Abstract
Dendrobium loddigesii has long been used in traditional folk medicine. The purpose of this study was to optimize the culture conditions for its protocorm-like bodies (PLBs) and explore their biological activities. The use of an air-lift bioreactor demonstrated superior PLB proliferation compared to agitated and solid culture methods. The optimal inoculum quantity of 30 g/vessel, cultured for 28 days in the bioreactor, yielded the highest PLB biomass production. Analysis of PLB extracts revealed that the ethyl acetate (EtOAc) extract exhibited the highest levels of flavonoids and alkaloids, as well as potent antioxidant activity demonstrated by DPPH free radical scavenging assay and reducing power. Furthermore, the antiproliferative effects of the PLB extracts were assessed using MTT assays, and the EtOAc extract showed significant efficacy by reducing cell viability by over 60% in the human colon carcinoma cell line SW620 at the highest tested concentration (200 μg/mL). Mechanistic analysis revealed the downregulation of key regulatory apoptosis genes, including survivin, p53, caspase-3, and caspase-9. These results demonstrate the potential of the bioreactor culture method for the efficient production of D. loddigesii PLBs and the biological activities of the EtOAc extract, suggesting its therapeutic potential.
1. Introduction
Dendrobium loddigesii Rolfe, a member of the Orchidaceae family, is a highly valuable medicinal plant predominantly found in the southwestern region of China [1]. In traditional folk medicine, the stem of D. loddigesii has been used to treat diabetes, alleviate heat-related ailments, moisturize the lungs, and relieve coughs [2]. The plant contains various compounds, including polysaccharides, alkaloids, and flavonoids [3]. These important secondary phytocompounds are typically extracted from whole plants or specific tissues.
Protocorm-like bodies (PLBs) were initially observed in the shoot-tip culture of Cymbidium orchids by Morel (1960). These regenerated structures share similarities in growth and structure with protocorms. PLB induction is an important technique for micropropagating Orchidaceae [4,5] and it has several advantages over traditional shoot and plantlet regeneration, including higher multiplication rates, long-term preservation, ease of differentiation into shoots, and the generation of secondary PLBs [6]. However, conventional PLB culture techniques fail to meet consumer demands, necessitating the development of new technologies for commercial production.
Bioreactors, characterized as low-cost autoclavable containers, offer several advantages for plant tissue culture. These advances can replace the manual solution of some difficulties in in vitro cultures, leading to decreased costs. Bioreactors facilitate the efficient uptake of inorganic and organic nutrients by cells, enabling the rapid accumulation of enriched active compounds [7,8]. Their utilization has been explored in the multiplication of plant tissues and organs in various plant species, including Cattleya forbesii [9], lemon grass [10], potato [11], and strawberry [12]. However, limited studies have investigated the proliferation and growth of PLBs in the Orchidaceae family, particularly in Dendrobium species, using bioreactors.
Atoms or molecules with one or more unpaired electrons are known as free radicals [13]. The three most significant oxygen-containing radicals in various disease situations, according to Lobo et al., are superoxide anion radicals, nitric oxide radicals, and peroxynitrite radicals [14]. Important macromolecules are attacked by free radicals, which causes cell death and homeostasis disturbance. In cells, free radicals are continually created. An antioxidant is a molecule that is sufficiently stable to provide an electron to a free radical that is raging and neutralizes it, so lowering the potential for harm. Due to their ability to scavenge free radicals, these antioxidants postpone or prevent cellular damage [15]. Some antioxidants are generated by the body’s natural metabolic processes. The diet also contains some softer antioxidants. Numerous synthetic phenolic antioxidants exist. The two primary antioxidants that are created are butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT), but some investigations have suggested that these compounds may have carcinogenic effects and may be harmful to cells at high doses [16]. Due to this restriction and their low toxicity and side effects, natural antioxidants are currently receiving a lot of interest [17].
Cancer is the second biggest cause of mortality in the Western world [18]. According to statistics, there are about 7.6 million civilian deaths from cancer globally on an annual basis. By 2030, this figure is predicted to rise to 13.1 million [19]. According to Torre et al., colorectal cancer (CRC) accounted for 10.0% of the estimated 14.1 million new cancer cases documented in 2012, making it the third most-often diagnosed disease globally. With 693,600 fatalities globally in 2012, it was also the third-leading cause of cancer-related deaths in women and the fourth-leading cause in men [20]. Rectal cancer accounts for around one-third of CRC, with over 450,000 new cases reported globally in 2008 [21]. Chemotherapy is normally the common method for the treatment of cancer. However, it is possible that the toxicity of chemotherapeutic medications occasionally poses a significant obstacle to the use of allopathy or conventional medicine in the treatment of cancer. On the other hand, plants continue to hold great promise for the development of novel medications and, as such, serve as a source of natural compounds that may offer chemical protection against cancer. As a mainstay of medical care, herbal remedies have been and still are utilized. The anticarcinogenic characteristics of plants have been the subject of several investigations. The use of natural substances derived from plants in the treatment of cancer has continued to attract encouraging interest [22].
In 1984, Marshall and Warren confirmed that gastritis and most stomach ulcer diseases are caused by Helicobacter pylori (spiral-shaped bacteria) infection, not by stress or spicy food as had been previously assumed. This bacterium is known to produce a toxin that may be potentiated by ammonia generated from urease. Since that time, many types of anti-H. pylori agents to treat H. pylori growth and its effects have been chemically synthesized or isolated from natural sources for the treatment of patients with gastroduodenal disorders. For example, the administration of a mixture of synthetic compounds such as bismuth subsalicylate, tetracycline, and metronidazole for eight weeks was found to inhibit the growth of H. pylori. Natural products, such as decurcin, decurcinol angelate, berberine, and magnolol, have been reported to demonstrate a moderate inhibition of H. pylori growth [23].
In China, some traditional healers use boiled Dendrobium loddigesii Rolfe extract for the treatment of various kinds of diseases, such as cancer and atrophic gastritis, and to alleviate heat-related ailments and they have obtained good results [2]. However, its biological activities have not yet been fully understood. Furthermore, D. loddigesii contains alkaloids [24] and flavonoids [25]. Alkaloids cure inflammation, cancer, and act as antioxidants. Flavonoids of D. loddigesii show good antioxidant, anticancer, and neuroprotective activities [26]. Unfortunately, wild populations of D. loddigesii are endangered due to overexploitation and habitat destruction, primarily driven by its medicinal importance [27,28]. Therefore, it is imperative to conserve D. loddigesii resources and explore alternative propagation methods. In vitro rapid propagation of D. loddigesii not only provides additional resource options but also helps protect this important plant species from overexploitation. Therefore, this study aimed to determine the optimal conditions for D. loddigesii PLB proliferation, including the effects of the culture method, growth curve, and inoculum density. Additionally, we evaluated the content of bioactive compounds (total phenolics, flavonoids, and alkaloids) as well as the antioxidant, anti-Helicobacter pylori, and antiproliferative activities of PLBs cultured in bioreactors.
2. Materials and Methods
2.1. Induction and Propagation of D. loddigesii PLBs
Dendrobium loddigesii Rolfe was collected from Hezhou, the Guangxi Zhuang Autonomous Region, China, in August 2022. Young shoot tips of D. loddigesii were selected and sterilized to induce PLBs. Explants were cultured in 50 mL Erlenmeyer flasks containing 20 mL of Sachs and Hicks (SH) medium supplemented with 2 mg/mL benzyladenine (BA), 2 mg/mL kinetin (KT), 1 mg/mL naphthaleneacetic acid (NAA), 30 g/L sucrose, and 7 g/L agar at pH 5.7. The cultures were maintained at 25 °C, 1600 lx illumination, and a 16/8 h light/dark cycle. After 7–8 weeks of continual subculture, PLBs were selected for further analysis.
2.2. PLB Proliferation
To determine the optimal culture conditions for the proliferation of D. loddigesii PLBs, due to the different volume of culture vessels, different inoculation quantities were added in proportion, as described by Mujib [28]. Eight pieces of PLBs (0.5 cm × 0.5 cm, 0.5 g) were placed in each 150 mL Erlenmeyer flask containing 50 mL of liquid SH medium supplemented with 2 mg/mL BA, 2 mg/mL KT, 1 mg/mL NAA, and 30 g/L sucrose. The flasks were incubated in a gyratory shaker at 100 rpm for 28 days. For solid cultures, the same medium composition was used but with the addition of 7 g/L agar. In bioreactor cultures, 30 g of PLB pieces (0.5 cm × 0.5 cm × 0.5 cm) were transferred into balloon-type air-lift bioreactors (Shanghai Precision & Scientific Instrument Co., Ltd., Shanghai, China) and submerged in 3 L of liquid medium for the entire culture period. All cultures were maintained under a 16/8 h light/dark cycle at 1600 lx and 25 °C.
2.3. PBL Growth Curve Analysis
PLBs (0.5 cm × 0.5 cm, 30 g) were transferred to bioreactors containing of SH medium with 2 mg/mL BA, 2 mg/mL KT, 1 mg/mL NAA, and 30 g/L sucrose. The growth of PLBs was monitored every 7 days for a total of 35 days. At each periodic harvest, the fresh weight (FW) and dry weight (DW) of PLBs were determined.
2.4. Influence of Inoculation Quantities on PLB Growth
PLBs with different inoculation quantities (20, 30, or 40 g/vessel) were transferred to bioreactors containing SH medium with 2 mg/mL BA, 2 mg/mL KT, 1 mg/mL NAA, and 30 g/l sucrose. Cultures were incubated under the previously described laboratory conditions. The FW, DW, and growth ratio were determined on the 28th day of incubation.
PLB FW was measured after blotting away the surface water. Fresh PLBs were dried at 50 °C for 24 h, and the PLB DWs were recorded. The growth ratio was calculated as follows: growth ratio = (FWb − FWa)/FWa; where FWa and FWb are the initial FW and harvested FW of the PLBs, respectively.
2.5. Extraction and Analysis of Alkaloids
PLBs were extracted three times with 100% methanol at room temperature. A rotary evaporator (Kulzer & Co., Wehrheim, Germany) was then used to concentrate the extract. The crude extract was then partitioned with hexane, ethyl acetate (EtOAc), and n-butanol (water-saturated BuOH) extracts after being suspended in distilled water. An amount of 5.0 mL of potassium hydrogen phthalate (pH = 4.5) buffer and 2.0 mL of 0.04% bromocresol were added to 5.0 mL of a 1 mg/mL sample in order to evaluate the sample’s total alkaloid content. Before sifting, the mixture stood for 40 min after being strongly shaken for 3 min. With the use of a UminV-vis spectrophotometer (Adobe, Seattle, WA, USA), the absorbance was determined at 415 nm [29].
2.6. Total Flavonoid Content Assessment
The total flavonoid content was measured, as described by Moreno et al. Amounts of 0.1 mL of 10% aluminum nitrate, 0.1 mL of 1 M potassium acetate, and 4.3 mL of ethanol were combined with 0.15 mL of the sample. The absorbance of the mixture was determined to be 415 nm after 40 min of room temperature reaction [30].
2.7. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Radical Scavenging Capacity
In brief, 0.1 mL of different concentrations of the samples were thoroughly mixed with 1 mL of 0.15 mM DPPH and 4 mL of 100% methanol, and the mixture was then incubated for 30 min at room temperature in the dark. At 519 nm, absorbance was measured. The DPPH• radical scavenging activity (%) was calculated as: , (Ai: absorbance of sample; Aj: absorbance of control; A0: absorbance of DPPH solution). The concentration at which the scavenging activity is 50% is known as the IC50 value [29].
2.8. Reducing Power Assay
The reducing power of the samples was measured, according to Oyaizu’s method (1986). Trisodium phosphate and 1% K3[Fe(CN)6] were each applied to 0.2 mL of the samples. At 50 °C, the reaction took place for 20 min. Following that, 0.1 mL of trichloroacetic acid, 0.4 mL of distilled water, and 50 L of ferric chloride were added. At 700 nm, absorbance was measured [30].
2.9. Anti-Helicobacter Pylori Assay
Agar dilution testing was used to detect anti-H. pylori. H. pylori was introduced into 10% bovine calf serum-infused Brucella broth. To make stock solutions containing 10 mg/mL, samples were dissolved in DMSO. An amount of 20 μL of each stock solution was added to 180 μL of H. pylori-containing media on a 96-well microassay plate. In order to produce a concentration range from 1000 to 8 g/mL, micrococcus medium at a concentration of 1 mg/mL was serially diluted using the twofold process. After that, the culture plates were incubated for 24 h. The preparation’s MIC was the lowest concentration at which the growth of bacteria was fully inhibited.
2.10. MTT Assay
The 293 (Human Embryonic Kidney), SW620 (Human colo carcinoma cell), A549 (Huma lung cancer cell), and AGS (Human gastric carcinoma cell) cells were obtained from the Korean Cell Line Bank. In DMEM that was supplemented with 10% FBS and 1% penicillin, 293 cells were maintained. At 37 °C, in an incubator with 5% CO2, SW620, AGS, and A549 cells were grown in RPMI 1640 with 10% fetal bovine serum and 10 g/mL of penicillin. The cells were seeded on 96-well plates at a density of 104 cells/well for the cell viability experiment, which was performed during a total of 24 h at 37 °C and 5% CO2. Then, each well received the diluted samples prepared in the medium. The supernatants were removed after 24 h, and 0.1 mL of MTT (0.5 mg/mL) solution was then added. DMSO (100 µL) was added to the mixture after 4 h to dissolve the formazan crystals. At 540 nm, the absorbance was measured.
2.11. Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
The SW620 cells were treated with the desired concentrations of the samples. Following that, cDNA was synthesized in accordance with the manufacturer’s instructions after total RNA was extracted using a Kit (iNtRON Biotechnology Inc., Seongnam, Republic of Korea). Using RT-PCR and primers (Table 1), variations in the loaded volumes of caspase-3, caspase-9, p53, and survivin were normalized by comparison with the β-actin control.

Table 1.
Sequences of primer used in RT-PCR analysis.
2.12. Statistical Analysis Method
Three replicates were used for all the experiments. The significance of the data, which were estimated and compared using Duncan’s multiple range tests at a 0.05 level of significance (p < 0.05), was analyzed using IBM SPSS Statistics 21.
3. Results and Discussion
3.1. Effect of Culture Methods on PLB Proliferation
Significant differences were observed among the culture methods, including solid, liquid (agitated flask), and bioreactor cultures in terms of PLB proliferation. The bioreactor culture demonstrated superior growth after 28 days, with a total FW of 352.1 g/vessel compared to 3.5 g/vessel and 1.9 g/vessel in the agitated flask and solid cultures, respectively. The growth ratio of PLBs in the bioreactor reached 10.7, which was 1.9 and 3.8 times higher than that of the agitated flask and solid cultures, respectively (Table 2). These findings indicate that the bioreactor culture method is well suited for promoting PLB proliferation. This may be attributed to the enhanced nutrient uptake facilitated by the immersion system, promoting efficient nutrient assimilation [31]. A previous study by Mujib et al. highlighted the advantages of liquid medium in bioreactors, which led to shorter “embryo-plantlet” recovery times compared to agar solidified medium [28].

Table 2.
Effect of culture method on PLB proliferation.
The accelerated propagation achieved through reduced time requirements holds promise for enhancing secondary metabolite synthesis in medicinally important plant genera. Bioreactors have also proven successful in scaling up vegetative propagation across a range of plant species. These include PLBs, shoots, adventitious roots [32], bulblets, cormlets, rhizomes, microtubers, and somatic embryos [33]. The bioreactor culture method demonstrated the superior growth and proliferation of Dendrobium loddigesii PLBs compared to solid and agitated flask cultures, highlighting its potential for enhancing nutrient uptake and promoting efficient nutrient assimilation.
3.2. Growth Curve Study of PLB Proliferation in a Bioreactor
The growth curve of PLBs in the bioreactor was studied for a period of 35 days. The biomass gradually increased up to 28 days and then declined (Figure 1). During the initial week, PLBs exhibited slow growth, followed by a log phase where the growth ratio reached 17.2 on the 28th day of incubation. The volume of PLBs also increased significantly during this period. However, no further growth was observed between days 28 and 35, and the PLBs exhibited a brown color. This decline in growth may be due to insufficient oxygen transfer in the culture medium as the biomass increased and nutrient depletion occurred. As a result, cells underwent lysis and autolysis. Therefore, the 28th day was selected as the optimal time point for inoculation in subsequent studies.
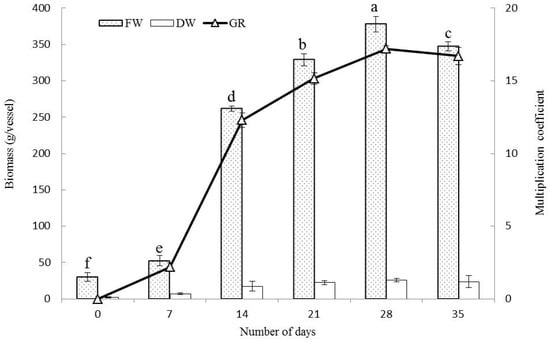
Figure 1.
Growth curve study of PLB proliferation in a bioreactor. Duncan’s Multiple Range Test at 5% level (DMRT, p < 0.05). Significance was indicated with different letters according to statistical analysis.
3.3. Influence of Inoculation Quantities on PLB Proliferation in a Bioreactor
To investigate the effects of different inoculation quantities on PLB proliferation, 20, 30, or 40 g of PLBs were inoculated in balloon-type air-lift bioreactors for the proliferation culture. The growth ratio was assessed as an indicator. Low (20 g) or high (40 g) inoculation quantities led to a decrease in the growth ratio. However, at an inoculation quantity of 30 g, the PLBs achieved a significant mass increase, with FWs and DWs reaching 360.7 g and 23.3 g per bioreactor after 28 days of incubation (Table 3). Inoculum quantity is a critical parameter that influences the scaling up of suspended plant cells, tissues, and organs. Generally, with a low inoculum quantity, explants grew rapidly but did not reach sufficiently high biomass levels in a short vegetative cycle. In contrast, high initial inoculum quantities resulted in an earlier onset of the declining phase and senescence of explants. Cui et al. reported that the growth of Hypericum perforatum L. adventitious roots decreased due to a high inoculum quantity, which limited the availability of oxygen and nutrients, resulting in poor growth and accumulation of phenolics and flavonoids [34]. Therefore, an inoculum quantity of 30 g/vessel is suitable for generating an optimal PLB biomass.

Table 3.
Effect of inoculation quantities on PLB growth during bioreactor culture.
3.4. Alkaloid Content in PLBs
As seen in the data presented in Table 4, we observed variations in the total alkaloid contents among different extracts of PLBs. Specifically, the ethyl acetate (EtOAc) extract had the highest content of total alkaloids at 0.86 mg/g followed by the BuOH and MeOH extracts at 0.53 mg/g and 0.42 mg/g, respectively. The aqueous and hexane extracts of PLBs did not contain detectable levels of total alkaloids, which is consistent with previous findings [22]. Alkaloids have been extensively studied for their beneficial health effects in treating inflammation and cancer and acting as antioxidants. These compounds can inhibit the formation of free radicals (e.g., ROS), increase the activity of antioxidant enzymes [35], and induce apoptosis in human colorectal cancer cells via the expression of proapoptotic proteins such as Bax, caspase-9, and caspase-3 [36]. Therefore, PLBs hold promise as alternative materials for extracting various alkaloids, including dendrobine, an alkaloid compound found in certain species of the Dendrobium genus.

Table 4.
The total alkaloid, flavonoid contents, and DPPH radical scavenging activity in extraction from PLBs.
3.5. Antioxidant Properties of PLBs
The influence of the extraction methods on the antioxidant activity of PLBs is presented in Table 4. Among the extracts, the EtOAc extract of PLBs demonstrated the strongest ability to reduce DPPH radicals (IC50, 48.81 ± 2.34 μg/mL), surpassing both other extracts and the positive control BHT (2,6-di-tert-butyl-4-methylphenol) (IC50, 80.81 ± 1.12 µg/mL) (Table 4). Additionally, the reducing power assay was conducted with various amounts of crude extract, revealing significant differences in reducing power among solvent extracts in a dose-dependent manner. The EtOAc fraction exhibited the highest reducing activity, with absorbance values of 0.15 at 0.3 mg/mL, 0.8 at 0.5 mg/mL, and 1.25 at 1 mg/mL (Figure 2). Our findings align with previous reports that the antioxidant capacity of banana flesh (M. paradisiaca) is positively correlated with flavonoid constituents [37]. Consistently, our results indicated that the EtOAc extract displayed the highest total flavonoid content (TFC: 19.58 ± 1.04 mg QE/g) compared to other extracts, while the aqueous extract exhibited the lowest TFC. These results affirm the significant antioxidant activity of PLB extracts. Yang et al. also observed the effectiveness of the EtOAc extract in DPPH radical scavenging and inhibition of platelet function when evaluating a polyphenol-rich fraction from Salvia miltiorrhiza Bunge. Furthermore, they reported a similar trend with a higher TFC in the EtOAc extract compared to the other extracts [38].
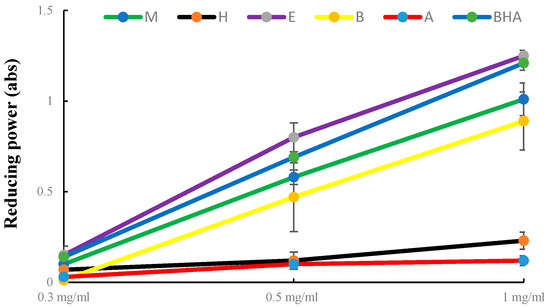
Figure 2.
Reducing power of extract from PLBs. M: Methanol, H: hexane, E: ethyl acetate, B: n-butanol, A: aqueous, BHA: butylated hydroxyanisole, data are the mean ± SE (n = 3).
3.6. Anti-Helicobacter Pylori Activity
The anti-H. pylori activity of the PLB extracts was assessed in the current investigation using the agar dilution approach (Table 5). Our findings showed that the EtOAc extract, which has an MIC value of 250 μg/mL, has the strongest anti-H. pylori activity among the different extracts. Marshall and Warren established that, contrary to what was previously believed, Helicobacter pylori (spiral-shaped bacterium) infection is the true cause of gastritis and the majority of stomach ulcer illnesses. It is known that this bacterium produces a toxin, which the ammonia produced by urease may enhance [39]. According to studies conducted by Han et al., natural compounds such as decurcin, decurcinol angelate, berberine, and magnolol showed a modest reduction in H. pylori development [23]. These results also align with the findings of our study as well. We found that the PLB EtOAc extract contains high levels of alkaloids, and these compounds may be effective against H. pylori. White rose petal extract (WRPE) in ethanol and ethyl acetate was studied for its antibacterial properties by Park et al. They also suggested that the WRPE-EtOAc fraction was particularly effective against H. pylori [26,40].

Table 5.
Anti-H. pylori Effect of Extraction from PLBs.
3.7. Antiproliferative Activity
The antitumor effects of various concentrations of the PLB extract were evaluated by assessing the cell viability of the SW620 (colorectal cancer), AGS (gastric cancer), A549 (lung cancer), and HEK 293 (control) cell lines using the MTT assay. Treatment with the PLB EtOAc extract resulted in a decrease in cell viability in the SW620 and AGS cell lines (Figure 3). Specifically, the moderate inhibition rate of antiproliferation was obtained in the EtOAc extract with a cell viability of 68.74% for the treatment on AGS cells. However, concentrations of 200 μg/ml of the EtOAc extract exhibited strong cytotoxic effects, suppressing the proliferation of SW620 cells by more than 50%. Notably, the extract did not induce cytotoxic effects on normal HEK 293 cells. Initial screening data showed that the other fractions (hexane, BuOH, and aqueous) did not demonstrate significant antiproliferative effects on the tested cancer cells. Considering the significant antiproliferative activity of the PLB EtOAc extract observed in the SW620 cell line, further investigation was conducted to explore the molecular mechanism.
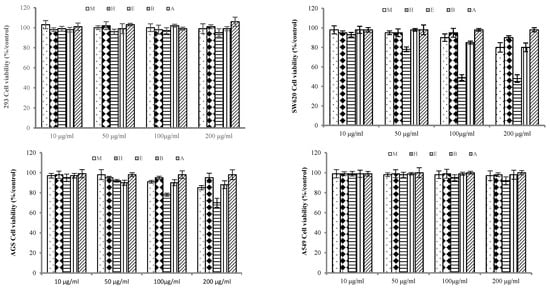
Figure 3.
Inhibitory effect of extract from PLBs on proliferation of cells.
To determine whether the inhibition of the proliferative response was associated with decreased cell viability, we evaluated the potential induction of apoptosis in the SW620 cell line, as described by Noriko [41]. RT-PCR results revealed that the PLB EtOAc extract induced a reduction in the expression of survivin, p53, caspase-3, and caspase-9 in SW620 cells compared to the untreated cells (Figure 4). These data suggested that one of the mechanisms involved in the inhibition of tumor cell proliferation is associated with the induction of cell death, including apoptosis.
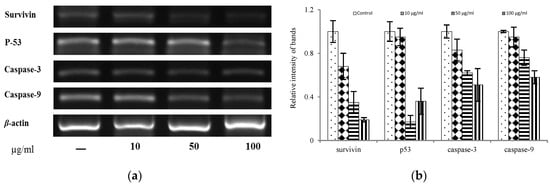
Figure 4.
The inhibition of gene expression of EtOAc extract from PLBs in SW620 (Human colon cancer cell): (a) Gel electrophoresis of amplified product of β-actin, survivin, p53, caspase-3, and caspase-9; (b) Quantitative data of panel A.
Caspase-3 and caspase-9 are associated with the modulation of the apoptotic pathway. Yamamoto et al. demonstrated that suppressing the survivin gene induces cell death through apoptosis or mitotic catastrophe, as survivin is widely distributed and functions as an effector molecule in the apoptosis process [42]. The p53 gene also interacts with the survivin gene in response to DNA damage caused by anticancer agents. Considering the results of this study, it is suggested that the PLB EtOAc extract might exert its anticancer properties through a reduction in the expression of apoptosis-related genes. In addition, reactive oxygen species have numerous pathological effects, such as lipid and protein peroxidations, DNA damage, and cellular degeneration, which cause cardiovascular disease, aging, cancer, inflammatory diseases, and a variety of other disorders [43]. It seems the PLB EtOAc extract might prevent or inhibit colorectal cancer because of its scavenger capability. Although from the results of this study it might be suggest that the PLB EtOAc extract exhibits potent antiproliferative properties and can induce apoptosis in SW620 cells, more detailed investigations concerning its antiproliferative mechanism as a supplement are required in future studies.
4. Conclusions
Our study establishes the optimal conditions for PLB production, utilizing a bioreactor with 30 g inoculum cultured for 28 days. This protocol promotes robust growth and increased volume of PLBs. The PLB EtOAc extracts exhibited remarkable bioactivity, including high levels of flavonoids and alkaloids, such as dendrobine. These extracts demonstrate potent antioxidant properties, effectively scavenging free radicals and displaying strong reducing power. Furthermore, they exhibit significant antiproliferative effects, particularly in the SW620 cell line, by downregulating key apoptotic genes, such as survivin, p53, caspase-3, and caspase-9.
These findings highlight the promising antioxidant and antiproliferative potential of PLBs, positioning them as a valuable source of phytochemicals with therapeutic implications. Future studies will focus on the purification and elucidation of the specific bioactive compounds present in PLB extracts and their underlying mechanisms of action. By further understanding the molecular pathways involved, we can uncover the full therapeutic potential of PLBs and their extracts. This research opens avenues for utilizing PLBs as a substitute for plant organs in producing phytochemical content and underscores their importance in the field of natural product research and drug discovery.
Author Contributions
Conceptualization, J.Y.; Methodology, M.J.K.; Writing—original draft, J.Y., Y.S.K. and E.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are contained within the article.
Acknowledgments
This research was supported by the Research Grant from Bioherb Research Institute, Kangwon National University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Editorial Committee of Flora Republicae Popularis Sinicae; Academic Press: Beijing, China, 1999.
- National Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China. Part 1; China Medical Science Press: Beijing, China, 2015. [Google Scholar]
- Xu, J.; Han, Q.B.; Li, S.L.; Chen, X.J.; Wang, X.N.; Zhao, Z.Z.; Chen, H.B. Chemistry, bioactivity and uality control of Dendrobium, a commonly used tonic herb in traditional Chinese medicine. Phytochem. Rev. 2013, 12, 341–367. [Google Scholar] [CrossRef]
- Mehbub, H.; Akter, A.; Akter, M.A.; Mandal, M.S.H.; Hoque, M.A.; Tuleja, M.; Mehraj, H. Tissue culture in ornamentals: Cultivation factors, propagation techniques, and its application. Plants 2022, 11, 3208. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.I.; Hsu, S.T.; Yeung, E.C. Orchid protocorm-like bodies are somatic embryos. Am. J. Bot. 2013, 100, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Mujib, A.; Ilah, A.; Aslam, J.; Fatima, S.; Siddiqui, Z.H.; Maqsood, M. Catharanthus roseus alkaloids: Application of biotechnology for improving yield. Plant Growth Regul. 2012, 68, 111–127. [Google Scholar] [CrossRef]
- Ekmekcigil, M.; Bayraktar, M.; Akkus, O.; Gurel, A. High-frequency protocorm like bodies and shoot regeneration through a combination of thin cell layer and RITA® temporary immersion bioreactor in Cattleya forbesii lindl. Plant Cell Tissue Organ Cult. 2019, 136, 451–464. [Google Scholar] [CrossRef]
- Quiala, E.; Barbón, R.; Capote, A.; Pérez, N.; Jiménez, E. In vitro mass propagation of cymbopogon citratus stapf., a medicinal gramineae. Methods Mol. Biol. 2016, 1391, 445–457. [Google Scholar]
- Ebadi, M.; Iranbakhsh, A.; Khaniki, G.B. Shoot micropropagation and microtuberization in potato (Solanum tuberosum L.) by the semi-continuous bioreactor. Pak. J. Biol. Sci. 2007, 10, 861–867. [Google Scholar] [CrossRef]
- Debnath, S.C. Developing a scale-up system for the in vitro multiplication of thidiazuron-induced strawberry shoots using a bioreactor. Can. J. Plant Sci. 2008, 88, 737–746. [Google Scholar] [CrossRef]
- Jiangseubchatveera, N.; Liawruangrat, S.; Teerawutgulrag, A.; Santiarworn, D.; Pyne, S.G.; Liawruangrath, B. Phytochemical screening, phenolic and flavonoid contents, antioxidant and cytotoxic activities of Graptophyllum pictum (L.) Griff. Fac. Sci. Med. Health Pap. Part A 2017, 44, 193–202. [Google Scholar]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Halliwell, B. How to characterize an antioxidant—An update. Biochem. Soc. Symp. 1995, 61, 73–101. [Google Scholar] [PubMed]
- Yu, R.; Mandlekar, S.; Kong, A.N.T. Molecular mechanism of butylated hydroxylanisole-induced toxicity: Induction of apoptosis through direct release of cytochrome c. Mol. Pharmacol. 2000, 58, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Ra, J.H.; Nakamura, M.; Herath, K.H.I.N.M.; Kim, I.S. Antioxidant and hepatoprotective effects of different ethanol concentrations in extraction from leaves of Sasa quelpaertensis Nakai. S. Afr. J. Bot. 2017, 112, 376–382. [Google Scholar] [CrossRef]
- Madhusudan, S.; Middleton, M.R. The emerging role of DNA repair proteins and predictive, prognostic and therapeutic target in cancer. Cancer Treat. Rev. 2005, 31, 603–617. [Google Scholar] [CrossRef]
- Minarni, M.; Julistiono, H.; Bermawie, M.; Riyanti, E.I.; Hasan, A.E.Z. Anticancer activity test of ethyl acetate extract od endophytic fungi isolated from soursop leaf (Annona muricata L.). Asian Pac. J. Trop. Med. 2017, 10, 566–571. [Google Scholar] [CrossRef]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Tamas, K.; Walenkamp, A.M.; Hospers, G.A. Rectal and colon cancer: Not just a different anatomic site. Cancer Treat. Rev. 2015, 41, 671–679. [Google Scholar] [CrossRef]
- Desai, A.G.; Qazi, G.N.; Ganju, R.K.; Tamer, M.E.; Singh, J. Medicinal plants and cancer chemoprevention. Curr. Drug Metab. 2008, 9, 581–591. [Google Scholar] [CrossRef]
- Han, Y.H.; Lee, K.D.; Lee, D.U. Anti-Helicobacter pylori activity and structure-activity relationships of berberine derivatives. Bull. Korean Chem. Soc. 2009, 30, 3147. [Google Scholar] [CrossRef]
- Wang, Z.J.; Jiang, W.M.; Liu, Y.Y.; Meng, X.X.; Peng, D.Y. Putative genes in alkaloid biosynthesis identified in Dendrobium officinale by correlating the contents of major bioactive metabolites with genes expression between Protocorm-like bodies and leaves. BMC Genom. 2021, 22, 579. [Google Scholar] [CrossRef]
- Singleton, V.L.; Roosi, J.A. Colorimetry of total phenoilcs with phosphomolypdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Li, C.H.; Qiu, J.; Ding, L.; Huang, M.Z.; Huang, S.R.; Yang, G.S. Anthocyanin biosynthesis regulation of DhMYB2 and DhbHLH1 in Dendrobium hybrids petals. Plant Physiol. Biochem. 2017, 112, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Cai, H.; Li, W.D.; Cai, B.C. Ultrafiltration coupled with high-performance liquid chromatography and quadrupole-time-of-flight mass spectrometry for screening lipase binders from different extracts of Dendrobium officinale. Anal. Bioanal. Chem. 2015, 407, 6081–6093. [Google Scholar] [CrossRef] [PubMed]
- Park, D.S.; Shin, K.H.; Choi, Y.J.; Kim, J.B.; Kim, Y.B. Antimicrobial activities of ethanol and butanol fractions of white rose petal extract. Regul. Toxicol. Pharmacol. 2016, 76, 57–62. [Google Scholar] [CrossRef]
- Yuan, Y.D.; Zhang, J.C.; Liu, X.; Meng, M.J.; Wang, J.P.; Lin, J. Tissue-specific transcriptome for Dendrobium officinale reveals genes involved in flavonoid biosynthesis. Genomics 2019, 112, 1781–1794. [Google Scholar] [CrossRef]
- Mujib, A.; Muzamil, A.; Isah, T. Somatic embryo mediated mass production of Catharanthus roseus in culture vessel (bioreactor)—A comparative study. Saudi J. Biol. Sci. 2014, 21, 442–449. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. J. Nat. 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reactions: Antioxidative activities of products of browning reaction prepared from glucosamine. J. Nutr. 1986, 44, 307–315. [Google Scholar]
- Sivakumar, G.; Kim, S.J.; Hahn, E.J.; Paek, K.Y. Optimizing environmental factors for large-scale multiplication of chrysanthemum (Chrysanthemum grandiflorum) in balloon-type bioreactor culture. In Vitro Cell. Dev. Biol. Plant 2005, 41, 822–825. [Google Scholar] [CrossRef]
- Kentsop, R.A.D.; Consonni, R.; Alfieri, M.; Laura, M.; Ottolina, G.; Mascheretti, I.; Mattana, M. Linum lewisii adventitious and hairy-roots cultures as lignan plant factories. Antioxidants 2022, 11, 1526. [Google Scholar] [CrossRef]
- Murthy, H.N.; Joseph, K.S.; Paek, K.Y.; Park, S.Y. Bioreactor systems for micropropagation of plants: Present scenario and future prospects. Front. Plant Sci. 2023, 14, 1159588. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.H.; Chakrabarty, D.; Lee, E.J.; Paek, K.Y. Production of adventitious roots and secondary metabolites by Hypericum perforatum L. in a bioreactor. Bioresour. Technol. 2010, 101, 4708–4716. [Google Scholar] [CrossRef] [PubMed]
- Indah, P.; Iman, P.M.; Dadan, S.S. A Review of Fibraurea tinctoria and its component, berberine, as an antidiabetic and antioxidant. Molecules 2023, 28, 1294. [Google Scholar]
- Mou, Z.M.; Zhao, Y.; Ye, F.; Shi, Y.; Kennelly, E.J.; Chen, S.Y.; Zhao, D. Identification, biological activities and biosynthetic pathway of dendrobium alkaloids. Front. Pharmacol. 2021, 12, 605994. [Google Scholar] [CrossRef]
- Oyeyinka, B.O.; Afolayan, A.J. Comparative and correlational evaluation of the phytochemical constituents and antioxidant activity of Musa sinensis L. and Musa paradisiaca L. fruit compartments (Musaceae). Sci. World J. 2020, 2020, 4503824. [Google Scholar] [CrossRef]
- Yang, S.A.; Im, N.K.; Ji, Y.J.; Yoo, D.C.; Hee, K.H.; Lee, I.S. Radical scavenging and inhibition of platelet function by a polyphenol rich fraction from Salvia miltiorrhiza Bunge. Nat. Prod. 2008, 1, 7–13. [Google Scholar] [CrossRef]
- Marshall, B.J.; Warren, J.R. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1983, 321, 1273–1275. [Google Scholar]
- Ke, H.L.; Wang, X.P.; Zhou, Z.; Wang, A.; Wu, Z.Y.; Zhang, Y.W. Effect of weimaining on apoptosis and Caspase-3 expression in a breast cancer mouse model. J. Ethnopharmacol. 2021, 264, 113363. [Google Scholar] [CrossRef] [PubMed]
- Noriko, O.; Nam, H.D.; Emi, K.; Akira, K.; Satoshi, I.; Chikao, M. Aqueous extract of Carica papaya leaves exhibits anti-tumor activity and immunomodulatory effects. J. Ethnopharmacol. 2010, 127, 760–767. [Google Scholar]
- Yamamoto, H.; Ngan, C.Y.; Monden, M. Cancer cells survive with survivin. Cancer Sci. 2008, 99, 1709–1714. [Google Scholar] [CrossRef]
- Habibi, R.M.; Mohammadi, R.A.; Delazar, A.; Halabian, R.; Soleimani, R.J.; Mehdipour, A. Effects of polygonum aviculare herbal extract on proliferation and apoptotic gene expression of MCF-7. DARU J. Pharm. Sci. 2021, 19, 326–331. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).