Effects of Long-Term Straw Returning and Nitrogen Fertilizer Reduction on Soil Microbial Diversity in Black Soil in Northeast China
Abstract
1. Introduction
2. Materials and Methods
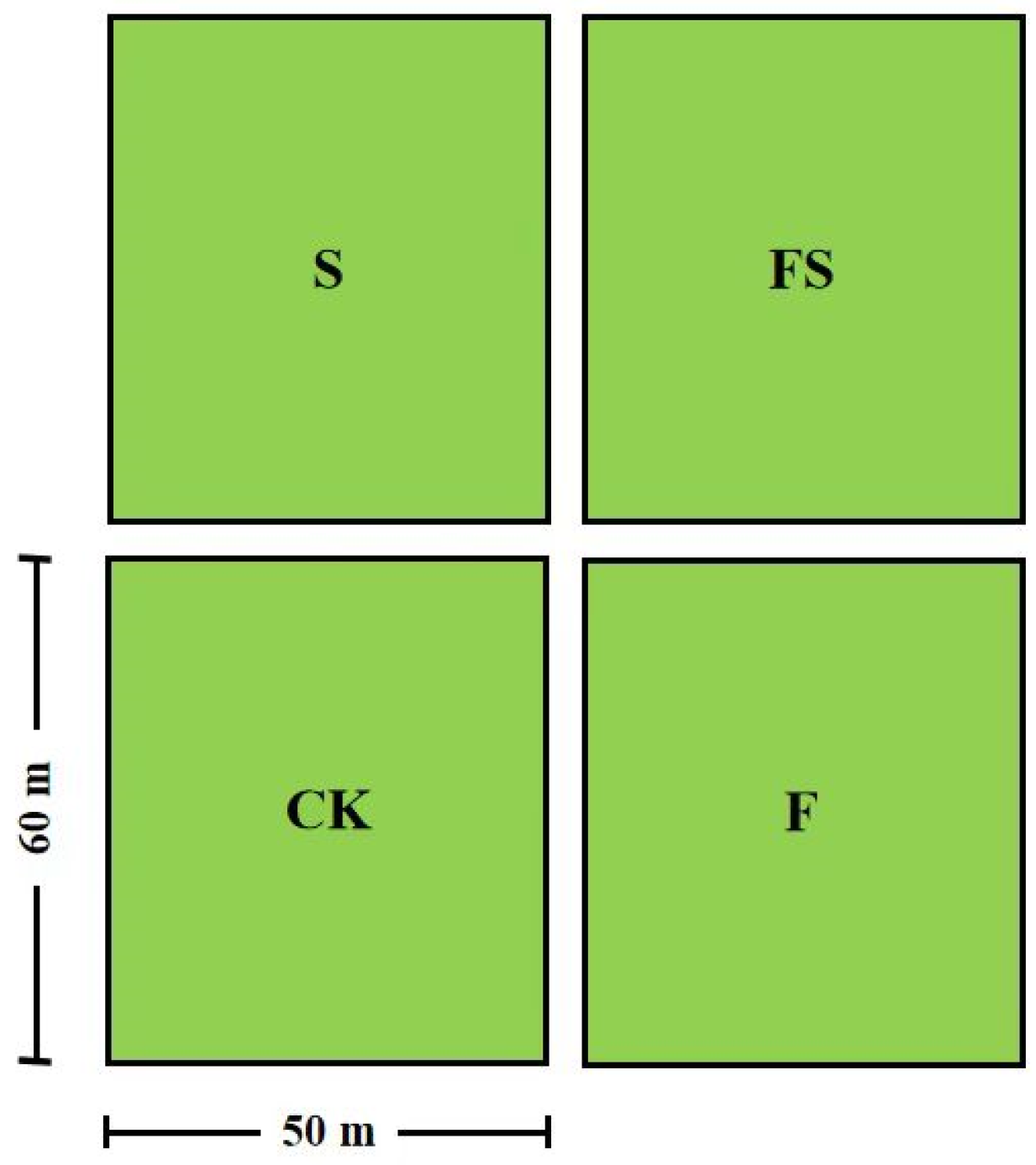
2.1. Experiment Site and Design
2.2. DNA Extraction and 16S rRNA Sequencing
2.3. Bioinformatics and Statistical Analysis
3. Results
3.1. Physicochemical Properties of the Soil Samples under Different Treatments
3.2. Alpha and Beta Diversity of Soil Bacteria
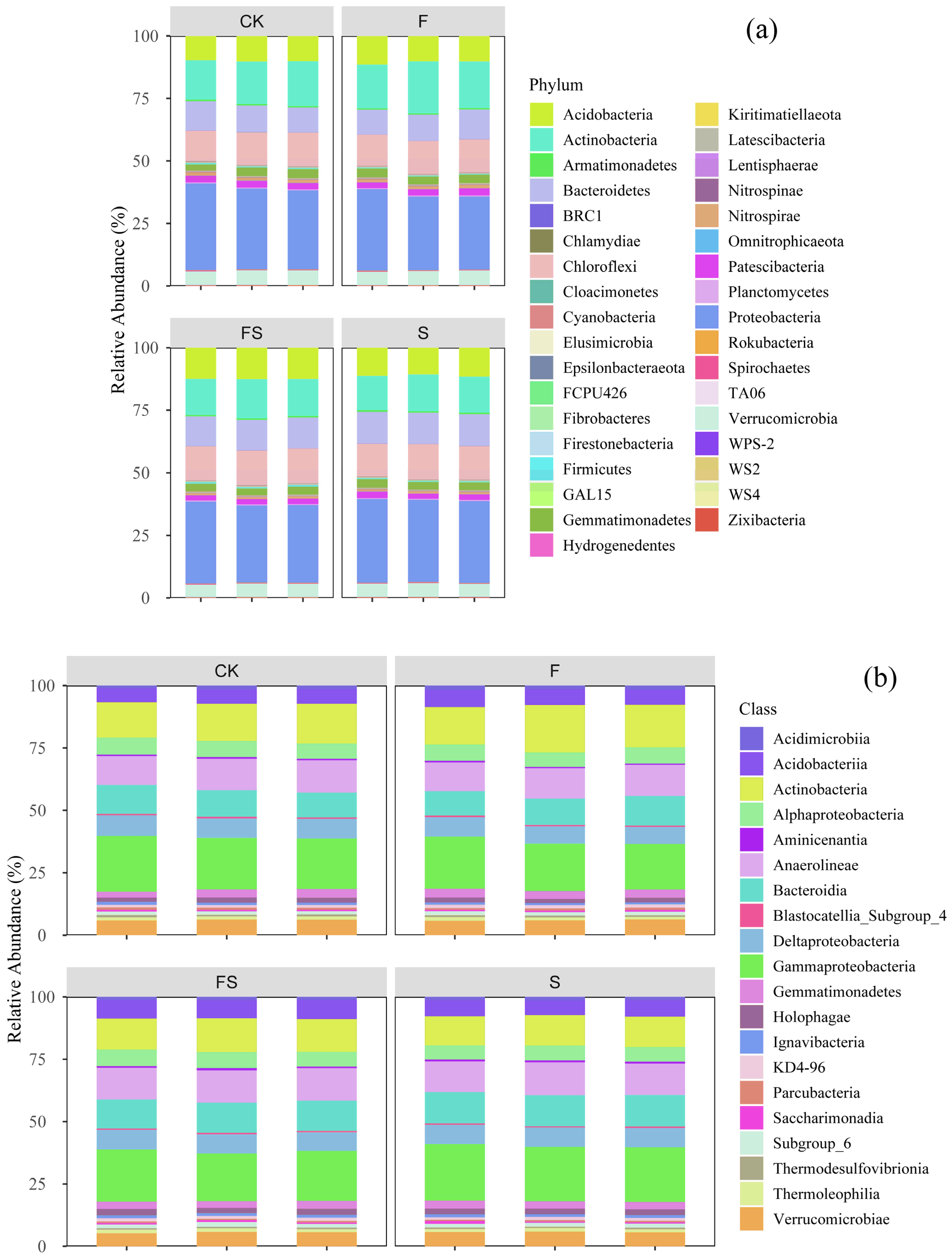
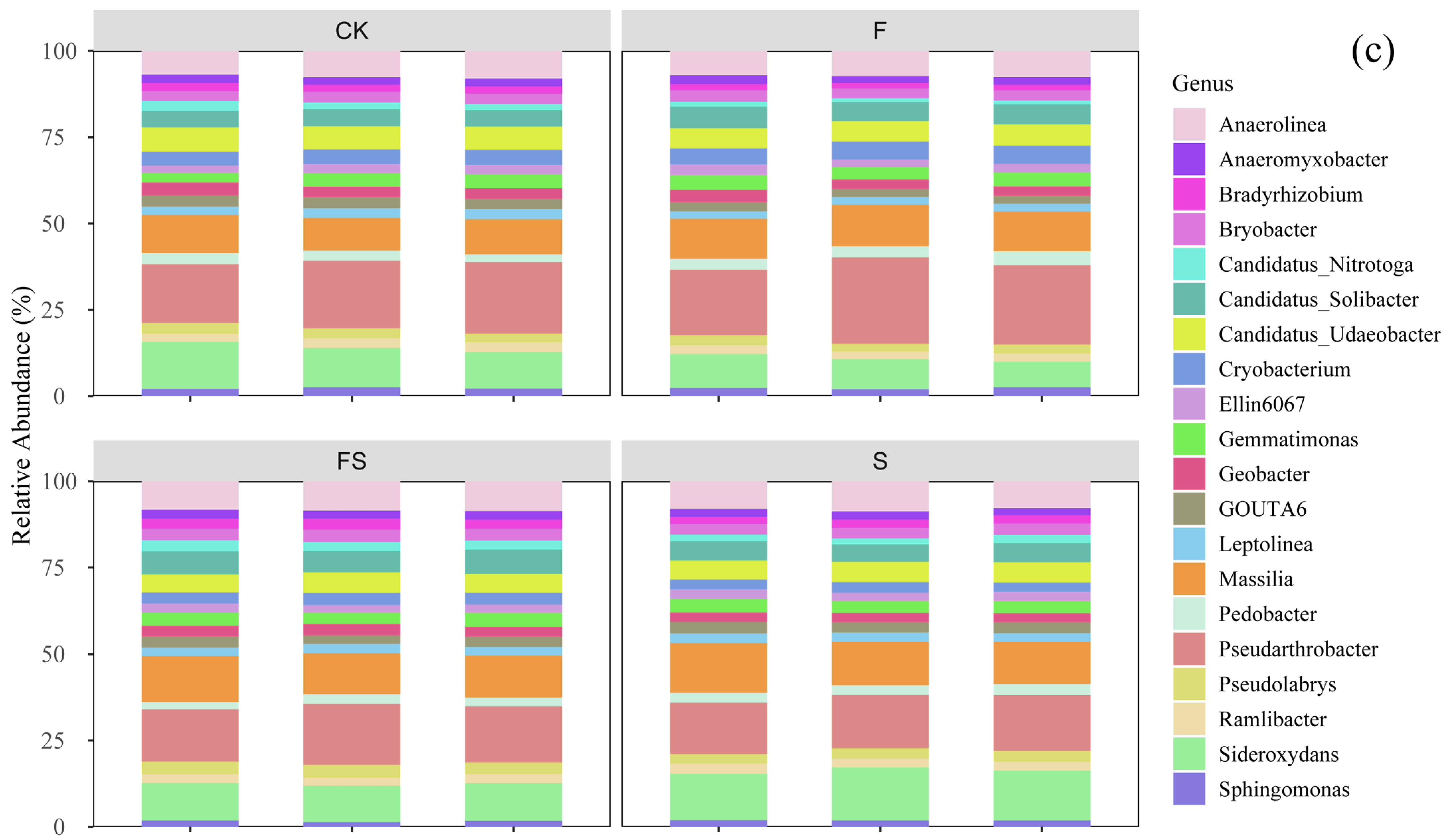
3.3. Community Structure of Soil Bacteria
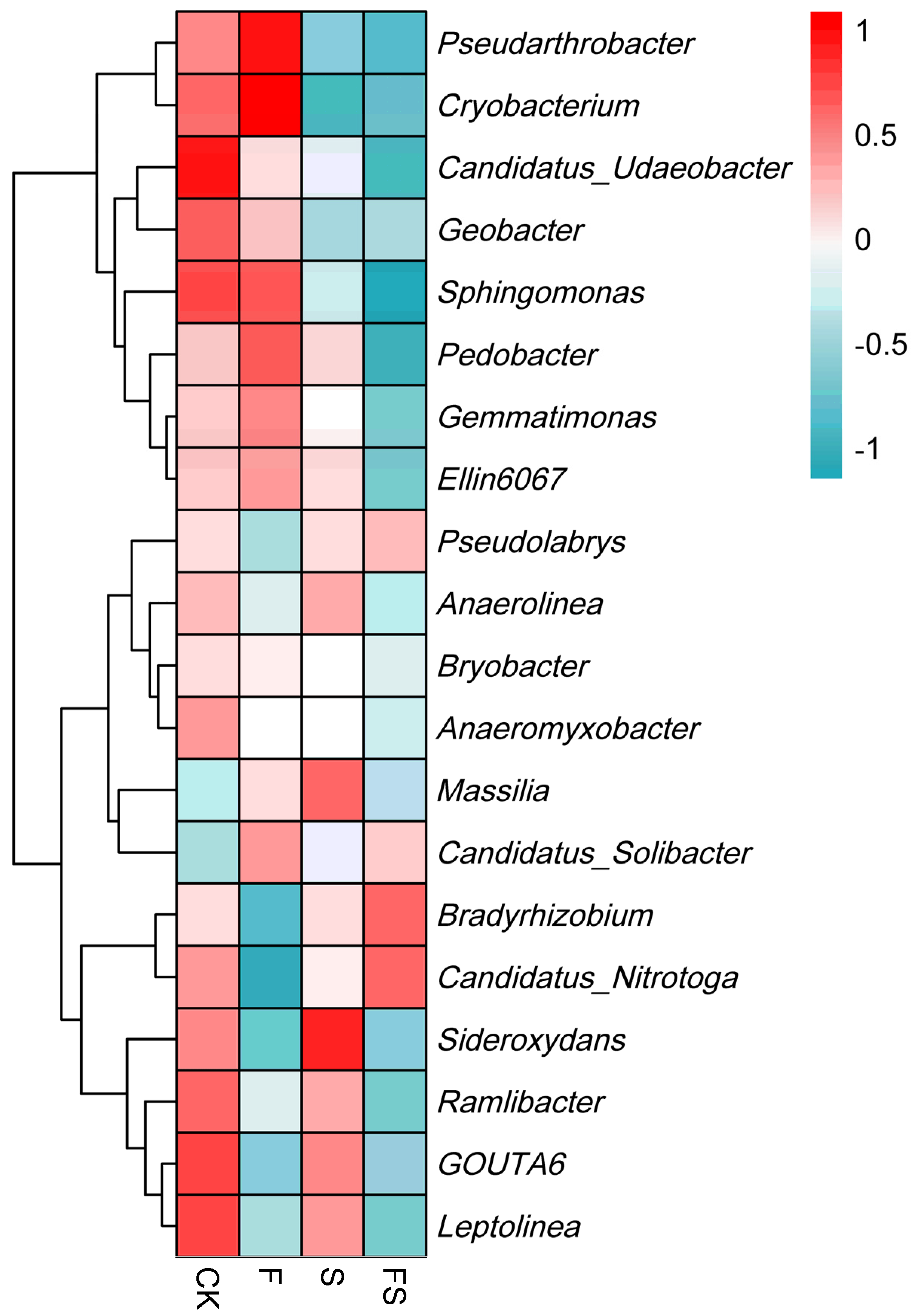
3.4. Indicator Species of Soil Bacteria
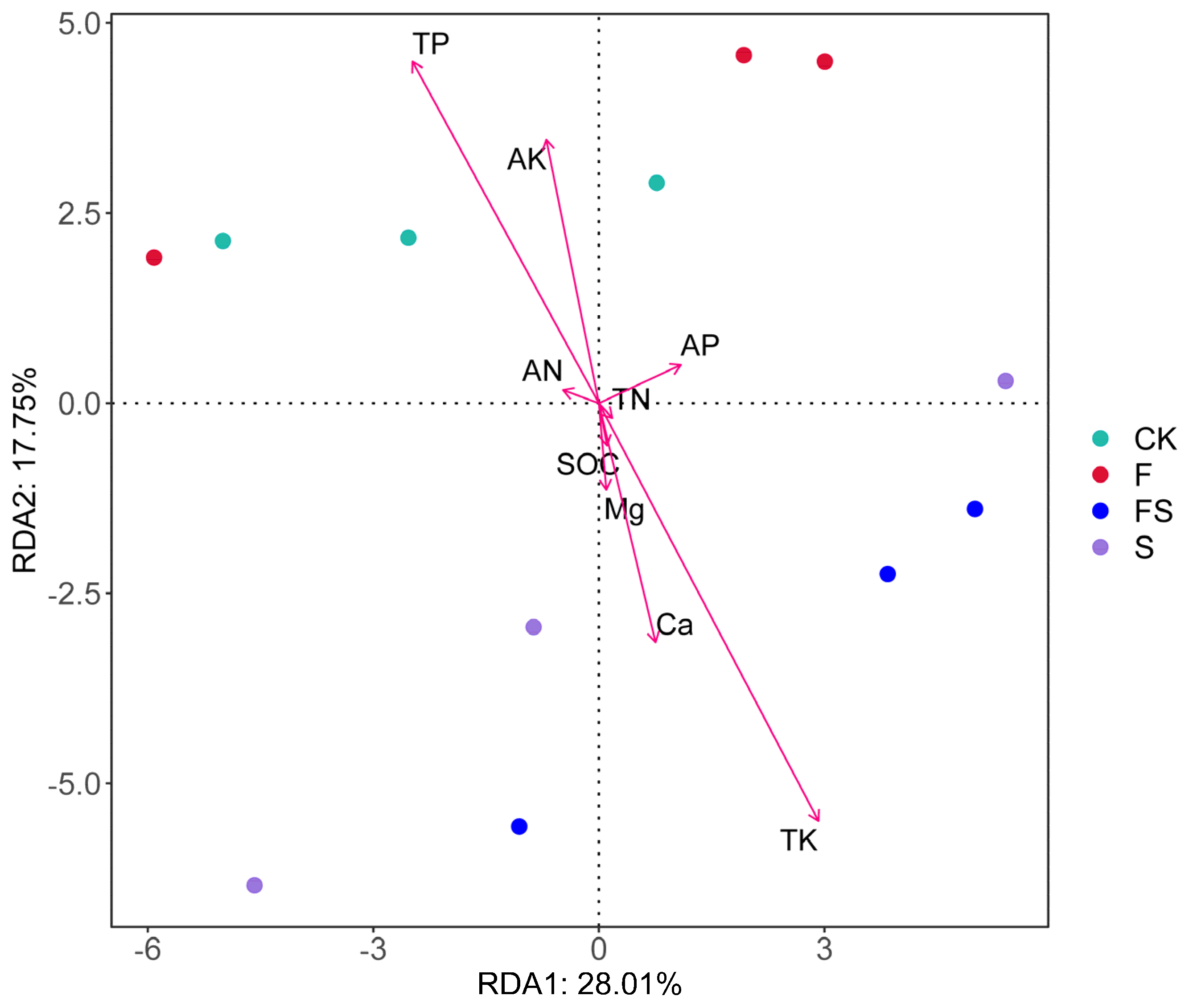
3.5. Effect of Soil Physicochemical Properties on Soil Bacterial Community Composition
3.6. Inferred Functionality of Soil Bacteria and Differences between the Experimental Treatments
4. Discussion
4.1. Changes in Soil Bacterial Community Diversity under Straw and Fertilizer Treatments
4.2. Driving Factors of Soil Bacterial Community Composition under Straw and Fertilizer Treatments
4.3. Changes of Soil Bacterial Functions between the Experimental Treatments
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ao, M.; Zhang, X.; Guan, Y. Research and Practice of Conservation Tillage in Black Soil Region of Northeast China. Bull. Chin. Acad. Sci. 2021, 36, 1203–1215. [Google Scholar]
- Zhou, M.; Liu, C.; Wang, J.; Meng, Q.; Yuan, Y.; Ma, X.; Liu, X.; Zhu, Y.; Ding, G.; Zhang, J.; et al. Soil aggregates stability and storage of soil organic carbon respond to cropping systems on Black Soils of Northeast China. Sci. Rep. 2020, 10, 265. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Wang, J.; Gao, J.; Zhu, P.; Cui, X.A.; Xu, M.; Zhou, B.; Lu, C. Estimation of soil organic carbon losses and counter approaches from organic materials in black soils of northeastern China. J. Soils Sediments 2020, 20, 1241–1252. [Google Scholar] [CrossRef]
- Rashid, M.; Hussain, Q.; Khan, K.S.; Alwabel, M.I.; Hayat, R.; Akmal, M.; Ijaz, S.S.; Alvi, S.; Rehman, O.U. Carbon-based slow-release fertilizers for efficient nutrient management: Synthesis, applications, and future research needs. J. Soil Sci. Plant Nutr. 2021, 21, 1144–1169. [Google Scholar] [CrossRef]
- Horrigan, L.; Lawrence, R.S.; Walker, P. How sustainable agriculture can address the environmental and human health harms of industrial agriculture. Environ. Health Perspect. 2002, 110, 445–456. [Google Scholar] [CrossRef]
- Berhane, M.; Xu, M.; Liang, Z.; Shi, J.; Wei, G.; Tian, X. Effects of long-term straw return on soil organic carbon storage and sequestration rate in North China upland crops: A meta-analysis. Glob. Chang. Biol. 2020, 26, 2686–2701. [Google Scholar] [CrossRef]
- Anning, D.K.; Qiu, H.; Zhang, C.; Ghanney, P.; Zhang, Y.; Guo, Y. Maize straw return and nitrogen rate effects on potato (Solanum tuberosum L.) performance and soil physicochemical characteristics in Northwest China. Sustainability 2021, 13, 5508. [Google Scholar] [CrossRef]
- Islam, M.U.; Guo, Z.; Jiang, F.; Peng, X. Does straw return increase crop yield in the wheat-maize cropping system in China? A meta-analysis. Field Crops Res. 2022, 279, 108447. [Google Scholar] [CrossRef]
- Zheng, Y.; Han, X.; Li, Y.; Yang, J.; Li, N.; An, N. Effects of biochar and straw application on the physicochemical and biological properties of paddy soils in northeast China. Sci. Rep. 2019, 9, 16531. [Google Scholar] [CrossRef]
- Halko, S.; Vershkov, O.; Horák, J.; Lezhenkin, O.; Boltianska, L.; Kucher, A.; Suprun, O.; Miroshnyk, O.; Nitsenko, V. Efficiency of combed straw harvesting technology involving straw decomposition in the soil. Agriculture 2023, 13, 655. [Google Scholar] [CrossRef]
- Abs, E.; Chase, A.B.; Allison, S.D. How do soil microbes shape ecosystem biogeochemistry in the context of global change? Environ. Microbiol. 2023, 25, 780–785. [Google Scholar] [CrossRef]
- Garaycochea, S.; Altier, N.A.; Leoni, C.; Neal, A.L.; Romero, H. Abundance and phylogenetic distribution of eight key enzymes of the phosphorus biogeochemical cycle in grassland soils. Environ. Microbiol. Rep. 2023, 1–18. [Google Scholar] [CrossRef]
- Bonilla, N.; Gutiérrez-Barranquero, J.A.; de Vicente, A.; Cazorla, F.M. Enhancing soil quality and plant health through suppressive organic amendments. Diversity 2012, 4, 475–491. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, J.; Liu, J.; Jin, J.; Liu, X.; Wang, G. Responses of ammonia-oxidizing bacterial communities to land-use and seasonal changes in Mollisols of Northeast China. Eur. J. Soil Biol. 2016, 74, 121–127. [Google Scholar] [CrossRef]
- Hao, M.; Hu, H.; Liu, Z.; Dong, Q.; Sun, K.; Feng, Y.; Ning, T. Shifts in microbial community and carbon sequestration in farmland soil under long-term conservation tillage and straw returning. Appl. Soil Ecol. 2019, 136, 43–54. [Google Scholar] [CrossRef]
- Su, Y.; Lv, J.L.; Yu, M.; Ma, Z.H.; Xi, H.; Kou, C.L.; He, Z.; Shen, A.L. Long-term decomposed straw return positively affects the soil microbial community. J. Appl. Microbiol. 2020, 128, 138–150. [Google Scholar] [CrossRef]
- Li, F.; Chen, L.; Zhang, J.; Yin, J.; Huang, S. Bacterial community structure after long-term organic and inorganic fertilization reveals important associations between soil nutrients and specific taxa involved in nutrient transformations. Front. Microbiol. 2017, 8, 187. [Google Scholar] [CrossRef]
- Zhao, S.; Qiu, S.; Xu, X.; Ciampitti, I.A.; Zhang, S.; He, P. Change in straw decomposition rate and soil microbial community composition after straw addition in different long-term fertilization soils. Appl. Soil Ecol. 2019, 138, 123–133. [Google Scholar] [CrossRef]
- Chen, Y.; Xin, L.; Liu, J.; Yuan, M.; Liu, S.; Jiang, W.; Chen, J. Changes in bacterial community of soil induced by long-term straw returning. Sci. Agric. 2017, 74, 349–356. [Google Scholar] [CrossRef]
- Shi, J.; Xu, F.; Wang, Z.; Stiverson, J.A.; Yu, Z.; Li, Y. Effects of microbial and non-microbial factors of liquid anaerobic digestion effluent as inoculum on solid-state anaerobic digestion of corn stover. Bioresour. Technol. 2014, 157, 188–196. [Google Scholar] [CrossRef]
- Jin, Z.; Shah, T.; Zhang, L.; Liu, H.; Peng, S.; Nie, L. Effect of straw returning on soil organic carbon in rice–wheat rotation system: A review. Food Energy Secur. 2020, 9, e200. [Google Scholar] [CrossRef]
- Ding, L.J.; Su, J.Q.; Sun, G.X.; Wu, J.S.; Wei, W.X. Increased microbial functional diversity under long-term organic and integrated fertilization in a paddy soil. Appl. Microbiol. Biotechnol. 2018, 102, 1969–1982. [Google Scholar] [CrossRef]
- Yeomans, J.C.; Bremner, J.M. A rapid and precise method for routine determination of organic carbon in soil. Commun. Soil Sci. Plant Anal. 1988, 19, 1467–1476. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, L.; Zuo, Y.; Liu, J.; Yu, J.; Yuan, F.; Wang, N.; He, L.; Wang, Y.; Guo, Z.; et al. Wetland reclamation homogenizes microbial properties along soil profiles. Geoderma 2021, 395, 115075. [Google Scholar] [CrossRef]
- Liu, S.; Yu, X. Nutrient cycle of planted forest of Pinus tabulaeformis in the Miyun Reservoir Watershed, Beijing. Front. For. China 2009, 4, 46–52. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, Version 21.0; IBM Corp.: Armonk, NY, USA, 2012.
- Qiu, S.; Gao, H.; Zhu, P.; Hou, Y.; Zhao, S.; Rong, X.; Zhou, W. Changes in soil carbon and nitrogen pools in a Mollisol after long-term fallow or application of chemical fertilizers, straw or manures. Soil Till Res. 2016, 163, 255–265. [Google Scholar] [CrossRef]
- Ma, P.; Nan, S.; Yang, X.; Qin, Y.; Ma, T.; Li, X.; Bodner, G. Macroaggregation is promoted more effectively by organic than inorganic fertilizers in farmland ecosystems of China—A meta-analysis. Soil Till Res. 2022, 221, 105394. [Google Scholar] [CrossRef]
- Cao, H.; Chen, R.; Wang, L.; Jiang, L.; Yang, F.; Zheng, S.; Wang, G.; Lin, X. Soil pH, total phosphorus, climate and distance are the major factors influencing microbial activity at a regional spatial scale. Sci. Rep. 2016, 6, 25815. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Zhou, X.; Guo, D.; Zhao, J.H.; Yan, L.; Feng, G.Z.; Gao, Q.; Yu, H.; Zhao, L.P. Soil pH is the primary factor driving the distribution and function of microorganisms in farmland soils in northeastern China. Ann. Microbiol. 2019, 69, 1461–1473. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, Q.; Li, Z.; Cheng, W.; Sun, J.; Guo, Z.; Li, Y.; Zhou, J.; Meng, D.; Li, H.; et al. Environmental factors shaping the diversity of bacterial communities that promote rice production. BMC Microbiol. 2018, 18, 51. [Google Scholar] [CrossRef] [PubMed]
- Chungopast, S.; Yodying, P.; Nomura, M. Effects of Cellulolytic Bacteria on Nitrogen-Fixing Bacteria, 16S rRNA, nifH Gene Abundance, and Chemical Properties of Water Hyacinth Compost. J. Soil Sci. Plant Nutr. 2021, 21, 768–779. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, L.; Li, Y.; Wang, C.; Zhang, W.; Wang, L.; Niu, L. Pollution gradients shape the co-occurrence networks and interactions of sedimentary bacterial communities in Taihu Lake, a shallow eutrophic lake. J. Environ. Manag. 2022, 305, 114380. [Google Scholar] [CrossRef]
- Shang, Q.; Ling, N.; Feng, X.; Yang, X.; Wu, P.; Zou, J.; Shen, Q.; Guo, S. Soil fertility and its significance to crop productivity and sustainability in typical agroecosystem: A summary of long-term fertilizer experiments in China. Plant Soil 2014, 381, 13–23. [Google Scholar] [CrossRef]
- Hu, X.; Liu, J.; Wei, D.; Zhou, B.; Chen, X.; Jin, J.; Wang, G. Long-term application of nitrogen, not phosphate or potassium, significantly alters the diazotrophic community compositions and structures in a Mollisol in northeast China. Res. Microbiol. 2019, 170, 147–155. [Google Scholar] [CrossRef]
- Grzyb, A.; Wolna-Maruwka, A.; Niewiadomska, A. The significance of microbial transformation of nitrogen compounds in the light of integrated crop management. Agronomy 2021, 11, 1415. [Google Scholar]
- Orchard, E.D.; Webb, E.A.; Dyhrman, S.T. Molecular analysis of the phosphorus starvation response in Trichodesmium spp. Environ. Microbiol. 2009, 11, 2400–2411. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, M.M.; Song, W.F.; Chen, R.F.; Zhao, X.Q.; Wen, S.L.; Zheng, Z.S.; Shen, R.F. Biogeographic patterns and co-occurrence networks of diazotrophic and arbuscular mycorrhizal fungal communities in the acidic soil ecosystem of southern China. Appl. Soil Ecol. 2021, 158, 103798. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Q.; Zhou, J.; Wei, Q. Illumina amplicon sequencing of 16S rRNA tag reveals bacterial community development in the rhizosphere of apple nurseries at a replant disease site and a new planting site. PLoS ONE 2014, 9, e111744. [Google Scholar] [CrossRef]
- Sun, R.B.; Guo, X.S.; Wang, D.Z.; Chu, H.Y. The impact of long-term application of chemical fertilizers and straw returning on soil bacterial community. Microbiol. China 2015, 42, 2049–2057. [Google Scholar]
- Jin, Y.T.; Li, X.F.; Cai, Y.; Hu, H.X.; Liu, Y.F.; Fu, S.W.; Zhang, B.R. Effects of straw returning with chemical fertilizer on soil enzyme activities and microbial community structure in rice-rape rotation. Environ. Sci. 2021, 42, 3985–3996. [Google Scholar]
- Inderjit. Soil microorganisms: An important determinant of allelopathic activity. Plant Soil 2005, 274, 227–236. [Google Scholar] [CrossRef]
- Delmont, T.O.; Quince, C.; Shaiber, A.; Esen, Ö.C.; Lee, S.T.; Rappé, M.S.; McLellan, S.L.; Lücker, S.; Eren, A.M. Nitrogen-fixing populations of Planctomycetes and Proteobacteria are abundant in surface ocean metagenomes. Nat. Microbiol. 2018, 3, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wei, Y.; Du, H.; Zhu, W.; Zhou, Y.; Yin, Y. Effects of intercropping between Morus alba and nitrogen fixing species on soil microbial community structure and diversity. Forests 2022, 13, 1345. [Google Scholar] [CrossRef]
- Amin, F.R.; Khalid, H.; El-Mashad, H.M.; Chen, C.; Liu, G.; Zhang, R. Functions of bacteria and archaea participating in the bioconversion of organic waste for methane production. Sci. Total Environ. 2021, 763, 143007. [Google Scholar] [CrossRef]
- Abdallah, R.Z.; Wegner, C.E.; Liesack, W. Community transcriptomics reveals drainage effects on paddy soil microbiome across all three domains of life. Soil Biol. Biochem. 2019, 132, 131–142. [Google Scholar] [CrossRef]
- Yu, D.; Wen, Z.; Li, X.; Song, X.; Wu, H.; Yang, P. Effects of straw return on bacterial communities in a wheat-maize rotation system in the North China Plain. PLoS ONE 2018, 13, e0198087. [Google Scholar] [CrossRef]
- Bai, N.; Zhang, H.; Zhou, S.; Sun, H.; Zhao, Y.; Zheng, X.; Li, S.; Zhang, J.; Lv, W. Long-term effects of straw return and straw-derived biochar amendment on bacterial communities in soil aggregates. Sci. Rep. 2020, 10, 7891. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Liu, W.; Bao, Y.; Zhang, J.; Petropoulos, E.; Li, Z.; Lin, X.; Feng, Y. Fertilization shapes a well-organized community of bacterial decomposers for accelerated paddy straw degradation. Sci. Rep. 2018, 8, 7981. [Google Scholar] [CrossRef] [PubMed]



| Soil Layer (cm) | pH | TN (g·kg−1) | TP (g·kg−1) | TK (g·kg−1) | AN (mg·kg−1) | AP (mg·kg−1) | AK (mg·kg−1) |
|---|---|---|---|---|---|---|---|
| 0–20 | 5.92 | 1.65 | 0.75 | 16.50 | 191.14 | 32.33 | 183.50 |
| Treatment | CK | F | S | FS |
|---|---|---|---|---|
| TN (g·kg−1) | 1.77 ± 0.05 b | 1.72 ± 0.03 b | 1.59 ± 0.07 c | 1.93 ± 0.02 a |
| TP (g·kg−1) | 0.96 ± 0.02 a | 0.86 ± 0.00 b | 0.74 ± 0.01 c | 0.74 ± 0.03 c |
| TK (g·kg−1) | 18.76 ± 0.37 c | 17.86 ± 0.28 d | 20.23 ± 0.13 b | 20.91 ± 0.34 a |
| AN (mg·kg−1) | 196.74 ± 4.05 c | 180.57 ± 3.06 c | 214.13 ± 1.53 a | 159.06 ± 8.53 d |
| AP (mg·kg−1) | 46.38 ± 0.53 b | 52.33 ± 0.21 a | 45.19 ± 3.53 b | 52.89 ± 1.87 a |
| AK (mg·kg−1) | 209.12 ± 1.96 b | 229.45 ± 1.46 a | 151.33 ± 2.68 d | 197.13 ± 4.10 c |
| Ca (g·kg−1) | 1.21 ± 0.05 b | 0.78 ± 0.02 c | 1.22 ± 0.04 b | 1.32 ± 0.02 a |
| Mg (g·kg−1) | 3.76 ± 0.04 a | 2.97 ± 0.05 d | 3.59 ± 0.04 b | 3.57 ± 0.10 b |
| SOC (g·kg−1) | 2.05 ± 0.10 a | 1.59 ± 0.10 c | 1.92 ± 0.01 b | 1.89 ± 0.08 b |
| pH | 6.17 ± 0.04 a | 5.89 ± 0.07 b | 6.14 ± 0.10 a | 6.26 ± 0.06 a |
| Treatment | ACE | Chao1 | Simpson | Shannon |
|---|---|---|---|---|
| CK | 1710 ± 1.1 a | 1710 ± 2.9 a | 0.0089 ± 0.0004 b | 6.2 ± 0.01 ab |
| F | 1710 ± 0.9 a | 1710 ± 1.1 a | 0.0112 ± 0.0016 a | 6.0 ± 0.060 b |
| S | 1711 ± 1.4 a | 1711 ± 1.4 a | 0.0076 ± 0.0001 bc | 6.3 ± 0.01 ab |
| FS | 1712 ± 2.0 a | 1712 ± 2.6 a | 0.0074 ± 0.0002 bc | 6.2 ± 0.02 a |
| OTU | ACE | Chao1 | Simpson | Shannon | |
|---|---|---|---|---|---|
| TN | 0.46 | 0.47 | 0.43 | 0.43 | −0.33 |
| TP | 0.04 | 0.07 | 0.08 | −0.33 | 0.50 |
| TK | 0.42 | 0.37 | 0.3 | 0.43 | −0.26 |
| AN | −0.04 | −0.05 | −0.04 | −0.42 | 0.62 * |
| AP | −0.09 | −0.08 | −0.06 | −0.11 | −0.10 |
| AK | −0.17 | −0.12 | −0.08 | −0.22 | 0.06 |
| Ca | 0.59 * | 0.55 * | 0.45 | 0.56 * | −0.23 |
| Mg | 0.64 * | 0.61 * | 0.52 * | 0.32 | 0.15 |
| SOC | 0.55 * | 0.51 | 0.41 | 0.41 | −0.01 |
| pH | 0.64 * | 0.61 * | 0.54 | 0.63 * | −0.33 |
| Phyla | Proteobacteria | Actinomycetota | Chloroflexi | Bacteroidetes | Acidobacteria |
| CK | 32,750 ± 4548.5 a | 16,779 ± 1909.7 a | 12,890 ± 1356.9 a | 8775 ± 1722.8 b | 9944 ± 903.7 b |
| F | 28,873 ± 7476.2 a | 17,700 ± 2355.0 a | 12,184 ± 1938.2 a | 10,047 ± 1270.7 ab | 10,054 ± 2689.0 ab |
| S | 32,226 ± 8612.7 a | 13,867 ± 3567.6 a | 13,085 ± 3189.9 a | 12,357 ± 3395.9 a | 10,968 ± 3333.8 a |
| FS | 27,189 ± 3828.5 a | 13,059 ± 2643.6 a | 11,926 ± 1899.5 b | 10,474 ± 1729.0 a | 10,763 ± 1760.1 a |
| Verrucomicrobia | Patescibacteria | Gemmatimonadetes | Nitrospirae | Armatimonadetes | |
| CK | 5584 ± 496.5 a | 2651 ± 294.6 a | 3060 ± 571.2 a | 1279 ± 212.3 a | 555 ± 42.4 a |
| F | 5174 ± 806.9 a | 2412 ± 260.6 a | 3166 ± 748.9 a | 1198 ± 231.3 a | 502 ± 117.5 a |
| S | 5136 ± 1232.7 a | 2245 ± 720.3 a | 3092 ± 876.9 a | 925 ± 254.0 ab | 581 ± 198.0 a |
| FS | 4457 ± 851.7 b | 1850 ± 310.0 b | 2602 ± 230.4 a | 739 ± 124.0 b | 492 ± 144.6 a |
| Class | Gammaproteobacteria | Actinobacteria | Bacteroidia | Anaerolineae | Deltaproteobacteria |
| CK | 19,438 ± 2770.6 a | 13,814 ± 1656.4 ab | 9866 ± 1499.6 a | 11,359 ± 1131.3 a | 7347 ± 1006.9 a |
| F | 17,030 ± 4502.2 a | 14,701 ± 1521.4 a | 9274 ± 1058.8 a | 10,476 ± 1606.9 a | 6349 ± 1756.3 a |
| S | 19,993 ± 5414.8 a | 10,904 ± 2887.0 ab | 11,353 ± 3109.8 a | 11,419 ± 2793.2 a | 6997 ± 1851.3 a |
| FS | 15,971 ± 1881.3 a | 10,475 ± 2012.1 b | 9561 ± 1594.2 a | 10,354 ± 1687.5 a | 6140 ± 987.0 a |
| Alphaproteobacteria | Verrucomicrobiae | Acidobacteriia | Gemmatimonadetes | Holophagae | |
| CK | 5965 ± 788.6 a | 5584 ± 496.5 a | 5196 ± 538.4 a | 2754 ± 536.7 a | 1780 ± 169.5 a |
| F | 5494 ± 1259.6 a | 5174 ± 806.9 a | 5610 ± 1449.8 a | 2900 ± 707.1 a | 1611 ± 436.9 a |
| S | 5236 ± 1361.3 a | 5136 ± 1232.7 a | 5668 ± 1802.2 a | 2758 ± 779.1 a | 2085 ± 599.9 a |
| FS | 5078 ± 965.2 a | 4457 ± 851.7 a | 5908 ± 776.9 a | 2321 ± 226.1 a | 1896 ± 105.1 a |
| Genera | Pseudarthrobacter | Sideroxydans | Massilia | Candidatus_Udaeobacter | Anaerolinea |
| CK | 7329.67 ± 807.77 ab | 4634.33 ± 1022.62 a | 3958.67 ± 729.66 a | 2640 ± 355.28 a | 2891.67 ± 310.054 a |
| F | 8138 ± 696.79 a | 3252 ± 984.33 a | 4339.33 ± 720.7 a | 2202.67 ± 315.65 ab | 2694.33 ± 372.22 a |
| S | 5587 ± 1601.14 b | 5180 ± 1261.95 a | 4686.67 ± 1322.84 a | 2077 ± 557.43 ab | 2910.33 ± 644.71 a |
| FS | 5196.67 ± 1595.22 b | 4114.75 ± 1191.49 a | 3895.33 ± 436.1 a | 1702 ± 391.28 b | 2651 ± 440.75 a |
| Candidatus_Solibacter | Cryobacterium | Geobacter | Pedobacter | Pseudolabrys | |
| CK | 1860.67 ± 171.45 a | 1643.67 ± 182.44 a | 1276.33 ± 282.91 a | 1106 ± 221.31 a | 1076.67 ± 214.65 a |
| F | 2172.33 ± 507.34 a | 1850 ± 218.35 a | 1148 ± 379.92 a | 1243.67 ± 120.97 a | 956.67 ± 272.24 a |
| S | 1965.33 ± 624.37 a | 1039.67 ± 230.37 b | 971.33 ± 255.01 a | 1083.33 ± 373.94 a | 1075.67 ± 328.05 a |
| FS | 2087.67 ± 181.79 a | 1102.67 ± 220.93 b | 975.33 ± 215.63 a | 777.67 ± 225.22 a | 1110.67 ± 215.4 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, F.; Zhang, D.; Chen, Y.; Wu, J.; Zhang, J. Effects of Long-Term Straw Returning and Nitrogen Fertilizer Reduction on Soil Microbial Diversity in Black Soil in Northeast China. Agronomy 2023, 13, 2036. https://doi.org/10.3390/agronomy13082036
Jiao F, Zhang D, Chen Y, Wu J, Zhang J. Effects of Long-Term Straw Returning and Nitrogen Fertilizer Reduction on Soil Microbial Diversity in Black Soil in Northeast China. Agronomy. 2023; 13(8):2036. https://doi.org/10.3390/agronomy13082036
Chicago/Turabian StyleJiao, Feng, Dongdong Zhang, Yang Chen, Jinhua Wu, and Junying Zhang. 2023. "Effects of Long-Term Straw Returning and Nitrogen Fertilizer Reduction on Soil Microbial Diversity in Black Soil in Northeast China" Agronomy 13, no. 8: 2036. https://doi.org/10.3390/agronomy13082036
APA StyleJiao, F., Zhang, D., Chen, Y., Wu, J., & Zhang, J. (2023). Effects of Long-Term Straw Returning and Nitrogen Fertilizer Reduction on Soil Microbial Diversity in Black Soil in Northeast China. Agronomy, 13(8), 2036. https://doi.org/10.3390/agronomy13082036






