Abstract
A variety of environmental issues are affecting crops all across the world, but rising temperatures are posing the greatest threat. High temperature has been found to drastically inhibit seedling emergence and cause leaf necrosis at the seedling stage, which results in poor plant stand and significantly decreased yields. Polyamines (PAs) are positively charged, low-molecular-weight aliphatic nitrogenous bases present in all living organisms and are involved in various biological processes in plant growth and development, including senescence and response to different abiotic stresses. Putrescine (Put) functions as a master growth regulator that promotes optimal plant development and greater stress tolerance. Here, the current study aimed to elucidate how Put (1 mM) functions in reducing the negative impacts of high temperature on four varieties of Brassica juncea (RH-1707, RH-1708, RH-1566 and RH-1999-42). Exposure of plants to high temperature resulted in decrease in growth parameters, chlorophyll content and relative water content. Simultaneously, increases were found in antioxidant enzymes, electrolyte leakage, lipid peroxidation, hydrogen peroxide content and stomatal density. High temperature more significantly affected varieties RH-1707 and RH-1708, while RH-1566 and RH-1999-42 showed lesser effects. Exogenous application of Put mitigated the negative impacts of high temperature by enhancing growth, chlorophyll content, relative water content and antioxidant enzyme activities and, simultaneously, it reduces oxidative damage and stomatal density. This study specifies that varieties RH-1707 and RH-1708 are sensitive whereas RH-1566 and RH-1999-42 are tolerant of high temperature and provides an insight into the effectiveness of Put in mitigating the effects of high temperature to a significant extent in B. juncea seedlings.
1. Introduction
Indian mustard (Brassica juncea) is an important oilseed crop being produced in tropical and subtropical areas as a cold season crop (6 °C to 27 °C). It is primarily cultivated in a variety of conditions, including rainfed and irrigated, early, timely, and late sowing, as well as solitary or mixed crop [1]. In India, the biggest rapeseed-mustard growing states are Rajasthan, Uttar Pradesh, Haryana and Madhya Pradesh, contributing 45.5%, 13.1%, 11.8% and 11.1%, respectively, to the national acreage during the past five years. With an average yield of 2058 kg ha−1, Haryana produced 1.25 million tonnes (13.42 percent of all India’s production) from 0.61 million hectares (9.78 percent of all India’s land) [2].
As a result of global climate change, high-temperature stress is becoming increasingly important for plant growth and development as it severely affects agricultural productivity [3]. With each degree centigrade rise in average temperature, agricultural productivity reduces by 17% [4]. Exposure to high temperatures can induce a wide range of morphological, anatomical, physiological and biochemical alterations in plants. These changes have significant impacts on plant growth and development and can result in a substantial decline in economic yield [5,6]. Among the essential phases of plant development, the early stages of seedling growth are one of the most important in stand establishment in many crops [7]. The exposure of plants to high temperatures during the early stages of growth retards the overall growth including yield. Complex effects of heat stress on crop plants include increased seedling mortality, reduced photosynthesis, senescence of the leaves, decreased pollen production and viability, seed abortion and ultimately lower grain quantity and weight [8]. High temperature has been found to drastically inhibit seedling emergence and cause leaf necrosis at the seedling stage, which results in poor plant stand and significantly decreased yields [9].
Due to their immobility, plants cannot evade stress; however, they employ various strategies such as early maturation, modifying the composition of membrane lipids, producing stress proteins, osmotic adjustment and the restoration of the redox balance of cells and homeostasis by modifying the antioxidant system [10]. The application of phytohormones, osmoprotectants and polyamines (PA) is found to effectively mitigate the effects of heat stress in plants. Polyamines are a class of small, polycationic and aliphatic metabolites that are ubiquitously present in all organisms. They participate in a range of physiological reactions within organisms [11]. In plant cells, the main polyamines are putrescine (Put), spermidine (Spd) and spermine (Spm) [12]. These polyamines can exist either in their free form or as conjugates bound to other molecules such as phenolic acids, proteins and nucleic acids [13]. The cationic nature of polyamines contributes to their biological activity [14,15]. They play a role in various cellular processes, including chromatin condensation, maintaining the structure of DNA, RNA processing, translation and activating proteins [16,17,18]. PAs work by regulating osmosis and detoxifying the cell by removing ROS by boosting antioxidant defense capacity or preventing ROS synthesis [19]. The involvement of PAs in plant growth and development, as well as enhancing their ability to withstand stress, is widely recognized [20,21,22].
Putrescine is the central product of the polyamine biosynthesis pathway [23]. It is produced through the decarboxylation of ornithine and arginine, catalyzed by the enzymes ornithine decarboxylase (ODC) and arginine decarboxylase (ADC), respectively [24]. It plays a significant role in providing tolerance to different abiotic stresses. Scavenging free radicals, controlling ABA levels, avoiding lipid peroxidation, maintaining cellular pH and ionic equilibrium and regulating cationic channels are among the key reported processes related to ability of Put to produce abiotic stress tolerance responses [25]. Several studies have shown the significant role of Put in enhancing abiotic stress tolerance such as drought [26,27,28], salinity [29,30], cold [31,32] and heat [33,34,35]. The effectiveness of polyamines (1 mM) in mitigating the harmful effects of high temperature has been extensively studied [36,37,38]. The role of polyamines in mitigating high-temperature stress in B. juncea is largely unexplored. As a result, this study was undertaken to investigate the acclimation response of B. juncea to high-temperature stress through the exogenous application of Put (1 mM). The aim was to understand how the exogenous application of Put may contribute to enhancing the plant’s tolerance to high temperatures.
2. Materials and Methods
2.1. Growth Conditions, Plant Materials, and Treatments
Four Indian mustard (Brassica juncea L. Czern. & Coss.) varieties RH-1707, RH-1708, RH-1566 and RH-1999-42 were utilized for the study. Seeds were obtained from Oilseeds Section, Department of Genetics and Plant Breeding, CCS Haryana Agricultural University, Hisar. Uniformly selected seeds were surface sterilized with 0.1% HgCl2 for one minute and then repeatedly washed with distilled water. Sowing was carried out in pots containing normal homogenized field soil in a greenhouse. Each variety was sown in triplicates. Seedlings were allowed to grow under controlled conditions (light- 75 W/m2, 65% RH, temp 25 ± 2 °C) and watered regularly for 21 days.
For heat treatment, seedlings were transferred to a growth chamber and exposed to a gradually elevating temperature of 38–40 °C (RH ~ 45–50%) for 3 h in light. Then the temperature was decreased to 25 °C (RH ~ 70%) and the entire cycle was repeated for 7 days. Water was not given during the period of heat stress. A control experiment was carried out in a greenhouse where optimum temperature (25 °C, RH ~ 70%) was maintained throughout the experiment. Put (1 mM) was sprayed on both sides of the leaves of seedlings using a manual sprayer two hours prior to exposing them to heat treatment. Similarly, control plants were sprayed with an equal volume of double-distilled water and Put (1 mM). There were four distinct treatments used in the experiment—(1) Control, (2) Put (1 mM), (3) High temperature (HT) (38–40 °C) and (4) HT + Put (1 mM). Following seven days of treatment, the treated plants were acclimatized for two days under a normal environment and leaves from each treatment were collected for subsequent analysis.
2.2. Determination of Growth Attributes
Growth parameters such as seedling length, fresh weight, dry weight and leaf area per plant were measured. Plants were gently removed from their pots and cleaned to remove the dirt and dust from the roots. The fresh weight of plants was recorded, and subsequently they were kept for drying in an oven at 80 °C to record dry weight. Seedling length refers to combined shoot length and root length which was determined by carefully harvesting seedlings from pots and measuring length using a standard scale. Leaf area was measured using a leaf area meter (LI 3000 Area meter, LICOR Ltd., Lincoln, NE, USA).
2.3. Chlorophyll Content
Chlorophyll and carotenoids were extracted by according to the method of [39,40] using 5 mL of methanol solution for each 50 mg of fresh leaf tissue, and absorbance was read at 480 nm, 645 nm and 663 nm. Chlorophyll content was calculated using the equations:
2.4. Plant Water Status
2.4.1. Relative Water Content
Leaf relative water content was calculated by using the method as described [41]. Leaf samples were collected and weighed immediately to measure weight. Leaves were placed separately in petri dishes filled with distilled water. Afterwards, the same leaves (fully turgid) were weighed again and placed in an oven at 85 °C for 72 h for drying and weighing and used to calculate relative water content percentage (RWC %) by the formula:
2.4.2. Electrolyte Leakage
Electrolyte leakage was analyzed using a conductivity meter according to the method described by [42]. Fresh leaves were cut into smaller pieces and then incubated in 20 mL of deionized water and kept overnight. Next day, the initial electrical conductivity (EC1) of the medium was measured. The samples were autoclaved for 30 min to release all the electrolytes, cooled, and then final electrical conductivity (EC2) was measured. The electrolyte leakage percentage was calculated as follows:
2.5. Antioxidant Enzyme Estimation
2.5.1. Preparation of Enzyme Extract
200 mg of leaf samples were homogenized in a mortar and pestle using a 0.1 M potassium phosphate buffer with a pH of (7.0). In a chilled centrifuge set at 4 °C, the homogenate was centrifuged for 30 min at 12,000 rpm. The supernatant was used to measure the activity of antioxidant enzymes, including superoxide dismutase (SOD) and catalase (CAT).
2.5.2. Superoxide Dismutase
The potential of SOD to prevent the photochemical reduction of nitro blue tetrazolium (NBT) was used to measure its activity [43]. Three mL of reaction mixture contained 50 mM of potassium phosphate buffer (pH 7.8), 13 mM of methionine, 25 mM of nitro blue tetrazolium (NBT), 2 μM of riboflavin, 0.1 mM of EDTA, 50 mM of sodium carbonate and 0.1 mL of enzyme extract. The test tubes were shaken properly to homogenize the reaction and the mixture was illuminated for 30 min. Absorbance was recorded at 560 nm.
2.5.3. Catalase
Catalase activity was estimated using the protocol given by [44]. Three mL of reaction mixture contained 0.1 mL of enzyme extract, 1.5 mL of 100 mM phosphate buffer (pH 7.0), 0.5 mL of 75 mM H2O2 and 950 μL of distilled water. The reduction in H2O2 absorbance (=39.4 mM1 cm−1) at 240 nm was used to calculate catalase activity. The enzyme activity was expressed as mM of H2O2 reduced/min/mg FW.
2.6. MDA Content
Lipid peroxidation was determined by using method [45]. Leaf tissue (500 mg) was homogenized in 10 mL of 0.1% (w/v) Trichloroacetic acid (TCA) and centrifuged for 20 min at 10,000 rpm. One ml of the supernatant was mixed with 4 mL of 0.5% Thiobarbituric acid (ΤΒA) diluted in 20% TCA, incubated in a water bath at 95 °C for 30 min and then cooled in an ice bath. The absorbance was measured at 532 and 600 nm. Lipid peroxidation was expressed as μ mols malondialdehyde (MDA)/g FW.
2.7. H2O2 Content
Hydrogen peroxide levels were determined according to [46]. An amount of 200 mg of leaf tissues was homogenized in an ice bath with 1 cc of TCA, 0.1%. After centrifuging the homogenate at 12,000× g for 15 min, 0.5 mL of the supernatant was added to 1 mL of 1 M potassium iodide (KI) and 0.5 mL of 10 mM potassium phosphate buffer (pH 7.0). At 390 nm, the supernatant’s absorbance was measured using the standard curve.
2.8. Histochemical Analysis
The method of Evans blue staining was employed to observe the death of cells in leaf tissue. This was performed by immersing fresh leaves in a solution consisting of 0.25% (w/v) aqueous solution of Evans blue [47]. Leaves were kept in the solution overnight and then bleached with 95% v/v ethanol. The occurrence of cell death could be observed through the presence of blue patches on the surface of the leaf.
To analyze the accumulation of superoxide ion (O2·−) through the histochemical staining method, leaves were stained using nitro blue tetrazolium (NBT) [48]. The samples from each treatment were immersed into 1 mg/mL of NBT solution prepared in 10 mM of phosphate buffer (pH 7.8). Leaves were illuminated until dark blue spots became visible. Subsequently, the leaves were treated with ethanol to remove the staining.
2.9. Stomatal Density
The stomatal density was evaluated by the method described by [49]. A fresh leaf sample was taken and gently washed with running tap water to remove any kind of dust particles. A coat of clear nail polish was applied on the abaxial (lower) surface of the leaf to obtain a suitable replica, then allowed to dry completely for 8–10 min. The replica was gently peeled off using a forceps and placed on the slide in such a manner that the imprinted surface was on the upper side. One or two drops of water were put on the slide and then covered with a coverslip. Similarly, replica preparations were performed for all the samples and observed under the light microscope. The total number of stomata visible in the circular view field of the microscope at a given magnification were counted. The total number of stomata from the lower surface of the leaf were recorded and the area of the circle under the microscopic view field was calculated by the formula: πr 2, where r is the radius of the circle (view field), i.e., ½ of the diameter of the circle.
2.10. Statistical Analysis
The data were analysed using a completely randomized block design (CRD). Two factorial analyses of variance were performed on the data using OPSTAT software (CCSHAU, Hisar, India; http://14.139.232.166/opstat/, accessed on 7 May 2023) to determine the significance of differences among the treatments at p < 0.05. Least significant difference (LSD) was calculated for the significant data to identify difference in the mean of the varieties (p < 0.05).
3. Results
3.1. Growth Parameters
3.1.1. Seedling Length
The growth of seedlings in all the studied varieties was reduced by high temperatures. Exogenous Put application resulted in significant enhancement, counteracting the negative impact (Figure 1). A significant decrease in seedling length was noticed in RH-1708 (36.01%) and RH-1707 (33.35%); however, a lesser decrease was shown by RH-1566 (22.94%) and RH-1999-42 (15.66%). The application of Put under normal temperature showed a slight increment in the length of seedlings in RH-1708 (10.75%), RH1707 (7.56%), RH-1566 (5.35%) and RH-1999-42 (4.12%). The application of Put mitigated the effects of high temperature, leading to improvements in RH-1708 (28.46%), Rh-1707 (26.78%), RH-1999-42 (17.39%) and RH-1566 (11.32%) (Figure 2).
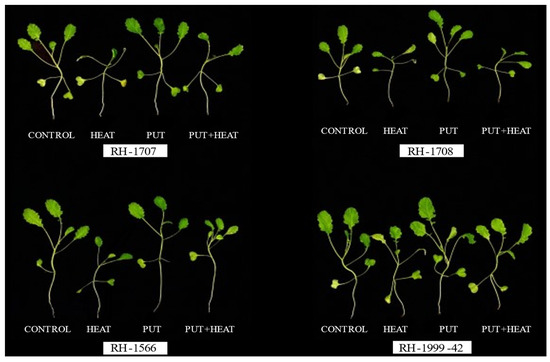
Figure 1.
Morphology of Brassica juncea seedlings under the effect of high temperature and Putrescine.
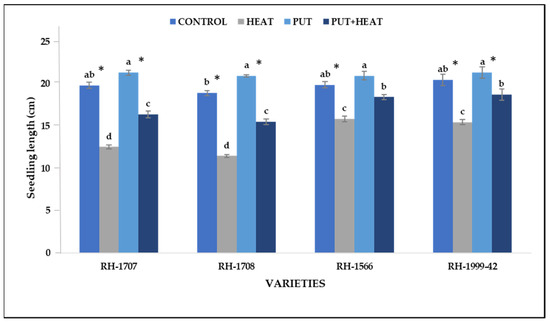
Figure 2.
Effect of high temperature and Putrescine on seedling length in Brassica juncea seedlings. Each bar represents the mean (n = 3) and the error bar indicates the standard deviation. Asterisk (*) indicates a significant difference between the treatments. Different alphabets for each mean show statistically significant differences between the varieties according to the least significant difference (LSD) test (p < 0.05).
3.1.2. Fresh Weight
Seedlings subjected to high-temperature stress experienced a decrease in their fresh weight. The reduction was more pronounced in RH-1708 (65.56%) and RH-1707 (60.39%). On the other hand, RH-1999-42 (34.1%) and RH-1566 (28.05%) exhibited a lesser decline in fresh weight. Put application led to a small improvement in fresh weight of seedlings under normal temperature, i.e., RH-1707 (17.4%), RH-1708 (16.7%), RH-1566 (14.88%) and RH-1999-42 (13.46%). Under high-temperature conditions, the exogenous application of Put led to a significant increase in the fresh weight of seedlings in RH-1708 (30.73%), RH-1707 (26.78%), RH-1999-42 (24.05%) and RH-1566 (18.15%) (Figure 3).
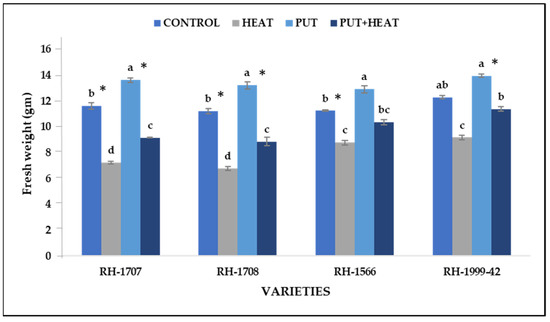
Figure 3.
Effect of high temperature and Putrescine on fresh weight in Brassica juncea seedlings. Each bar represents the mean (n = 3) and the error bar indicates the standard deviation. Asterisk (*) indicates significant difference between the treatments. Different alphabets for each mean show statistically significant differences between the varieties according to the least significant difference (LSD) test (p < 0.05).
3.1.3. Dry Weight
The effect of high temperature on dry weight of seedlings was comparable to that on fresh weight. Under high temperature, the most significant reduction was shown by varieties RH-1708 (59.46%) and RH-1707 (56.87%), whereas varieties RH-1999-42 (39.12%) and RH-1566 (33.23%) were less affected. Under normal temperature conditions, a minimal increase in dry weight occurred after the application of Put in all the treated varieties, as seen in RH-1708 (8.3%), RH-1566 (8.01%), RH-1707 (6.62%) and RH-1999-42 (3.55%). However, under high temperature, exogenous application of Put led to an increment in the dry weight of varietiesRH-1708 (25.26%), RH-1707 (23.61%), RH-156 (20.16%) and RH-1999-42 (15.17%) (Figure 4).
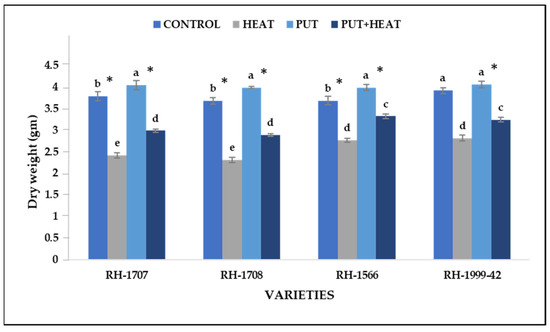
Figure 4.
Effect of high temperature and Putrescine on dry weight in Brassica juncea seedlings. Each bar represents the mean (n = 3) and the error bar indicates the standard deviation. Asterisk (*) indicates significant difference between the treatments. Different alphabets for each mean show statistically significant differences between the varieties according to the least significant difference (LSD) test (p < 0.05).
3.1.4. Leaf Area per Plant
After being exposed to high temperatures, the plants exhibited a visible reduction in leaf area and displayed symptoms resembling chlorosis. However, the application of Put helped alleviate these symptoms (Figure 5).

Figure 5.
Morphology of Brassica juncea leaves under the effect of high temperature and Putrescine.
High-temperature stress reduces the leaf area per plant substantially. The reduction was more prominent in RH-1708 (68.96%) and RH-1707 (54.29%) than in RH-1999-42 (27.62%) and RH-1566 (17.73%). Under normal temperature, application of Put led to a slight increase in leaf area in RH-1708 (13.06%), RH-1707 (12.41%), RH-1566 (9.8%) and RH-1999-42 (8.76%). The combined treatment of plants with both high temperature and Put resulted in a reduction in the adverse effects caused by high-temperature stress. This combined treatment helped to maintain leaf area more efficiently, particularly in variety RH-1708 (33.07%) followed by RH-1707 (27.92%), RH-1999-42 (19.31%) and RH-1566 (9.98%) (Figure 6).
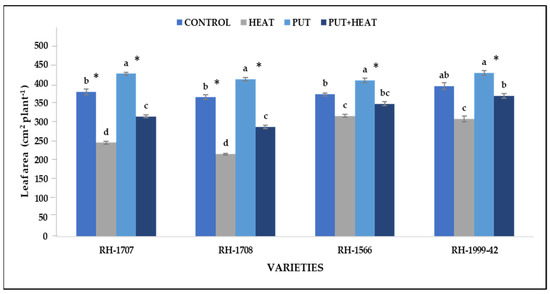
Figure 6.
Effect of high temperature and Putrescine on leaf area plant−1 in Brassica juncea seedlings. Each bar represents the mean (n = 3) and the error bar indicates the standard deviation. Asterisk (*) indicates significant difference between the treatments. Different alphabets for each mean show statistically significant differences between the varieties according to the least significant difference (LSD) test (p < 0.05).
3.2. Chlorophyll Content
High-temperature stress leads to a sharp decline in chlorophyll content in varietiesRH-1708 (64.86%) and RH-1707 (55.28%) whereas RH-1566 (24.5%) and RH-1999-42 (21.25%) were less affected. Under controlled conditions, exogenous application of Put led to a slight increase in chlorophyll content in RH-1999-42 (12.61%), RH-1566 (9.64%), RH-1707 (6.23%) and RH-1708 (5.32%). However, combined treatment of both Put and high temperature led to a considerable improvement in chlorophyll content as was seen in RH-1707 (27.88%), RH-1708 (20.73%), RH-1999-42 (27.57%) and RH-1566 (18.29%) (Figure 7).
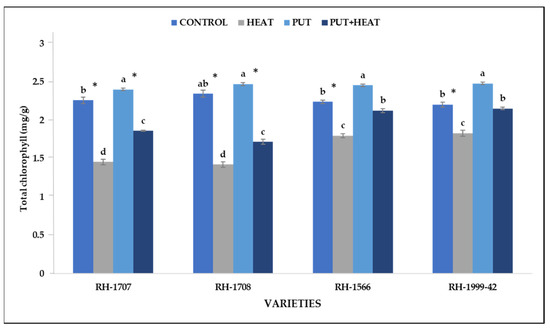
Figure 7.
Effect of high temperature and Putrescine on chlorophyll content in Brassica juncea seedlings. Each bar represents the mean (n = 3) and the error bar indicates the standard deviation. Asterisk (*) indicates significant difference between the treatments. Different alphabets for each mean show statistically significant differences between the varieties according to the least significant difference (LSD) test (p < 0.05).
3.3. Plant Water Status
3.3.1. Relative Water Content (RWC)
Under high temperature, the RWC content was significantly reduced, more substantially in varieties RH-1708 (64.86%) and RH-1707 (55.28%) whereas varieties RH-1566 (24.58%) and RH-1999-42 (20.25%) showed a lesser decline. The exogenous application of Put at normal temperature led to a slight increase in RWC in varieties RH-1999-42 (12.61%), RH-1566 (9.64%), RH-1707 (6.23%) and RH-1708 (5.32%). However, under high temperature, Put application shows a more efficient increase in the water status of plants, as was seen in varieties RH-1707 (27.88%), RH-1708 (20.73%), RH-1566 (18.29%) and RH-1999-42 (17.57%) (Figure 8).
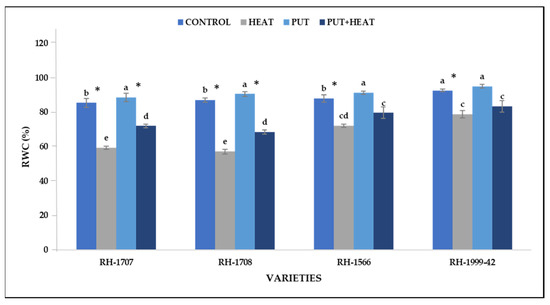
Figure 8.
Effect of high temperature and Putrescine on RWC in Brassica juncea seedlings. Each bar represents the mean (n = 3) and the error bar indicates the standard deviation. Asterisk (*) indicates significant difference between the treatments. Different alphabets for each mean show statistically significant differences between the varieties according to the least significant difference (LSD) test (p < 0.05).
3.3.2. Electrolyte Leakage
All the studied varieties experienced an elevation in electrolyte leakage, but a more significant increase was noticed in varieties RH-1708 (61.45%) and RH-1707 (55.87%) than in RH-1999-42 (32.38%) and RH-1566 (24.17%). The exogenous application of Put at normal temperature led to a slight increase in electrolyte leakage in varieties RH-1999-42 (13.76%), RH-1707 (10.21%), RH-1566 (8.93%) and RH-1708 (8.66%). The damage caused to membranes due to high temperature was considerably reduced by exogenous application of Put, as was seen in varieties RH-1707 (23.29%), RH-1999-42 (21.92%), RH-1566 (8.93%) and RH-1708 (8.66%) (Figure 9).
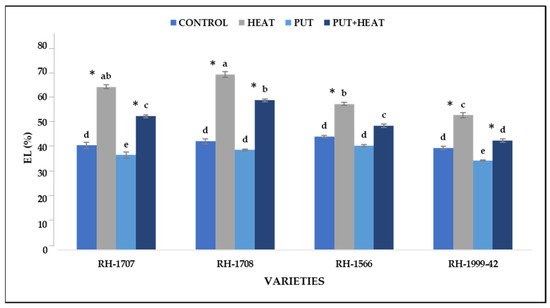
Figure 9.
Effect of high temperature and Putrescine on electrolyte leakage in Brassica juncea seedlings. Each bar represents the mean (n = 3) and the error bar indicates the standard deviation. Asterisk (*) indicates significant difference between the treatments. Different alphabets for each mean show statistically significant differences between the varieties according to the least significant difference (LSD) test (p < 0.05).
3.4. Antioxidant Enzyme Activity
3.4.1. Superoxide Dismutase
Under high-temperature conditions, the activity of superoxide dismutase (SOD) was observed to increase in all the stuided varieties. A more significant increase was seen in varieties RH-1999-42 (44.63%) and RH-1566 (39.88%) than in varieties RH-1707 (28.92%) and RH-1708 (26.12%). The activity of SOD was higher among plants sprayed with Put in the absence of heat stress than in control plants, as seen in varieties RH-1708 (19.08%), RH-1707 (19.93%), RH-1999-42 (15.82%) and RH-1566 (11.42%). The activity increased greatly in seedlings subjected to combined heat stress and Put treatment. RH-1999-42 (50.06%) and RH-1566 (46.98%) show a more significant increase than RH-1707 (30.92%) and RH-1708 (28.16%) (Figure 10).
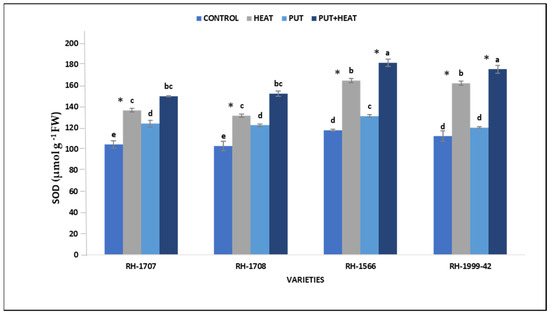
Figure 10.
Effect of high temperature and Putrescine on SOD in Brassica juncea seedlings. Each bar represents the mean (n = 3) and the error bar indicates the standard deviation. Asterisk (*) indicates significant difference between the treatments. Different alphabets for each mean show statistically significant differences between the varieties according to the least significant difference (LSD) test (p < 0.05).
3.4.2. Catalase
Under high temperature, the activity of catalase was observed to be enhanced. RH-1999-42 showed a more pronounced increase in catalase activity (60.84%) under high-temperature conditions compared to RH-1566 (53.18%), RH-1708 (44.43%) and RH-1707 (40.19%). Under controlled conditions, exogenous application of Put led to a slight increment in catalase content in varieties RH-1999-42 (16.27%), RH-1707 (14.61%), RH-1566 (10.48%) and RH-1708 (8.67%). When Put was applied in combination with high temperature, the maximum increase in catalase activity was observed in RH-1999-42 (70.76%), surpassing the increase in RH-1566 (59.64%), RH-1708 (56.29%) and RH-1707 (50.24%) (Figure 11).
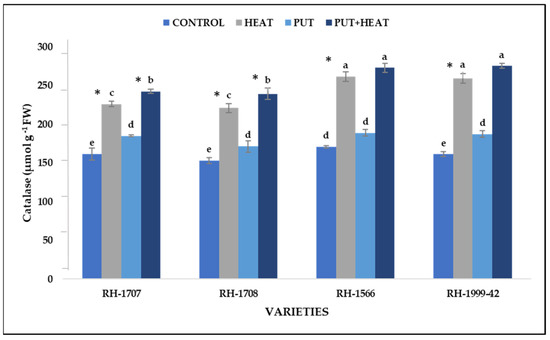
Figure 11.
Effect of high temperature and Putrescine on catalase in Brassica juncea seedlings. Each bar represents the mean (n = 3) and the error bar indicates the standard deviation. Asterisk (*) indicates significant difference between the treatments. Different alphabets for each mean show statistically significant differences between the varieties according to the least significant difference (LSD) test (p < 0.05).
3.5. Lipid Peroxidation
Under high-temperature conditions, all the studied varieties exhibited an increase in lipid peroxidation, with the maximum increase in RH-1708 (71.98%) and RH-1707 (67.14%). RH-1566 (48.03%) and RH-1999-42 (47.07%) showed an increase but to a lesser extent. Under normal temperature, the application of Put resulted in a more efficient reduction of MDA levels in variety RH-1999-42 (30.59%) in comparison to RH-1707 (24.01%), RH-1566 (15.37%), and RH-1708 (7.3%). The exogenous application of Put in combination with high temperature resulted in a reduction of MDA content in RH-1707 (27.02%), RH-1708 (26.73%), RH-1566 (13.54%) and RH-1999-42 (11.74%) (Figure 12).
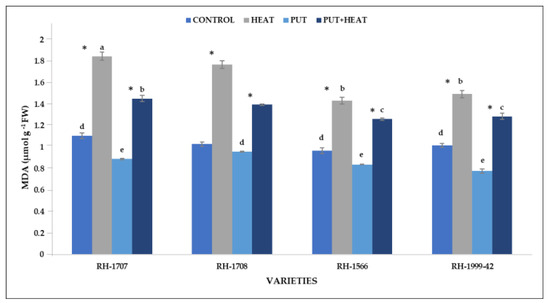
Figure 12.
Effect of high temperature and Putrescine on MDA in Brassica juncea seedlings. Each bar represents the mean (n = 3) and the error bar indicates the standard deviation. Asterisk (*) indicates significant difference between the treatments. Different alphabets for each mean show statistically significant differences between the varieties according to the least significant difference (LSD) test (p < 0.05).
3.6. H2O2 Content
Under high temperature, H2O2 content increased in all four varieties. RH-1708 (51.34%) and RH-1707 (45.81%) showed a more noteworthy increase than RH-1999-42 (30.54%) and RH-1566 (26.21%). Under normal temperature, a decrease in H2O2 content was observed in seedlings treated with exogenous Put. Specifically, in RH-1999-42, there was the maximum reduction (27.25%), followed by RH-1708 (14.85%), RH-1707 (10.03%), and RH-1566 (9.29%). The application of Put along with high temperature was functional in reducing the H2O2 content as was seen in all the varieties, RH-1708 (21.95%), RH-1707 (20.32%), RH-1566 (18.68%) and RH-1999-42 (15.26%) (Figure 13).
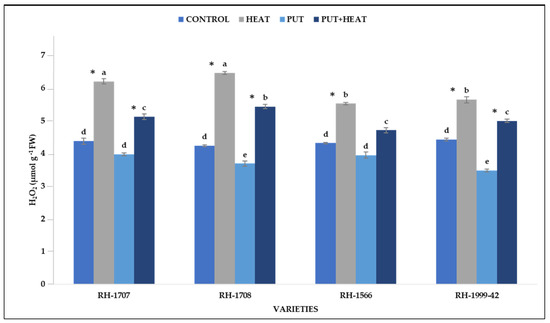
Figure 13.
Effect of high temperature and Putrescine on H2O2 in Brassica juncea seedlings. Each bar represents the mean (n = 3) and the error bar indicates the standard deviation. Asterisk (*) indicates significant difference between the treatments. Different alphabets for each mean show statistically significant differences between the varieties according to the least significant difference (LSD) test (p < 0.05).
3.7. Histochemical Analysis
The staining process using Evans blue dye revealed the rapid initiation of cell death, which was indicated by the appearance of blue-colored spots on the leaf surfaces. These spots were more noticeable in leaves exposed to high temperatures compared to the control group. The presence of blue spots indicated the irreversible uptake of Evans blue stain by the dying cells. However, when high-temperature conditions were combined with the application of Put, there were significantly fewer blue spots observed on the leaves (Figure 14A).

Figure 14.
Histochemical analysis in leaves of Brassica juncea exposed to high temperature and Putrescine (1 mM). (A). Cell death visualization using Evans blue staining method (blue color indicates death of cells), (B). Localization of superoxide radical by NBT staining (blue colored spots reflect reduction of NBT to formazan).
Formation of dark blue colored spots of formazan due to reduction of NBT by O2·− was seen distributed all over the surface of leaves under high temperature, which clearly marked the accumulation of superoxide ions. When leaves were treated with Put under normal temperature conditions, there were very few spots observed, suggesting a reduction in the accumulation of superoxide ions. Seedlings that were subjected to the combined treatment of Put and high temperature exhibited a diminished accumulation of O2·−. This was evidenced by a reduction in the number of dark blue spots (Figure 14B).
3.8. Stomatal Density
Under high-temperature stress, an increase in stomatal density was observed (Figure 15). RH-1708 exhibited the most substantial increase (37.84%), followed by RH-1707 (29.41%), while varieties RH-1999-42 (19.63%) and RH-1566 (10.58%) showed a lesser increase. No significant change in stomatal density was observed in seedlings treated with Put under normal temperature conditions. However, application of Put under high temperature reduced the density of stomata in leaves to some extent. The maximum percentage reduction was seen in RH-1708 (18.7%) followed by RH-1707 (9.19%) and RH-1999-42 (6.17%). No significant change was observed in RH-1566 (0.2%) (Figure 16).

Figure 15.
Stomatal density in leaves of Brassica juncea under high temperature and Putrescine.
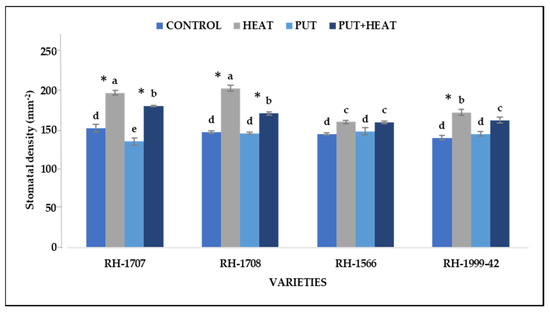
Figure 16.
Effect of high temperature and Putrescine on stomatal density in Brassica juncea seedlings. Each bar represents the mean (n = 3) and the error bar indicates the standard deviation. Asterisk (*) indicates significant difference between the treatments. Different alphabets for each mean show statistically significant differences between the varieties according to the least significant difference (LSD) test (p < 0.05).
4. Discussion
Indian mustard (Brassica juncea L.) is one of the most important oilseed crops in the country and it occupies a considerable acreage among the Brassica group of oilseed crops. One of the most harmful stresses for plant growth and development is the steadily rising ambient temperature. Heat stress has a number of effects on biological functions, either directly or indirectly through the alteration of the environment. In Brassica species, high temperatures during growth and developmental phases can cause substantial yield losses [50,51]. There are several pieces of evidence that exogenous application of polyamines such as Put, spermidine and spermine protects plants against the damage caused by various types of abiotic stresses [52,53]. Polyamines are the endogenous plant growth regulators, or they may act as intracellular messengers that encourage a number of physiological and biochemical processes in response to high temperature. As a result, they increase tolerance of plants to stresses by modifying growth and development. [54,55].
In the present study, the effects of high temperature on four varieties of B. juncea, namely RH-1707, RH-1708, RH-1566 and RH-1999-42, were analyzed, and various morpho-physiological and biochemical parameters were studied. This study revealed that high-temperature stress significantly reduced various growth-related parameters such as seedling length, fresh weight, dry weight and leaf area. The most prominent decrease was shown by variety RH-1708, followed by RH-1707. The impact of exogenous application of Put shows an improvement in these growth-related parameters which suggests its effectiveness in ameliorating heat-induced morphological damage. Our results were in agreement with the findings of El Bassiouny et al. [56] and Hassanein et al. [34] in wheat and Amooaghaie et al. in soybean [36].
One of the crucial characteristics of plants with improved tolerance and better performance under high temperatures is reportedly their RWC [57]. Elevated temperatures led to a notable decrease in the RWC across all the examined varieties. However, RH-1566 and RH-1999-42 exhibited a smaller decline. Our results are in agreement with work on rice [58] and wheat [59]. The decrease in leaf water content might affect plant metabolism and decrease plant growth and biomass. The decrease in RWC of the leaves may be related to the reduction in the quantity, mass and development of the roots during heat stress, which eventually restricts the provision of water and nutrients to the plant’s above-ground portions [60]. In this study, the exogenous application of Put substantially improved the water status of leaves, which is in accordance with the findings of Gupta et al. in wheat under heat stress [61].
High-temperature stress is known to cause a loss of leaf pigment in plants and seriously impairs photosynthetic processes. In the present study, it was observed that all four varieties experienced a reduction in chlorophyll content when exposed to high temperatures. The impact was particularly noticeable in RH-1708 and RH-1707, demonstrating a more pronounced effect. Chlorophyll breakdown or the suppression of chlorophyll production may be responsible for the reduction in chlorophyll content [62]. The exogenous application of Put reduced heat-induced chlorophyll degradation in our study. Put is known to increase leaf chlorophyll levels and protect thylakoid membranes via a chlorophyll-protein complex site [63]. Similarly to our findings, Put reduced the heat-induced damage to chlorophyll pigments in wheat [57]. Additionally, the generation of harmful oxygen species and a decrease in antioxidant defense are the causes of the impact of high temperatures on pigments and other photosynthetic machinery [64]. Thus, there might be a correlation between decrease in chlorophyll content and increase EL, MDA and H2O2 content. Our result revealed that varieties with higher EL, MDA and H2O2 content had lower chlorophyll content also. RH-1707 and RH-1708 showed much higher oxidative damage, which may reveal their heat sensitivity. However, the other two varieties, RH-1566 and RH-1999-42, had lowered EL, MDA and H2O2 content, indicating that these varieties could maintain their membrane integrity under heat stress. Maintaining membrane stability and integrity under stress conditions is a key element of tolerance and is necessary for continued photosynthetic and respiratory function [65]. RH-1566 and RH-1999-42 may be heat-tolerant as a result of lower oxidative damage. Polyamines play a crucial role in preserving the integrity of the membrane by lowering electrolyte leakage and MDA levels [66]. In the present study, a decline in the content of EL, MDA and H2O2 was seen after the application of Put. Our results correlated with the findings of Islam et al. [67] in Beta vulgaris, Bhattacharjee and Mukherjee in Amaranthus lividus seedlings [68] and Nagesh and Devaraj in Phaseolus vulgaris [69], wherein Put-treated plants experienced less oxidative stress than untreated plants, as seen by decreased electrolyte leakage and lesserH2O2 and MDA buildup. This is mostly because of the increased antioxidant enzyme activities that regulate ROS homeostasis.
A significant rise in antioxidant enzyme levels was observed when plants were subjected to high-temperature stress or treated with Put. This can be considered as a mechanism employed by the plants for the detoxification of ROS. RH-1566 and RH-1999-42 exhibited a greater increase in antioxidant enzyme concentration under heat stress compared to the other two varieties. This observation suggests that RH-1566 and RH-1999-42 possess a greater ability to detoxify oxidative damage. Interestingly, a boost was seen in SOD and catalase enzyme activity after the application of Put in all the studied varieties. Polyamines are known to have the ability to increase the activities of ROS-scavenging antioxidants which results in reducing heat-induced oxidative damage and creates more balanced conditions [70]. Put is known to reduce the oxidative damage by direct scavenging of free radicals and by indirectly elevating the contents of antioxidants [71]. Similar increases in antioxidants under high temperature and Put application were reported by Jing et al. [72] in Triticum aestivum and Zhao et al. [73] in Cabernet Sauvignon seedlings. This indicates that exogenous application of Put substantially improved high-temperature-induced damage (Figure 17).
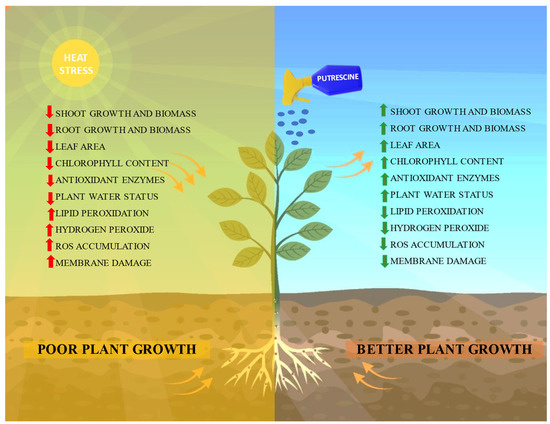
Figure 17.
Schematic diagram displaying the role of Putrescine in mitigating the adverse effects of high-temperature stress in plants. Application of Putrescine improved plant growth by enhancing antioxidants, water relation, membrane stabilization and photosynthetic pigments, while reducing oxidative stress markers.
Histochemical analysis was conducted to assess the levels of superoxide ions using NBT staining, and localized cell death was monitored using Evans blue dye. The leaves were more extensively stained with blue spots under high-temperature treatment in both the staining procedures. The staining was more prominent in RH-1707 and RH-1708 showing more oxidative damage. However, much less accumulation was seen in leaves exposed to combined treatment of exogenous Put and high temperature. The overproduction of ROS at high temperatures might be attributed to imbalanced antioxidant enzyme activity and the expression of associated genes, which has the principal consequence of causing heat-induced stress [74]. Damage to cellular components such proteins, nucleic acids, lipids and metabolites as a consequence of an imbalance between ROS and antioxidants (both enzymatic and nonenzymatic) eventually affects cell viability and leads to cell death [75,76].
In response to high temperature, a rapid closure of stomata, an increase in stomatal density and reduction in cell size have been reported [77]. In the present study, high-temperature stress increased stomatal density in the leaves of B. juncea; consequently Put application reduced it to some extent. However, no significant difference was seen in Put-treated and control plants at normal temperature. A more efficient increase in stomatal density was seen in RH-1707 and RH-1708. Stomatal density increases as external temperature increases, because to reduce cell and tissue damage caused by heat stress stomata are newly formed [78]. In maize, an increase in stomatal density was reported, which is associated with the need of cooling through transpiration, but an excessive increase can also lead to an increase in leaf temperature [79]. Similar results were obtained from the study of lettuce seedlings under high-temperature stress. The stomatal density increased under high temperature treatment and was significantly inhibited after spraying with exogenous spermidine. This revealed that under high-temperature stress, lettuce seedling stomatal density rose, and water transpiration loss increased, which is likely to generate more drought stress. Exogenous spermidine might effectively restrict stomatal density growth while preventing fast water loss via transpiration. It prevents dehydration in lettuce seedlings under high-temperature stress, hence preventing drought stress [80]. The exogenous application of Put exhibited several beneficial effects in mitigating the adverse impacts of high-temperature stress on the growth, physiological, and biochemical processes of B. juncea. As a result, the plants treated with Put demonstrated improved performance compared to the untreated plants when exposed to high-temperature stress.
5. Conclusions
High temperature affects growth, morpho-physio and biochemical parameters in B. juncea. The more pronounced effect was seen in varieties RH-1708 and RH-1707 as evidenced by reduction in growth rate, water status, membrane stability, more ROS accumulation and increased stomatal density under high temperature. However, varieties RH-1566 and RH-1999-42 performed better under high temperature by maintaining sufficient membrane integrity and accumulation of antioxidants to reduce oxidative damage. Thus, RH-1708 and RH-1707 were most sensitive and RH-1566 and RH-1999-42 were tolerant of high-temperature stress. These varieties can be further used to examine the molecular mechanisms of heat tolerance in B. juncea. Exogenous application of Put restored tissue integrity, maximized levels of antioxidant enzymes which have been associated in reducing oxidants (H2O2 and O2·−) and their harm in terms of ionic leakage (EL), lipid peroxidation (MDA) and other oxidative damage. Therefore, exogenously applied Put could be regarded as an effective bioactive stimulant that mitigates high-temperature stress damage by minimizing membrane damage, enhancing antioxidative enzyme activities, resolving ionic imbalance, promoting plant growth and production and, consequently, promoting production of B. juncea under extreme temperatures.
Author Contributions
Conceptualization, N.L. and P.S.; methodology, P.S.; software, Y.A. and P.S.; validation, N.L., Y.A. and A.Z.; formal analysis, P.S.; investigation, P.S. and. N.L. resources, A.M.A.-E.; and H.O.E.; data curation, P.S.; writing—original draft preparation, P.S., N.L., Y.A. and A.Z.; writing—review and editing, A.Z. and N.L. and A.G.; visualization, P.S.; supervision, N.L.; project administration, N.L.; funding acquisition, A.M.A.-E. and H.O.E. All authors have read and agreed to the published version of the manuscript.
Funding
CSIR-UGC Junior Research Fellowship, and Researchers Supporting Project number (RSPD2023R676), King Saud University.
Data Availability Statement
All data are present with the article.
Acknowledgments
The author (P.S.) would like to thank CSIR-UGC for Junior Research Fellowship and oilseeds section Department of Genetics and Plant Breeding, CCS Haryana Agricultural University, Hisar for providing seeds. The authors extend their sincere appreciation to Researchers Supporting Project number (RSPD2023R676), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Singh, D.; Balota, M.; Collakova, E.; Isleib, T.G.; Welbaum, G.E.; Tallury, S.P. Heat stress related physiological and metabolic traits in peanut seedlings. Peanut Sci. 2016, 43, 24–35. [Google Scholar] [CrossRef]
- DAC. Agricultural Statistics at a Glance; Directorate of Economics and Statistics, Department of Agriculture, Cooperation and Farmers Welfare, Ministry of Agriculture and Farmers Welfare, Government of India: New Delhi, India, 2019; pp. 72–73. [Google Scholar]
- Asthir, B. Protective mechanisms of heat tolerance in crop plants. J. Plant Interact. 2015, 10, 202–210. [Google Scholar] [CrossRef]
- Lobel, G.H.; Asner, P.K. Effect of high temperature on crop growth and yield. Prog. Agric. Eng. Sci. 2003, 49, 23–99. [Google Scholar]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Porter, J.R. Rising temperatures are likely to reduce crop yields. Nature 2005, 43, 166–174. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Bassel, G.W. Seed vigor and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef]
- Nadeem, M.; Li, J.; Wang, M.; Shah, L.; Lu, S.; Wang, X.; Ma, C. Unraveling field crops sensitivity to heat stress: Mechanisms, approaches, and future prospects. Agronomy 2018, 8, 128. [Google Scholar] [CrossRef]
- Azharudheen, T.M.; Yadava, D.K.; Singh, N.; Vasudev, S.; Singh, R.; Prabhu, K.V. A study on the thermo-tolerance at germination and seedling stage in Indian Mustard [Brassica Juncea (L.) Czern&Coss]. Int. J. Agric. Food Sci. 2013, 4, 589–594. [Google Scholar]
- Valliyodan, B.; Nguyen, H.T. Understanding regulatory networks and engineering for enhanced drought tolerance in plants. Curr. Opin. Plant Biol. 2006, 9, 189–195. [Google Scholar] [CrossRef]
- Bohra, A.; Sanadhya, D.; Bhatia, D.S. Polyamines: Metabolism and Role in Abiotic Stress Amelioration. J. Plant Sci. Res. 2015, 31, 183–195. [Google Scholar]
- Mustafavi, S.H.; Badi, H.N.; Sekara, A.; Mehrafarin, A.; Janda, T.; Ghorbanpour, M.; Rafiee, H. Polyamines and their possible mechanisms involved in plant physiological processes and elicitation of secondary metabolites. Acta Physiol. Plant. 2018, 40, 102. [Google Scholar] [CrossRef]
- Falasca, G.; Franceschetti, M.; Bagni, N.; Altamura, M.M.; Biasi, R. Polyamine biosynthesis and control of the development of functional pollen in kiwifruit. Plant Physiol. Biochem. 2010, 48, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Galston, A.W.; Kaur Sawhney, R.; Altabella, T.; Tiburcio, A.F. Plant polyamines in reproductive activity and response to abiotic stress. Acta Bot. 1997, 110, 197–207. [Google Scholar] [CrossRef]
- Bais, H.P.; Ravishankar, G.A. Role of polyamine in the ontogeny of plants and their biotechnological applications. Plant Cell Tissue Organ Cult. 2002, 69, 1–34. [Google Scholar] [CrossRef]
- Galston, A.W.; Sawhney, R.K. Polyamines in plant physiology. Plant Physiol. 1990, 94, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance. Planta 2010, 231, 1237–1249. [Google Scholar] [CrossRef]
- Childs, A.C.; Mehta, D.J.; Gerner, E.W. Polyamine-dependent gene expression. Cell. Mol. Life Sci. 2003, 60, 1394–1406. [Google Scholar] [CrossRef]
- Ouyang, J.; Song, C.; Chen, D. Research progress on heat-tolerance mechanism and transports of polyamines in plant. Mol. Plant Breed. 2017, 15, 3286–3294. [Google Scholar]
- Spormann, S.; Soares, C.; Teixeira, J.; Fidalgo, F. Polyamines as key regulatory players in plants under metal stress—A way for an enhanced tolerance. Ann. Appl. Biol. 2021, 178, 209–226. [Google Scholar] [CrossRef]
- Antoniou, C.; Zarza, X.; Gohari, G.; Panahirad, S.; Filippou, P.; Tiburcio, A.F.; Fotopoulos, V. Involvement of polyamine metabolism in the response of Medicago truncatula genotypes to salt stress. Plants 2021, 10, 269. [Google Scholar] [CrossRef]
- Sundararajan, S.; Sivakumar, H.P.; Nayeem, S.; Rajendran, V.; Subiramani, S.; Ramalingam, S. Influence of exogenous polyamines on somatic embryogenesis and regeneration of fresh and long-term cultures of three elite indica rice cultivars. Cereal Res. Commun. 2021, 49, 245–253. [Google Scholar] [CrossRef]
- Walters, D.R. Polyamines and plant disease. Phytochemistry 2003, 64, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Tiburcio, A.F.; Altabella, T.; Bitrián, M.; Alcázar, R. The roles of polyamines during the lifespan of plants: From development to stress. Planta 2014, 240, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Polyamines and abiotic stress tolerance in plants. Plant Signal. Behav. 2010, 5, 26–33. [Google Scholar] [CrossRef]
- Gupta, S.; Agarwal, V.P.; Gupta, N.K. Efficacy of putrescine and benzyladenine on photosynthesis and productivity in relation to drought tolerance in wheat (Triticum aestivum L.). Physiol. Mol. Biol. Plants 2012, 18, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, L.; Yang, R.; Han, Y.; Hao, J.; Liu, C.; Fan, S. Effects of exogenous putrescine on the ultrastructure of and calcium ion flow rate in lettuce leaf epidermal cells under drought stress. Hortic. Environ. Biotechnol. 2019, 60, 479–490. [Google Scholar] [CrossRef]
- Hassan, N.; Ebeed, H.; Aljaarany, A. Exogenous application of spermine and putrescine mitigate adversities of drought stress in wheat by protecting membranes and chloroplast ultra-structure. Physiol. Mol. Biol. Plants 2020, 26, 233–245. [Google Scholar] [CrossRef]
- Quinet, M.; Ndayiragije, A.; Lefèvre, I.; Lambillotte, B.; Dupont-Gillain, C.C.; Lutts, S. Putrescine differently influences the effect of salt stress on polyamine metabolism and ethylene synthesis in rice cultivars differing in salt resistance. J. Exp. Bot. 2010, 61, 2719–2733. [Google Scholar] [CrossRef]
- Xiong, F.; Liao, J.; Ma, Y.; Wang, Y.; Fang, W.; Zhu, X. The protective effect of exogenous putrescine in the response of tea plants (Camellia sinensis) to salt stress. HortScience 2018, 53, 1640–1646. [Google Scholar] [CrossRef]
- Mustafavi, S.H.; Shekari, F.; Abbasi, A. Putrescine improve low temperature tolerance of fennel (Foeniculum vulgare Mill.) seeds. Cercet. Agron. Mold. 2015, 48, 69–76. [Google Scholar] [CrossRef]
- Jankovska-Bortkevic, E.; Gavelienè, V.; Šveikauskas, V.; Mockevičiūtė, R.; Jankauskienè, J.; Todorova, D.; Sergiev, I.; Jurkonienè, S. Foliar application of polyamines modulates winter oilseed rape responses to increasing cold. Plants 2020, 9, 179. [Google Scholar] [CrossRef]
- Asthir, B.; Deep, A. Thermotolerance and antioxidant response induced by putrescine and heat acclimation in wheat seedlings. Seed Sci. Biotechnol. 2011, 5, 42–46. [Google Scholar]
- Hassanein, R.A.; El-Khawas, S.A.; Ibrahim, S.K.; El-Bassiouny, H.M.; Mostafa, H.A.; Abd El-Monem, A.A. Improving the thermotolerance of wheat plant by foliar application of arginine or putrescine. Pak. J. Bot. 2013, 45, 111–118. [Google Scholar]
- Das, A.; Karwa, S.; Taunk, J.; Bahuguna, R.N.; Chaturvedi, A.K.; Kumar, P.; Chinnusamy, V.; Pal, M. Putrescine exogenous application alleviates oxidative stress in reproductive tissue under high temperature in rice. Plant Physiol. Rep. 2021, 26, 381–391. [Google Scholar] [CrossRef]
- Amooaghaie, R.; Moghym, S. Effect of polyamines on thermotolerance and membrane stability of soybean seedling. Afr. J. Biotechnol. 2011, 10, 9673–9679. [Google Scholar]
- Fu, X.Z.; Xing, F.; Wang, N.Q.; Peng, L.Z.; Chun, C.P.; Cao, L.; Ling, L.L.; Jiang, C.L. Exogenous spermine pretreatment confers tolerance to combined high-temperature and drought stress in vitro in trifoliate orange seedlings via modulation of antioxidative capacity and expression of stress-related genes. Biotechnol. Biotechnol. Equip. 2014, 28, 192–198. [Google Scholar] [CrossRef]
- Jahan, M.S.; Hasan, M.M.; Alotaibi, F.S.; Alabdallah, N.M.; Alharbi, B.M.; Ramadan, K.M.; Bendary, E.S.; Alshehri, D.; Jabborova, D.; Al-Balawi, D.A.; et al. Exogenous putrescine increases heat tolerance in tomato seedlings by regulating chlorophyll metabolism and enhancing antioxidant defense efficiency. Plants 2022, 11, 1038. [Google Scholar] [CrossRef]
- Nobosse, P.; Fombang, E.N.; Mbofung, C.M. Effects of age and extraction solvent on phytochemical content and antioxidant activity of fresh Moringa oleifera L. leaves. Food Sci. Nutr. 2018, 6, 2188–2198. [Google Scholar] [CrossRef]
- Yang, A.; Larsen, T.W.; Tume, R.K. Carotenoid and retinol concentrations in serum, adipose tissue and liver and carotenoid transport in sheep, goats and cattle. Aust. J. Agric. Res. 1992, 4, 1809–1817. [Google Scholar] [CrossRef]
- Smart, R.E.; Bingham, G.E. Rapid estimates of relative water content. Plant Physiol. 1974, 53, 258–260. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Heath, R.L.; Packer, L. Photo peroxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Kurteva, M.; Tsonev, T. Effects of simulated acid rain on the photosynthetic characteristics of Phaseolus vulgaris L. Photosynthetica 1998, 34, 523–535. [Google Scholar] [CrossRef]
- Romero-Puertas, M.C.; Rodríguez-Serrano, M.; Corpas, F.J.; Gomez, M.D.; Del Rio, L.A.; Sandalio, L.M. Cadmium-induced subcellular accumulation of O2·− and H2O2 in pea leaves. Plant Cell Environ. 2004, 27, 1122–1134. [Google Scholar] [CrossRef]
- Wang, J.; Sun, P.P.; Chen, C.L.; Wang, Y.; Fu, X.Z.; Liu, J.H. An arginine decarboxylase gene PtADC from Poncirus trifoliata confers abiotic stress tolerance and promotes primary root growth in Arabidopsis. J. Exp. Bot. 2011, 62, 2899–2914. [Google Scholar] [CrossRef]
- Paul, V.; Sharma, L.; Pandey, R.; Meena, R.C. Measurements of stomatal density and stomatal index on leaf/plant surfaces. In Proceedings of the ICAR Sponsored Training Programme for Technical Staff of ICAR Institutes on―Physiological Techniques to Analyze the Impact of Climate Change on Crop Plants, New Delhi, India, 16–25 January 2017; p. 27. [Google Scholar]
- Angadi, S.V.; Cutforth, H.W.; Miller, P.R.; McConkey, B.G.; Entz, M.H.; Brandt, S.A.; Volkmar, K.M. Response of three Brassica species to high temperature stress during reproductive growth. Can. J. Plant Sci. 2000, 80, 693–701. [Google Scholar] [CrossRef]
- Morrison, M.J.; Stewart, D.W. Heat stress during flowering in summer Brassica. Crop Sci. 2002, 42, 797–803. [Google Scholar] [CrossRef]
- Todorova, D.; Katerova, Z.; Alexieva, V.; Sergiev, I. Polyamines–possibilities for application to increase plant tolerance and adaptation capacity to stress. Genet. Plant Physiol. 2015, 5, 123–144. [Google Scholar]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef] [PubMed]
- Kusano, T.; Berberich, T.; Tateda, C.; Takahashi, Y. Polyamines: Essential factors for growth and survival. Planta 2008, 228, 367–381. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, A.I.; Mohamed, A.H.; Rafudeen, M.S.; Omar, A.A.; Awad, M.F.; Mansour, E. Polyamines mitigate the destructive impacts of salinity stress by enhancing photosynthetic capacity, antioxidant defense system and upregulation of calvin cycle-related genes in rapeseed (Brassica napus L.). Saudi J. Biol. Sci. 2022, 29, 3675–3686. [Google Scholar] [CrossRef]
- El Bassiouny, H.M.S.; Mostafa, H.A. Physiological responses of wheat plant to foliar treatments with arginine or putrescine. Aust. J. Basic Appl. Sci. 2008, 2, 1390–1403. [Google Scholar]
- Dhanda, S.S.; Sethi, G.S.; Behl, R.K. Indices of drought tolerance in wheat genotypes at early stages of plant growth. J. Agron. Crop Sci. 2004, 190, 6–12. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Saud, S.; Hassan, S.; Tanveer, M. A combined application of biochar and phosphorus alleviates heat-induced adversities on physiological, agronomical and quality attributes of rice. Plant Physiol. Biochem. 2016, 103, 191–198. [Google Scholar] [CrossRef]
- Hameed, A.; Goher, M.; Iqbal, N. Heat Stress-Induced Cell Death, Changes in Antioxidants, Lipid Peroxidation, and Protease Activity in Wheat Leaves. J. Plant Growth Regul. 2012, 31, 283–291. [Google Scholar] [CrossRef]
- Bañon, S.; Fernandez, J.A.; Franco, J.A.; Torrecillas, A.; Alarcón, J.J.; Sánchez-Blanco, M.J. Effects of water stress and night temperature preconditioning on water relations and morphological and anatomical changes of Lotus creticus plants. Sci. Hortic. 2004, 101, 333–342. [Google Scholar] [CrossRef]
- Gupta, S.; Sharma, M.L.; Gupta, N.K.; Kumar, A. Productivity enhancement by putrescine in wheat (Triticum aestivum L.). Physiol. Mol. Biol. Plants 2003, 9, 279–282. [Google Scholar]
- Dutta, S.; Mohanty, S.; Tripathy, B.C. Role of Temperature Stress on Chloroplast Biogenesis and Protein Import in Pea. Plant Physiol. 2009, 150, 1050–1061. [Google Scholar] [CrossRef]
- Besford, R.; Richardson, C.; Campos, J.; Tiburcio, A. Effect of polyamines on stabilization of molecular complexes in thylakoid membranes of osmotically stressed oat leaves. Planta 1993, 189, 201–206. [Google Scholar] [CrossRef]
- Kumar, S.; Thakur, P.; Kaushal, N.; Malik, J.A.; Gaur, P.; Nayyar, H. Effect of varying high temperatures during reproductive growth on reproductive function, oxidative stress and seed yield in chickpea genotypes differing in heat sensitivity. Arch. Agron. Soil Sci. 2013, 59, 823–843. [Google Scholar] [CrossRef]
- Bajji, M.; Kinet, J.M.; Lutts, S. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul. 2002, 36, 61–70. [Google Scholar] [CrossRef]
- Khalil, S.I.; El-Monem, H.M.S.; Hassanein, R.A.; Mostafa, H.A.; El-Khawas, S.A.; Abd El-Monem, A.A. Antioxidant Defense System in Heat Shocked Wheat Plants Previously Treated with Arginine or Putrescine. Aust. J. Basic Appl. Sci. 2009, 3, 1517–1526. [Google Scholar]
- Islam, M.J.; Uddin, M.J.; Hossain, M.A.; Henry, R.; Begum, M.K.; Sohel, M.A.T.; Mou, M.A.; Ahn, J.; Cheong, E.J.; Lim, Y.-S. Exogenous Putrescine Attenuates the Negative Impact of Drought Stress by Modulating Physio-Biochemical Traits and Gene Expression in Sugar Beet (Beta vulgaris L.). PLoS ONE 2022, 17, e0262099. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Mukherjee, A.K. Implications of reactive oxygen species in heat shock induced germination and early growth impairment in Amaranthus lividus L. Biol. Plant. 2003, 47, 517–522. [Google Scholar] [CrossRef]
- Nagesh Babu, R.; Devaraj, V.R. High temperature and salt stress response in French bean (Phaseolus vulgaris). Aust. J. Crop Sci. 2008, 2, 40–48. [Google Scholar]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Mahmud, J.A.; Suzuki, T.; Fujita, M. Insights into spermine-induced combined high temperature and drought tolerance in mung bean: Osmoregulation and roles of antioxidant and glyoxalase system. Protoplasma 2017, 254, 445–460. [Google Scholar] [CrossRef]
- Mafakheri, A.; Siosemardeh, A.; Bahramnejad, B.; Struik, P.C.; Sohrabi, Y. Effect of drought stress and subsequent recovery on protein, carbohydrate contents, catalase and peroxidase activities in three chickpea (‘Cicer arietinum’) cultivars. Aust. J. Crop Sci. 2011, 5, 1255–1260. [Google Scholar]
- Jing, J.G.; Guo, S.Y.; Li, Y.F.; Li, W.H. Effects of polyamines on agronomic traits and photosynthetic physiology of wheat under higher temperature stress. Photosynthetica 2019, 57, 912–920. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Pan, X.; Jiang, Q.; Xi, Z. Exogenous putrescine alleviates drought stress by altering reactive oxygen species scavenging and biosynthesis of polyamines in the seedlings of Cabernet Sauvignon. Front. Plant Sci. 2021, 12, 767992. [Google Scholar] [CrossRef] [PubMed]
- Rout, J.R.; Sahoo, S.L. Antioxidant enzyme gene expression in response to copper stress in Withania somnifera L. Plant Growth Regul. 2013, 71, 95–99. [Google Scholar] [CrossRef]
- Xie, X.; He, Z.; Chen, N.; Tang, Z.; Wang, Q.; Cai, Y. The roles of environmental factors in regulation of oxidative stress in plant. BioMed Res. Int. 2019, 2019, 9732325. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.M.; Jibran, R.; Zhou, Y.; Albert, N.W.; Brummell, D.A.; Jordan, B.R.; Bowman, J.L.; Schwinn, K.E. The evolution of flavonoid biosynthesis: A bryophyte perspective. Front. Plant Sci. 2020, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Chen, Y.J.; Zhang, H.L.; Fu, P.L.; Fan, Z.X. Stronger cooling effects of transpiration and leaf physical traits of plants from a hot dry habitat than from a hot wet habitat. Funct. Ecol. 2017, 31, 2202–2211. [Google Scholar] [CrossRef]
- Shen, H.F.; Zhao, B.; Xu, J.J.; Liang, W.; Huang, W.M.; Li, H.H. Effects of heat stress on changes in physiology and anatomy in two cultivars of Rhododendron. S. Afr. J. Bot. 2017, 112, 338–345. [Google Scholar] [CrossRef]
- Zhao, W.; Sun, Y.; Kjelgren, R.; Liu, X. Response of stomatal density and bound gas exchange in leaves of maize to soil water deficit. Acta Physiol. Plant. 2015, 37, 1704. [Google Scholar] [CrossRef]
- Yang, X.; Han, Y.; Hao, J.; Qin, X.; Liu, C.; Fan, S. Exogenous spermidine enhances the photosynthesis and ultrastructure of lettuce seedlings under high-temperature stress. Sci. Hortic. 2022, 291, 110570. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).