Optimal Substrate Moisture Content for Kiwifruit (Actinidia valvata Dunn) Seedling Growth Based on Analyses of Biomass, Antioxidant Defense, and Photosynthetic Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Culture Conditions
2.2. Substrate Moisture Content, Irrigation, and Plant Growth
2.3. Measurement of Chlorophyll Content and Photosynthetic Parameters
2.4. Measurement of Leaf Relative Water Content and Cell Membrane Stability
2.5. Measurement of Antioxidant Enzyme Activity and Lipid Peroxidation
2.6. Date Analysis and Statistics
3. Results
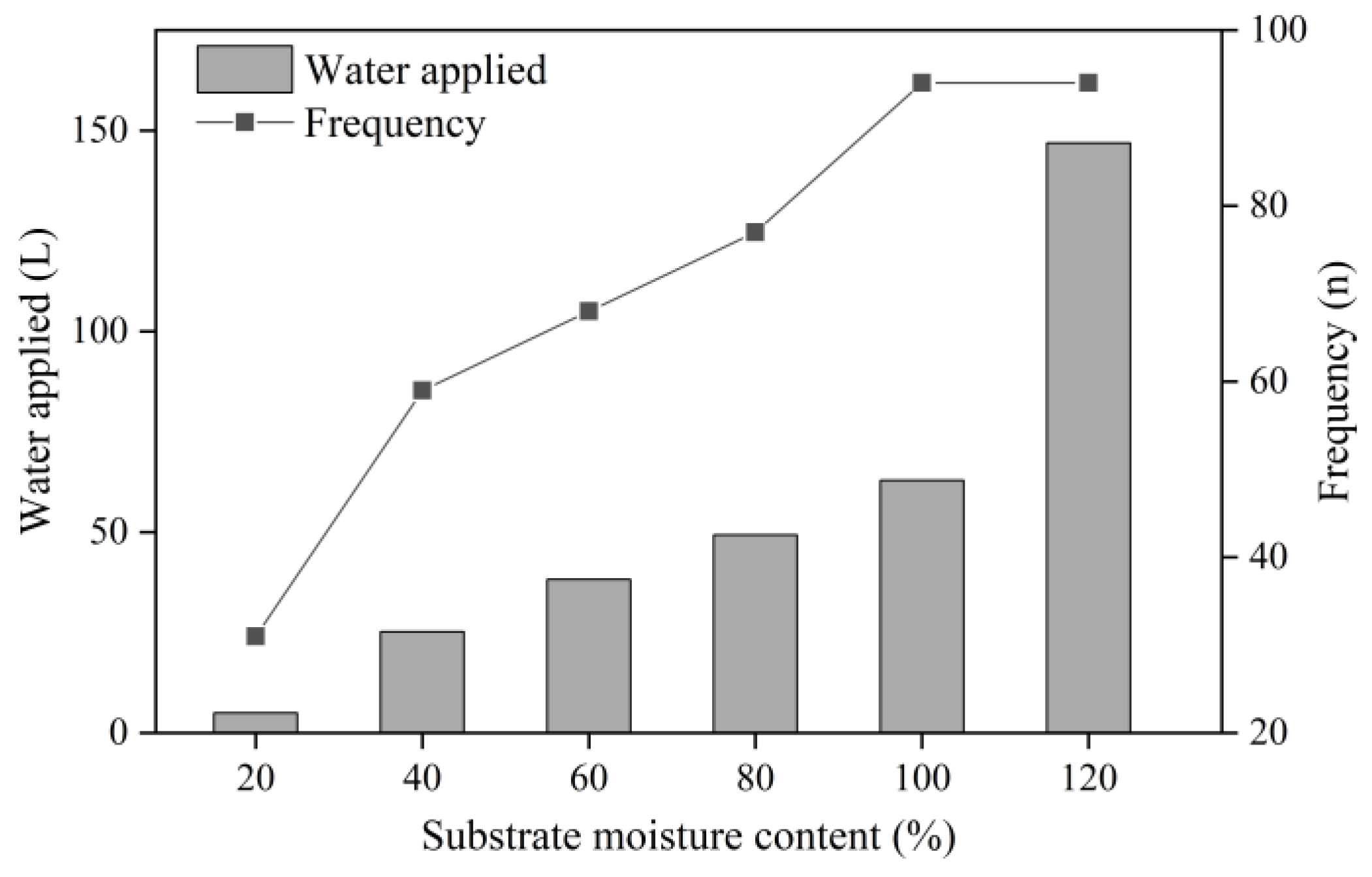
3.1. Total Irrigation Volume and Irrigation Frequency
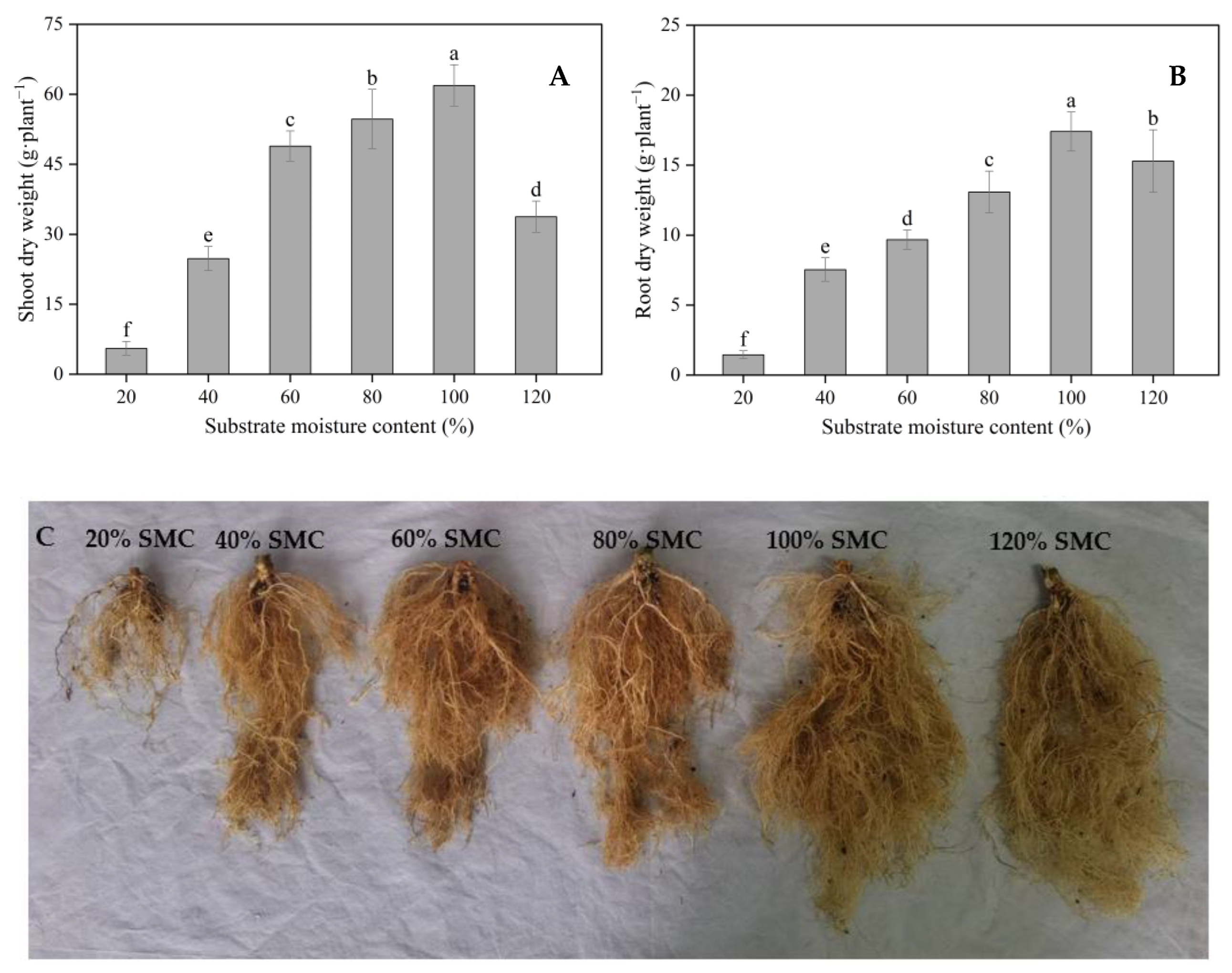
3.2. Plant Growth Parameters
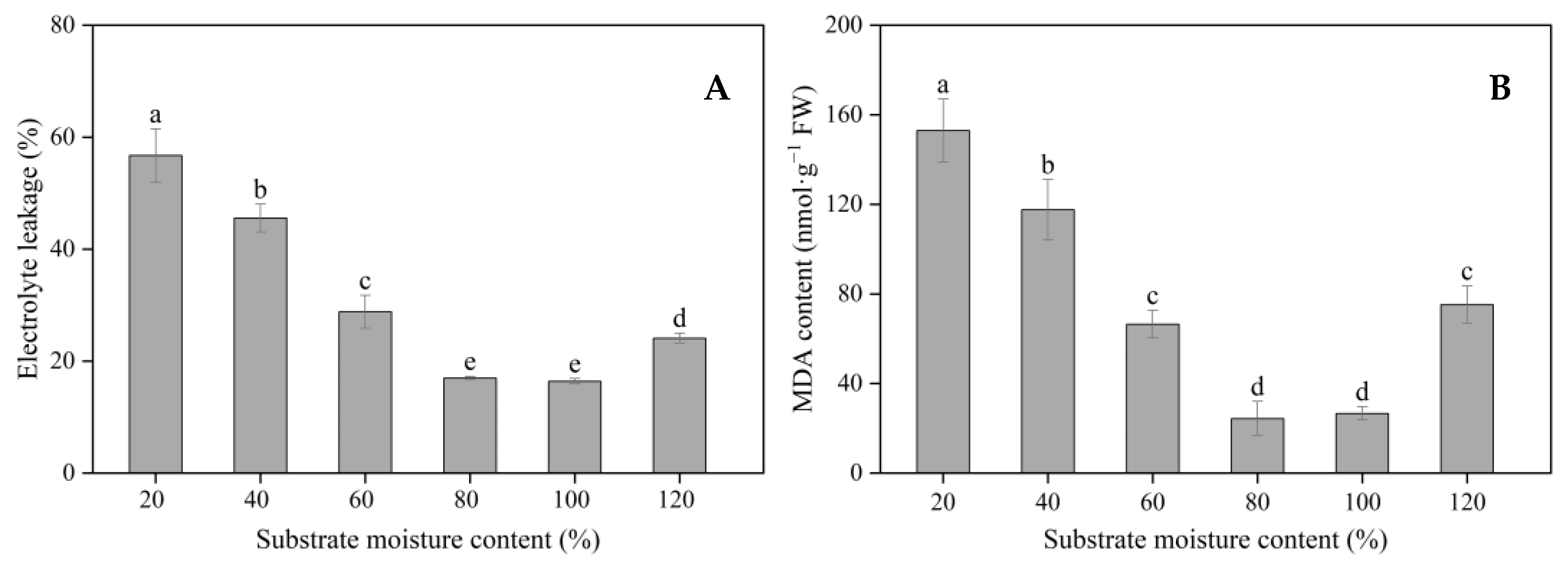
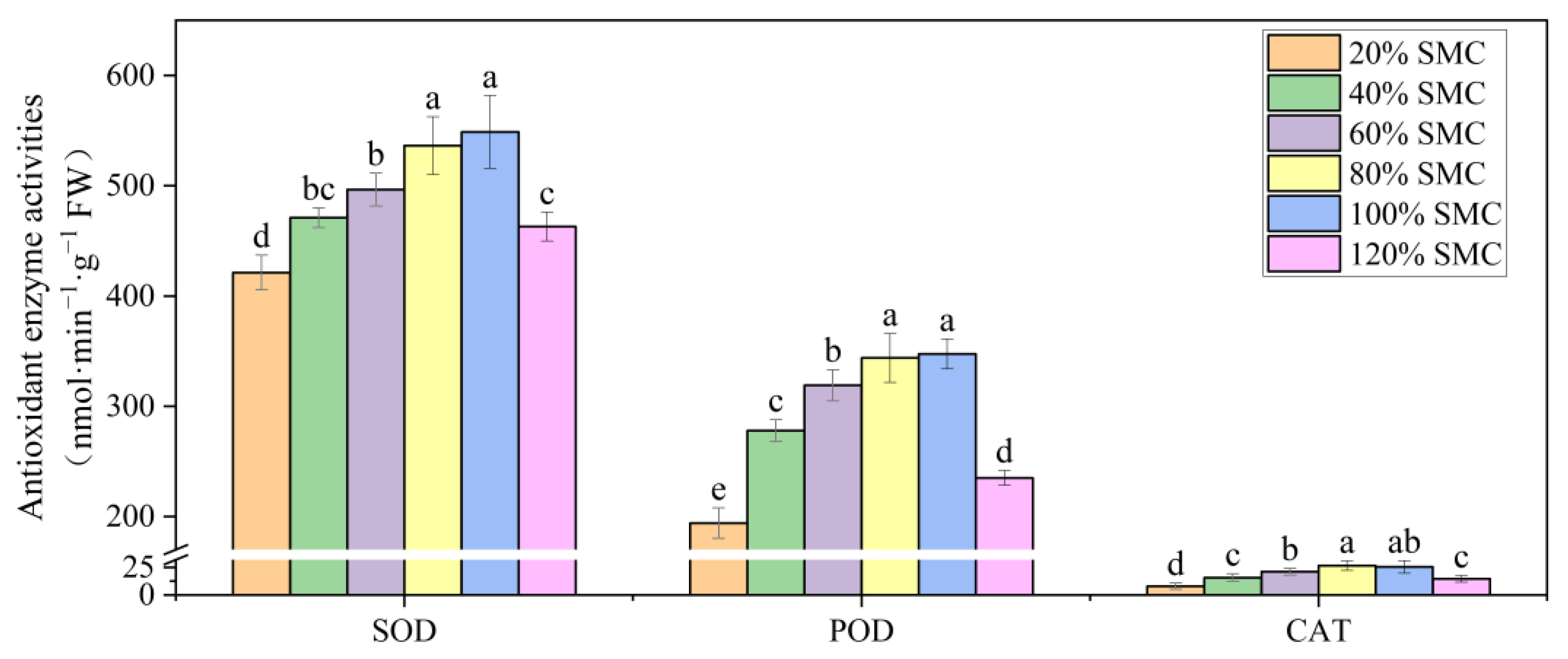
3.3. Cell Membrane Stability and Antioxidant Enzyme Activities
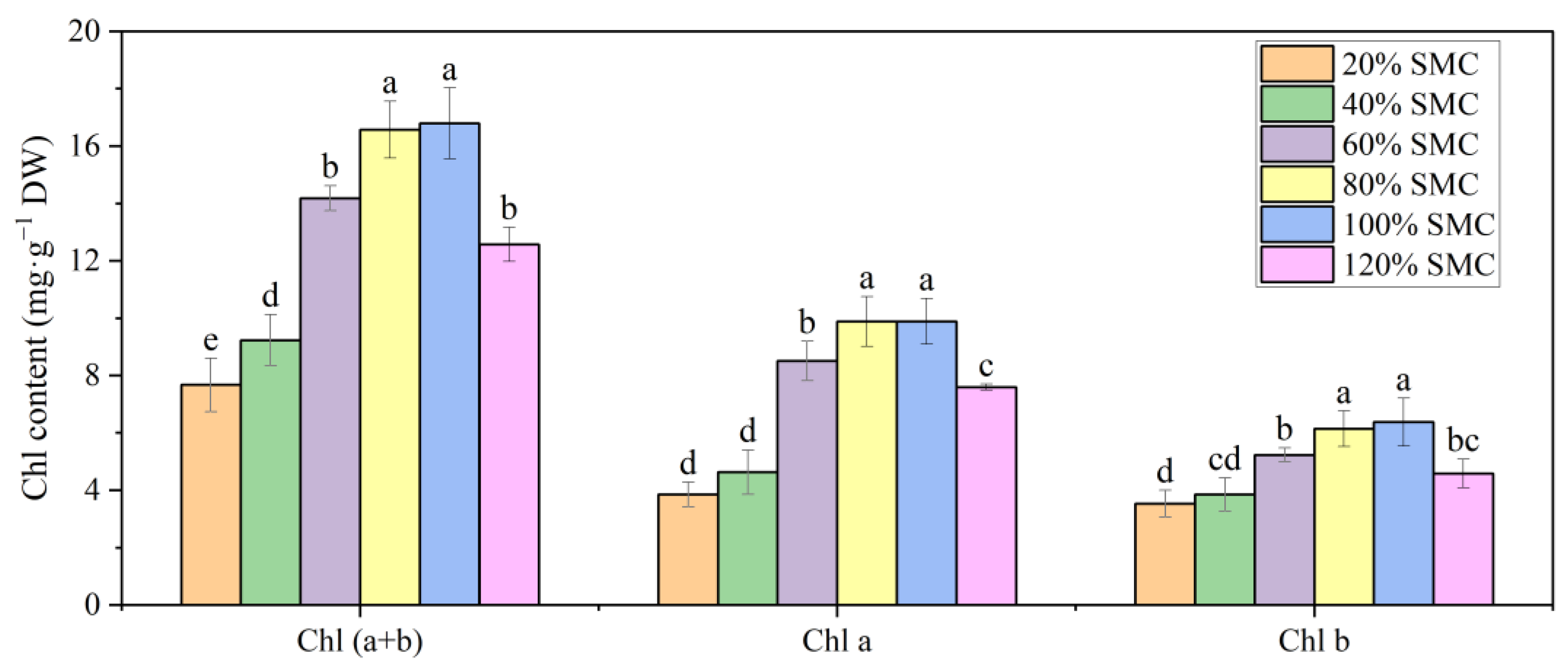
3.4. Leaf Water Status and Chlorophyll Content
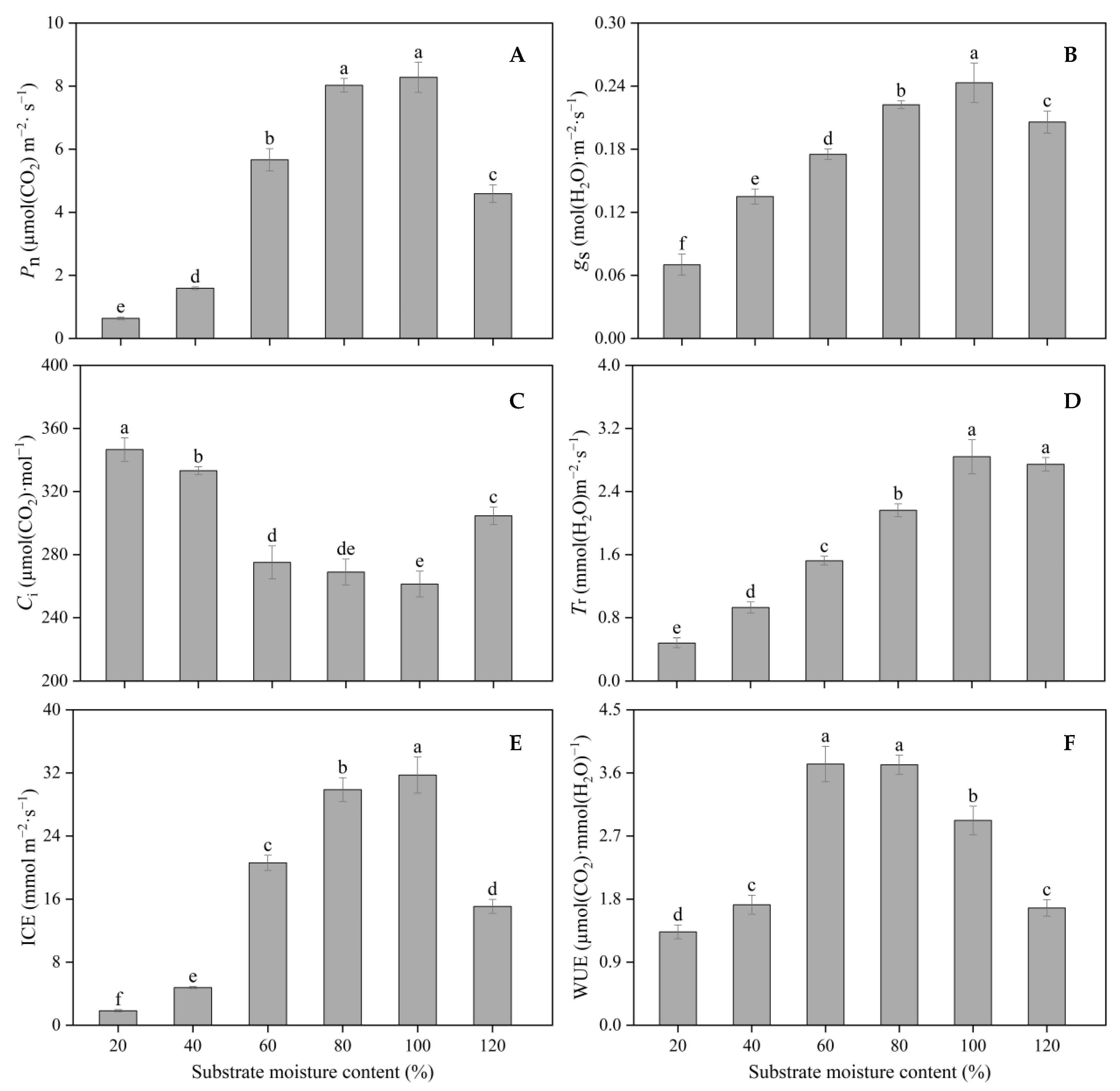
3.5. Photosynthetic Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Richardson, D.P.; Ansell, J.; Drummond, L.N. The nutritional and health attributes of kiwifruit: A review. Eur. J. Nutr. 2018, 57, 2659–2676. [Google Scholar] [CrossRef] [PubMed]
- Satpal, D.; Kaur, J.; Bhadariya, V.; Sharma, K. Actinidia deliciosa (Kiwi fruit): A comprehensive review on the nutritional composition, health benefits, traditional utilization, and commercialization. J. Food Process Preserv. 2021, 45, e15588. [Google Scholar] [CrossRef]
- Gao, Y.B.; Jiang, Z.Y.; Shi, M.Q.; Zhou, Y.F.; Huo, L.Q.; Li, X.L.; Xu, K. Comparative transcriptome provides insight into responding mechanism of waterlogging stress in Actinidia valvata Dunn. Gene 2022, 845, 146843. [Google Scholar] [CrossRef] [PubMed]
- Abid, M.; Gu, S.C.; Zhang, Y.J.; Sun, S.H.; Li, Z.; Bai, D.F.; Sun, L.M.; Qi, X.J.; Zhong, Y.P.; Fang, J.B. Comparative transcriptome and metabolome analysis reveal key regulatory defense networks and genes involved in enhanced salt tolerance of Actinidia (kiwifruit). Hortic. Res. 2022, 9, uhac189. [Google Scholar] [CrossRef]
- Barrett, G.E.; Alexander, P.D.; Robinson, J.S.; Bragg, N.C. Achieving environmentally sustainable growing media for soilless plant cultivation systems–A review. Sci. Hortic. 2016, 212, 220–234. [Google Scholar] [CrossRef]
- Valenzano, V.; Parente, A.; Serio, F.; Santamaria, P. Effect of growing system and cultivar on yield and water-use efficiency of greenhouse-grown tomato. J. Hortic. Sci. Biotech. 2008, 83, 71–75. [Google Scholar] [CrossRef]
- Heller, C.R.; Nunez, G.H. Preplant fertilization increases substrate microbial respiration but does not affect southern highbush blueberry establishment in a coconut coir-based substrate. HortScience 2022, 57, 17–21. [Google Scholar] [CrossRef]
- Ortiz-Delvasto, N.; Garcia-Ibañez, P.; Olmos-Ruiz, R.; Bárzana, G.; Carvajal, M. Substrate composition affects growth and physiological parameters of blueberry. Sci. Hortic. 2023, 308, 111528. [Google Scholar] [CrossRef]
- Yang, H.Y.; Wu, Y.Q.; Duan, Y.K.; Zhang, C.H.; Huang, Z.J.; Wu, W.L.; Lyu, L.F.; Li, W.L. Metabolomics combined with physiological and transcriptomic analyses reveal regulatory features associated with blueberry growth in different soilless substrates. Sci. Hortic. 2022, 302, 111145. [Google Scholar] [CrossRef]
- Kilinc, S.S.; Ertan, E.; Seferoglu, S. Effects of different nutrient solution formulations on morphological and biochemical characteristics of nursery fig trees grown in substrate culture. Sci. Hortic. 2007, 113, 20–27. [Google Scholar] [CrossRef]
- Şirin, U.; Ertan, E.; Ertan, B. Growth substrates and fig nursery tree production. Sci. Agric. 2010, 67, 633–638. [Google Scholar] [CrossRef]
- Cai, X.Y.; Starman, T.; Niu, G.H.; Hall, C. The effect of substrate moisture content on growth and physiological responses of two landscape roses (Rosa hybrida L.). HortScience 2014, 49, 741–745. [Google Scholar] [CrossRef]
- Xia, P.G.; Guo, H.B.; Zhao, H.G.; Jiao, J.; Deyholos, M.K.; Yan, X.J.; Liu, Y.; Liang, Z.S. Optimal fertilizer application for Panax notoginseng and effect of soil water on root rot disease and saponin contents. J. Ginseng Res. 2016, 40, 38–46. [Google Scholar] [CrossRef]
- Fulcher, A.F.; Buxton, J.W.; Geneve, R.L. Developing a physiological-based, on-demand irrigation system for container production. Sci. Hortic. 2012, 138, 221–226. [Google Scholar] [CrossRef]
- Jahromi, N.B.; Fulcher, A.; Walker, F.; Altland, J. Photosynthesis, growth, and water use of Hydrangea paniculata ‘Silver Dollar’ using a physiological-based or a substrate physical properties-based irrigation schedule and a biochar substrate amendment. Irrig. Sci. 2020, 38, 263–274. [Google Scholar] [CrossRef]
- Cifre, J.; Bota, J.; Escalona, J.M.; Medrano, H.; Flexas, J. Physiological tools for irrigation scheduling in grapevine (Vitis vinifera L.): An open gate to improve water-use efficiency? Agric. Ecosyst. Environ. 2005, 106, 159–170. [Google Scholar] [CrossRef]
- Nadal, M.; Flexas, J. Variation in photosynthetic characteristics with growth form in a water-limited scenario: Implications for assimilation rates and water use efficiency in crops. Agric. Water Manag. 2019, 216, 457–472. [Google Scholar] [CrossRef]
- Wu, J.J.; Wang, J.Y.; Hui, W.K.; Zhao, F.Y.; Wang, P.Y.; Su, C.Y.; Gong, W. Physiology of plant responses to water stress and related genes: A Review. Forests 2022, 13, 324. [Google Scholar] [CrossRef]
- Zou, J.N.; Jin, X.J.; Zhang, Y.X.; Ren, C.Y.; Zhang, M.C.; Wang, M.X. Effects of melatonin on photosynthesis and soybean seed growth during grain filling under drought stress. Photosynthetica 2019, 57, 512–520. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, J.; Oh, M.M. Determination of adequate substrate water content for mass production of a high value-added medicinal plant, Crepidiastrum denticulatum (Houtt.) Pak & Kawano. Agronomy 2020, 10, 388. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, K.; Wang, Y.Q.; Zhang, Z.P.; Lu, F.; Yu, H.Q.; Zou, J.Q. Changes in photosynthetic and chlorophyll fluorescence characteristics of sorghum under drought and waterlogging stress. Photosynthetica 2019, 57, 1156–1164. [Google Scholar] [CrossRef]
- Bañón, D.; Lorente, B.; Bañón, S.; Ortuño, M.F.; Sánchez-Blanco, M.J.; Alarcón, J.J. Control of substrate water availability using soil sensors and effects of water deficit on the morphology and physiology of Potted Hebe andersonii. Agronomy 2022, 12, 206. [Google Scholar] [CrossRef]
- An, S.K.; Lee, H.B.; Kim, J.; Kim, K.S. Efficient water management for Cymbidium grown in coir dust using a soil moisture sensor-based automated irrigation system. Agronomy 2021, 11, 41. [Google Scholar] [CrossRef]
- Ozkur, O.; Ozdemir, F.; Bor, M.; Turkan, I. Physiochemical and antioxidant responses of the perennial xerophyte Capparis ovata Desf. to drought. Environ. Exp. Bot. 2009, 66, 487–492. [Google Scholar] [CrossRef]
- Jia, L.T.; Qin, X.; Lyu, D.G.; Qin, S.J.; Zhang, P. ROS production and scavenging in three cherry rootstocks under short-term waterlogging conditions. Sci. Hortic. 2019, 257, 108647. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—a practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Shin, Y.K.; Bhandari, S.R.; Jo, J.S.; Song, J.W.; Lee, J.G. Effect of drought stress on chlorophyll fluorescence parameters, phytochemical contents, and antioxidant activities in lettuce seedlings. Horticulturae 2021, 7, 238. [Google Scholar] [CrossRef]
- Qiu, S.F.; Yang, L.Q.; Zhao, H.; Huang, D.F.; Tang, D.Q. Effects of water holding capacity in substrate on growth characters and comprehensive quality of Brassica campestris L. ssp. Chinensis cv. Ziyoucai. Acta Agric. Zhejiangensis 2016, 28, 195–199. [Google Scholar]
- González-Vega, R.I.; Cárdenas-López, J.L.; López-Elías, J.A.; Ruiz-Cruz, S.; Reyes-Díaz, A.; Perez-Perez, L.M.; Cinco-Moroyoqui, F.J.; Robles-Zepeda, R.E.; Borboa-Flores, J.; Del-Toro-Sánchez, C.L. Optimization of growing conditions for pigments production from microalga Navicula incerta using response surface methodology and its antioxidant capacity. Saudi J. Biol. Sci. 2021, 28, 1401–1416. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Blum, A.; Ebercon, A. Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Sci. 1981, 21, 43–47. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Van Iersel, M.W.; Dove, S.; Kang, J.G.; Burnett, S.E. Growth and water use of petunia as affected by substrate water content and daily light integral. Hortscience 2010, 45, 277–282. [Google Scholar] [CrossRef]
- Burnett, S.E.; van Iersel, M.W. Morphology and irrigation efficiency of Gaura lindheimeri grown with capacitance sensor-controlled irrigation. Hortscience 2008, 43, 1555–1560. [Google Scholar] [CrossRef]
- Bohnert, H.J.; Jensen, N.R.G. Adaptations to Environmental Stresses. Plant Cell 1995, 7, 1099–1111. [Google Scholar] [CrossRef]
- Khaleghi, A.; Naderi, R.; Brunetti, C.; Maserti, B.E.; Salami, S.A.; Babalar, M. Morphological, physiochemical and antioxidant responses of Maclura pomifera to drought stress. Sci. Rep. 2019, 9, 19250. [Google Scholar] [CrossRef]
- Jiao, P.P.; Wu, Z.H.; Wang, X.; Jiang, Z.B.; Wang, Y.Q.; Liu, H.; Qin, R.; Li, Z.J. Short-term transcriptomic responses of Populus euphratica roots and leaves to drought stress. J. For. Res. 2021, 32, 841–853. [Google Scholar] [CrossRef]
- Zhao, Q.Z.; Guo, J.; Shu, M.; Wang, P.; Hu, S.J. Impacts of drought and nitrogen enrichment on leaf nutrient resorption and root nutrient allocation in four Tibetan plant species. Sci. Total Environ. 2020, 723, 138106. [Google Scholar] [CrossRef]
- Goodger, J.Q.D.; Sharp, R.E.; Marsh, E.L.; Schachtman, D.P. Relationships between xylem sap constituents and leaf conductance of well-watered and water-stressed maize across three xylem sap sampling techniques. J. Exp. Bot. 2005, 56, 2389–2400. [Google Scholar] [CrossRef] [PubMed]
- Schachtman, D.P.; Goodger, J.Q.D. Chemical root to shoot signaling under drought. Trends Plant Sci. 2008, 13, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Kreuzwieser, J.; Rennenberg, H. Molecular and physiological responses of trees to waterlogging stress. Plant Cell Environ. 2014, 37, 2245–2259. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhong, Y.P.; Bai, D.F.; Lin, M.M.; Qi, X.J.; Fang, J.B. Comparative analysis of physiological traits of three Actinidia valvata Dunn genotypes during waterlogging and post-waterlogging recovery. Hortic. Environ. Biotechnol. 2020, 61, 825–836. [Google Scholar] [CrossRef]
- Arbona, V.; Manzi, M.; de Ollas, C.; Gómez-Cadenas, A. Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 4885–4911. [Google Scholar] [CrossRef]
- Bettaieb, I.; Hamrouni-Sellami, I.; Bourgou, S.; Limam, F.; Marzouk, B. Drought effects on polyphenol composition and antioxidant activities in aerial parts of Salvia officinalis L. Acta Physiol. Plant. 2011, 33, 1103–1111. [Google Scholar] [CrossRef]
- Bowler, C.; Montagu, M.V.; Inzé, D. Superoxide dismutase and stress tolerance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 83–116. [Google Scholar] [CrossRef]
- Jiang, M.Y.; Zhang, J.H. Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J. Exp. Bot. 2002, 53, 2401–2410. [Google Scholar] [CrossRef]
- Li, Z.; Shi, P.; Peng, Y. Improved drought tolerance through drought preconditioning associated with changes in antioxidant enzyme activities, gene expression and osmoregulatory solutes accumulation in White clover (Trifolium repens L.). Plant Omics. 2013, 6, 481–489. [Google Scholar]
- Zheng, J.W.; Fang, C.Y.; Ru, L.; Sun, N.; Liu, Y.Y.; Huang, Y.P.; Wang, Y.H.; Zhu, Z.J.; He, Y. Role of glutathione-ascorbate cycle and photosynthetic electronic transfer in alternative oxidase-manipulated waterlogging tolerance in watermelon seedlings. Horticulturae 2021, 7, 130. [Google Scholar] [CrossRef]
- DaCosta, M.; Huang, B.R. Changes in antioxidant enzyme activities and lipid peroxidation for bentgrass species in response to drought stress. J. Am. Soc. Hortic. Sci. 2007, 132(30, 319–326. [Google Scholar] [CrossRef]
- Irfan, M.; Hayat, S.; Hayat, Q.; Afroz, S.; Ahmad, A. Physiological and biochemical changes in plants under waterlogging. Protoplasma 2010, 241, 3–17. [Google Scholar] [CrossRef]
- Puyang, X.H.; An, M.Y.; Xu, L.X.; Han, L.B.; Zhang, X.Z. Antioxidant responses to waterlogging stress and subsequent recovery in two Kentucky bluegrass (Poa pratensis L.) cultivars. Acta Physiol. Plant. 2015, 37, 197. [Google Scholar] [CrossRef]
- Vaseva, I.; Akiscan, Y.; Simova-Stoilova, L.; Kostadinova, A.; Nenkova, R.; Anders, I.; Feller, U.; Demirevska, K. Antioxidant response to drought in red and white clover. Acta Physiol. Plant. 2012, 34, 1689–1699. [Google Scholar] [CrossRef]
- Rood, S.B.; Nielsen, J.L.; Shenton, L.; Gill, K.M.; Letts, M.G. Effects of flooding on leaf development, transpiration, and photosynthesis in narrowleaf cottonwood, a willow-like poplar. Photosynth. Res. 2010, 104, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.d.A.; Jifon, J.L.; dos Santos, C.M.; Jadoski, C.J.; da Silva, J.A.G. Photosynthetic capacity and water use efficiency in sugarcane genotypes subject to water deficit during early growth phase. Braz. Arch. Biol. Technol. 2013, 56, 735–748. [Google Scholar] [CrossRef]
- Liu, B.B.; Li, M.; Li, Q.M.; Cui, Q.Q.; Zhang, W.D.; Ai, X.Z.; Bi, H.G. Combined effects of elevated CO2 concentration and drought stress on photosynthetic performance and leaf structure of cucumber (Cucumis sativus L.) seedlings. Photosynthetica 2018, 56, 942–952. [Google Scholar] [CrossRef]
- Tian, L.X.; Li, J.; Bi, W.S.; Zuo, S.Y.; Li, L.J.; Li, W.L.; Sun, L. Effects of waterlogging stress at different growth stages on the photosynthetic characteristics and grain yield of spring maize (Zea mays L.) Under field conditions. Agric. Water Manag. 2019, 218, 250–258. [Google Scholar] [CrossRef]
- Herzog, M.; Striker, G.G.; Colmer, T.D.; Pedersen, O. Mechanisms of waterlogging tolerance in wheat—A review of root and shoot physiology. Plant Cell Environ. 2016, 39, 1068–1086. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Díaz-Espejo, A.; Flexas, J.; Galmés, J.; Warren, C.R. Importance of mesophyll diffusion conductance in estimation of plant photosynthesis in the field. J. Exp. Bot. 2009, 60, 2271–2282. [Google Scholar] [CrossRef]
- Xia, J.B.; Zhao, Z.G.; Sun, J.K.; Liu, J.T.; Zhao, Y.Y. Response of stem sap flow and leaf photosynthesis in Tamarix chinensis to soil moisture in the Yellow River Delta, China. Photosynthetica 2017, 55, 368–377. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant 2016, 38, 102. [Google Scholar] [CrossRef]
- Jedmowski, C.; Ashoub, A.; Brüeggemann, W. Reactions of Egyptian landraces of Hordeum vulgare and Sorghum bicolor to drought stress, evaluated by the OJIP fluorescence transient analysis. Acta Physiol. Plant 2013, 35, 345–354. [Google Scholar] [CrossRef]
- Oukarroum, A.; Madidi, S.E.; Schansker, G.; Strasser, R.J. Probing the responses of barley cultivars (Hordeum vulgare L.) by chlorophyll a fluorescence OLKJIP under drought stress and re-watering. Environ. Exp. Bot. 2007, 60, 438–446. [Google Scholar] [CrossRef]
- Silva, M.d.A.; Jifon, J.L.; Sharma, V.; da Silva, J.A.G.; Caputo, M.M.; Damaj, M.B.; Guimarães, E.R.; Ferro, M.I.T. Use of physiological parameters in screening drought tolerance in sugarcane genotypes. Sugar Tech 2011, 13, 191–197. [Google Scholar] [CrossRef]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 414–421. [Google Scholar] [CrossRef]
- Cai, Y.F.; Zhang, S.B.; Hu, H.; Li, S.Y. Photosynthetic performance and acclimation of Incarvillea delavayi to water stress. Biol. Plant. 2010, 54, 89–96. [Google Scholar] [CrossRef]

| SMC (%) | Fv/Fm | Fv/F0 | ETR | qp | Y(II) | NPQ |
|---|---|---|---|---|---|---|
| 20 | 0.68 ± 0.05 c | 2.16 ± 0.37 d | 20.91 ± 1.95 c | 0.81 ± 0.02 d | 0.47 ± 0.03 c | 0.89 ± 0.16 a |
| 40 | 0.73 ± 0.01 b | 2.68 ± 0.18 c | 21.49 ± 0.42 bc | 0.86 ± 0.02 bc | 0.49 ± 0.01 c | 0.88 ± 0.07 a |
| 60 | 0.77 ± 0.01 a | 3.33 ± 0.25 b | 24.33 ± 0.87 a | 0.88 ± 0.01 ab | 0.55 ± 0.02 a | 0.69 ± 0.07 b |
| 80 | 0.79 ± 0.01 a | 3.75 ± 0.22 a | 25.25 ± 0.33 a | 0.90 ± 0.02 a | 0.57 ± 0.01 a | 0.64 ± 0.07 b |
| 100 | 0.79 ± 0.01 a | 3.77 ± 0.37 a | 25.05 ± 0.27 a | 0.88 ± 0.01 ab | 0.57 ± 0.01 a | 0.59 ± 0.04 b |
| 120 | 0.72 ± 0.03 b | 2.63 ± 0.38 c | 22.77 ± 1.11 b | 0.84 ± 0.03 c | 0.52 ± 0.03 b | 0.67 ± 0.08 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, D.-D.; Chen, D.-G.; Xu, K.-W.; Penttinen, P.; You, H.-Y.; Liao, H.-P.; Yang, R.; Chen, Y.-X. Optimal Substrate Moisture Content for Kiwifruit (Actinidia valvata Dunn) Seedling Growth Based on Analyses of Biomass, Antioxidant Defense, and Photosynthetic Response. Agronomy 2023, 13, 1858. https://doi.org/10.3390/agronomy13071858
Peng D-D, Chen D-G, Xu K-W, Penttinen P, You H-Y, Liao H-P, Yang R, Chen Y-X. Optimal Substrate Moisture Content for Kiwifruit (Actinidia valvata Dunn) Seedling Growth Based on Analyses of Biomass, Antioxidant Defense, and Photosynthetic Response. Agronomy. 2023; 13(7):1858. https://doi.org/10.3390/agronomy13071858
Chicago/Turabian StylePeng, Dan-Dan, Da-Gang Chen, Kai-Wei Xu, Petri Penttinen, Hao-Yu You, Hui-Ping Liao, Ran Yang, and Yuan-Xue Chen. 2023. "Optimal Substrate Moisture Content for Kiwifruit (Actinidia valvata Dunn) Seedling Growth Based on Analyses of Biomass, Antioxidant Defense, and Photosynthetic Response" Agronomy 13, no. 7: 1858. https://doi.org/10.3390/agronomy13071858
APA StylePeng, D.-D., Chen, D.-G., Xu, K.-W., Penttinen, P., You, H.-Y., Liao, H.-P., Yang, R., & Chen, Y.-X. (2023). Optimal Substrate Moisture Content for Kiwifruit (Actinidia valvata Dunn) Seedling Growth Based on Analyses of Biomass, Antioxidant Defense, and Photosynthetic Response. Agronomy, 13(7), 1858. https://doi.org/10.3390/agronomy13071858









