Abstract
Withania coagulans (Stocks) Dunal is used in traditional medicine to treat diseases and has numerous pharmacological properties due to its biological compounds. The plant is a subshrub native to Asia, especially the tropical and temperate regions of western Asia. Its medicinal effects derive from its biological components, which are linked to human health. Conventional medicine uses these compounds to treat a variety of diseases, such as neurological issues, diabetes, and asthma. The long-term benefits of W. coagulans necessitate conservation strategies and plant biotechnological techniques such as micropropagation, synthetic seed, cell suspension, and hairy root elicitation technology, and genetic transformation can all play significant roles in conservation and sustainable utilization of the biological compounds for clinical uses. The objective of this review is to provide a comprehensive overview of the W. cogaulans medicinal properties, potential applications, and innovative approaches for sustainable utilization, making it a unique contribution to the existing body of knowledge. Multi-omics methods for the production of withanolides were also examined in order to gain a better understanding of the genome structure, prospective genes, and candidate proteins involved in the production.
1. Introduction
The success of the primary healthcare system depends on the accessibility of the right medications. A possible function for medicinal plants has always existed, and they have long been employed as part of a traditional treatment system. A significant medicinal plant in the Solanaceae family known as Withania coagulans (Stocks) Dunal may play a part in the primary healthcare system. W. coagulans, also known as Rishyagandha, Indian cheese maker, or paneer bandh, is native to Asia, particularly the tropical and temperate regions of western Asia, including Afghanistan. It can also be found on the Indian subcontinent, which includes Nepal and the northwest region of India [1] (Figure 1). It occurs in Nepal and the northwest of India in the Indian subcontinent [2]. The National Medical Plant Board (NMBP) recognizes 24 species of Withania, but two of them stand out for their valuable medical properties [3]. These two species are known as Withania coagulans and W. somnifera, also known as ashwagandha. Both species contain valuable metabolites known as withanolides (WTDs), whose therapeutic potential has been extensively researched [1,2,3,4,5,6,7]. W. coagulans is used for a variety of medicinal purposes, so permanent stocks have been overexploited, and the species is endangered. In addition, the polygamous nature of the flowers, excessive natural harvesting, ineffective cultivation methods, self-incompatibility, and limited seed environment are factors that have brought the species to the brink of extinction [4,5]. Overall, the scenario emphasizes the necessity to investigate biotechnological methods for the conservation, propagation, and increased production of secondary metabolites in W. coagulans.
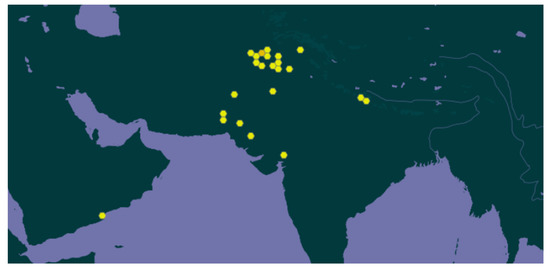
Figure 1.
Geographical distribution of W. coagulans (source: https://www.gbif.org/species/3801167, accessed on 16 May 2023).
Biotechnology can play a significant role in conserving the plant and increasing its secondary metabolite production in W. coagulans. A combination of in vitro propagation, genetic engineering, and biological processes can ensure the sustainable use of this important medicinal plant and reduce the overexploitation that has led to its endangerment. These techniques can also increase the production of secondary metabolites that have medicinal properties, which can meet the increasing demand for natural remedies. Despite the interest of numerous researchers in W. coagulans [6,7], there is currently a lack of integration between the fields of ethnomedicine, pharmacology, and biotechnology. This lack of integration hinders the development of a comprehensive platform that can bring together the knowledge from these fields to fully understand the potential applications of the plant. By developing a more holistic understanding of the plant, it is possible to identify new potential applications, optimize the biotechnological techniques for enhanced secondary metabolite production, and ultimately contribute to the conservation of this important medicinal plant.
The aim and purpose of this study are to provide a comprehensive overview of the medicinal properties of W. coagulans and to investigate its potential applications in the treatment of various diseases. The study will highlight the numerous pharmacological properties of the plant, which are due to its biological compounds that contribute to its therapeutic effects in traditional medicine. In addition, the study will emphasize the importance of long-term conservation strategies for W. coagulans due to their medicinal significance. The study discusses various plant biotechnology techniques such as micropropagation, synthetic seed, cell suspension, hairy root culture, and genetic transformation to achieve this. These techniques are considered essential for the sustainable use of W. coagulans biological compounds for clinical applications. In addition, the study addresses multi-omics methods used for the production of withanolides, the bioactive compounds of W. coagulans. By using multi-omics approaches, researchers hope to gain a better understanding of the genome structure, candidate genes, and candidate proteins involved in the production of these valuable compounds. Moreover, we assess the safety of W. coagulans by examining the available toxicological and pharmacological data, which have been crucial in identifying potential adverse effects of the plant and ensuring its safe use. The proposed review includes new information that will be of great interest to researchers in the subject. The latest study results are included to provide readers with up-to-date information and keep them informed. This study will help to understand its therapeutic properties, put conservation measures into practice, and explore biotechnological methods for better long-term use. Additionally, the study adds to what is already known about W. coagulans, expands the commercial potential for biotech enterprises, and addresses health issues related to diseases such as neurological disorders, diabetes, and asthma. By providing useful information, innovative approaches, and business opportunities in the field of plant medicine and natural products research, the study has the potential to benefit researchers, farmers, and biotech companies.
2. Ethnobotanical Profile
Withania coagulans is a small bushy gray shrub, 60–120 cm tall, with dense gray or yellowish-white branches [8]. The plant has lanceolate-oblong leaves (2.5–7.5 cm long and 1.5 cm broad) and yellowish, dioecious, polygamous flowers (0.7–1.2 cm across) in axillary cymose racemes [8]. Such as other Solanaceae berries, the fruits (0.7–1.2 cm in diameter) are orange-red, spherical, smooth, and enclosed by a leathery calyx [9]. The seeds are dark brown, glabrous, fruity-smelling, and ear-shaped [8,9]. The plant flowers from November to April and bears fruit from January to May [10]. The extended ripening period and unisexual flowering hinder the commercial development of this seed-propagated plant [11]. For years, Withania coagulans has been utilized in primary care as a medicine source, with each plant portion having its unique therapeutic purpose (Table 1).

Table 1.
Ethnobotanical uses of W. coagulans in traditional system of medicine.
3. Phytochemical Profile
Due to the widespread usage of W. coagulans in classical medicine and the subsequent commercialization in modern medicine, numerous studies have been conducted to identify, isolate and characterize various phytochemicals from different parts of the plant: among these, steroid lactones, tannins, flavonoids, terpenoids, iridoids, alkaloids and others have been found [18,19,20,21]. The presence of aerial WTDs in W. coagulans, as demonstrated by Mishra et al. [22], suggests that the extraction process is cheaper and easier compared with W. somnifera, making it a more suitable candidate for WTDs production. Moreover, the plant’s berries are a rich source of essential oils, amino acids, and alkaloids, including a milk-coagulating enzyme called Indian cheese maker [23]. WTDs-C-28 steroid lactones are the main chemical ingredient. They are found in plant leaves and roots (0.001 to 0.5% dry weight) [20]. W. coagulans produces various WTD derivatives, including withanaloid-a, physalins, withanolides-glycosides, withaphysalins, acnistins, perculactones, jabrols, D-aromatic WTDs, and coagulin L, its most important component [24,25,26,27]. Multiple studies have reported the isolation of 138 WTDs, 13 alkaloids, and many sitoindosides (withanolide derivatives) from various parts of the plant, including the aerial parts, roots, and berries [15,18,19,28,29,30,31,32,33,34,35,36]. Isolated compounds and their pharmacological activities have been shown in Figure 2 and Table 2.
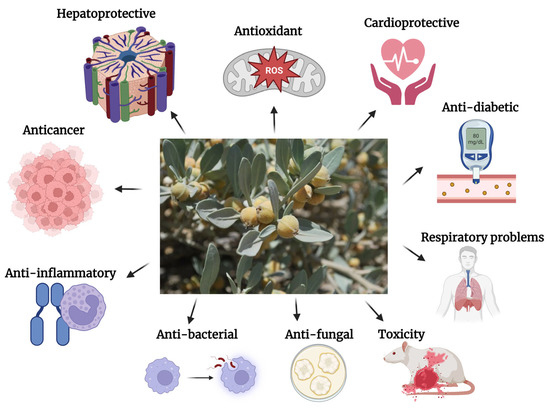
Figure 2.
Different pharmacological activities of W. coagulans [source (figure in the center): Wikimedia commons; Creative Commons Attributions-Share Alike 4.0 International license; Attribution: AhmadHB] [This figure was created with https://biorender.com/ (accessed on 16 May 2023)].

Table 2.
Important bioactive compounds isolated from Withania coagulans.
4. Breeding and Cultivation Potential of W. coagulans
Effective agronomic practices and cultivation methods must be developed to ensure a steady supply of W. coagulans. These include optimizing soil conditions, water management, and proper cropping practices to increase crop growth and yield. Agronomists can study the plant’s growth patterns, nutrient requirements, and environmental conditions to develop specific guidelines for farmers growing W. coagulans. In addition, traditional breeding techniques can be used to develop W. coagulans cultivars with higher levels of medicinal compounds. By selecting and breeding plants that have higher concentrations of beneficial compounds, such as withanolides, breeders can create cultivars with improved therapeutic potential. This approach can play a critical role in developing high-yielding and pharmacologically active W. coagulans cultivars. However, the breeding and cultivation of W. coagulans are limited due to several factors, including the polygamous nature of the flower, self-incompatibility, challenges in domestication, low seed germination and viability, biological constraints, etc. [4,5]. These circumstances caused W. coagulans’ natural population to be overexploited and then depleted, which could be a troublesome signal in the field of agronomy. Despite these limitations, there is a growing interest in traditional and alternative medicine worldwide, which could lead to increasing demand for medicinal plants such as W. coagulans in the future. In addition, as research on the plant’s therapeutic properties and cultivation techniques progresses, there may be more incentives for farmers and growers to explore its cultivation potential and sustainable utilization of its available germplasm. By integrating agronomy, breeding, cultivation, and biotechnological processes, researchers and practitioners can work toward sustainable production, conservation, and utilization of W. coagulans for its therapeutic properties, benefiting both traditional medicine and modern health care systems.
5. Biotechnological Intervention for the Conservation and Withanolide Production
As a result of human development activities in the Himalayan region, several populations of medicinal plants are on the edge of extinction. W. cogulans is one such plant with a wide range of medical applications and a declining natural population. Ineffective growth techniques, the gathering of wild polygamodioecious blossoms, self-incompatibility, and a lack of opportunities for seed generation are to blame for the loss of the natural variety of this exceptional germplasm [4,5,49]. The plant has not been able to keep up with the survival rate due to its dry lifestyle and extremely poor regeneration rate, and the species is currently regarded as severely endangered [50,51]. As a result, it is no longer practicable to harvest this plant species sustainably. This requires the development of workable solutions that can address the challenges facing the plant’s survival and ultimately contribute to the betterment of humanity. Biotechnological interventions offer a range of tools and approaches that can be used to conserve and enhance biodiversity. By using biotechnology to address the challenges facing the planet, we can ensure the continued availability of ecosystem services and natural resources, improve human well-being and contribute to sustainable development. In order to retain the genetic identity of plants, particularly those that are challenging to cultivate using conventional procedures, biotechnological approaches, such as micropropagation techniques, have played a larger role and received increasing attention from scientists. An adequate supply of W. coagulans plants can be made available with a methodical micropropagation strategy. The micropropagation of this plant has been the subject of extensive study, and a number of studies have been released. This section emphasizes the potential benefits of micropropagation for W. coagulans and the need for further research in this area to ensure the sustainable use of this important medicinal plant.
5.1. In Vitro Propagation in Withania coagulans
The two methods of in vitro propagation that are most frequently used are direct and indirect organogenesis. When micropropagation is carried out utilizing shoot tip, node, and axillary bud cultures, clonal integrity is primarily kept. However, clonal fidelity can be lost as a result of callus-mediated indirect organogenesis or, to a lesser extent, somatic embryogenesis. The callus tissue is genetically unstable and can accumulate genetic changes, leading to a loss of clonal fidelity. This can result in variations in plant growth, morphology, and secondary metabolite production. In contrast, direct organogenesis involves the use of pre-existing plant parts, such as shoot tips, to directly form new shoots, bypassing the formation of callus tissue and thus reducing the risk of clonal fidelity loss [52,53]. Any effective micropropagation method must begin with the explant’s source and disinfection. As it aims to eliminate surface contaminants and endophytic microorganisms from the explants. These contaminants can interfere with the growth and development of the explants and potentially lead to the failure of the entire micropropagation process. Successful disinfection of explants enables the growth of healthy and uncontaminated plant material, which in turn can lead to the production of genetically identical plants in large numbers. Therefore, the success of the micropropagation protocol largely depends on the effectiveness of explant disinfection. The choice of disinfectant used during micropropagation can vary depending on the plant species and tissue being cultured. However, some commonly used disinfectants include sodium hypochlorite (bleach), ethanol, Teepol, Bavistin, and hydrogen peroxide. The concentration and duration of treatment can also vary depending on the disinfectant and tissue being used. It is important to optimize the disinfection protocol to effectively kill any contaminants while minimizing damage to the explant tissue. The information on the treatment of explant from W. coagulans has been collected and organized in Table 3.

Table 3.
Different sterilization procedures for explants of W. cogulans.
The success of a micropropagation protocol is highly dependent on the culture medium and culture conditions. Shoot and root development depends on the nutrients and growth regulators that the culture medium contains to promote cell division, elongation, and differentiation. Depending on the plant species and stage of development, the composition of the medium may change, so it is critical to adjust the medium for each situation. In addition, the growth and development of plant cells and tissues can be affected by pH, sucrose, and agar, which are important elements in tissue culture [62]. The pH of the culture medium is crucial for the growth and development of plant cells. Depending on the plant species and stage of development, a different pH range is ideal. In general, most plant species thrive in a pH range of 5.5 to 6.5. pH levels outside of this range can potentially be harmful to cells and result in less than optimal growth and development [63,64]. For instance, many critical elements, such as calcium, magnesium, and phosphorus, are less accessible and may become insoluble at low pH levels, restricting their uptake by the plant cells. The availability of some elements, such as iron and manganese, may decrease at high pH levels as a result of precipitation or molecule complex action. Extreme pH levels can also damage proteins and cellular membranes, which results in cell death and slowed growth. Overall, the availability and uptake of different molecules by the plant cells are affected by the ionization state of the medium, which is affected by the pH of the culture medium.
Sucrose serves as a carbon source in the nutrient medium and provides plant cells with the energy they need for cell growth and development [65]. It also helps regulate the osmotic pressure of the medium. The concentration of sucrose can affect the rate of cell elongation and division, as well as the differentiation of cells into shoots or roots. The osmotic potential of the culture medium is affected by sucrose concentration, which can affect the ability of cells to absorb water, grow, and develop. In addition, sucrose can act as a signaling molecule to control metabolic pathways and gene expression that are important for plant growth and development [66]. Depending on the type of plant and its developmental stage, there are different ideal sucrose concentrations. In the nutrient medium, other sugars, such as fructose and glucose, can also be used as carbon sources [67]. While they have similar effects on cell growth and development as sucrose, their ideal amounts may vary. The gelling material agar is frequently utilized to provide plants with a reliable foundation for growth in tissue culture. Explants’ capacity to expand and mature can be influenced by the amount of agar present in the culture media [68,69]. A medium that is harder and more solid due to a higher agar content may be advantageous for certain plant species that need a strong rootstock for proper growth and development. Utilizing more agar can help lower the risk of infection because it forms a physical barrier that keeps germs from getting to the explants. A higher agar concentration, on the other hand, can make it harder for roots to pierce the medium and lessen the efficacy of rooting. A softer medium with less agar, on the other hand, can be better for the roots but is also more prone to infection. Agar’s function in tissue culture is to support the growth of plant cells and tissues physically while controlling the cells’ access to nutrients, water, and gases. Depending on the plant species and tissue type, the culture medium’s amount of agar can be adjusted for the explants’ best growth and development; however, a commonly used concentration of agar in tissue culture is 0.8% to 1.0% (w/v).
To promote the best possible growth and development of cells and tissues in tissue culture, pH, sucrose, and agar concentrations must be carefully adjusted. The ideal concentrations of these elements must be changed during culture, as they depend on the plant species and growth stage. In addition to the nutrient medium, the culture conditions, such as temperature, light intensity, photoperiod, humidity, and ventilation, also play a crucial role in the success of micropropagation [70,71,72]. The pace of enzymatic processes and membrane fluidity, for instance, can be influenced by temperature in plant cells, which can then have an impact on cell elongation and division [73]. Similar to this, the effects of light and photoperiod on plant tissue culture include the perception of light signals by certain photoreceptors, activation of signaling pathways downstream, and modification of physiological processes and gene expression to aid in growth and development [74]. These circumstances may affect the explants’ growth and development as well as their rate of shoot proliferation, germination, and acclimation. To achieve successful micropropagation and to produce healthy and vigorous plantlets for subsequent field planting or commercial production, the nutrient media and culture conditions must be properly tuned. In the instance of W. coagulans, we obtained data on the culture conditions, pH level, sucrose and agar content, and type of nutrient medium. The data is grouped in Table 4 and Table 5 for both direct and indirect organogenesis. Additionally, Figure 3A–D displays a micropropagation protocol developed by our team.

Table 4.
Direct organogenesis in W. coagulans.

Table 5.
Indirect organogenesis in W. coagulans.

Figure 3.
Micropropagation protocol and synthetics seed production in Withania coagulans (source: unpublished culture photographs from our group). (A) Explant inoculation, (B,C) shoot multiplication, (D) in vitro rooting, and (E,F) synthetic seeds.
5.2. Synthetic Seed Production
Synthetic seed technology is another approach that has revolutionized the concept and possibilities of plant propagation and conservation. Somatic embryos, shoot buds, nodes or shoot tips, and leaf segments are encapsulated using this technology and used as seeds after sowing under in vitro or ex vitro conditions. According to [77], it has excellent potential for germplasm exchange and conservation, micropropagation, ease of handling, reduced time and space requirements, and cost-effectiveness. This method has been used for the conservation of a number of medicinal plants, but the conservation and propagation of W. cogulans by synthetic seed has received less attention. Figure 3E,F show artificial seeds produced by enclosing nodal and shoot tip explants [4], demonstrating the production of synthetic seeds from W. coagulans micro explants by alginate encapsulation with 3% sodium alginate and 100 mM CaCl2·2H2O with a maximum growth of 96% on a regeneration medium, i.e., 0.75% MS agar gel medium with an addition of 0.57 µM IAA and 1.11 µM BA. They demonstrated the efficacy of in vitro regeneration of plantlets for rapid propagation, short-term storage, and germplasm distribution. The encapsulated explants showed a 72% revival of plantlets on regeneration media after sterile storage at 4 °C for 60 days. The resulting synthetic seedlings successfully took root and adapted to their environment. However, the use of synthetic seed technologies for the maintenance and propagation of W. cogulans has not been adequately studied. Therefore, further research is needed to develop a technique for synthesizing synthetic seed from the nodule and shoot tip explants of W. cogulans and to evaluate the effectiveness of this strategy for large-scale production. The research could have important implications for the conservation and ethical use of W. cogulans and also help develop new techniques for plant propagation, conservation, and secondary metabolite production.
6. Withanolide Production In Vitro
Plants create bioactive substances known as secondary metabolites as part of their defense system against biotic and abiotic stress. These substances have a variety of pharmacological properties that have been demonstrated, including antibacterial, antioxidant, anticancer, anti-inflammatory, and immunomodulatory effects. As a result, the pharmaceutical industry has made in vitro secondary metabolite generation a key topic of research. Secondary metabolites can be produced in vitro, which has a number of benefits over conventional plant-based extraction techniques. First off, it makes it possible to quickly and in huge quantities produce the needed chemicals. The ability to produce homogenous, standardized chemicals with consistent quality and purity is its second benefit. Thirdly, it lessens the requirement for plant material, which can be scarce or challenging to obtain. Additionally, the in vitro creation of secondary metabolites offers the chance to improve the production of particular compounds or to develop whole new compounds via genetic engineering and biosynthetic pathway alteration. Moreover, the pharmaceutical industry has a lot to gain from the in vitro creation of secondary metabolites as a source of new compounds for drug discovery and development, as well as for the mass manufacture of existing compounds with high quality and consistency. Numerous techniques, such as cell suspension culture, hairy root culture, elicitation, bioreactors, and genetic engineering, can be used to carry out the procedure. The choice of method relies on the kind of plant and the desired secondary metabolite, and each method has advantages and limits of its own. The natural phytocompounds of W. coagulans, also known as WTDs, are said to be the most potent natural compounds of the plant. In this section, we discuss the rationale for the various in vitro techniques used with W. coagulans for the production of secondary metabolites.
6.1. Thin Cell Layer (TCL) Culture and Elicitation
A helpful technique for obtaining secondary metabolites from plants is a thin cell layer (TCL) culture. This involves growing thin sections of plant tissue on a solid or liquid medium, usually 0.1 to 0.5 mm thick. Comparing TCL culture to whole plant cultures or other in vitro methods, one advantage is the possibility of greater production of secondary metabolites. The small size of the tissue sections and the large surface area to volume ratio accelerate the flux of nutrients and metabolites, promoting biosynthesis and leading to an accumulation of secondary metabolites [78]. In order to obtain the greatest amount of shoot multiplication, Tripathi et al. [60] used three explants of W. coagulans, including node, shoot apical meristem, and TCL explant, and inoculated on the ideal concentration consisting of MS with BA 2.0 mg L−1 and NAA at 0.5 mg L−1. When the regenerated shoots were inoculated in MS media supplemented with 2 mg L−1 IBA, the best response in terms of rooting was obtained. After a period of acclimatization, the regenerated plants were successfully transplanted to the outdoor setting in the field. Withaferin A and withanolide A quantification from mother plants growing in the field (wild type) as well as regenerated plants, were studied. When compared with the mother plant, HPLC analysis of the TCL cloned plants revealed an increase in withaferin A content of 1.4-fold and withanolide A content of 1.6-fold [60]. The use of these approaches for the generation of secondary metabolites has, however, received very little research in the species of Withania. Although TCL culture is a promising technique for obtaining secondary metabolites with potential applications in the pharmaceutical, nutraceutical, and agrochemical industries. The TCL technique has some restrictions and difficulties, notwithstanding any possible benefits [79]. The challenge of preserving the cultures’ physiological and biochemical stability over long stretches of time is a significant restriction. The frequent subculturing necessary for TCL cultivation might cause somaclonal variation, which may modify how secondary metabolites are produced. Another difficulty is the potential for microbial contamination of cultures, which can hinder cell growth and metabolism and the overall production of secondary metabolites.
Elicitation, which involves putting plants under stress to increase the production of secondary metabolites in plants or microorganisms, has a lot of promise for use in both industrial and medical settings. Elicitors are biotic or abiotic molecules that are members of various chemical compound classes with different structures (Figure 4).
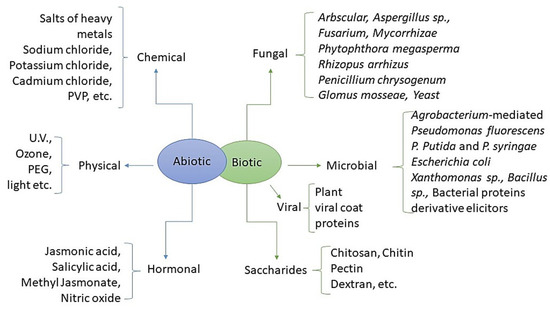
Figure 4.
Types of elicitors [80].
Elicitors have the ability to increase or induce a certain secondary metabolite’s production, and variables such as types of elicitors, concentrations, treatment duration, and nutrient medium are important for the success of this process. Elicitors act as signals for elicitor-specific receptors on the plant cell membrane, which in turn triggers the start of a signal transduction cascade that changes the expression level of the genes and associated transcription factors of the secondary metabolic pathways, boosting synthesis and accumulation [81,82]. According to Maurya et al. [83], the addition of salicylic acid to the culture medium at concentrations of 150 and 200 µM dramatically increased the number of photosynthetic pigments, increased biomass, and produced secondary metabolites such as proline, phenol, anthocyanin, and antioxidant enzymes [83]. Further studies showed a clear correlation between the production of secondary metabolites in W. coagulans cultures and the expression of mRNA involved in the control of metabolic pathways. However, UV-B exposure in vitro increased secondary metabolites such as carotenoids, anthocyanin, phenol, and proline, while negatively affecting chlorophyll concentration and photosynthetic apparatus of W. coagulans [84]. In addition, transcript levels of genes involved in WTD production were found to be excessively high. Although there has been little research on W. coagulans, elicitation has demonstrated considerable promise in increasing the production of secondary metabolites in plants and microorganisms. In the case of W. coagulans, the development of high-proficiency protocols for the synthesis of WTDs utilizing various elicitors is still necessary. When establishing the protocol, it is also important to take into account the protocol’s limitations, such as the specificity of the elicitors, difficulties with optimization, and potential toxicity.
6.2. Cell Suspension and Root Culture
Cell suspension culture is a type of plant tissue culture that involves growing cells or tiny clumps of cells in a liquid medium. High biomass production, homogeneous culture, reduced labor and space requirements, and reduced contamination risk are some of the benefits that make this technique a powerful tool for the production of valuable compounds from plant cells, with significant potential in the pharmaceutical, agriculture, and biotechnology industries [85]. The fascinating thing about in vitro culture is that, unlike plants cultivated in the field, cell suspension culture is not affected by environmental factors that strongly influence plant metabolism [86]. The fundamental step in any cell culture is the induction of callus formation, which then serves as an ideal means to study the metabolic pathways of the plant as well as a source for the utilization of the desired metabolite of the plant. Mirjalili and Esmaeili [76] showed a higher rate of callus production from leaf explants (25.0–96.0%) followed by intermodal (23.2–85.4%) segments of W. coagulans on a nutrient medium containing MS + 2.5 mg/L 2.4-D + 0.5 mg/L BAP. Calli induction from a leaf explant was used to establish a cell suspension culture, and the medium MS, supplemented with 1.5 mg/L IAA + 0.5 mg/L BA, showed a higher rate of biomass formation in a cell suspension culture. The growth curve of the cell suspension culture of W. coagulans resembled a sigmoid curve, with an initial moderate growth rate followed by a substantial increase in biomass with a 3.5-fold increase in fresh weight and dry weight in a period of only 28 days. After 28 days of growth, the cells began to turn brown and ceased further development. In the fourth week of culture, metabolite production of WTDs, especially withaferin A and withanolide A, increased by 0.08 ± 0.003 and 21 ± 0.4 g/L, respectively [76].
W. coagulans leaf explant was used to compare withaferin A production by cell suspension and root culture [87]. Callus was induced on MS supplemented with 2.0 mg/L 2, 4-D + 0.5 mg/L Kn, and adventitious roots were directly generated from leaf segments at half-strength MS with 2.0 mg/L IBA. It was observed that the accumulation of withaferin A was higher in adventitious roots (21.4 ± 1.67 at 4 weeks and 66.73 ± 0.86 in 8-week-old cultures) than in cell suspension cultures (6.62 ± 2.01) [87]. One possible explanation is that suspension cultures are constantly in motion and lack the tissue differentiation and organization that occurs in intact plants. Root culture, on the other hand, preserved the morphological and functional characteristics of roots while growing in a stationary environment more conducive to secondary metabolite accumulation than suspension culture. Moreover, root cultures showed higher expression of genes involved in secondary metabolite production compared with suspension cultures [88]. Another study indicated that half-strength MS + 4.93 µM IBA + 2.46 µM IAA supplementation was optimum for the greatest biomass growth and withanolide production from adventitious root culture [89]. After HPTLC, the highest amount of withanolide A (204.98 ± 0.87 g/L DW) and withaferin A (227.15 ± 0.57 g/L DW) accumulation was observed. However, suspension and traditional root culture are less commonly used procedures in W. cogulans than in other Withania species. Furthermore, there are several limitations to traditional root culture, such as slow growth rate, cost and complexity, and genotypic heterogeneity [90].
However, compared with traditional root culture, hairy root culture has a number of advantages, such as faster growth, genetic stability, and high productivity [91]. In addition, hairy root cultures can be easily scaled up and produce large amounts of secondary metabolites while reducing the risk of pathogen contamination. Most medicinal plants are known for accumulating secondary metabolites in their roots [92]. For this reason, A. rhizogenes-mediated hairy root culture has been of critical importance in overcoming the harmful harvest of medicinal plants for many years. Hairy root culture has gained popularity and is considered an important biotechnological application as it provides a new platform to meet the needs of the pharmaceutical industry. The leaf portion of W. coagulans was utilized to inoculate with A. tumefaciens strain C58C1 (pRiA4) [34]. As a result, the roots underwent a transformation that allowed them to produce the two main bioactive components of the Withania species, withanolide A and withaferin A. The hair roots obtained showed two morphologies: normal hair roots (HR) with faster growth potential and lower withanolide accumulation and callus-like roots (CR) with a high capacity to produce withanolides. By PCR analysis, the aux1 gene of pRiA4 was found in all roots with callus-like morphology. However, only 12.5% of the roots with the characteristic hairy root shape had this gene. This suggests that aux genes have a significant influence on the shape of the modified roots. According to time-course studies of withanolide production, withaferin A accumulated most heavily toward the end of the culture period, while withanolide A accumulated most heavily during the early stages of culture. Some modified root lines, such as HR112 and CR26, showed a strong ability to make withanolides, especially the important medicine withanolide A, in a larger bioreactor. Despite the use of hairy root culture technology, W. coagulans has received less attention when it comes to research on the production of secondary metabolites than other species, such as W. somnifera. Cutting the WTDs reliance on W. somnifera and creating W. cogulans as a parallel option for the WTDs required a lot of attention. In addition, while hairy root culture has many advantages as a culture system, it also has some limitations that need to be considered when choosing a culture system for a specific application.
6.3. Application of Genetic Engineering and Omics Techniques in Secondary Metabolites Production
Plant cell genomes may be precisely and selectively modified thanks to the fast-developing field of genetic engineering. With the advent of genetic engineering, it is now possible to introduce genes for important enzymes involved in the biosynthesis of secondary metabolites into plant cells, resulting in the production of secondary metabolites of high value (Figure 5). Omics technologies such as genomics, transcriptomics, proteomics, and metabolomics offer several advantages for secondary metabolite improvement in vitro. Some of these advantages are the identification of candidate genes and prediction of gene function, comprehensive analysis of metabolites, etc. Mishra et al. developed an effective agrobacterium-mediated genetic transformation protocol using W. coagulans leaf explants [93]. The transformation method was made with the help of the Agrobacterium strain LBA4404, which had the binary vector pIG121Hm with the β-glucuronidase gene (gusA) under the control of the CaMV35S promoter. Explants were exposed to an Agrobacterial inoculum for 20 min and then cultured for three days on a medium to which 100 M acetosyringone had been added and on MS medium with 10.0 μM BA + 8.0 μM IAA and 50.0 mg L kanamycin, shoot bud induction, and differentiation happened after three selection cycles. After making roots for a week in a medium with 2.5 μM IBA, the long shoots were moved to a hormone-free half MS for two weeks to finish the process. Under ideal conditions, they achieved 100% frequency of transient GUS expression and 5% constant transformation efficiency. The presence of gusA and nptII genes in transgenic T0 plants confirmed the transgenic event. The putative transgenic W. coagulans plants showed histochemical GUS expression. TLC revealed that similar types of withanolides were found in both the transgenic and nontransgenic regenerated plants.
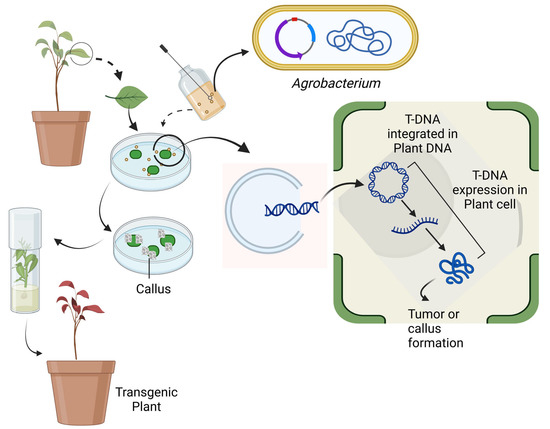
Figure 5.
Agrobacterium-mediated genetic transformation [this figure was created with https://biorender.com/ (accessed on 16 May 2023)].
Squalene is a common precursor in the biosynthesis of steroid and triterpenoid compounds in plants and is produced by the enzyme squalene synthase (SS) by dimerization of two molecules of farnesyl diphosphate overexpress the squalene gene in W. coagulans hairy root culture [94]. T-DNA from Agrobacterium rhizogenes A4 and the SS1 gene encoding SS from Arabidopsis thaliana under the CaMV35S promoter was used. The formation of withanolide and accumulation of phytosterols in the modified hairy roots were examined, and the results were compared with those of control roots containing only the T-DNA from pRiA4. The ability of the modified roots to produce more phytosterols and withanolides was closely correlated with the level of transgene expression, demonstrating the value of overexpressing 35SS1 to increase triterpenoid biosynthesis [94]. Tryptophan decarboxylase (EC 4.1.1.28) decarboxylates tryptophan in a way that depends on pyridoxal-5′-phosphate (PLP) to make tryptamine, which can then be used in a number of metabolite biosynthesis pathways with an indolyl component. According to a recent study of specific indolyl metabolites in Withania species, tryptophan decarboxylase (TDC) may play a dominant functional role in the genome of these species by facilitating the production of the indolyl precursor molecule tryptamine [95]. This metabolic investigation led to the discovery and cloning of a complete TDC cDNA sequence in the aerial tissue of W. coagulans. The 1506 bp open reading frame (ORF) of the functional WcTDC gene encodes a 502 amino acid protein with a predicted molecular mass and pI of 56.38 kDa and 8.35, respectively. Escherichia coli was used to produce the gene, and the recombinant enzyme was affinity purified to homogeneity to understand its catalytic kinetics. The enzyme (WcTDC) was specifically designed to catalyze the decarboxylation of tryptophan or, to a lesser extent, its counterpart (5-hydroxytryptophan) and had a much higher Km value for tryptophan than for pyridoxal 5′-phosphate. The ability of the herb to adapt to hot, dry environments—its main natural habitat—was demonstrated by the observed optimal catalytic function of the enzyme on the slightly basic side of the pH scale and at slightly higher temperatures. This is the first study on the cloning and characterization of a recombinant enzyme from W. coagulans heterologously expressed, and it serves as a basis for further research on the biosynthesis of withanamides.
Transcriptome sequencing of W. coagulans micropropagated plants’ leaves and roots produced 292,074 and 16,474 high-quality reads from a total of 8.08 and 6.35 GB of raw data, of which 267,119 and 15,758 unigenes were found in WcL and WcR, respectively [96]. Furthermore, annotations for 40.6% WcL and 55.05% WcR unigenes were made utilizing multiple databases. In gene ontology, the categories of metabolic process and cellular components were found to be dominating, and a total of 20,927 WcL and 2474 WcR unigenes were mapped to various biological pathways. The discovery of genes involved in the production of the precursor of withanolide, 24-methylenecholesterol, was made easier by the KEGG categorization. Withanolide precursor biosynthesis genes were all present only in WcL, indicating de novo production, while the absence of some rate-limiting enzymes in WcR showed salvage routes for withanolide biosynthesis. The putative genes GTs, MTs, and CYP450s were discovered to be involved in the conversion of 24-methylenecholesterol to various withanolides. The details of the enzymes responsible for the manufacture of tissue-specific withanolides were further elucidated by the differential expression of these genes. The differential manufacture of withanolides was validated by withanolide profiling using HPLC analysis, and their accumulation in both tissues at relatively low concentrations supported their biosynthesis via root salvage mechanisms.
7. Safety Evaluation
Consumption of herbal products has steadily increased over the last three decades, and safety testing is required for conventional medicines, which is an important step for the pharmaceutical industry. Nowadays, herbal supplements derived from medicinal herbs are gaining popularity as a source of nutrients and to maintain health [97]. However, they are generally considered safe due to their natural origin and are available over the counter [98]. However, in most cases, consumers are not very aware of the side effects of the herb taken without medical instruction. Due to limited studies, physicians are also unaware of the potential health risk associated with herbal medicine [99]. There is increasing evidence of the toxicity of various herbal extracts, and understanding herb-drug interactions is an important issue [100]. The traditional use of Withania has a long history, but there is a lack of information on the safety evaluation of W. coagulans. However, studies that are available did not report any toxic effects of the extract nor observed a significant difference in body weight and body temperature between the control and the treated subjects [16,100]. Moreover, efforts are needed to identify and evaluate the toxicity associated with this plant. Nevertheless, a careful clinical study to clarify the various mechanisms is needed to confirm the efficacy and safety of its uses in traditional medicine. Hussain et al. pointed out the potential plant-drug interactions of 123 medicinal plants used as ingredients in many herbal products such as herbal supplements, beverages, dietary supplements, etc., and noted that prolonged or excessive consumption of these herbal products may lead to adverse side effects [101]. According to the report, W. somnifera proved to be a mild activator of human pregnane X receptor (PXR), a potent activator of arylhydrocorban receptor (AhR), and showed an inhibitory effect on the activity of cytochrome P450 3A4 (CYP3A4) and CYP1A2 [101]. Another study reported that the high concentration of withaferin A can damage red blood cells [12], while the ingestion of root extracts, e.g., ashwagandha root, showed no toxicity in animals even at higher doses. In addition, some studies also suggest that ashwagandha increases the risk of miscarriage in pregnant women. The demand for this medicinal plant is widely recognized, but the need for rigorous evaluation of their safety is still essential work to be conducted as much as possible to close the gap between their toxicity and medicinal value [33].
8. Conclusions and Future Prospects
The significance of W. coagulans in terms of their secondary metabolites, their pharmacological value, and the methods employed to improve these phytocompounds are reviewed in this paper. As the demand for natural remedies increases, it is possible that additional Withania species with medicinal properties will be discovered. Due to its significant use, this plant is in high demand in both domestic and international markets. As a result, the plant has been overexploited, and its environment has been destroyed. Innovative conservation and propagation tools for W. coagulans are needed to keep up with supply and demand in the commercial market. Increasing commercial production can be aided by biotechnological measures such as micropropagation, synthetic seed synthesis, cryopreservation, and bioreactors. A deeper understanding of the withanolides biosynthesis pathway may also lead to better metabolic engineering of withanolide to produce them in vitro culture, for example, cell suspension, the root and hairy root culture, etc. Multi-omics approaches can be a promising tool to achieve this goal for a deeper understanding of the complex network of the withanolide biosynthesis pathway, and, thereby, the possibilities of precisely manipulating the pathways through different genome editing techniques. No toxicity of W. coagulans has been detected in toxicological tests to date. However, thorough side effect studies are needed to prove the safety of W. coagulans. Clinical trials are needed to demonstrate that W. coagulans is useful in medicine. In summary, W. coagulans has been extensively studied in terms of its taxonomy, regional distribution, ethnobotany, phytochemical analysis, bioactivity, and biotechnological approaches. The current information and new findings of this review will be of great benefit to researchers in the field by providing a better understanding of the therapeutic properties of the plant, implementation of conservation measures, and exploration of biotechnological methods for long-term sustainable use. It also expands the commercial potential for biotech companies and addresses health problems related to neurological disorders, diabetes, and asthma, contributing to the advancement of plant medicine and natural products research. There are still many areas, such as clinical trials, bioactivity and mechanism of action, optimization of biotechnological processes, conservation strategies, and economic and sociocultural impacts, that need to be covered to achieve breakthrough results from cutting-edge research.
Author Contributions
Conceptualization, Z.A. and A.S. (Anwar Shahzad); writing—original draft preparation, Z.A. and A.S. (Arjumend Shaheen); writing—review and editing, Z.A., A.S. (Arjumend Shaheen), A.W., S.T., S.u.R., A.U., I.B.G. and M.R.; supervision A.S. (Anwar Shahzad); funding, Y.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by The National Natural Science Foundation for Scholars of China (31870595) and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Data Availability Statement
The authors declare that they have no competing interests. No humans or animals are involved in this study.
Acknowledgments
We want to acknowledge BioRender.com. Figure 2 and Figure 5 was created with https://biorender.com/ (accessed on 16 May 2023).
Conflicts of Interest
The author declares that they have no financial/or non-financial competing interests.
References
- Pandey, I.; Nama, K.S. Withania coagulans (Stocks) Dunal: A Rare Ethnomedicinal Plant of the Western Rajasthan Desert. Int. J. Pharmaceut. Biomed. Res. 2015, 2, 34–40. [Google Scholar]
- Barad, R.; Soni, P.; Upadhyay, S.; Upadhyay, U. Withania coagulans and Psidium guajava: An Overview. Int. Res. J. Pharm. Appl. Sci. 2013, 3, 42–47. [Google Scholar]
- Rathore, M.S.; Khatri, K.; Kheni, J.; Shekhawat, N.S. Biotechnological Advancement in an Important Medicinal Plant, Withania coagulans: An Overview and Recent Updates. In Biotechnological Approaches for Medicinal and Aromatic Plants; Kumar, N., Ed.; Springer: Singapore, 2018; pp. 445–465. [Google Scholar]
- Bhandari, M.M. Flora of Indian Desert; MPS Reports: Jodhpur, India, 1990. [Google Scholar]
- Gilani, S.A.; Kikuchi, A.; Watanabe, K.N. Genetic Variation within and among Fragmented Populations of Endangered Medicinal Plant, Withania coagulans (Solanaceae) from Pakistan and Its Implications for Conservation. Afr. J. Biotechnol. 2009, 8, 2948–2958. [Google Scholar]
- Gupta, R.; Sonawane, T.; Pai, S. An Overview on Pharmaceutical Properties and Biotechnological Advancement of Withania coagulans. Adv. Tradit. Med. 2021, 22, 673–683. [Google Scholar] [CrossRef]
- Khan, M.I.; Maqsood, M.; Saeed, R.A.; Alam, A.; Sahar, A.; Kieliszek, M.; Miecznikowski, A.; Muzammil, H.S.; Aadil, R.M. Phytochemistry, Food Application, and Therapeutic Potential of the Medicinal Plant (Withania coagulans): A Review. Molecules 2021, 26, 6881. [Google Scholar] [CrossRef]
- Gupta, P.C. Withania coagulans Dunal—An Overview. Int. J. Pharm. Sci. 2012, 12, 68–71. [Google Scholar]
- Srivastav, A.K.; Das, P. Phytochemical Extraction and Characterization of Roots of Withania somnifera for Its Anti-Bacterial, Antioxidant, Anti-Inflammation and Analgesic Activity. Int. J. Innov. Res. Dev. 2014, 7, 22–33. [Google Scholar]
- Hemalatha, S.; Kumar, R.; Kumar, M. Withania coagulans Dunal: A Review. Pharmacogn. Rev. 2008, 2, 351–358. [Google Scholar]
- Ciddi, V. Withaferin A from Cell Cultures of Withania somnifera. Indian J. Pharm. Sci. 2010, 68, 490–492. [Google Scholar] [CrossRef]
- Jilani, K.; Adrian, L.; Mohanad, Z.; Nazneen, S.; Florian, L. Withaferin A-stimulated Ca2+ Entry, Ceramide Formation and Suicidal Death of Erythrocytes. Toxicol. Vitr. 2013, 27, 52–58. [Google Scholar] [CrossRef]
- Subramanian, S.S.; Sethi, P.D.; Glotter, E.; Kirson, I.; Lavie, D. 5, 20α(R)-Dihydroxy-6α,7α-Epoxy-1-Oxo-(5α) with a-2,24-Dienolide, A new steroidal lactone from Withania coagulans. Phytochemistry 1971, 10, 685–688. [Google Scholar] [CrossRef]
- Khare, C.P. Indian Medicinal Plants; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Prabu, P.C.; Panchapakesan, S.; Raj, C.D. Acute and Sub-Acute Oral Toxicity Assessment of the Hydroalcoholic Extract of Withania somnifera Roots in Wistar Rats. Phytother. Res. 2013, 27, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, M.; Qureshi, R.; Ikram, M.; Ahmad, M.S.; Jabeen, B.; Asi, M.R.; Khan, J.A.; Ali, S.; Lilge, L. In Vitro Anticancer Activities of Withania coagulans against HeLa, MCF-7, RD, RG2, and INS-1 Cancer Cells and Phytochemical Analysis. Integr. Med. Res. 2018, 7, 184–191. [Google Scholar] [CrossRef]
- Adnan, M.; Bibi, R.; Azizullah, A.; Andaleeb, R.; Mussarat, S.; Tariq, A.; Ullah, R.; Elsalam, N.M.; Khan, A.L.; Begum, S. Ethnomedicinal Plants Used against Common Digestive Problems. Afr. J. Tradit. Complement. Altern. Med. 2015, 12, 99–117. [Google Scholar] [CrossRef]
- Atta-ur-Rahman, A.; Abbas, S.; Dur-e-Shawar, N.A.; Jamal, A.S.; Choudhary, M.I. New Withanolides from Withania spp. J. Nat. Prod. 1993, 56, 1000–1006. [Google Scholar] [CrossRef]
- Neogi, P.; Kawai, M.; Butsugan, Y.; Mori, Y.; Suzuki, M. Withacoagin, a New Withanolide from Withania coagulans Roots. Bull. Chem. Soc. Jpn. 1988, 61, 4479–4481. [Google Scholar] [CrossRef]
- Kapoor, L. Handbook of Ayurvedic Medicinal Plants: Herbal Reference Library, 1st ed.; CRC Press: Boca Raton, FL, USA, 2001; Volume 2. [Google Scholar]
- Jain, R.; Kachhwaha, S.; Kothari, S. Phytochemistry, Pharmacology, and Biotechnology of Withania somnifera and Withania coagulans: A Review. J. Med. Plants Res. 2012, 6, 5388–5399. [Google Scholar]
- Mishra, J.; Dash, A.K.; Mishra, S.N.; Gupta, A.K. Withania coagulans in Treatment of Diabetics and Some Other Diseases: A Review. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 1251. [Google Scholar]
- Gupta, V.; Keshari, B.B. Withania coagulans Dunal (Paneer Doda): A Review. Int. J. Ayurvedic Herb. Med. 2013, 3, 1330–1336. [Google Scholar]
- Atta-ur-Rahman, A.; Choudhary, M.I.; Yousaf, M.; Gul, W.; Qureshi, S. New Withanolides from Withania coagulans. Chem. Pharm. Bull. 1998, 46, 1853–1856. [Google Scholar] [CrossRef]
- Maryam, K.; Mehrana; Mitra, N. Remedial Use of Withanolides from Withania Coagolans (Stocks). Adv. Life Sci. 2012, 2, 6–19. [Google Scholar]
- Adebajo, A.; Ayoola, O.; Iwadewa, E.; Akindahunsi, A.; Omisore, N.; Adewunmi, C.; Adenowo, T. Antitrichomonal, Biochemical and Toxicological Activities of Methanolic Extract and Some Carbazole Alkaloids Isolated from the Leaves of Murraya koenigii Growing in Nigeria. Phytomedicine 2006, 13, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Samadi, A.K.; Tong, X.; Mukerji, R.; Zhang, H.; Timmermann, B.N.; Cohen, M.S. Withaferin A, a Cytotoxic Steroid from Vassobia brewiflora, Induces Apoptosis in Human Head and Neck Squamous Cell Carcinoma. J. Nat. Prod. 2010, 73, 1476–1481. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.S.; Sethi, P.D. Withaferin-A from the Roots of Withania coagulans. Curr. Sci. 1969, 38, 267–268. [Google Scholar]
- Atta-ur-Rahman, A.; Dur-e-Shahwar, N.A.; Choudhary, M.I. Withanolides from Withania coagulans. Phytochemistry 2003, 63, 387–390. [Google Scholar] [CrossRef]
- Atta-ur-Rahman, A.; Shabbir, M.; Yousaf, M.; Qureshi, S.; Dur-e-Shahwar, N.A.; Naz, A.; Choudhary, M.I. Three Withanolides from Withania coagulans. Phytochemistry 1999, 52, 1361–1364. [Google Scholar] [CrossRef]
- Atta-ur-Rahman, M.; Shabbir, D.-E.-S.; Choudhary, M.I.; Voelter, W.; Hohnholz, D. New Steroidal Lactones from Withania coagulans. Heterocycles 1998, 47, 1005–1012. [Google Scholar]
- Velde, V.V.; Lavie, D.; Budhiraja, R.D.; Sudhir, S.; Garg, K.N. Potential Biogenetic Precursors of Withanolides from Withania coagulans. Phytochemistry 1983, 22, 2253–2257. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Dur-e-Shahwar, N.A.; Parveen, Z.; Jabbar, A.; Ali, I.; Atta-ur-Rahman, A. Antifungal Steroidal Lactones from Withania coagulanc. Phytochemistry 1995, 40, 1243–1246. [Google Scholar] [CrossRef]
- Mirjalili, M.H.; Moyano, E.; Bonfill, M.; Cusido, R.M.; Palazon, J. Steroidal Lactones from Withania somnifera, an Ancient Plant for Novel Medicine. Molecules 2009, 14, 2373–2393. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-M.; Gao, S.; Bunting, D.P.; Gunatilaka, A.A.L. Unusual Withanolides from Aeroponically Grown Withania somnifera. Phytochemistry 2011, 72, 518–522. [Google Scholar] [CrossRef]
- Nur-e-Alam, M.; Yousaf, M.; Qureshi, S.; Baig, I.; Nasim, S. A Novel Dimeric Podophyllotoxin-Type Lignan and a New Withanolide from Withania coagulans. Helv. Chim. Acta 2003, 86, 607–614. [Google Scholar] [CrossRef]
- Maurya, R.; Singh, A.B.; Srivastava, A.K. Coagulanolide, a Withanolide from Withania coagulans Fruits and Antihyperglycemic Activity. Bioorg. Med. Chem. Lett. 2008, 18, 6534–6537. [Google Scholar] [CrossRef] [PubMed]
- Hemalatha, S.; Wahi, A.K.; Singh, P.N.; Chansouria, J.P. Hypoglycemic Activity of Withania coagulans Dunal in Streptozotocin-Induced Diabetic Rats. J. Ethnopharmacol. 2004, 93, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-F.; Ma, L.; Sun, L.-J.; Ali, M.; Arfan, M.; Liu, J.-W.; Hu, L.-H. Immunosuppressive Withanolides from Withania coagulans. Chem. Biodivers. 2009, 6, 141. [Google Scholar] [CrossRef]
- Maher, S.; Choudhary, M.I.; Saleem, F.; Rasheed, S.; Waheed, I.; Halim, S.A.; Azeem, M.; Abdullah, I.B.; Froeyen, M.; Mirza, M.U.; et al. Isolation of Antidiabetic Withanolides from Withania coagulans Dunal and Their In Vitro and In Silico Validation. Biology 2020, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Ojha, A.; Srivastava, N. In Vitro Studies on Organophosphate Pesticides Induced Oxidative DNA Damage in Rat Lymphocytes. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 761, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Jogee, P.S.; Agarkar, G.; Santos, C.A. Anticancer Activities of Withania somnifera: Current Research, Formulations, and Future Perspectives. Pharm. Biol. 2016, 54, 189–197. [Google Scholar] [CrossRef]
- Budhiraja, R.D.; Sudhir, S.; Garg, K.N. Cardiovascular Effects of a Withanolide from Withania coagulans, Dunal Fruits. Indian J. Physiol. Pharmacol. 1983, 27, 129–134. [Google Scholar]
- Budhiraja, R.D.; Garg, K.N.; Sudhir, S.; Arora, B. Protective effect of 3-ss-hydroxy-2, 3-dihydrowithanolide F against CCl4-induced hepatotoxicity. Planta Medica 1986, 52, 28–29. [Google Scholar] [CrossRef]
- Budhiraja, R.D.; Sudhir, S.; Garg, K.N. Anti-inflammatory activity of 3 β-Hydroxy-2, 3-dihydro-withanolide F. Planta Med. 1984, 50, 134–136. [Google Scholar] [CrossRef] [PubMed]
- Bahr, V.; Hansel, R. Immunomodulating properties of 5, 20a (R)-dihydroxy-6a,7a-epoxy-1-oxo(5a)-witha-2,24-dienolide and solasodine. Planta Med. 1982, 44, 32–33. [Google Scholar] [CrossRef] [PubMed]
- Ramaiah, P.A.; Lavie, D.; Budhiraja, R.D.; Sudhir, S.; Garg, K.N. Spectroscopic Studies on a Withanolide from Withania coagulans. Phytochemistry 1984, 23, 143–149. [Google Scholar] [CrossRef]
- Sethi, P.D.; Subramanian, S. Steroidal Constituents of Withania coagulans Roots. Indian J. Pharm. 1971, 38, 22–23. [Google Scholar]
- Chapin, F.S., III; Zavaleta, E.S.; Eviner, V.T.; Naylor, R.L.; Vitousek, P.M.; Reynolds, H.L. Consequences of Changing Biodiversity. Nature 2000, 405, 234–242. [Google Scholar] [CrossRef]
- Jain, R.; Sinha, A.; Jain, D.; Kachhwaha, S.; Kothari, S.L. Adventitious Shoot Regeneration and In Vitro Biosynthesis of Steroidal Lactones in Withania coagulans (Stocks) Dunal. Plant Cell Tissue Organ Cult. 2010, 105, 135–140. [Google Scholar] [CrossRef]
- Rathore, M.S.; Shekhawat, S.; Kaur, G.; Singh, R.P.; Shekhawat, N.S. Micropropagation of Vegetable Rennet (Withania coagulans [Stocks] Dunal)-A Critically Endangered Medicinal Plant. J. Sustain. For. 2012, 31, 727–746. [Google Scholar] [CrossRef]
- Shahzad, A.; Sharma, S.; Parveen, S.; Saeed, T. Historical Perspective and Basic Principles of Plant Tissue Culture. In Plant Biotechnology: Principles and Applications; Abdin, M., Kiran, U., Kamaluddin, A., Eds.; Springer: Singapore, 2017; pp. 3–13. ISBN 978-981-10-2961-5. [Google Scholar]
- Shahzad, A.; Parveen, S.; Sharma, S.; Shaheen, A.; Saeed, T. Plant Tissue Culture: Applications in Plant Improvement and Conservation. In Plant Biotechnology: Principles and Applications; Abdin, M., Kiran, U., Kamaluddin, A., Eds.; Springer: Singapore, 2017; pp. 15–35. ISBN 978-981-10-2961-5. [Google Scholar]
- Jain, R.; Sinha, A.; Kachhwaha, S.; Kothari, S.L. Micropropagation of Withania coagulans (Stocks) Dunal: A Critically Endangered Medicinal Herb. J. Plant Biochem. Biotechnol. 2009, 18, 249–252. [Google Scholar] [CrossRef]
- Valizadeh, J.; Valizadeh, M. Development of Efficient Micropropagation Protocol for Withania coagulans (Stocks) Dunal. Afr. J. Biotechnol. 2011, 10, 7611–7616. [Google Scholar]
- Castilla, A.M. Micropropagation of Withania frutescens: One Way to Recover Reduced or Extinct Plant Populations in the Balearic Islands. Bolletí De La Soc. D’història Nat. De Les Balear. 2011, 54, 67–74. [Google Scholar]
- Sharma, N.; Sachdeva, P.; Dhiman, M.; Koshy, E.P. Comparative Evaluation of In Vitro Regeneration Potential of Seeds of W. somnifera and W. coagulans. Biotechnol. Int. 2015, 8, 21–33. [Google Scholar]
- Joshi, H.; Nekkala, S.; Soner, D.; Kher, M.M.; Nataraj, M. In Vitro Shoot Multiplication of Withania coagulans (stocks) Dunal. Plant Tissue Cult. Biotechnol. 2016, 26, 187–195. [Google Scholar] [CrossRef][Green Version]
- Rathore, M.S.; Mastan, S.G.; Yadav, P.; Bhatt, V.D.; Shekhawat, N.S.; Chikara, J. Shoot Regeneration from Leaf Explants of Withania coagulans (Stocks) Dunal and Genetic Stability Evaluation of Regenerates with RAPD and ISSR Markers. S. Afr. J. Bot. 2016, 102, 12–17. [Google Scholar] [CrossRef]
- Tripathi, D.; Rai, K.K.; Rai, S.K.; Rai, S.P.J. An Improved Thin Cell Layer Culture System for Efficient Clonal Propagation and In Vitro Withanolide Production in a Medicinal Plant Withania coagulans Dunal. Ind. Crops Prod. 2018, 119, 172–182. [Google Scholar] [CrossRef]
- Ghahremani, A.; Ganji Moghadam, E.; Tatari, M.; Khosroyar, S. Effect of Type and Concentration of Growth Regulators on the Proliferation and Marcotting of Withania coagulans Medical Plant. Iran. J. Hortic. Sci. 2020, 51, 2. [Google Scholar]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Hoque, A.; Islam, S. pH management in plant tissue culture: A review. J. Plant Biotechnol. 2021, 48, 241–250. [Google Scholar]
- Padmavathy, S.; Rajeswari, S. Role of pH in Plant Tissue Culture. In Plant Tissue Culture; Springer: Singapore, 2021; pp. 61–81. ISBN 978-981-15-8641-0. [Google Scholar]
- Jahan, M.S.; Rabbani, M.G. The Effect of Sucrose Concentration on Shoot Induction and Multiplication of Cucumis melo var. Dudaim via Tissue Culture Technique. Plant Cell Biotechnol. Mol. Biol. 2020, 21, 24–31. [Google Scholar]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar Sensing and Signaling in Plants: Conserved and Novel Mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, A.; Prasad, B.C.N. Role of Carbon Sources on In Vitro Plant Growth and Development. In Plant Growth and Health Promoting Bacteria; Springer: Singapore, 2017; pp. 33–47. [Google Scholar]
- Zhou, X.; Yu, Q.; Yao, Y.; Liu, X.; Zhang, M.; Zhang, Y. Effects of Agar Concentration and Plant Growth Regulators on In Vitro Shoot Regeneration of Sweet Cherry (Prunus avium L.). Horticulturae 2020, 55, 352–356. [Google Scholar]
- He, L.; Huang, X.; Li, X.; Li, Y.; Liu, Y.; Xu, Z. Agar and Activated Charcoal Concentrations Affect Shoot Regeneration from Hypocotyl Explants of Nitraria tangutorum Bobr. J. For. Res. 2021, 32, 1119–1128. [Google Scholar]
- George, E.F.; Hall, M.A.; De Klerk, G.J. Plant Propagation by Tissue Culture: Volume 1. The Background; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008; ISBN 978-1-4020-5005-7. [Google Scholar]
- Li, Y.; Wang, J.; Li, S.; Li, J.; Du, H.; Zhao, C. Effects of Light Quality and Photoperiod on In Vitro Regeneration and Growth of Lonicera macranthoides. Plant Cell Tissue Organ Cult. 2021, 10, 315–324. [Google Scholar]
- Ahmad, Z.; Teixeira da Silva, J.A.; Shahzad, A. Biotechnological Interventions in Bamboo Plants. Plant Cell Tissue Organ Cult. 2023, 153, 459–487. [Google Scholar] [CrossRef]
- Rahimi, S.; Bahmani, R.; Tabarzad, M. Temperature Effect on Callus Induction, Regeneration, and Antioxidant Enzymes Activity of Stevia rebaudiana Bertoni. Plants 2021, 10, 375–382. [Google Scholar]
- Jiang, Y.; Song, X.; Li, J.; Dai, X. Light Quality and Photoperiod Affect Shoot Proliferation and Rooting of Two Pear Rootstocks In Vitro. Front. Plant Sci. 2021, 12, 721311. [Google Scholar]
- Dehvari, N.P.; Abbaspour, H.H.; Asare, M.H.; Saadatmand, S. An Efficient Protocol for In Vitro Regeneration from the Nodal Explants of Withania coagulans (Stocks) Dunal: A Valuable Medicinal Herb. Acta Agric. Slov. 2021, 117, 1–7. [Google Scholar]
- Mirjalili, M.H.; Esmaeili, H. Callus Induction and Withanolides Production through Cell Suspension Culture of Withania coagulans (Stocks) Dunal. J. Med. Plants 2022, 21, 79–91. [Google Scholar] [CrossRef]
- Sharma, S.; Shahzad, A.; Teixeira da Silva, J.A. Synseed Technology—A Complete Synthesis. Biotechnol. Adv. 2013, 31, 186–207. [Google Scholar] [CrossRef]
- Van, K.T.T. Thin Cell Layer Concept. In Thin Cell Layer Culture System: Regeneration and Transformation Applications; Nhut, D.T., Van Le, B., Tran Thanh Van, K., Thorpe, T., Eds.; Springer: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Azad, M.O.K.; Al-Forkan, M.; Rana, M.M. Thin Cell Layer Culture: Features and Applications in Plant Biotechnology. Plant Cell Tissue Organ Cult. 2021, 10, 485–510. [Google Scholar]
- Chamkhi, I.; Benali, T.; Aanniz, T.; El Menyiy, N.; Guaouguaou, F.; El Omari, N.; El-Shazly, M.; Zengin, G.; Bouyahya, A. Plant-Microbial Interaction: The Mechanism and the Application of Microbial Elicitor Induced Secondary Metabolites Biosynthesis in Medicinal Plants. Plants 2021, 67, 269–295. [Google Scholar] [CrossRef]
- Ganie, I.B.; Ahmad, Z.; Shahzad, A.; Zaushintsena, A.; Neverova, O.; Ivanova, S.; Wasi, A.; Tahseen, S. Biotechnological Intervention and Secondary Metabolite Production in Centella asiatica L. Plants 2022, 11, 2928. [Google Scholar] [CrossRef] [PubMed]
- Jeyasri, R.; Muthuramalingam, P.; Karthick, K. Methyl Jasmonate and Salicylic Acid as Powerful Elicitors for Enhancing the Production of Secondary Metabolites in Medicinal Plants: An Updated Review. Plant Cell Tissue Organ Cult. 2023; in press. [Google Scholar] [CrossRef] [PubMed]
- Maurya, B.; Rai, K.K.; Pandey, N.; Sharma, L.; Goswami, N.K.; Rai, S.P. Influence of Salicylic Acid Elicitation on Secondary Metabolites and Biomass Production in In-Vitro Cultured Withania coagulans (L.) Dunal. Plant Arch. 2019, 19, 1308–1316. [Google Scholar]
- Tripathi, D.; Meena, R.P.; Pandey-Rai, S. Short term UV-B radiation mediated modulation of physiological traits and withanolides production in Withania coagulans (L.) Dunal under in-vitro condition. Physiol. Mol. Biol. Plants 2021, 27, 1823–1835. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.; Ming, Q.L.; Lin, B.; Rahman, K.; Zheng, C.J.; Han, T.; Qin, L.P. Medicinal Plant Cell Suspension Cultures: Pharmaceutical Applications and High-Yielding Strategies for the Desired Secondary Metabolites. Crit. Rev. Biotechnol. 2016, 36, 215–232. [Google Scholar] [CrossRef]
- Rao, S.R.; Ravishankar, G.A. Plant Cell Cultures: Chemical Factories of Secondary Metabolites. Biotechnol. Adv. 2002, 20, 101–153. [Google Scholar]
- Bagheri, A.; Valizadeh, M.; Sharifi, A.; Senthil, K. Evaluation of Withaferin a Content in Different Accessions and in In Vitro Cultures of Withania coagulans (Stocks) Dunal. Iran. J. Med. Aromat. Plants 2016, 16, 231–243. [Google Scholar]
- Singh, R.; Kalia, R.K.; Sharma, R.; Singh, R.; Dhawan, A.K. Hairy Root Culture: A Biotechnological Approach for Secondary Metabolite Production. Eng. Life Sci. 2015, 15, 1–11. [Google Scholar]
- Murugesan, P.A.; Senthil, K.A. Influence of Auxin on Biomass Production and Withanolide Accumulation in Adventitious Root Culture of Indian Rennet: Withania coagulans. Asian J. Pharm. Clin. Res. 2017, 10, 131–134. [Google Scholar] [CrossRef]
- Murthy, H.N.; Dandin, V.S.; Zhong, J.J.; Paek, K.Y. Strategies for Enhanced Production of Plant Secondary Metabolites from Cell and Organ Cultures. Prod. Biomass Bioact. Compd. Using Bioreact. Technol. 2014, 118, 471–508. [Google Scholar]
- Sharma, M.; Kaur, C.; Katnoria, J.K.; Nagpal, A.K. Hairy Root Cultures: An Efficient Plant-Based System for the Production of Secondary Metabolites. Crit. Rev. Biotechnol. 2021, 41, 28–48. [Google Scholar]
- Flores, H.E.; Vivanco, J.M.; Loyola-Vargas, V.M. “Radicle” Biochemistry: The Biology of Root Specific Metabolism. Trends Plant Sci. 1999, 4, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Sangwan, R.S.; Bansal, S.; Sangwan, N.S. Efficient genetic transformation of Withania coagulans (Stocks) Dunal mediated by Agrobacterium tumefaciens from leaf explants of In Vitro multiple shoot culture. Protoplasma 2013, 250, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Mirjalili, M.H.; Moyano, E.; Bonfill, M. Overexpression of the Arabidopsis thaliana Squalene Synthase Gene in Withania coagulans Hairy Root Cultures. Biol. Plant. 2011, 55, 357–360. [Google Scholar] [CrossRef]
- Jadaun, J.S.; Sangwan, N.S.; Narnoliya, L.K.; Tripathi, S.; Sangwan, R.S. Withania coagulans Tryptophan Decarboxylase Gene Cloning, Heterologous Expression, and Catalytic Characteristics of the Recombinant Enzyme. Protoplasma 2017, 254, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Bohra, M.; Sharma, K.; Kachhwaha, S.; Kothari, S.; Jain, R. Leaves and roots of In Vitro cultured Withania coagulans adopt different pathways for withanolide biosynthesis: A comparative transcriptome study. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Husain, I.; Bala, K.; Khan, I.A.; Khan, S.I. A Review on Phytochemicals, Pharmacological Activities, Drug Interactions, and Associated Toxicities of Licorice (Glycyrrhiza sp.). Food Front. 2021, 2, 449–485. [Google Scholar] [CrossRef]
- Raizner, A.E.; Cooke, J.P. Dietary Supplements: Facts and Fallacies. Methodist Debakey Cardiovasc. J. 2019, 15, 169–170. [Google Scholar] [CrossRef]
- Fasinu, P.S.; Bouic, P.J.; Rosenkranz, B. An Overview of the Evidence and Mechanisms of Herb-Drug Interactions. Front. Pharmacol. 2012, 3, 69. [Google Scholar] [CrossRef]
- Yasir, M.; Shrivastava, R.; Jain, P.; Das, D. Hypoglycemic and Antihyperglycemic Effects of Different Extracts and Combinations of Withania coagulans Dunal and Acacia arabica Lamk in Normal and Alloxan-Induced Diabetic Rats. Phcog. Commun. 2012, 2, 61–66. [Google Scholar] [CrossRef]
- Husain, I.; Dale, O.R.; Martin, K.; Gurley, B.J.; Adams, S.J.; Avula, B.; Chittiboyina, A.G.; Khan, I.A.; Khan, S.I. Screening of Medicinal Plants for Possible Herb-Drug Interactions through Modulating Nuclear Receptors, Drug-Metabolizing Enzymes and Transporters. J. Ethnopharm. 2023, 301, 115822. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).