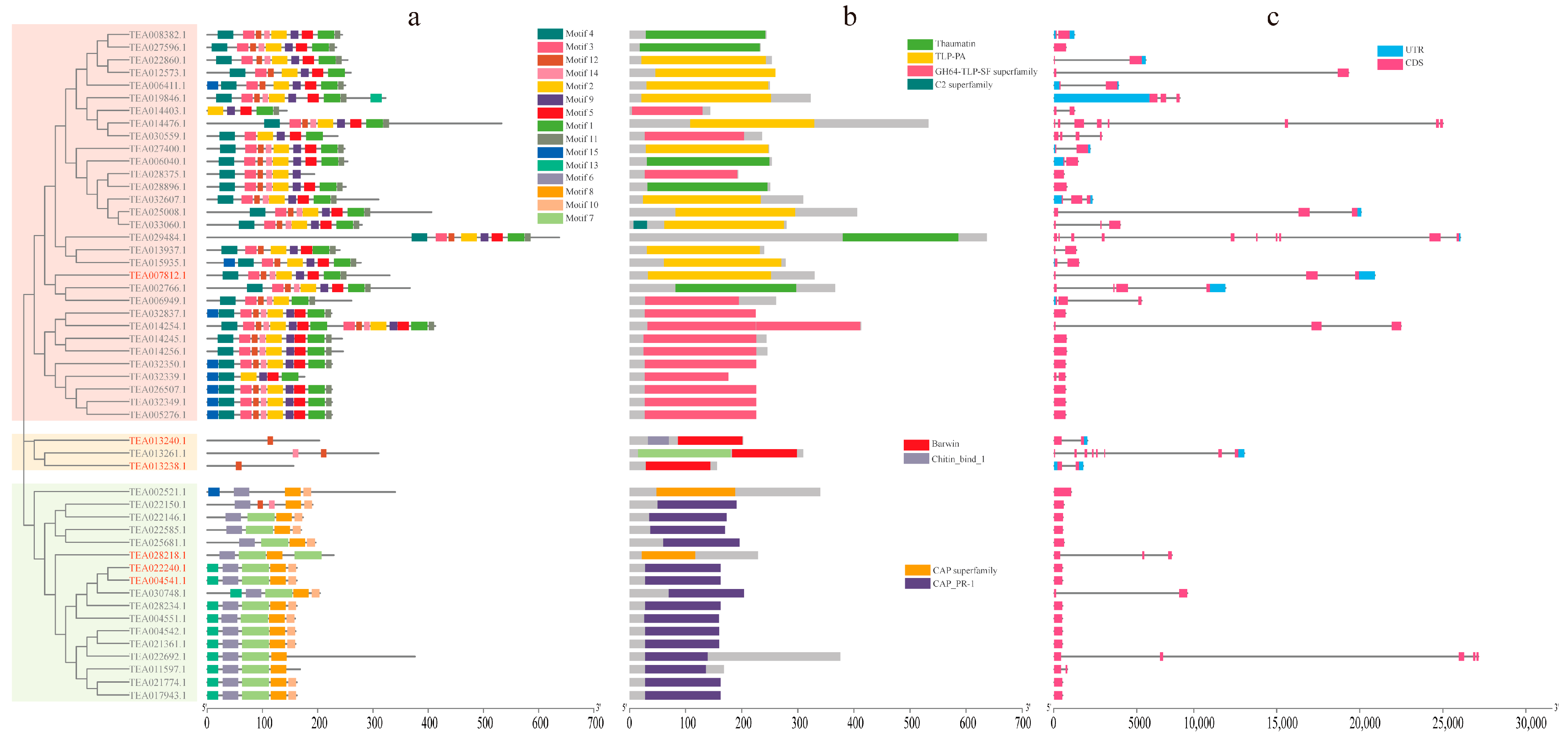
3.4. Gene Structures and Conserved Protein Motifs of CsPRs
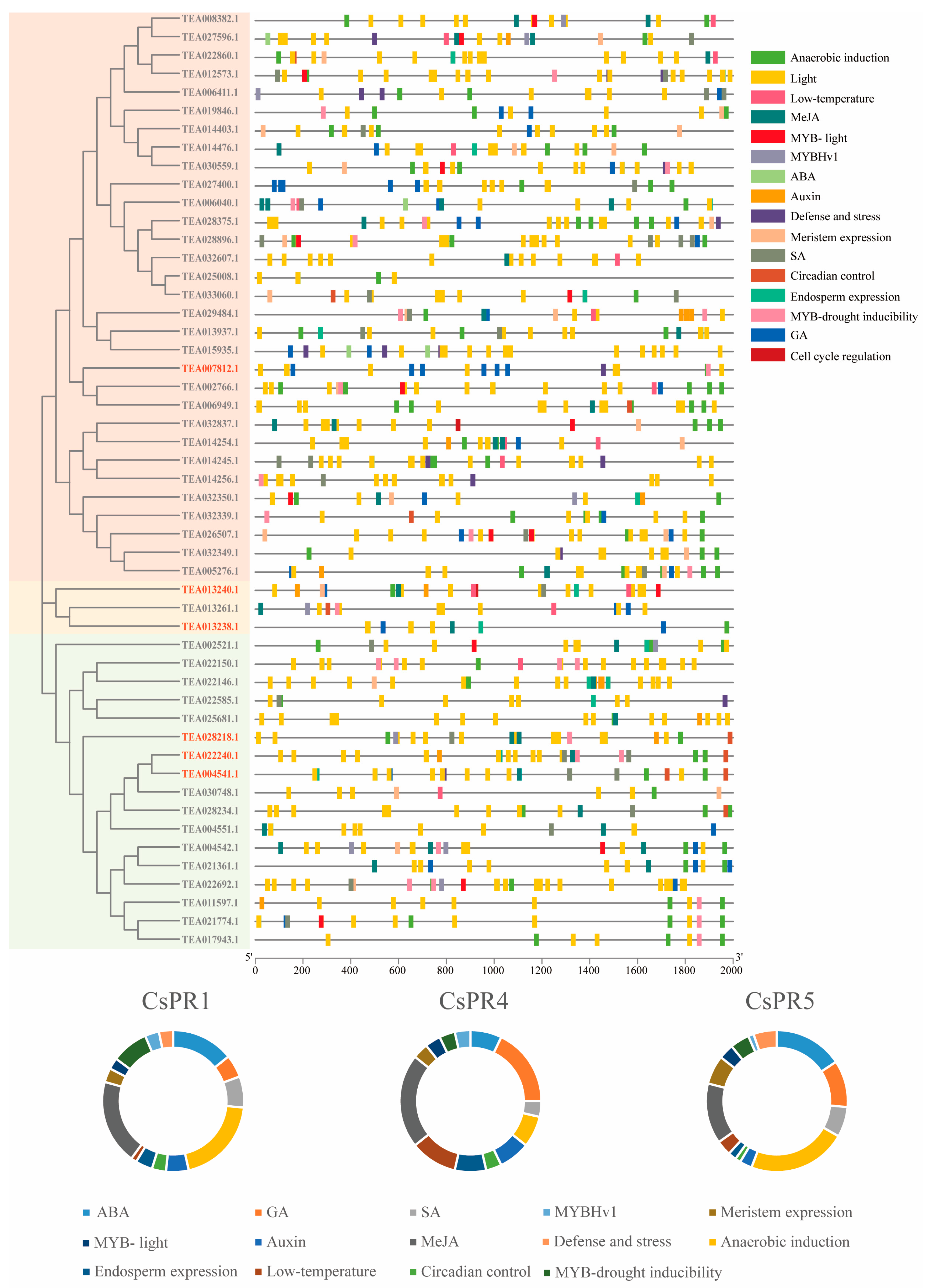
3.5. Analysis of Cis Elements of the CsPR Genes
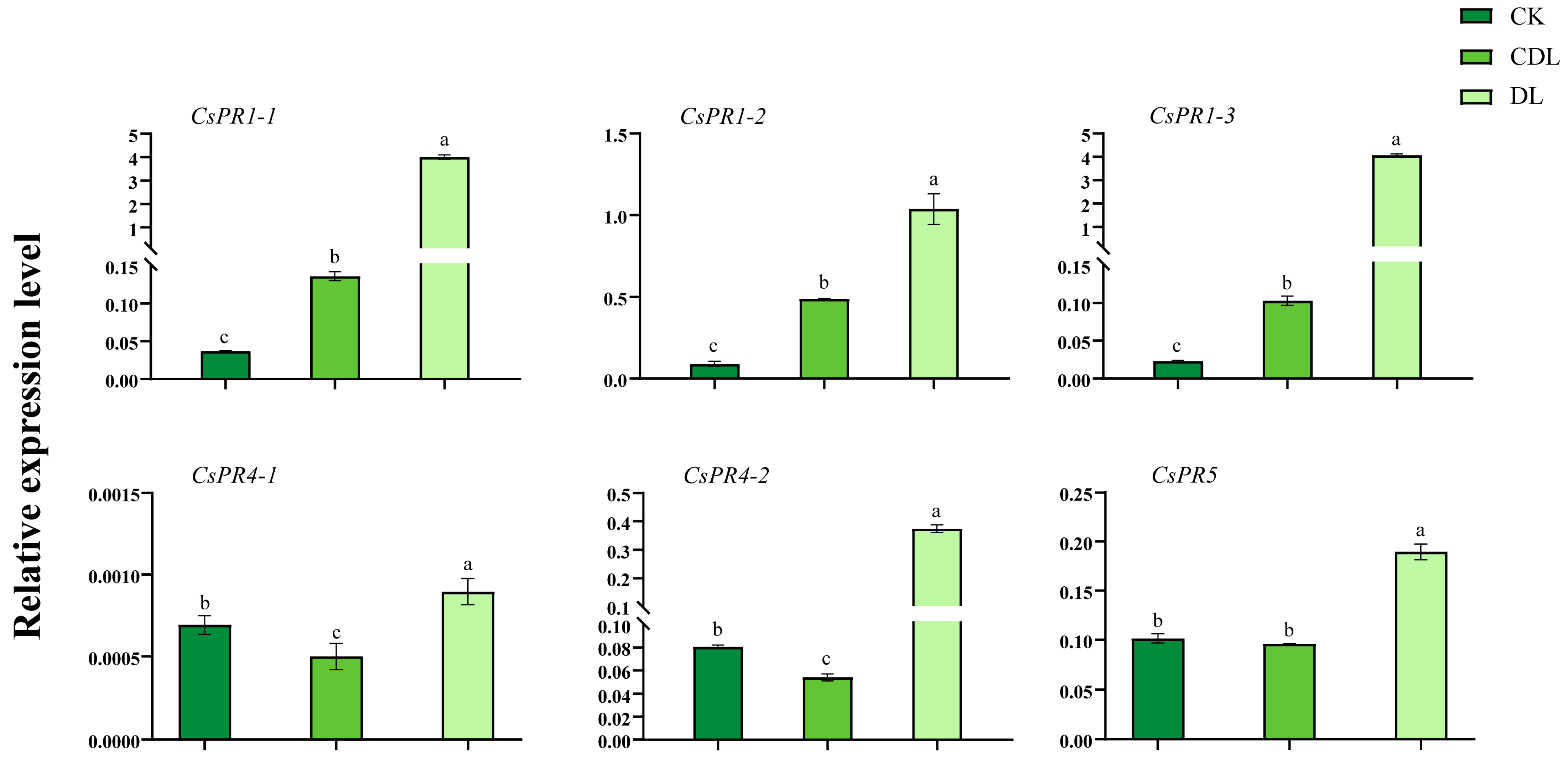
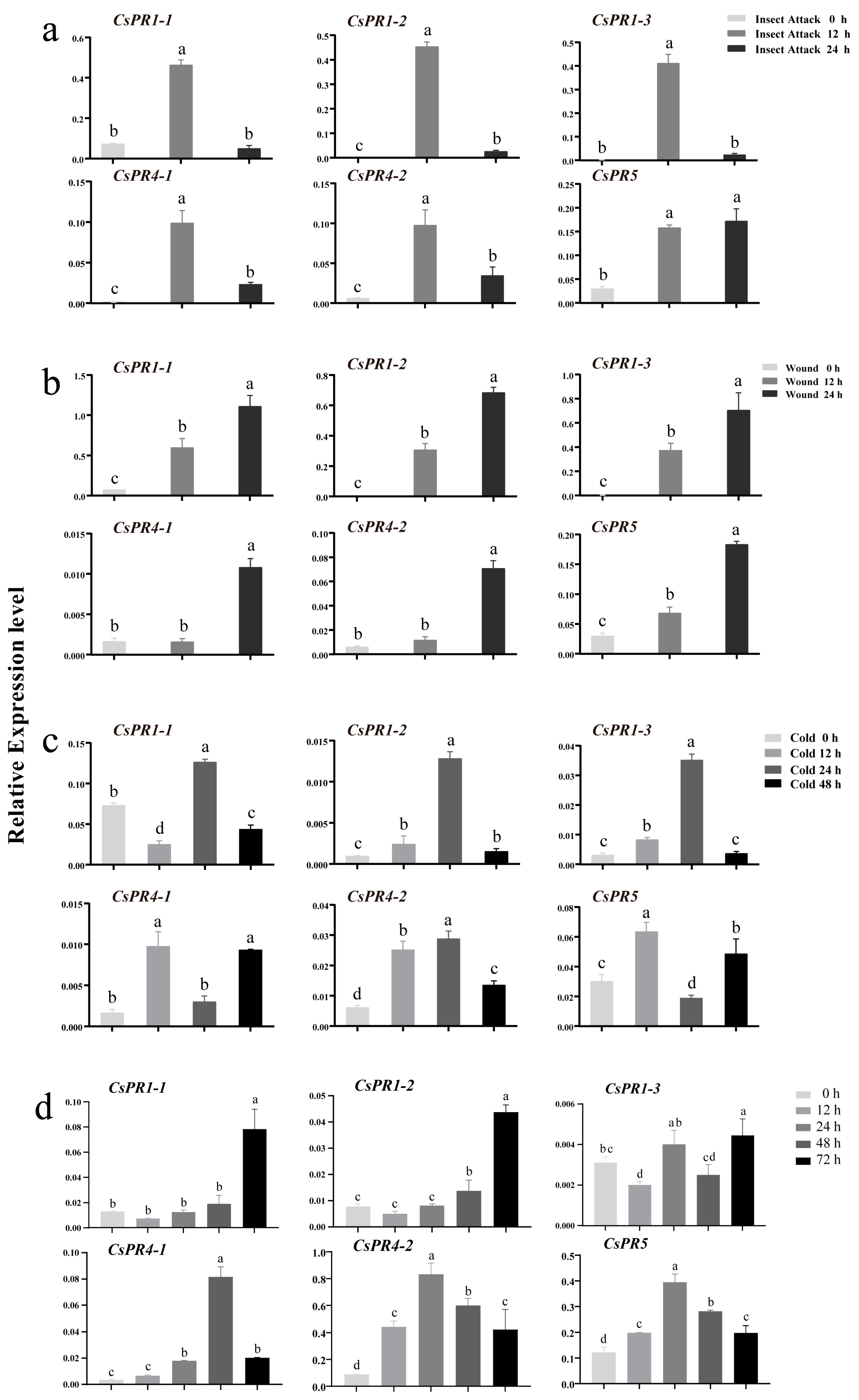
3.6. CsPR Gene Expression in Variegated Tea Leaves and Expression Profiles under Biotic and Abiotic Stresses
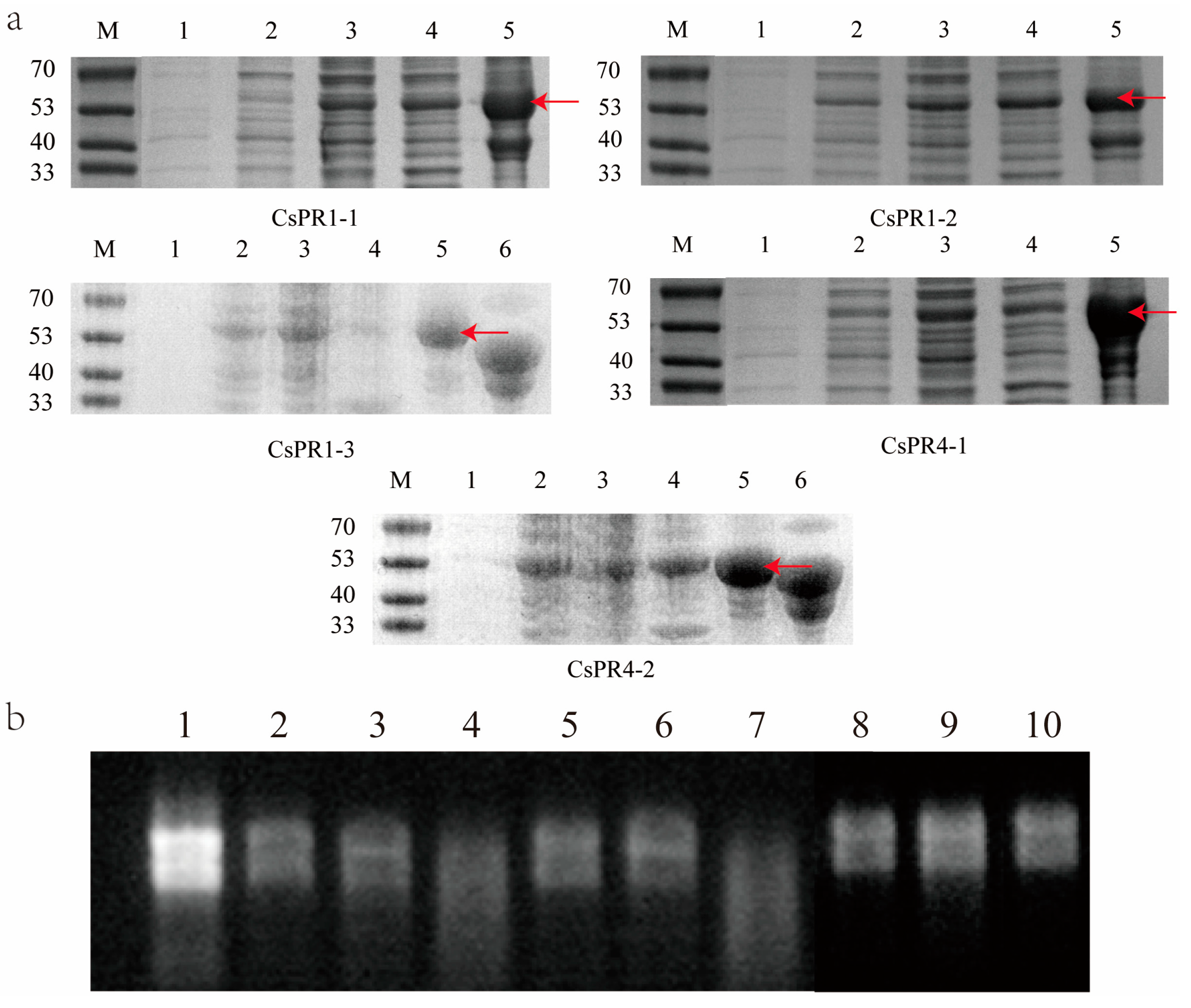
3.7. Expression and Ribonuclease Activity Determination of the Recombinant CsPR1, CsPR4, and CsPR5 Proteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ali, S.; Ganai, B.A.; Kamili, A.N.; Bhat, A.A.; Mir, Z.A.; Bhat, J.A.; Tyagi, A.; Islam, S.T.; Mushtaq, M.; Yadav, P. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol. Res. 2018, 212, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lee, H.J.; Jang, M.G.; Kwon, W.S.; Kim, S.Y.; Yang, D. Cloning and characterization of pathogenesis-related protein 4 gene from Panax ginseng. Russ. J. Plant Physiol. 2014, 61, 664–671. [Google Scholar] [CrossRef]
- Gaudet, D.A.; Laroche, A.; Frick, M.; Huel, R.; Puchalski, B. Cold induced expression of plant defensin and lipid transfer protein transcripts in winter wheat. Physiol. Plant. 2010, 117, 195–205. [Google Scholar] [CrossRef]
- Buchanan-Wollaston, V.; Earl, S.; Harrison, E.; Mathas, E.; Navabpour, S.; Page, T.; Pink, D. The molecular analysis of leaf senescence–a genomics approach. Plant Biotechnol. J. 2003, 1, 3–22. [Google Scholar] [CrossRef]
- van Loon, L.C.; Rep, M.; Pieterse, C.M. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef]
- Van Loon, L. Polyacrylamide disc electrophoresis of the soluble leaf proteins from Nicotiana tabacum var.‘Samsun’and ‘Samsun NN’: IV. Similarity of qualitative changes of specific proteins after infection with different viruses and their relationship to acquired resistance. Virology 1975, 67, 566–575. [Google Scholar] [PubMed]
- Van Loon, L.C. Occurrence and properties of plant pathogenesis-related proteins. In Pathogenesis-Related Proteins in Plants; CRC Press: Boca Raton, FL, USA, 1999; pp. 1–19. [Google Scholar]
- Sels, J.; Mathys, J.; De Coninck, B.M.; Cammue, B.P.; De Bolle, M.F. Plant pathogenesis-related (PR) proteins: A focus on PR peptides. Plant Physiol. Biochem. 2008, 46, 941–950. [Google Scholar] [CrossRef]
- Anisimova, O.K.; Shchennikova, A.V.; Kochieva, E.Z.; Filyushin, M.A. Pathogenesis-related genes of PR1, PR2, PR4, and PR5 families are involved in the response to Fusarium infection in garlic (Allium sativum L.). Int. J. Mol. Sci. 2021, 22, 6688. [Google Scholar] [CrossRef]
- AlHudaib, K.A.; Alanazi, N.A.; Ghorbel, M.; El-Ganainy, S.M.; Brini, F. Isolation and Characterization of a Novel Pathogenesis-Related Protein-1 Gene (AvPR-1) with Induced Expression in Oat (Avena sativa L.) during Abiotic and Hormonal Stresses. Plants 2022, 11, 2284. [Google Scholar] [CrossRef]
- Hejgaard, J.; Jacobsen, S.; Bjørn, S.E.; Kragh, K.M. Antifungal activity of chitin-binding PR-4 type proteins from barley grain and stressed leaf. FEBS Lett. 1992, 307, 389–392. [Google Scholar] [CrossRef]
- Van Damme, E.J.; Charels, D.; Roy, S.; Tierens, K.; Barre, A.; Martins, J.C.; Rougé, P.; Van Leuven, F.; Does, M.; Peumans, W.J. A gene encoding a hevein-like protein from elderberry fruits is homologous to PR-4 and class V chitinase genes. Plant Physiol. 1999, 119, 1547–1556. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pereira Menezes, S.; de Andrade Silva, E.M.; Matos Lima, E.; Oliveira de Sousa, A.; Silva Andrade, B.; Santos Lima Lemos, L.; Peres Gramacho, K.; da Silva Gesteira, A.; Pirovani, C.P.; Micheli, F. The pathogenesis-related protein PR-4b from Theobroma cacao presents RNase activity, Ca2+ and Mg2+ dependent-DNase activity and antifungal action on Moniliophthora perniciosa. BMC Plant Biol. 2014, 14, 161. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-C.; Lin, J.-H.; Chua, A.C.; Chung, T.-Y.; Tsai, I.-C.; Tzen, J.T.; Chou, W.-M. Cloning and expression of pathogenesis-related protein 4 from jelly fig (Ficus awkeotsang Makino) achenes associated with ribonuclease, chitinase and anti-fungal activities. Plant Physiol. Biochem. 2012, 56, 1–13. [Google Scholar] [CrossRef]
- van der Wel, H.; Loeve, K. Isolation and characterization of thaumatin I and II, the sweet-tasting proteins from Thaumatococcus daniellii Benth. Eur. J. Biochem. 1972, 31, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Kong, Q.; An, P.; Ren, X. The function and mechanism of pathogenesis-related 5 protein resistance in cherry tomato in response to Alternaria alternata. Food Biotechnol. 2018, 32, 178–190. [Google Scholar] [CrossRef]
- Muoki, R.C.; Paul, A.; Kaachra, A.; Kumar, S. Membrane localized thaumatin-like protein from tea (CsTLP) enhanced seed yield and the plant survival under drought stress in Arabidopsis thaliana. Plant Physiol. Biochem. 2021, 163, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, M.; Liang, N.; Yan, H.; Wei, Y.; Xu, X.; Liu, J.; Xu, Z.; Chen, F.; Wu, G. Molecular cloning and function analysis of the stay green gene in rice. Plant J. 2007, 52, 197–209. [Google Scholar] [CrossRef]
- Næsted, H.; Holm, A.; Jenkins, T.; Nielsen, H.B.; Harris, C.A.; Beale, M.H.; Andersen, M.; Mant, A.; Scheller, H.; Camara, B. Arabidopsis VARIEGATED 3 encodes a chloroplast-targeted, zinc-finger protein required for chloroplast and palisade cell development. J. Cell Sci. 2004, 117, 4807–4818. [Google Scholar] [CrossRef] [PubMed]
- Asakura, Y.; Hirohashi, T.; Kikuchi, S.; Belcher, S.; Osborne, E.; Yano, S.; Terashima, I.; Barkan, A.; Nakai, M. Maize mutants lacking chloroplast FtsY exhibit pleiotropic defects in the biogenesis of thylakoid membranes. Plant Cell 2004, 16, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Abbo, S.; Berger, J.; Turner, N.C. Evolution of cultivated chickpea: Four bottlenecks limit diversity and constrain adaptation. Funct. Plant Biol. 2003, 30, 1081–1087. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, N.; Zhang, Y.; Yu, Y.; Liu, S. Genome-wide characterization and expression analysis of pathogenesis-related 1 (PR-1) gene family in tea plant (Camellia sinensis (L.) O. Kuntze) in response to blister-blight disease stress. Int. J. Mol. Sci. 2022, 23, 1292. [Google Scholar] [CrossRef] [PubMed]
- Acharya, K.; Pal, A.K.; Gulati, A.; Kumar, S.; Singh, A.K.; Ahuja, P.S. Overexpression of Camellia sinensis thaumatin-like protein, CsTLP in potato confers enhanced resistance to Macrophomina phaseolina and Phytophthora infestans infection. Mol. Biotechnol. 2013, 54, 609–622. [Google Scholar] [CrossRef]
- Lu, M.; Li, Y.; Jia, H.; Xi, Z.; Gao, Q.; Zhang, Z.-Z.; Deng, W.-W. Integrated proteomics and transcriptome analysis reveal a decreased catechins metabolism in variegated tea leaves. Sci. Hortic. 2022, 295, 110824. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, L.; Hou, Y.; Wang, P.; Yang, H.; Wei, C. Differential transcriptome analysis of leaves of tea plant(Camellia sinensis) provides comprehensive insights into the defense responses to Ectropis oblique attack using RNA-Seq. Funct. Integr. Genom. 2016, 16, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ye, T.; Sun, Q.; Niu, T.; Zhang, J. Arbuscular mycorrhizal fungus alleviates anthracnose disease in tea seedlings. Front. Plant Sci. 2023, 13, 1058092. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Derbyshire, M.K.; Gonzales, N.R.; Lu, S.; Chitsaz, F.; Geer, L.Y.; Geer, R.C.; He, J.; Gwadz, M.; Hurwitz, D.I. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2015, 43, D222–D226. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Xia, E.; Tong, W.; Hou, Y.; An, Y.; Chen, L.; Wu, Q.; Liu, Y.; Yu, J.; Li, F.; Li, R. The reference genome of tea plant and resequencing of 81 diverse accessions provide insights into its genome evolution and adaptation. Mol. Plant 2020, 13, 1013–1026. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef] [PubMed]
- Chou, K.-C.; Shen, H.-B. Plant-mPLoc: A top-down strategy to augment the power for predicting plant protein subcellular localization. PLoS ONE 2010, 5, e11335. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, H.; Zhu, B.; Li, J.; Yang, T.; Zhang, Z.-Z.; Deng, W.-W. Molecular and biochemical characterization of jasmonic acid carboxyl methyltransferase involved in aroma compound production of methyl jasmonate during black tea processing. J. Agric. Food Chem. 2021, 69, 3154–3164. [Google Scholar] [CrossRef]
- Li, X.; Xia, B.; Jiang, Y.; Wu, Q.; Wang, C.; He, L.; Peng, F.; Wang, R. A new pathogenesis-related protein, LrPR4, from Lycoris radiata, and its antifungal activity against Magnaporthe grisea. Mol. Biol. Rep. 2010, 37, 995–1001. [Google Scholar] [CrossRef]
- Lu, S.; Friesen, T.L.; Faris, J.D. Molecular characterization and genomic mapping of the pathogenesis-related protein 1 (PR-1) gene family in hexaploid wheat (Triticum aestivum L.). Mol. Genet. Genom. 2011, 285, 485–503. [Google Scholar] [CrossRef]
- El-Kereamy, A.; El-Sharkawy, I.; Ramamoorthy, R.; Taheri, A.; Errampalli, D.; Kumar, P.; Jayasankar, S. Prunus domestica pathogenesis-related protein-5 activates the defense response pathway and enhances the resistance to fungal infection. PLoS ONE 2011, 6, e17973. [Google Scholar] [CrossRef]
| Protein Symbol | Length (aa) | MW (kD) | pI | SP | SL | GRAVY | Sequence ID |
|---|---|---|---|---|---|---|---|
| CsPR1−1 | 162 | 17.88 | 6.86 | Y | Vacuole | −0.297 | TEA022240.1 |
| CsPR1−2 | 168 | 18.53 | 8.10 | Y | Vacuole | −0.354 | TEA028218.1 |
| CsPR1−3 | 162 | 17.87 | 8.62 | Y | Vacuole | −0.345 | TEA004541.1 |
| CsPR1−4 | 191 | 21.53 | 9.16 | Y | Vacuole | −0.340 | TEA022150.1 |
| CsPR1−5 | 170 | 19.25 | 9.22 | Y | Vacuole | −0.375 | TEA022585.1 |
| CsPR1−6 | 173 | 18.60 | 6.81 | Y | Vacuole | −0.227 | TEA022146.1 |
| CsPR1−7 | 160 | 17.58 | 4.96 | Y | Vacuole | −0.235 | TEA004542.1 |
| CsPR1−8 | 376 | 41.14 | 4.88 | Y | Vacuole | −0.574 | TEA022692.1 |
| CsPR1−9 | 196 | 22.60 | 6.09 | Y | Vacuole | −0.479 | TEA025681.1 |
| CsPR1−10 | 159 | 17.52 | 8.47 | Y | Vacuole | −0.270 | TEA004551.1 |
| CsPR1−11 | 204 | 22.59 | 5.66 | N | Vacuole | −0.257 | TEA030748.1 |
| CsPR1−12 | 162 | 18.26 | 9.16 | Y | Vacuole | −0.399 | TEA028234.1 |
| CsPR1−13 | 162 | 17.93 | 8.74 | Y | Vacuole | −0.213 | TEA021774.1 |
| CsPR1−14 | 162 | 17.93 | 8.74 | Y | Vacuole | −0.213 | TEA017943.1 |
| CsPR1−15 | 168 | 18.08 | 4.90 | Y | Vacuole | −0.239 | TEA011597.1 |
| CsPR1−16 | 160 | 17.61 | 5.38 | Y | Vacuole | −0.269 | TEA021361.1 |
| CsPR1−17 | 340 | 38.28 | 10.07 | Y | Vacuole | −1.089 | TEA002521.1 |
| CsPR4−1 | 203 | 21.45 | 6.23 | Y | Vacuole | −0.300 | TEA013240.1 |
| CsPR4−2 | 156 | 16.82 | 4.53 | Y | Cell wall | 0.128 | TEA013238.1 |
| CsPR4−3 | 310 | 34.43 | 5.35 | N | Cytoplasm | −0.034 | TEA013261.1 |
| CsPR5−1 | 328 | 34.22 | 4.74 | N | Cytoplasm | 0.089 | TEA007812.1 |
| CsPR5−2 | 310 | 32.61 | 4.70 | Y | Cytoplasm | −0.073 | TEA032607.1 |
| CsPR5−3 | 144 | 15.79 | 5.22 | N | Cytoplasm | −0.062 | TEA014403.1 |
| CsPR5−4 | 533 | 58.55 | 6.85 | N | Cytoplasm | −0.020 | TEA014476.1 |
| CsPR5−5 | 244 | 25.74 | 6.05 | Y | Cytoplasm | 0.046 | TEA008382.1 |
| CsPR5−6 | 323 | 34.52 | 4.60 | Y | Cytoplasm | −0.011 | TEA019846.1 |
| CsPR5−7 | 226 | 24.17 | 4.75 | Y | Vacuole | −0.146 | TEA026507.1 |
| CsPR5−8 | 249 | 25.61 | 4.53 | Y | Cytoplasm | 0.019 | TEA027400.1 |
| CsPR5−9 | 194 | 20.46 | 8.62 | Y | Cytoplasm | 0.046 | TEA028375.1 |
| CsPR5−10 | 254 | 28.00 | 8.60 | N | Cytoplasm | −0.374 | TEA022860.1 |
| CsPR5−11 | 240 | 25.97 | 5.29 | Y | Cytoplasm | 0.053 | TEA013937.1 |
| CsPR5−12 | 250 | 27.03 | 9.00 | Y | Cytoplasm | 0.004 | TEA006411.1 |
| CsPR5−13 | 278 | 30.17 | 8.62 | N | Cytoplasm | −0.049 | TEA015935.1 |
| CsPR5−14 | 225 | 24.61 | 9.08 | Y | Vacuole | −0.312 | TEA032837.1 |
| CsPR5−15 | 367 | 38.73 | 4.63 | N | Cytoplasm | −0.187 | TEA002766.1 |
| CsPR5−16 | 251 | 26.57 | 5.75 | Y | Cytoplasm | 0.112 | TEA028896.1 |
| CsPR5−17 | 234 | 25.01 | 8.73 | N | Cytoplasm | −0.047 | TEA027596.1 |
| CsPR5−18 | 176 | 18.90 | 4.73 | Y | Cytoplasm | 0.069 | TEA032339.1 |
| CsPR5−19 | 226 | 24.19 | 4.65 | Y | Vacuolar | −0.152 | TEA032349.1 |
| CsPR5−20 | 226 | 24.61 | 5.09 | Y | Vacuolar | −0.304 | TEA032350.1 |
| CsPR5−21 | 236 | 25.74 | 5.14 | Y | Vacuolar | −0.005 | TEA030559.1 |
| CsPR5−22 | 406 | 41.93 | 4.32 | Y | Cytoplasm | −0.086 | TEA025008.1 |
| CsPR5−23 | 260 | 27.67 | 8.92 | N | Nuclear | −0.323 | TEA012573.1 |
| CsPR5−24 | 244 | 25.94 | 3.83 | Y | Vacuolar | −0.061 | TEA014245.1 |
| CsPR5−25 | 413 | 44.90 | 8.34 | N | cytoplasmic | −0.577 | TEA014254.1 |
| CsPR5−26 | 246 | 26.22 | 4.54 | Y | Vacuolar | −0.105 | TEA014256.1 |
| CsPR5−27 | 637 | 70.56 | 8.49 | Y | Vacuolar | 0.133 | TEA029484.1 |
| CsPR5−28 | 254 | 26.19 | 4.67 | Y | Vacuolar | 0.139 | TEA006040.1 |
| CsPR5−29 | 261 | 27.11 | 4.98 | Y | Vacuolar | 0.094 | TEA006949.1 |
| CsPR5−30 | 280 | 29.71 | 5.03 | N | Cytoplasmic | −0.371 | TEA033060.1 |
| CsPR5−31 | 226 | 24.14 | 4.97 | Y | Vacuolar | −0.140 | TEA005276.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, Z.; Jia, H.; Li, Y.; Ma, J.; Lu, M.; Wang, Z.; Kong, D.; Deng, W.-W. Identification and Functional Analysis of PR Genes in Leaves from Variegated Tea Plant (Camellia sinensis). Agronomy 2024, 14, 156. https://doi.org/10.3390/agronomy14010156
Xi Z, Jia H, Li Y, Ma J, Lu M, Wang Z, Kong D, Deng W-W. Identification and Functional Analysis of PR Genes in Leaves from Variegated Tea Plant (Camellia sinensis). Agronomy. 2024; 14(1):156. https://doi.org/10.3390/agronomy14010156
Chicago/Turabian StyleXi, Zuguo, Huiyan Jia, Yifan Li, Jinqing Ma, Mengqian Lu, Zhihui Wang, Dexu Kong, and Wei-Wei Deng. 2024. "Identification and Functional Analysis of PR Genes in Leaves from Variegated Tea Plant (Camellia sinensis)" Agronomy 14, no. 1: 156. https://doi.org/10.3390/agronomy14010156
APA StyleXi, Z., Jia, H., Li, Y., Ma, J., Lu, M., Wang, Z., Kong, D., & Deng, W.-W. (2024). Identification and Functional Analysis of PR Genes in Leaves from Variegated Tea Plant (Camellia sinensis). Agronomy, 14(1), 156. https://doi.org/10.3390/agronomy14010156






