Abstract
The chickpea (Cicer arieitnum L.) is an important food legume crop of the family Fabaceae with high protein levels that is widely grown in rainfed areas prone to drought stress. It is a self-pollinated cool season crop with a true diploid (2n = 16) nature. It is relatively cheap and a high source of protein. About 90% of the chickpea crop is grown by the use of residual moisture in the soil without depending on irrigation. In the present study, two varieties of chickpea, namely ICC 4958 and HC-6, were grown under three field capacities (FC) (100% FC, 50% FC and 25% FC). Samples were taken three times, i.e., 15, 30 and 45 days after sowing (DAS). Parameters such as morphological, physiological, biochemical, metabolite and protein profiling of the two varieties were completed. Morphological parameters such as shoot length (14.2%), number of branches (20.7%), number of leaves (17.5%) and yield (56%) declined as the drought level increased and other characteristics such as root length (9.7%), number of flowers (24.5%) and number of pods (34.4%) increased as drought stress progressed. Physiological parameters such as relative water content (RWC) (13.5%), cell membrane stability (CMS) (29.6%) and chlorophyll content decreased, whereas electrolyte conductivity (EC) (38%) increased in both the varieties as field capacity decreased. Biochemical parameters such as proline (54.75%), sugar (15.2%), glycine betaine (32.25%), superoxide dismutase (SOD) (49.5%), catalase (CAT) (50.5%), ascorbate peroxidase (APX) (44.9%) and glutathione reductase (GR) (49%) increased as drought stress increased. Metabolite analyses of, for example, MDA (malondialdehyde) content (30.5%), total anthocyanin (36.3%), flavonoid content (26%) and phenolic content (29.5%) increased as drought progressed. We also performed protein profiling of the two varieties using SDS-PAGE (sodium dodecyl-sulfate polyacrylamide gel electrophoresis) to differentiate the expression analysis of the two varieties.
1. Introduction
Among all the grain legumes consumed by people, the chickpea (Cicer arietinum L.) is one of the oldest cultivated food legumes nutritionally, agronomically and economically in the world. Throughout India, it is cultivated in an area of 17.85 million hectares with an annual production of over 17 Tg [1]. It is an annual, self-pollinating, diploid (2n = 2x = 16) cool season pulse crop with a genome size of 931 Mb [2,3]. It is mainly grown in Africa, South America and the Indian subcontinent. It is the world’s second most important food legume, representing an important component of the small-scale crop production in the arid and semiarid tropical regions of the world [4,5]. Among chickpea-growing countries, India alone contributes about 70% of the world’s total production [6,7,8].
Global warming and climate change present a major challenge to human beings due to the heavy dependence on natural resources which affects crop productivity and may degrade cultivable land [3,9]. Environmental stresses such as high temperature, cold, drought, salinity, alkalinity, UV and pathogen infections cause various serious effects on plants, and these stresses are the primary cause of crop losses worldwide by more than 50% [10]. In many regions, drought stress or water scarcity is a significant environmental barrier to the yield of many economically significant food crops [11,12]. Approximately 42% of India’s total land area is experiencing drought, with 6% of that region experiencing severely dry conditions, according to the Drought Early Warning System (DEWS) report for 2019. The extent of drought is uncertain because it relies on several factors such as precipitation frequency, evaporation level and soil moisture retention capability [13]. Water deficiency in plants impairs the regular growth of plants, interferes with water relations and influences the WUE of plants. Water limiting conditions results in impaired cell elongation mainly because of poor water flow from xylem to nearby cells [14]. Water deficit conditions change a plant’s growth pattern by preventing both qualitative and quantitative growth [15,16].
Drought generally affects overall crop performance starting from germination, and eventually manifests in grain yield and quality with losses of up to 40–50% in crop productivity, mainly in areas lacking satisfactory and constant rainfall. An estimated 33 percent of the world’s chickpea production is lost as a result of drought stress [17]. The effects of drought stress on chickpea growth have been revealed by some researchers with morphological, physiological, biochemical and molecular parameters [18,19]. Plants respond to stress by reprogramming their proteins to ensure a steady-state of vital metabolic processes, despite the fact that chickpea manageability in water deficit settings is generally great, there is a need to increase drought tolerant characteristics to boost plant efficiency in a water deficit climate. The actions that take place in important functional molecules such as proteins must be defined in order to gather more useful knowledge to understand the molecular mechanisms causing dehydration stress. In recent years, proteomics is being used as a robust technique to study the changes in protein accumulation in different plant tissues under stress conditions. Proteins are a functional product of a gene and studying at the protein level is useful to understand the mechanism and find the suitable protein and gene for further improvement of the crop.
2. Materials and Methods
2.1. Site Description
The experiments were conducted during the rabi season of 2021 and 2022 in the Department of Molecular Biology, Biotechnology and Bioinformatics, CCSHAU, Hisar. The soil was sandy loam in texture with a low status of organic matter. All the plants were grown in pots in greenhouse conditions with a controlled temperature and relative humidity.
2.2. Experimental Design and Treatments
The experiment was laid out in a factorial experiment design. All treatments including two varieties and 3 drought stress levels were performed as a completely randomized design (CRD) with 3 replications. In each replication, 4 pots for each treatment were placed which were used during 15, 30 and 45 days of sowing (DAS) and yield. A total of 12 pots for each replication (4 pots × 3 treatments) and 36 pots (12 pots × 3 replications) for each variety were placed. For two varieties, 72 pots were used, which were placed as per the experimental design.
2.3. Seed Sowing and Seedling Growth
Seeds of uniform size were selected and pre-treated with 1% Bavistin solution for a period of 1 min. Treated seeds of both the varieties were sown in protrays of 5 × 5 × 20 cm dimensions with coco-peat as the medium. They were allowed to germinate and grow in the protrays for 10 days. All the protrays were watered uniformly to obtain uniform growth at the seedling stage. Seedlings with similarities in growth factors such as stem length, root length and number of branches were selected. Then, they were transferred to plastic pots with 2.5 kg of soil.
2.4. Drought Stress Application and Management
Drought was maintained in pots using the gravimetric method. Firstly, the weight of the pot with soil was noted down. Pots were allowed to completely saturate, i.e., all the macro and micro pores in the soil filled with water. Pots were left overnight to attain the field capacity (FC), i.e., water left only in the micro pores which was completely available to the plants, and the weight was noted down as final weight.
Amount of water retained by the soil = FW − IW
IW—Initial weight of the pot with soil.
FW—Final weight of the pot with soil after complete saturation of water.
The initial weight of the pot and soil was subtracted from the final weight; this gave the amount of water that was retained by the soil. In total, 100% FC was the complete amount of water retained by the soil, 50% FC was half the amount of water retained by 100% FC and half of this was 25% FC. In all, 100% FC (Control), 50% FC (T1) and 25% FC (T2) were maintained based on the above calculations. All the pots were watered by weighing and adding water on a daily basis for all the treatments. Pots were maintained under greenhouse conditions with controlled temperatures and relative humidity.
2.5. Varieties and Drought Treatments
Two varieties (ICC 4958 and HC-6) of chickpea along with 3 conditions including T1-Control (100% Field Capacity), T2-50% Field Capacity and T3-25% Field Capacity (FC) were included in the study. For chickpeas, 25% FC was considered as the extreme drought condition. ICC 4958 was a drought-resistant chickpea genotype obtained from the International Crops Research Institute for Semi-Arid Tropics (ICRISAT), India. HC-6 was released from CCSHAU, Haryana, and had moderate tolerance to drought conditions. Seeds were collected from Pulses Section, CCSHAU.
2.6. Sample Collection
Sampling was conducted at 5, 20 and 35 days of drought application in pots, i.e., 15, 30 and 45 days after sowing (DAS). Destructive sampling was conducted to observe root length. For every sampling, 9 pots were used from each variety. A total of 54 pots of 72 pots were used for all the samplings. The remaining 18 pots were left for yield.
2.7. Morphological Parameters
The morphology of two varieties of chickpea plants was recorded on 15, 30 and 45 DAS and photographs were taken accordingly. Morphophysiological characteristics such as plant height, number of branches/plant, number of leaves/branch, number of flowers/plant, number of pods/plant, days to maturity and yield were recorded.
2.8. Physiological Parameters
2.8.1. Relative Water Content (RWC) (%)
The relative water content of the leaf was calculated by using the method as described by Smart and Bingham [20]. The fresh weight of the samples was taken, and leaves were allowed to soak in water overnight before taking the turgid weight. Samples were dried to obtain the dry weight. The RWC (%) of leaves was calculated according to the formula given below:
RWC (%) = (Fresh weight − Dry weight/Turgid weight − Dry weight) × 100
2.8.2. Electrolyte Leakage (EL) (%)
Membrane injury was analyzed according to the method described by Dionisio-Sese and Tobit [21]. The electrolyte leakage was determined by using a conductivity meter. Fresh leaves (30 mg) were incubated in 25 mL of deionized water and kept overnight. The next day, the initial electrical conductivity of the medium EC1 was measured. The samples were autoclaved for 30 min, and then the final electrical conductivity EC2 was measured. The percent electrolyte leakage was calculated as follows:
Electrolyte leakage (%) = (EC1/EC2) × 100
Cell membrane thermo-stability was calculated using the formula:
CMT = 1 − % injury = 1 − (EC1/EC2) × 100
2.8.3. Chlorophyll Content
The chlorophyll content in leaves was measured according to the protocol given by Lichtenthaler and Wellburn [22]. The optical density was read at λ = 663, 646 nm using 80% acetone as a blank by a spectrophotometer (GENESYS 180 UV-Visible Spectrophotometer).
The content of chlorophyll A, chlorophyll B and total chlorophyll (mg g−1) was calculated using the following formulae:
Chlorophyll A = (12.21 OD663 − 2.81 OD646) × V/1000W;
Chlorophyll B = (20.13 OD646 − 5.03 OD663) × V/1000W;
Total Chlorophyll = Chlorophyll A + Chlorophyll B;
2.9. Biochemical Parameters
Various biochemical parameters such as proline content, total sugar content, glycine betaine content and enzyme activity of super oxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX) and glutathione reductase (GR) were analyzed.
2.9.1. Proline
Proline content was estimated by using the method described by Bates et al. [23]. This absorbance was recorded at 520 nm by using toluene as blank.
2.9.2. Total Sugar
Total sugar content was estimated by using the method described by Dubois et al. [24]. Absorbance was taken at 630 nm using 80% methanol as blank.
2.9.3. Glycine Betaine Content
Glycine betaine content was estimated by using the method described by Grieve and Grattan [25]. The optical density of the lower organic layer was recorded at 365 nm. Different concentrations of glycine betaine were used to make a standard graph.
2.9.4. Enzyme Assays
The procedure of enzyme extraction was completed under 4 °C. Fresh leaves (200 mg) were ground in chilled 5 mL of phosphate buffer (0.1 M, pH 7.5) and ethylene diamine tetra acetic acid (EDTA, 0.5 mM). The homogenate was centrifuged at 4000 rpm for 20 min at 4 °C. The resulting supernatant was used for the assay of different enzymes.
Superoxide Dismutase (SOD) (EC 1.15.1.1)
SOD activity was determined by measuring its ability to inhibit the photochemical reduction of nitroblue tetrazolium chloride, as described by Beyer and Fridovich [26]. Absorbance of the reaction mixture was read at 560 nm using a spectrophotometer.
Catalase (CAT) (EC 1.11.1.6)
CAT activity was measured by the method of Aebi [27]. CAT activity was expressed as μΜ H2O2 g−1 FW. For the calculation of CAT enzyme activity, the extinction coefficient of 0.036 mM−1 cm−1 was used.
Ascorbate Peroxidase (APX) (EC 1.11.1.11)
APX activity was measured by the method of Nakano and Asada [28]. The APX was assayed as a decrease in absorbance at 290 nm using a spectrophotometer. For the calculation of APX enzyme activity, the extinction coefficient of 2.8 mM−1 cm−1 was used.
Glutathione Reductase (GR) (EC 1.6.4.2)
GR activity was assayed by the method of Foyer and Halliwell [29]. The decrease in absorbance was measured at 340 nm for 2 min using a spectrophotometer. The extinction coefficient of 6.2 mM−1 cm−1 was used for the calculation of GR activity.
2.10. Metabolite Analysis
2.10.1. Lipid Peroxidation
MDA is a product of lipid peroxidation and was measured by the thiobarbituric acid (TBA) reaction with the method described by Heath and Packer [30].
The absorbance of the reaction mixture was read at 532 nm and the value for non-specific absorption at 600 nm was subtracted. The MDA content of the sample was calculated using the formula:
where
MDA content (nmol g−1 FW) = [(A532 − A600)/155]
A532 is the absorbance at 532 nm;
A600 is the absorbance at 600 nm, the correction for non-specific turbidity; and 155 mM−1 cm−1 is the molar extinction coefficient for MDA.
2.10.2. Total Anthocyanin
Total anthocyanin content was recorded with the method of Hodges and Nozzolillo [31]. The absorbance at 530 nm and 600 nm was measured with the help of the spectrophotometer. The anthocyanin content of the sample was expressed by cyaniding-3-glucoside (CGE) and calculated by using the following equation:
where
A530nm and A600nm are the absorbance at 530 and 600 nm, respectively;
V is the total volume of extract;
Mw is the relative molecular weight of CGE (449.4);
ε is the molar extinction coefficient (29,600 M−1 cm−1);
m is the mass of the sample.
2.10.3. Phenolic Content
The total phenolic concentration was determined according to the method of Julkenen-Titto [32]. Readings were taken at 750 nm using a spectrophotometer. The standard curve was made using gallic acid, and the phenolic content was expressed as gallic acid equivalents (GAE).
2.10.4. Flavanoid Content
The content of flavonoids was determined using the method of Quettier et al. [33].
The same procedure was repeated for the standard solution of quercetin, and the calibration line was constructed. The content of flavonoids in extracts was expressed in terms of the quercetin equivalent (QE).
2.10.5. Carotenoid Content
The carotenoid content was measured according to the protocol of Lichtenthaler and Wellburn [22]. The optical density was read at λ = 470 nm using 80% acetone as a blank by a spectrophotometer. Carotenoid content (μg g−1 FW) was calculated using the following equation:
Carotenoids (μg g−1 FW) = (1000 OD470 − 3.27 Chlorophyll a − 104 Chlorophyll b)/229
2.11. Protein Profiling
Protein concentrations of samples were determined using the Bradford assay, and bovine serum albumin was used as the standard [34].
SDS-PAGE
Protein was separated on SDS PAGE with a discontinuous buffer system used as described by Laemmli [35]. In brief, the leaf tissues were homogenized in the buffer (phosphate buffer), and the supernatants were added with equal volumes of 2 × gel loading dye. The mixture was heated at 100 °C for 3–5 min and loaded on 12% SDS-PAGE gel. The protein sample was stored at −20 °C until used for electrophoresis.
2.12. Statistical Analysis
The data were analyzed using a completely randomized block design (CRD). A three-factorial analysis of variance (ANOVA), appropriate for the design, was carried out to determine the significance of differences among the treatments for each of the parameters. Using the OPSTAT software (http://14.139.232.166/opstat/, accessed on 17 May 2023), the data were examined (CCSHAU, Hisar, India) at a 5% and 1% level of significance for comparing the means, and the critical difference (CD) was determined.
3. Results
3.1. Morphological Parameters
Morphological characteristics such as shoot length, root length, number of branches/plant, number of leaves/plant, number of flowers/plant and number of pods/plant were noted after each sampling (15, 30 and 45 days after sampling).
The difference in plant height between the two varieties at different sampling times and also the morphology of seeds in different treatments were observed (Figure 1).
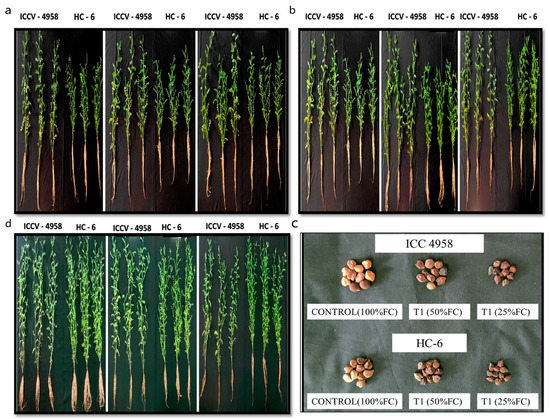
Figure 1.
Morphological parameters: (a) at 15 DAS; (b) at 30 DAS; (c) at 45 DAS; (d) seed.
3.1.1. Shoot Length
Under drought conditions, the shoot length decreased in both varieties. The difference was more prominent at 45 DAS in both the varieties. A decrease of 8.2% and 13.05% over the control was observed in both the varieties at 15 and 30 DAS, respectively. In ICC 4958, we observed a decrease (3.7% in 50% FC and 27% in 25% FC) in comparison to the control; whereas in HC-6, we observed a decrease (16% in both 50% FC and 25% FC) in comparison to the control (Figure 2).
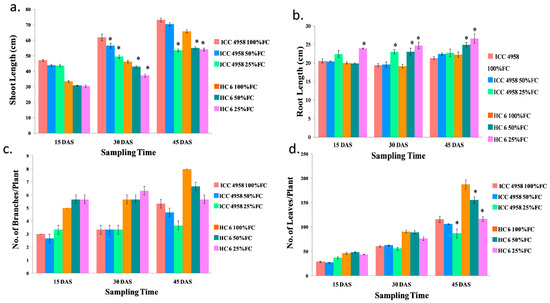
Figure 2.
Effect of drought stress in two varieties on (a) shoot length; (b) root length; (c) number of branches/plant; and (d) number of leaves/plant. * shows the significance level with respect to control.
3.1.2. Root Length
From the initial stages of drought application, we have noticed an increase in root length at 25% FC in both the varieties. The increase in root length became more prominent as the drought progressed. A significant increase of 57% in ICC 4958 and 13.6% in HC-6 was noticed at 45 DAS compared to the control (Figure 2).
3.1.3. Number of Branches/Plant
An increase in the number of branches was observed at the initial stages but in later stages, the numbers decreased as compared to the control. An increase of 15% was observed at 15 and 30 DAS in both the varieties. At 45 DAS, in ICC 4958 we observed a decrease (12.5% in 50% FC and 31% in 25% FC) over the control; whereas in HC-6, we observed a decrease (16% in 50% FC and 29% in 25% FC) compared to the control (Figure 2).
3.1.4. Number of Leaves/Plant
Under drought conditions, the number of leaves/plant decreased as the field capacity (FC) decreased. A decrease of 9.4% and 6.75% was observed at 15 and 30 DAS, respectively. In ICC 4958, we observed a decrease (8.6% fold in 50% FC and 25% in 25% FC) in comparison to the control; in HC-6, we observed a similar decrease (17.2% in 50% FC and 38% in 25% FC) over the control (Figure 2).
3.1.5. Number of Flowers/Plant
The number of flowers/plant increased as the drought progressed in both varieties. No flowers were observed in HC-6 at 15 DAS. An increase of 26% in ICC 4958 and 28% HC-6 was observed at 30 DAS. At 45 DAS, there was an increase (25% in 50% FC and 56.8% in 25% FC) in ICC 4958, and an increase (18.3% in 50% FC and 21% in 25% FC) in HC-6 compared to the control was observed (Figure 3).
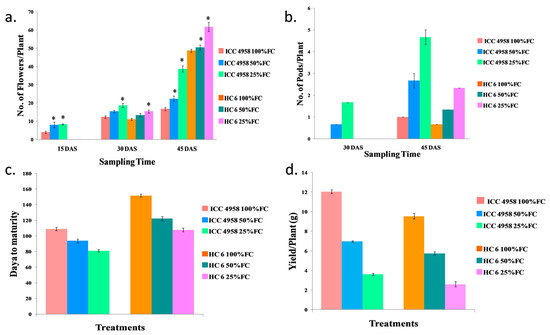
Figure 3.
Effect of drought stress in two varieties on (a) number of flowers/plant; (b) number of pods/plant; (c) days to maturity; and (d) yield/plant. * shows the significance level with respect to control.
3.1.6. Number of Pods/Plant
At 15 DAS, no pods were observed. At 30 DAS, pods were observed in 50% FC and 25% FC. At 45 DAS, there was an increase (62.5% in 50% FC and 78.5% in 25% FC) in ICC 4958, and an increase (49.9% in 50% FC and 71.4% in 25% FC) in HC-6 over the control was noticed (Figure 3).
3.1.7. Days to Maturity
Under drought conditions, plants attained maturity earlier than the control. In ICC 4958, days to maturity decreased (15% in 50% FC and 19% in 25% FC) over the control and in HC-6, days to maturity decreased (19% in 50% FC and 29% in 25% FC) compared to normal control conditions (Figure 3).
3.1.8. Yield/Plant (g)
Under drought conditions, yield/plant decreased as the drought progressed. In ICC 4958, yield decreased (42% in 50% FC and 70% in 25% FC) over the control, and in HC-6, yield decreased (39.8% in 50% FC and 72.77% in 25% FC) more than the control (Figure 3).
3.2. Physiological Parameters
Physiological parameters such as relative water content (RWC), electrolyte leakage (EL), cell membrane stability (CMS), chlorophyll A, chlorophyll B and total chlorophyll were performed after each sampling (15, 30 and 45 days after sampling).
3.2.1. Relative Water Content (RWC)
The relative water content in both varieties decreased as the drought progressed. At 15 DAS, there was a decrease (6.65% in 50% FC and 16.8% in 25% FC) in comparison to the control in both varieties. At 30 DAS, there was a decrease (11.8% in 50% FC and 36.5% in 25% FC) over the control in ICC 4958, and a similar decrease (7.02% in 50% FC and 14.4% in 25% FC) over the control in HC-6. At 45 DAS, there was a decrease (6% in 50% FC and 8.9% in 25% FC) compared to the control in both ICC 4958 and HC-6 (Figure 4).
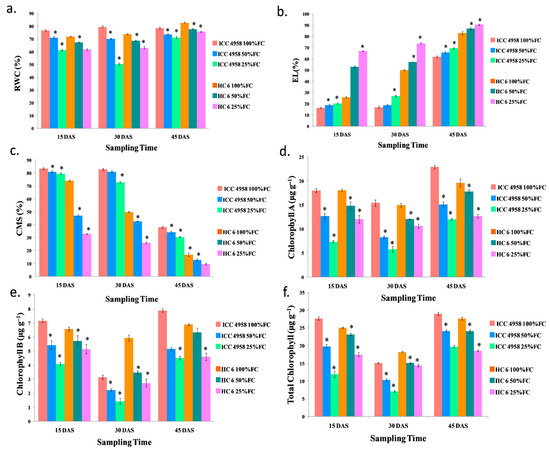
Figure 4.
Effect of drought stress in two varieties on (a) relative water content (RWC); (b) electrolyte leakage (EL); (c) cell membrane stability (CMS); (d) chlorophyll A; (e) chlorophyll B; and (f) total chlorophyll. * shows the significance level with respect to control.
3.2.2. Electrolyte Leakage (EL)
Electrolyte leakage increased compared to the control in both of the varieties. At 15 DAS, there was an increase (13% in 50% FC and 18.7% in 25% FC) in ICC 4958 and also an increase (51% in 50% FC and 61.3% in 25% FC) in HC-6 when compared to the control. At 30 DAS, there was an increase (11.05% in 50% FC and 34.6% in 25% FC) over the control in both varieties. At 45 DAS, there was also an increase (5.2% in 50% FC and 9.5% in 25% FC) over the control in both ICC 4958 and HC-6 (Figure 4).
3.2.3. Cell Membrane Stability (CMS)
Cell membrane stability is the inverse of electrolyte leakage. As the drought progressed, the values decreased. At 45 DAS, there was a decrease (31.5% in 50% FC and 20% in 25% FC) over the control in ICC 4958, and a similar decrease (23.5% in 50% FC and 43.5% in 25% FC) over the control in HC-6 was observed (Figure 4).
3.2.4. Chlorophyll
Chlorophyll content was expressed under chlorophyll A, B and total chlorophyll.
Chlorophyll A
Chlorophyll A content decreased in both the varieties under drought conditions. At 15 DAS, in ICC 4958, chlorophyll A decreased (29.5% in 50% FC and 59.4% in 25% FC) over the control, and in the HC-6 variety, chlorophyll A decreased (18% in 50% FC and 34.6% in 25% FC) over the control. At 30 DAS, chlorophyll A decreased (46.37% in 50% FC and 65.8% in 25% FC) compared to the control in ICC 4958, and in the HC-6 variety, chlorophyll A decreased (19.4% in 50% FC and 32.8% in 25% FC) compared to the control. At 45 DAS, chlorophyll A decreased (33.85% in 50% FC and 45.8% in 25% FC) in ICC 4958, and it also decreased (8.9% in 50% and 35.8% in 25% FC) in the HC-6 variety in comparison to the control (Figure 4).
Chlorophyll B
Under drought situations, chlorophyll B content decreased in both of the varieties. At 15 DAS, in ICC 4958, chlorophyll B decreased (24.3% in 50% FC and 42.97% in 25% FC) over the control, and in the HC-6 variety, chlorophyll B decreased (12.6% in 50% FC and 23.3% in 25% FC) over the control. At 30 DAS, chlorophyll B decreased (29% in 50% FC and 54.4% in 25% FC) in ICC 4958 and it also decreased (41.28% in 50% FC and 53.9% in 25% FC) in the HC-6 variety compared to the control. At 45 DAS, in ICC 4958, chlorophyll B decreased (34.66% in 50% FC and 43.52% in 25% FC) over the control, and in the HC-6 variety, chlorophyll B decreased (7.94% in 50% and 35.48% in 25% FC) over the control (Figure 4).
Total Chlorophyll
Under drought conditions, total chlorophyll content decreased in both varieties. At 15 DAS, in ICC 4958, total chlorophyll decreased (28.46% in 50% FC and 56.79% in 25% FC) over the control, and in the HC-6 variety, total chlorophyll decreased (7.3% in 50% FC and 30.1% in 25% FC) over the control. At 30 DAS, total chlorophyll decreased (31.7% in 50% FC and 53.6% in 25% FC) in ICC 4958 and it also decreased (16.9% in 50% FC and 22.4% in 25% FC) in the HC-6 variety compared to the control. At 45 DAS, total chlorophyll decreased (16.8% in 50% FC and 33% in 25% FC) in ICC 4958, and a similar decrease (12.9% in 50% and 20.2% in 25% FC) in the HC-6 variety was also observed compared with the control (Figure 4).
3.3. Biochemical Parameters
Biochemical parameters such as proline content, total sugar content, glycine betaine content and enzyme activity of superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX) and glutathione reductase (GR) were assayed after each sampling (15, 30 and 45 days after sampling).
3.3.1. Proline
Under drought conditions, the proline content increased in both of the varieties. At 15 DAS, there was an increase (20.77% in 50% FC and 37.26% in 25% FC) in ICC 4958, and a similar increase (50.93% in 50% FC and 69.8% in 25% FC) in HC-6 was observed compared to the control. At 30 DAS, proline increased (47.58% in 50% FC and 81.36% in 25% FC) in ICC 4958 and it also increased (67.48% in 50% FC and 88.4% in 25% FC) in HC-6 compared to the control. At 45 DAS, there was an increase (40.723% in 50% FC and 68.883 in 25% FC) over the control in ICC 4958, and there was an increase (18.63% in 50% FC and 62.38% in 25% FC) over the control in HC-6 (Figure 5).
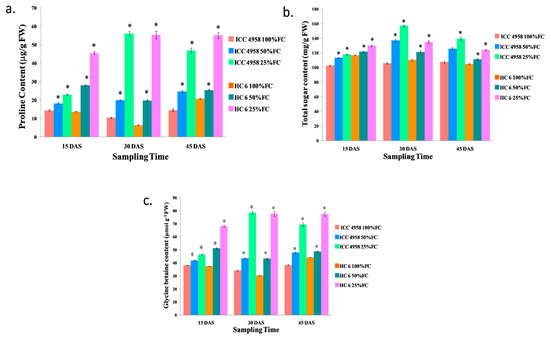
Figure 5.
Effect of drought stress in two varieties on (a) proline content; (b) total sugar content; and (c) glycine betaine content. * shows the significance level with respect to control.
3.3.2. Total Sugar
Total sugar content increased in both varieties under drought conditions. At 15 DAS, in ICC 4958, there was an increase (9.57% in 50% FC and 12.89% in 25% FC) over the control, and in HC-6, there was an increase (4.11% fold in 50% FC and 10.02% in 25% FC) over the control. At 30 DAS, there was an increase (22.68% in 50% FC and 32.7% in 25% FC) in ICC 4958, and a similar increase (9.04% in 50% FC and 18.2% in 25% FC) in HC-6 was observed compared to the control. At 45 DAS, in ICC 4958, there was an increase (14.57% in 50% FC and 23% in 25% FC) over the control, and in HC-6, there was an increase (5.9% in 50% FC and 15.6% in 25% FC) over the control (Figure 5).
3.3.3. Glycine Betaine
Under drought conditions, glycine betaine content increased in both varieties. At 15 DAS, in ICC 4958, there was an increase (8.754% in 50% FC and 17.85% in 25% FC) over the control, and in HC-6, there was an increase (26.88% in 50% FC and 41.05% in 25% FC) over the control. At 30 DAS, there was an increase (21.11% in 50% FC and 61.25% in 25% FC) in ICC 4958, and an increase (29.76% in 50% FC and 60.9% in 25% FC) in HC-6 was noticed compared to the control. At 45 DAS, in ICC 4958, there was an increase (19.83% in 50% FC and 44.91% in 25% FC) compared to the control, and in HC-6, there was an increase (9.4% in 50% FC and 42.91% in 25% FC) compared to the control (Figure 5).
3.3.4. Enzyme Activity
Superoxide Dismutase (SOD)
The superoxide dismutase activity of both the varieties showed an increase under drought stress. At 15 DAS, the enzyme activity increased (50.45% in 50% FC and 72.33% in 25% FC) in ICC 4958 and a similar increase (45.78% in 50% FC and 71.9% in 25% FC) in HC-6 was observed over the control. At 30 DAS, in ICC 4958, the enzyme activity increased (38.16% in 50% FC and 55.45% in 25% FC) over the control, and in HC-6 the enzyme activity also increased (45.12% in 50% FC and 54.7 in 25% FC) over the control. At 45 DAS, the enzyme activity decreased compared to 30 DAS due to progressive exposure to drought. In ICC 4958, the enzyme activity increased (18.61% in 50% FC and 30.7% in 25% FC), and in HC-6, the enzyme activity increased (42.5% in 50% FC and 63.83% in 25% FC) compared to the control (Figure 6).
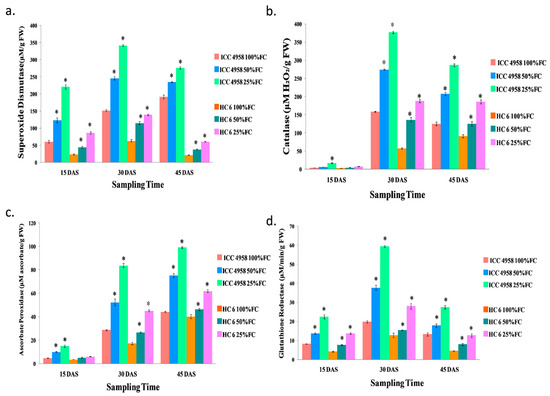
Figure 6.
Effect of drought stress in two varieties on (a) superoxide dismutase; (b) catalase; (c) ascorbate peroxidase; and (d) glutathione reductase. * shows the significance level with respect to control.
Catalase (CAT)
The catalase activity of both varieties showed an increase under drought stress. At 15 DAS, in ICC 4958, the enzyme activity increased (37.65% in 50% FC and 76.87% in 25% FC) over the control, and in HC-6, the enzyme activity also increased (30.22% in 50% FC and 58.92% in 25% FC) over the control. The activity of catalase was high at 30 DAS in both the varieties. In ICC 4958, the enzyme activity increased (42.08% in 50% FC and 57.85% in 25% FC), and in HC-6, the enzyme activity increased (57.3% in 50% FC and 69.1% in 25% FC) in comparison to the control. At 45 DAS, in ICC 4958, the enzyme activity increased (39.5% in 50% FC and 56.2% in 25% FC), and in HC-6, the enzyme activity increased (27.02% in 50% FC and 51% in 25% FC) over the control (Figure 6).
Ascorbate Peroxidase (APX)
The ascorbate peroxidase activity of both the varieties showed an increase under drought stress. At 15 DAS, in ICC 4958, the enzyme activity increased (51.78% in 50% FC and 67.67% in 25% FC) over the control, and in HC-6, the enzyme activity also increased (28.9% in 50% FC and 38.7% in 25% FC) over the control. At 30 DAS, the enzyme activity increased (45.15% in 50% FC and 65.67% in 25% FC) in ICC 4958 and the enzyme activity increased (28.9% in 50% FC and 38.7% in 25% FC) in HC-6 compared to the control. At 45 DAS, in ICC 4958, the enzyme activity increased (41.2% in 50% FC and 55.4% in 25% FC), and in HC-6, the enzyme activity increased (13.1% in 50% FC and 35.1% in 25% FC) in comparison to the control (Figure 6)
Glutathione Reductase (GR)
The glutathione reductase activity of both the varieties showed an increase under drought stress. At 15 DAS, in ICC 4958, the enzyme activity increased (39.64% in 50% FC and 63.2% in 25% FC), and in HC-6, the enzyme activity also increased (45% in 50% FC and 69.1% in 25% FC) compared to the control. At 30 DAS, in ICC 4958, the enzyme activity increased (47.32% in 50% FC and 66.6% in 25% FC) over the control, and in HC-6, the enzyme activity also increased (17.37% in 50% FC and 54.4% in 25% FC) over the control. At 45 DAS, the enzyme activity decreased compared to 30 DAS due to progressive exposure to drought in both of the varieties. In ICC 4958, the enzyme activity increased (25.53% in 50% FC and 51.82% in 25% FC), and in HC-6, the enzyme activity also increased (43% in 50% FC and 64% in 25% FC) in comparison to the control (Figure 6).
3.4. Metabolite Analysis
A metabolite analysis of MDA content, total anthocyanin, carotenoids, flavonoids and phenolics was conducted after each sampling (15, 30 and 45 days after sampling).
3.4.1. MDA
Under drought conditions, the MDA content increased in both varieties. At 15 DAS, an increase (16.9% in 50% FC and 37.5% in 25% FC) in ICC 4958 and a similar increase (13.4% in 50% FC and 32.4% in 25% FC) in HC-6 were observed over the control. At 30 DAS, MDA increased (17.3% in 50% FC and 28.5% in 25% FC) in ICC 4958, and a similar increase (38% in 50% FC and 48.7% in 25% FC) in HC-6 was observed over the control. At 45 DAS, we observed an increase (14.17% in 50% FC and 49.7% in 25% FC) in ICC 4958 and a similar increase (27.5% in 50% FC and 40.7% in 25% FC) in HC-6 in comparison to the control (Figure 7).
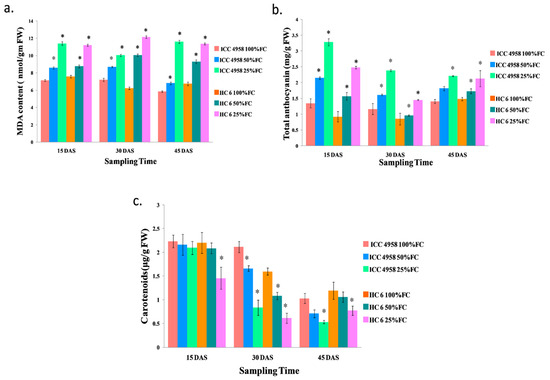
Figure 7.
Effect of drought stress in two varieties on (a) malondialdehyde content; (b) total anthocyanin content;and (c) carotenoid content. * shows the significance level with respect to control.
3.4.2. Total Anthocyanin
The total anthocyanin content increased in both the varieties under drought conditions. At 15 DAS, we observed an increase (37.15% in 50% FC and 58.8% in 25% FC) in ICC 4958 and a similar increase (41% in 50% FC and 62.68% in 25% FC) in HC-6 compared to the control. At 30 DAS, we observed an increase (27.5% in 50% FC and 62.68% in 25% FC) in ICC 4958 and a similar increase (11.3% in 50% FC and 41.25% in 25% FC) in HC-6 over the control. At 45 DAS, we observed an increase (22.5% in 50% FC and 36.2% in 25% FC) over the control in ICC 4958, and a similar increase (14.5% in 50% FC and 30.5% in 25% FC) over the control in HC-6 (Figure 7).
3.4.3. Carotenoid Content
Under drought conditions, the carotenoid content decreased in both varieties. At 15 DAS, in ICC 4958, the carotenoid content decreased (3.13% in 50% FC and 6% in 25% FC), and in the HC-6 variety, the carotenoid content also decreased (5.22% in 50% FC and 33.8% in 25% FC) over the control. At 30 DAS, it decreased (21.6% in 50% FC and 60.5% in 25% FC) in ICC 4958 and a similar decrease (32.4% in 50% FC and 61.6% in 25% FC) in HC-6 was observed over the control. At 45 DAS, in ICC 4958, the carotenoid content decreased (31.2% in 50% FC and 48.3% in 25% FC) over the control, and in the HC-6 variety, the carotenoid content decreased (10.8% in 50% and 35.3% in 25% FC) over the control (Figure 7).
3.4.4. Flavonoid
The flavonoid content (mg quercetin equivalents (QE)) increased in both of the varieties as the drought progressed. At 15 DAS, we observed an increase (18.35% in 50% FC and 33.8% in 25% FC) in ICC 4958 and a similar increase (16.8% in 50% FC and 36.15% in 25% FC) in HC-6 compared to the control conditions. At 30 DAS, we observed an increase (23.8% in 50% FC and 42.5% in 25% FC) over the control in ICC 4958, and a similar increase (11% in 50% FC and 22.7% in 25% FC) over the control in HC-6. At 45 DAS, we observed an increase (4.32% in 50% FC and 30.8% in 25% FC) in ICC 4958 and a similar increase (32.6% in 50% FC and 37.2% in 25% FC) in HC-6 in comparison to the control (Figure 8).
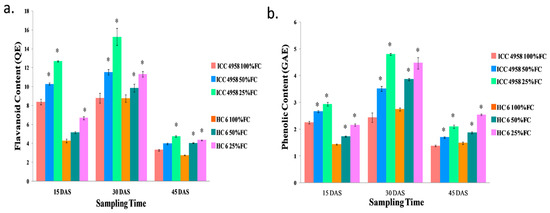
Figure 8.
Effect of drought stress in two varieties on (a) flavonoid content and (b) phenolic content. * shows the significance level with respect to control.
3.4.5. Phenolic
The phenolic content (mg gallic acid equivalents (GAE)) increased in both the varieties as drought progressed. At 15 DAS, we observed an increase (15.21% in 50% FC and 23.33% in 25% FC) in ICC 4958 and a similar increase (17.4% in 50% FC and 33.6% in 25% FC) in HC-6 as compared to the control. At 30 DAS, we observed an increase (30.6% in 50% FC and 49.2% in 25% FC) over the control in ICC 4958, and a similar increase (29% in 50% FC and 38.6% in 25% FC) over the control in HC-6. At 45 DAS, we observed an increase (18.6% in 50% FC and 34.3% in 25% FC) in ICC 4958 and a similar increase (21% in 50% FC and 42% in 25% FC) in HC-6 over the control (Figure 8).
3.5. Protein Profiling
Protein content and profiling were performed after each sampling (15, 30 and 45 days after sampling). Figure 9 shows the effect of moisture levels on the protein content in two varieties.
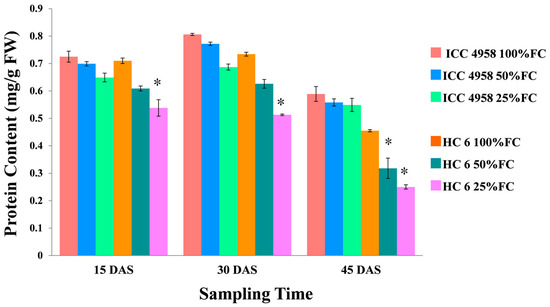
Figure 9.
Effect of drought stress in two varieties on protein content. * shows the significance level with respect to control.
3.5.1. Total Protein
Under drought conditions, the protein content decreased in both varieties. At 15 DAS, in ICC 4958, there was a decrease (3.5% in 50% FC and 10.4% in 25% FC) over the control, and in the HC-6 variety, there was a decrease (14.22% in 50% FC and 24.22% in 25% FC) over the control. At 30 DAS, there was a decrease (4.2% in 50% FC and 14.7% in 25% FC) in ICC 4958 and a similar decrease (14.7% in 50% FC and 30.1% in 25% FC) in HC-6 compared to the control. At 45 DAS, in ICC 4958, there was a decrease (5.26% in 50% FC and 6.79% in 25% FC) over the control, and in the HC-6 variety, there was a decrease (25.4% in 50% and 44.1% in 25% FC) over the control (Figure 9).
3.5.2. SDS-PAGE
Gel image (Figure 10): the expression level of peptides at 57, 32, 24 and 11 kDa showed a decrease in band intensity at 25% FC in both varieties. From the histograms, the intensity of bands can be compared with the control (100% FC). A clear difference pattern in protein changes was seen between the ICC 4958 and HC-6 varieties on the polyacrylamide gels from the presence or absence of bands to the varied intensity of expression. The absence or presence of some bands may also indicate a functional involvement stress response. Furthermore, 57 kDa, 32 kDa and 24 kDa proteins were resolved on the gel and appeared as dark bands, and their intensity decreased during stressed conditions. The results showed that the protein expression in ICC 4958 has decreased at 25% FC, and in HC-6, the decrease was observed at 50% and 25% FC.
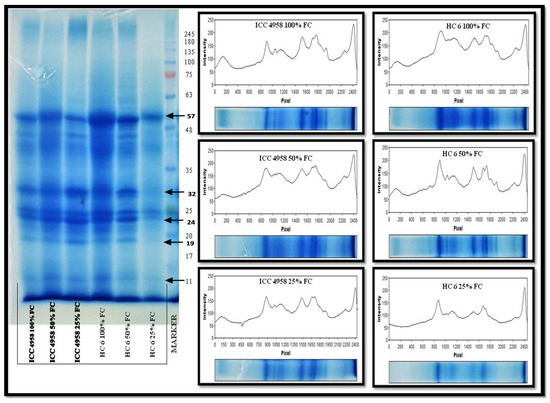
Figure 10.
SDS-PAGE gel showing expression profile of two varieties at 15 days after sowing (DAS).
The gel image (Figure 11) showed that the expression level of peptides at 57 kDa, 24 kDa, 19 kDa and 11 kDa showed a decrease in band intensity at 25% FC in both of the varieties. From the histograms, the intensity of bands can be compared with the control (100% FC). Flat and small peaks were observed in ICC 4958 at 25% FC and in HC-6 at 50% and 25% FC. The differences in protein patterns were significantly observed at 25% FC. The absence or presence of some bands may also indicate a functional involvement in the stress response. The 57 kDa and 19 kDa proteins were resolved on the gel and appeared as dark bands and their intensity decreased during stressed conditions. The results showed that the protein expression in ICC 4958 decreased at 25% FC, and in HC-6, the decrease was observed at 50% and 25% FC.
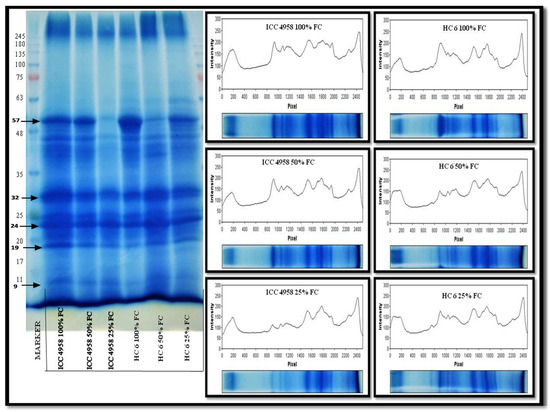
Figure 11.
SDS-PAGE gel showing the expression profile of two varieties at 30 days after sowing (DAS).
The gel image (Figure 12) showed that the expression level of peptides at 57 kDa, 24 kDa, 19 kDa and 11 kDa showed a decrease in band intensity at 25% FC in both of the varieties. In ICC 4958, we observed an increase in band intensity at 25% FC. In HC-6, the flat and small peaks were observed in 50% and 25% FC. A band at 32 kDa, which was prominent at the earlier stages, did not appear at 45 DAS, indicating a functional involvement in the stress response. The 57 kDa, 19 kDa and 11 kDa proteins were resolved on the gel and appeared as dark bands and their intensity decreased during stressed conditions. The results showed that the protein expression in ICC 4958 decreased at 25% FC, and in HC-6, the decrease was observed at 50% and 25% FC.
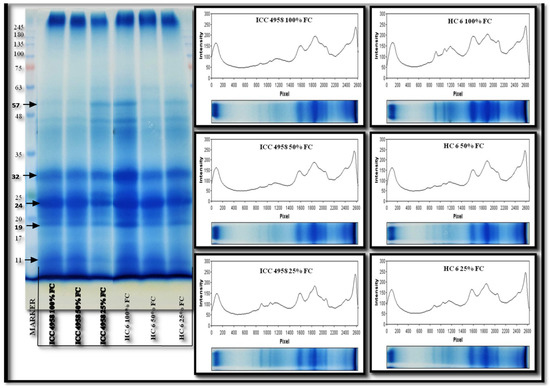
Figure 12.
SDS-PAGE gel showing expression profile of two varieties at 45 days after sowing (DAS).
4. Discussion
Chickpea (Cicer arietinum L.) is one of the stress-loving crops mostly grown in South-East Asia. Moisture stress-induced terminal drought remains a major threat to chickpea production in a rainfed-based cropping system [5,36,37]. The chickpea is one of the highly nutritive crops included in the diet for its high protein content [38]. Even under drought conditions, in tolerant varieties, the nutritive property is less affected due to its natural tolerance property. The natural drought-tolerant nature is due to many morpho-physio and biochemical changes in the plant occurring when prone to drought [39]. There are some varieties which can tolerate drought and grow normally; however, there are some varieties which cannot tolerate drought conditions, which can drastically affect their growth and yield. When sensitive varieties are prone to severe drought conditions, their nutritive properties will be affected, thus leading to nutrient deficiency if consumed [40].
The present investigation was undertaken to generate information on drought tolerance in two chickpea varieties, and its effect on various characteristics. We evaluated the two varieties (ICC 4958 and HC-6) under three drought conditions at three different growth stages. The parameters we evaluated were the morphological, physiological, biochemical, metabolite and protein profile of the two varieties. Under morphological parameters, we have taken plant height, number of branches/plant, number of leaves/plant, number of flowers/plant, number of pods/plant, days to maturity and yield/plant. Under physiological parameters, we have taken relative water content (RWC), electrolyte leakage (EL), cell membrane stability (CMS) and chlorophyll content (chlorophyll A, B and total chlorophyll). Under biochemical parameters, we have performed experiments for proline content, total sugar content, glycine betaine content and enzyme assays, namely, superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX) and glutathione reductase (GR). Under the metabolite analysis, we have performed MDA content, total anthocyanin, flavonoid content, phenolic content and carotenoids. We also performed protein profiling of the two varieties using SDS-PAGE.
4.1. Morphological Parameters
The morphological analysis showed that the effect of drought stress appeared to be higher in HC-6 than in ICC 4958. In deficient water conditions, the plant changes itself through changing its various pathways [19,41,42]. The reduction in plant height could be attributed to a decline in the cell enlargement and more leaf senescence in the plant underwater stress [43,44]. Karim et al. [45] stated that shoot length diminished under water shortage conditions, which was similar to our study where shoot length decreased in both the varieties. Naeem et al. [46] and Baloch et al. [47] discovered that a long root length executed better in water shortage conditions, yet the yield diminished. The plant can get by with longer root length in water shortfall conditions; however, the plant will have a critical low yield. Similarly, in our study, the root length increased as drought progressed, and it also resulted in lower yields. Hussain et al. [48] stated that water deficit affected more in the flowering stage than in the vegetative stage, initial pods were reduced in length and size, and all generative parts of the plant owing to water deficit resulted in damaging of the crop. Drought during flower induction shortens the flowering period and causes flower abortion [49], which can be clearly observed under the parameter number of the flowers/plant. Drought in critical stages such as pre-flowering and flowering causes a noticeable yield reduction [50]. Farooq et al. [51] and Hussain et al. [52] reported a similar decrease in the number of branches, leaves and flowers. Ulemale et al. [53] and Korbu et al. [5] stated that under drought conditions, plants tend to complete their life cycle, thus reducing vegetative growth, i.e., branches and leaves, and their studies in chickpeas showed similar results. A decrease in the seed yield per plant, the weight of 100 seeds and seed protein content in the plants was observed by Hasan et al. [54], and similar observations were also seen in the present study.
4.2. Physiological Parameters
The physiological changes observed could be the result of the deleterious effect of water deficit on important metabolic processes as well as the responses of various defense mechanisms by the plant under drought stress [55]. In the present study, we observed a decline in relative water content as drought stress increased. A high level of relative water content increases the performance of the plant in water deficit conditions [56,57,58]. Most studies have shown decreased relative water content and leaf water potential in response to drought stress [59,60,61,62,63]. Varieties that maintain higher relative water content under drought stress are believed to be more tolerant and give a higher yield than others [62].
Changes in the permeability of membranes may occur due to leakage from cells occurring due to damage to the components of the membrane in the lipid matrix [64]. Enhanced electrolyte leakage is considered as a symptom of stress-induced damage [65,66,67]. This often arises due to the generation of ROS, mainly H2O2, which is a long-lived molecule, and an increase in electrolyte leakage is an indication of sensitivity [68]. Shafiq et al. [69] reported that electrolyte leakage increased in maize cultivars exposed to drought stress similar to the present study. Cell membrane thermostability measures were conducted to estimate the percentage of injury during drought conditions [70]. Membrane stability was significantly reduced as indicated by an increased level of electrolyte leakage under drought stress [67,71]. The low injury index indicated the stability and resilience ability of the plant under water shortage [19].
Chlorophyll content decreased in both the varieties at 15, 30 and 45 days after sowing (DAS). Hussain et al. [48] and Shafiq et al. [69] reported that chlorophyll pigments declined in both maize cultivars under both drought stress regimes. A decrease in the chlorophyll content would be due to impaired biosynthesis or a breakdown of chlorophyll pigments, oxidative stress and also observed in drought-stressed plants [72,73,74,75,76]. Nyachiro et al. [77] and Karim et al. [45] reported that moisture stress imposed up to the flowering and pod formation stage significantly decreased chlorophyll A and chlorophyll B content. The total chlorophyll content significantly decreased in all varieties under drought stress, but the reduction was not as great in tolerant varieties [55]. A reduction in chlorophyll content has been reported in drought-stressed cotton [78], Catharanthus roseus [79], chickpea [80], mustard [81] and broad bean [82,83].
4.3. Biochemical Parameters
Upon exposure to stress, osmolytes such as proline, sugars, trehalose, glycine betaine and polyamines accumulate in many plants [84,85,86] and promote osmoregulation, serving a protective role [87,88]. Similar results of high levels of osmolytes were observed in both the varieties. High levels of compatible solutes enabled the plant to maintain low water potential [89], thereby allowing water uptake by the plants.
The accumulation of osmolytes such as proline during stress is well documented [90,91], and it has been known for a long time that the concentration of proline increases in a large variety of plants under drought stress. In addition to its role as an osmolyte, proline may also protect the protein structure and membranes from damage and reduce enzyme denaturation [92]. Seifikalhor et al. [93] reported that in the tolerant cultivar, the leaf proline content increased depending on the severity of the water deficit. In the sensitive cultivar, the proline content also increased in response to the water deficit, though this increase was not only relatively small but largely independent of water stress severity.
Sánchez et al. [94] and Hoekstra et al. [95] suggested that soluble sugars led to drought tolerance in plants as they acted as an osmoprotectant under water stress conditions. Soluble sugars not only act as metabolic resources and structural constituents of cells, but also function as signals, regulating many processes related with plant growth and development under water deficit conditions [96]. Khan et al. [97] reported that a clear increase in leaf sugar content was evident in both varieties of chickpea, and it led to an osmotic balance under stress conditions.
The high accumulation of glycine betaine or proline is considered as a prospective indicator of stress tolerance [98,99,100]. Shahet al. [101] screened various drought-tolerant chickpea cultivars and concluded that there was a significant increase in the amount of glycine betaines which significantly decreased the yield. Lv et al. [102] stated that glycine betaine not only protected the integrity of the cell membrane from drought stress damage, but also was involved in osmotic adjustment. Shafiq et al. [69] proposed that the positive role of glycine betaine helped in enhancing drought stress tolerance by upregulating mechanisms involved in growth and yield under drought conditions. These reports are similar to the results obtained in the present study, where glycine betaine increased as the drought progressed.
ROS production is stimulated under drought conditions and the deleterious effects are seen on biological membranes [103]. Major ROS scavenging enzymes in plants include superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX) and glutathione reductase (GR) [104]. In the present study, the enzyme activity was higher under drought conditions and lower under normal conditions. Mohammadi et al. [50] stated that the maximum activity of antioxidant enzymes was found in the drought stress condition, and the minimum activity was found in normal irrigation. The results from Amman [105], Saei [106] and Shafei [107] showed similar trends supporting our results, i.e., increased activity under drought conditions.
Rasool et al. [83] reported that a significant increase in SOD activity was observed in the chickpea varieties, suggesting that SOD may function as a ROS scavenger, by converting O2− to H2O2. The findings of Nazar et al. [100] showed similar results: SOD activity increased with stress in Vigna radiata. Mafakheri et al. [108] reported that CAT activity increased in drought-resistance cultivars significantly. High activities of catalase are important for plants to tolerate stresses. Similar results were reported by Rasool et al. [83], stating that CAT activity increased in varieties which were more efficient in scavenging H2O2. Nounjan et al. [109] observed an increase in APX activity in rice seedlings under stress. Eyidogan and Öz [110] reported that the APX and GR activities were significantly increased in chickpea leaves under stress.
4.4. Metabolite Analysis
In the present study, we observed an increase in the activity of metabolites such as MDA, anthocyanin, flavonoids and phenolics in response to drought stress. Lipid peroxidation estimated as MDA content was generally increased at drought stress compared to optimal conditions due to the increased damage caused by ROS. Gunes et al. [63] reported that the MDA content of the chickpea cultivars increased as the stress progressed. Eyidogan and Öz [110] studied the increased levels of H2O2 and MDA, indicating a higher level of damage under drought stress.
Higher anthocyanin content is related to higher scavenging ability [111]. Many studies have shown that drought stress causes a significant increase in the anthocyanin level including Arabidopsis [112], cotton [113], chickpea [114] and barley [115]. In the present investigation, increased anthocyanin indicated that under drought conditions, its activity enhanced, which might cause adaptation to drought.
Non-enzymatic antioxidants, such as phenols and flavonoids, are secondary metabolites that are involved in scavenging ROS and prevent damage to biomolecules [116]. Phenolics and flavonoid contents are strong antioxidants, which play an important role in the scavenging of free radicals against drought-induced oxidative damage [48,117]. In this study, we observed enhanced levels of phenols and flavonoids which may provide tolerance under drought conditions. Reddy et al. [118] reported that in higher plants, phenols and flavonoids increased under drought stress. The carotenoid content decreased under the water-deficient condition due to the production of ROS in the thylakoids that damaged plants by oxidative damage. Furthermore, progressive decreases in the moisture stress level resulted in a significant decrease in carotenoid content [45].
4.5. Protein Profiling
In the present study, protein content decreased under drought conditions. Studying proteins offers an occasion to categorize patterns of protein accumulation during stress perception, adaptation and cell defense [119]. Drought stress impacts actively on protein-related processes such as synthesis and degradation [120]. Drought stress significantly reduced the soluble protein content in the leaves of chickpeas [48], which was observed in both of the varieties. Faghani et al. [121] reported that the integrated physiology and proteomic analysis provided a better insight into the molecular responses of plants during drought. Protein degradation might be the result of the increased activity of protease or other catabolic enzymes, which were activated under drought stress, or due to the fragmentation of proteins due to the toxic effects of reactive oxygen species resulting in reduced protein content [108]. A decrease in the protein concentration would be a typical symptom of oxidative stress and has frequently been observed in drought stressed plants [72,122,123]. SDS-PAGE was used to determine the molecular weight (MW) of protein subunits and to identify subunits in relation to chickpea drought stress. Lande et al. [124] reported that chickpeas have also been found to respond to abiotic stress by the altered expression of many structural proteins and the proteins involved in stress adaptation, notably the ROS catabolizing enzymes using the proteomic approach.
5. Conclusions
From the present study, it is highly evident that plants respond differently to various drought stress treatments. Among the morphological characteristics studied, root length showed higher significance under drought conditions, which helped the plants to thrive under drought conditions. RWC and chlorophyll content can also be used to study various effects under drought conditions as they are primarily affected when the plant is exposed to water deficit conditions. Proline, total sugar content and enzyme activity are the biochemical characteristics that can be used to differentiate the tolerant from susceptible varieties. The activity of SOD, CAT, APX and GR increased under drought conditions irrespective of the variety, indicating that they play a key role under drought conditions by eliminating the ROS and help in providing resistance against drought conditions. During prolonged drought conditions, secondary metabolites can also be affected. An analysis of metabolites such as phenols, flavonoids, malondialdehyde and other pigments such as anthocyanin and carotenoids revealed that they could also be used as indicators for drought as stress levels progressed. The protein content decreased, indicating the activity of ROS during drought stress. Histograms showed a significant difference between the treatments. The presence or absence of protein bands indicated some specific function related to drought tolerance.
Extreme drought conditions affected the plant in terms of overall growth and yield in both of the varieties (Figure 13). Hence, under minimal drought conditions, both of the varieties produced the optimum yield. As a comparison among all the parameters, ICC 4958 performed better for most of the characteristics than HC-6, indicating that it is a better drought tolerant variety.
Figure 13.
Schematic presentation of drought stress on various parameters in chickpea.
Author Contributions
Conceptualization, Y.K.S., Y.A. and N.L.; methodology, Y.A., Y.K.S. and N.L.; software, Y.A. and N.L.; validation, Y.A., N.L. and A.Z.; formal analysis, Y.K.S., Y.A. and N.L.; investigation, Y.K.S., Y.A., N.L. and A.Z.; resources, Y.K.S., Y.A., M.A.R., H.O.E., S.R.M.S., E.A.M. and N.L.; data curation, Y.K.S., K.M., Y.A. and N.L.; writing—original draft preparation, Y.K.S., Y.A., M.A.R., A.Z., K.M. and N.L.; writing—review and editing, Y.A., A.Z., N.L. and M.A.R.; visualization, Y.K.S., Y.A. and N.L.; supervision, Y.K.S., Y.A. and N.L.; project administration, N.L., H.O.E., S.R.M.S. and E.A.M.; funding acquisition, H.O.E., S.R.M.S., M.A.R. and E.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
Researchers Supporting Project number (RSPD2023R743), King Saud University.
Data Availability Statement
The data will be available upon request from the corresponding author.
Acknowledgments
The authors express their gratitude to their respective institutions and Indian Council of Agricultural Research—National Talent Fellowship (ICAR-NTS) for their support and fellowships, respectively. The authors extend their sincere appreciation to Researchers Supporting Project number (RSPD2023R743), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAOSTAT. 2019. Available online: http://www.fao.org/faostat (accessed on 11 November 2019).
- Graham, P.H.; Vance, C.P. Legumes: Importance and constraints to greater use. Plant Physiol. 2003, 131, 872–877. [Google Scholar] [CrossRef]
- Sarmah, B.K.; Acharjee, S.; Sharma, H.C. Chickpea: Crop improvement under changing environment conditions. In Improving Crop Productivity in Sustainable Agriculture; Wiley: Hoboken, NJ, USA, 2012; pp. 361–380. [Google Scholar]
- Varshney, R.K.; Thudi, M.; Nayak, S.N.; Gaur, P.M.; Kashiwagi, J.; Krishnamurthy, L.; Jaganathan, D.; Koppolu, J.; Bohra, A.; Tripathi, S.; et al. Genetic dissection of drought tolerance in chickpea (Cicer arietinum L.). Theor. Appl. Genet. 2004, 127, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Korbu, L.; Fikre, A.; Tesfaye, K.; Funga, A.; Bekele, D.; Ojiewo, C.O. Response of chickpea to varying moisture stress conditions in Ethiopia. Agrosyst. Geosci. Environ. 2022, 5, e20234. [Google Scholar] [CrossRef]
- Singh, U.; Singh, B. Tropical grain legumes as important human foods. Econ. Bot. 1992, 46, 310–321. [Google Scholar] [CrossRef]
- Merga, B.; Haji, J. Economic importance of chickpea: Production, value, and world trade. Cogent Food Agric. 2019, 5, 1615718. [Google Scholar] [CrossRef]
- Manjunatha, L.; Puyam, A.; Prema, G.U.; Sanjay Bandi, M.; Kumar, R.; Keerthi, M.C.; Dixit, G.P.; Kavitha, T.R. Chickpea Biotic Stresses. In Genomic Designing for Biotic Stress Resistant Pulse Crops; Springer: Berlin/Heidelberg, Germany, 2022; pp. 117–159. [Google Scholar]
- Solomon, S.; Manning, M.; Marquis, M.; Qin, D. Climate Change 2007—The Physical Science Basis: Working Group I Contribution to the Fourth Assessment Report of the IPCC; Cambridge University Press: Cambridge, UK, 2007; Volume 7. [Google Scholar]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 2009, 9, 306. [Google Scholar] [CrossRef]
- Farooq, M.; Gogoi, N.; Barthakur, S.; Baroowa, B.; Bharadwaj, N.; Alghamdi, S.S.; Siddique, K.H. Drought stress in grain legumes during reproduction and grain filling. J. Agron. Crop Sci. 2017, 203, 81–102. [Google Scholar] [CrossRef]
- Gupta, S.; Mishra, S.K.; Misra, S.; Pandey, V.; Agrawal, L.; Nautiyal, C.S.; Chauhan, P.S. Revealing the complexity of protein abundance in chickpea root under drought-stress using a comparative proteomics approach. Plant Physiol. Biochem. 2022, 151, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Bodner, G.; Nakhforoosh, A.; Kaul, H.P. Management of crop water under drought: A review. Agron. Sustain. Dev. 2015, 35, 401–442. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wang, Z.; Kim, W.S. Effect of drought stress on shoot growth and physiological response in the cut rose ‘charming black’at different developmental stages. Hortic. Environ. Biotechnol. 2019, 60, 1–8. [Google Scholar] [CrossRef]
- Parkash, V.; Singh, S. A review on potential plant-based water stress indicators for vegetable crops. Sustainability 2020, 12, 3945. [Google Scholar] [CrossRef]
- Varshney, R.K.; Hiremath, P.J.; Lekha, P.; Kashiwagi, J.; Balaji, J.; Deokar, A.A.; Vadez, V.; Xiao, Y.; Srinivasan, R.; Gaur, P.M.; et al. A comprehensive resource of drought-and salinity-responsive ESTs for gene discovery and marker development in chickpea (Cicer arietinum L.). BMC Genom. 2009, 10, 532. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Shankar, R.; Thakkar, B.; Kudapa, H.; Krishnamurthy, L.; Mantri, N.; Varshney, R.K.; Bhatia, S.; Jain, M. Transcriptome analyses reveal genotype-and developmental stage-specific molecular responses to drought and salinity stresses in chickpea. Sci. Rep. 2016, 6, srep19228. [Google Scholar] [CrossRef]
- Rashid, K.A.; Akhtar, M.U.; Cheema, K.L.; Rasool, I.R.; Zahid, A.F.; Hussain, A.M.; Aqeel, M.U.; Anwar, S.A.; Amin, M.; Qadeer, Z.; et al. Development of Selection Criteria for Assessment of Chickpea (Cicer arientum L.) On Physio-Morphic Attributes under Drought Stress at Seedling Stage and Maturity. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 98–109. [Google Scholar]
- Smart, R.E.; Bingham, G.E. Rapid estimates of relative water content. Plant Physiol. 1974, 53, 258–260. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Analysis 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Beyer Jr, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Hodges, D.M.; Nozzolillo, C. Anthocyanin and anthocyanoplast content of cruciferous seedlings subjected to mineral nutrient deficiencies. J. Plant Physiol. 1996, 147, 749–754. [Google Scholar] [CrossRef]
- Julkunen-Tiitto, R. Phenolic constituents in the leaves of northern willows: Methods for the analysis of certain phenolics. J. Agric. Food Chem. 1985, 33, 213–217. [Google Scholar] [CrossRef]
- Quettier-Deleu, C.; Gressier, B.; Vasseur, J.; Dine, T.; Brunet, C.; Luyckx, M.; Cazin, M.; Cazin, J.C.; Bailleul, F.; Trotin, F. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J. Ethnopharmacol. 2000, 72, 35–42. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Korbu, L.; Tafes, B.; Kassa, G.; Mola, T.; Fikre, A. Unlocking the genetic potential of chickpea through improved crop management practices in Ethiopia. A review. Agron. Sustain. Dev. 2020, 40, 13. [Google Scholar] [CrossRef]
- Kumar, P.; Boora, K.S.; Kumar, N.; Batra, R.; Goyal, M.; Sharma, K.D.; Yadav, R.C. Traits of significance for screening of chickpea (Cicer arietinum L.) genotypes under terminal drought stress. J. Agrometeorol. 2018, 20, 40–45. [Google Scholar] [CrossRef]
- Malunga, L.N.; Bar-El, S.D.; Zinal, E.; Berkovich, Z.; Abbo, S.; Reifen, R. The potential use of chickpeas in development of infant follow-on formula. Nutr. J. 2014, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Gaur, P.M.; Jukanti, A.K.; Samineni, S.; Chaturvedi, S.K.; Basu, P.S.; Babbar, A.; Jayalakshmi, V.; Nayyar, H.; Devasirvatham, V.; Mallikarjuna, N.; et al. Climate change and heat stress tolerance in chickpea. In Climate Change and Plant Abiotic Stress Tolerance; Wiley: Hoboken, NJ, USA, 2013; pp. 837–856. [Google Scholar]
- Farooq, M.; Rizwan, M.; Nawaz, A.; Rehman, A.; Ahmad, R. Application of natural plant extracts improves the tolerance against combined terminal heat and drought stresses in bread wheat. J. Agron. Crop Sci. 2017, 203, 528–538. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef] [PubMed]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Manivannan, P.; Jaleel, C.A.; Sankar, B.; Kishorekumar, A.; Somasundaram, R.; Lakshmanan, G.A.; Panneerselvam, R. Growth, biochemical modifications and proline metabolism in Helianthus annuus L. as induced by drought stress. Colloids Surf. B Biointerfaces 2007, 59, 141–149. [Google Scholar] [CrossRef]
- Shakeel, A.A.; Xiao-yu, X.; Long-chang, W.; Muhammad, F.S.; Chen, M.; Wang, L. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]
- Karim, A.M.A.; Sarker, U.K.; Hasan, A.K.; Islam, N.; Uddin, M.R. Physiological and Biochemical Responses of Chickpea (Cicer arietinum L.) Genotypes to Different Moisture Stresses. Turk. J. Field Crops 2022, 27, 1–9. [Google Scholar] [CrossRef]
- Naeem, M.K.; Ahmad, M.; Shah, M.K.N.; Kamran, M.; Iqbal, M.S. Character association and path analysis of osmotic adjustment, growth and physiological traits in wheat. JAPS J. Anim. Plant Sci. 2016, 26, 680–685. [Google Scholar]
- Baloch, M.J.; Dunwell, J.; Khakwani, A.A.; Dennett, M.; Jatoi, W.A.; Channa, S.A. Assessment of wheat cultivars for drought tolerance via osmotic stress imposed at early seedling growth stages. J. Agric. Res. 2012, 50, 299–310. [Google Scholar]
- Hussain, I.; Rasheed, R.; Ashraf, M.; Mohsin, M.; Ali, S.; Rashid, A.; Akram, M.; Nisar, J.; Riaz, M. Foliar Applied Acetylsalicylic Acid InducedGrowth and Key-Biochemical Changes in Chickpea (Cicer arietinum L.) Under Drought Stress. Dose-Response 2020, 18, 1559325820956801. [Google Scholar] [CrossRef]
- Fang, X.; Turner, N.C.; Yan, G.; Li, F.; Siddique, K.H. Flower numbers, pod production, pollen viability, and pistil function are reduced and flower and pod abortion increased in chickpea (Cicer arietinum L.) under terminal drought. J. Exp. Bot. 2010, 61, 335–345. [Google Scholar] [CrossRef]
- Mohammadi, A.; Habibi, D.; Rohami, M.; Mafakheri, S. Effect of drought stress on antioxidant enzymes activity of some chickpea cultivars. Am-Eurasian J. Agric. Environ. Sci. 2011, 11, 782–785. [Google Scholar]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K.H.M. Drought stress in plants: An overview. In Plant Responses to Drought Stress; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–33. [Google Scholar]
- Hussain, M.; Farooq, S.; Hasan, W.; Ul-Allah, S.; Tanveer, M.; Farooq, M.; Nawaz, A. Drought stress in sunflower: Physiological effects and its management through breeding and agronomic alternatives. Agric. Water Manag. 2018, 201, 152–166. [Google Scholar] [CrossRef]
- Ulemale, C.S.; Mate, S.N.; Deshmukh, D.V. Physiological indices for drought tolerance in chickpea (Cicer arietinum L.). World J. Agric. Sci. 2013, 9, 123–131. [Google Scholar]
- Hasan, S.A.; Hayat, S.; Ali, B.; Ahmad, A. 28-Homobrassinolide protects chickpea (Cicer arietinum) from cadmium toxicity by stimulating antioxidants. Environ. Pollut. 2008, 151, 60–66. [Google Scholar] [CrossRef]
- Talebi, R.; Ensafi, M.H.; Baghebani, N.; Karami, E.; Mohammadi, K. Physiological responses of chickpea (Cicer arietinum) genotypes to drought stress. Environ. Exp. Biol. 2013, 11, 9–15. [Google Scholar]
- Atteya, A.M. Alteration of water relations and yield of corn genotypes in response to drought stress. Bulg. J. Plant Physiol. 2003, 29, 63–76. [Google Scholar]
- Bilal, M.; Rashid, R.M.; Rehman, S.U.; Iqbal, F.; Ahmed, J.; Abid, M.A.; Ahmed, Z.; Hayat, A. Evaluation of wheat genotypes for drought tolerance. J. Green Physiol. Genet. Genom. 2015, 1, 11–21. [Google Scholar]
- Liu, H.; Searle, I.R.; Mather, D.E.; Able, A.J.; Able, J.A. Morphological, physiological and yield responses of durum wheat to pre-anthesis water-deficit stress are genotypedependent. Crop Pasture Sci. 2015, 66, 1024–1038. [Google Scholar] [CrossRef]
- Siddique, M.R.B.; Hamid, A.I.M.S.; Islam, M.S. Drought stress effects on water relations of wheat. Bot. Bull. Acad. Sin. 2000, 41, 35–39. [Google Scholar]
- Fu, J.; Huang, B. Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ. Exp. Bot. 2001, 45, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Terzi, R.; Kadioglu, A. Drought stress tolerance and the antioxidant enzyme system. Acta Biol. Crac. Ser. Bot. 2006, 48, 89–96. [Google Scholar]
- Bayoumi, T.Y.; Eid, M.H.; Metwali, E.M. Application of physiological and biochemical indices as a screening technique for drought tolerance in wheat genotypes. Afr. J. Biotechnol. 2008, 7, 2341–2352. [Google Scholar]
- Gunes, A.I.; Adak, M.S.; Bagci, N.; Cicek, E.G.; Eraslan, F. Effect of Drought Stress Implemented at Pre- or Post-Anthesis Stage on Some Physiological Parameters as Screening Criteria in Chickpea Cultivars. Russ. J. Plant Physiol. 2008, 55, 59–67. [Google Scholar] [CrossRef]
- Scotti-Campos, P.; Pham-Thi, A.T.; Semedo, J.N.; Pais, I.P.; Ramalho, J.C.; do Céu Matos, M. Physiological responses and membrane integrity in three Vigna genotypes with contrasting drought tolerance. Emir. J. Food Agric. 2013, 25, 1002–1013. [Google Scholar] [CrossRef]
- Feng, Z.; Guo, A.; Feng, Z. Amelioration of chilling stress by triadimefon in cucumber seedlings. Plant Growth Regul. 2003, 39, 277–283. [Google Scholar] [CrossRef]
- Munne-Bosch, S.; Penuelas, J. Photo-and antioxidative protection, and a role for salicylic acid during drought and recovery in field-grown Phillyrea angustifolia plants. Planta 2003, 217, 758–766. [Google Scholar] [CrossRef]
- Farooq, M.; Basra, S.M.A.; Wahid, A.; Cheema, Z.A.; Cheema, M.A.; Khaliq, A. Physiological role of exogenously applied glycine betaine to improve drought tolerance in fine grain aromatic rice (Oryza sativa L.). J. Agron. Crop Sci. 2008, 194, 325–333. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signaling transduction. Annu. Rev. Plant Biol. 2004, 55, 373. [Google Scholar] [CrossRef]
- Shafiq, S.; Akram, N.A.; Ashraf, M.; García-Caparrós, P.; Ali, O.M.; Latef, A.A.H.A. Influence of glycine betaine (natural and synthetic) on growth, metabolism and yield production of drought-stressed maize (Zea mays L.) plants. Plants 2021, 10, 2540. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, J.A.; Karim, M.A.; Khaliq, Q.A.; Ahmed, A.U. Effect of drought stress on bio-chemical change and cell membrane stability of soybean genotypes. Bangladesh J. Agric. Res. 2017, 42, 475–485. [Google Scholar] [CrossRef]
- Simova-Stoilova, L.; Demirevska, K.; Petrova, T.; Tsenov, N.; Feller, U. Antioxidative protection in wheat varieties under severe recoverable drought at seedling stage. Plant Soil Env. 2008, 54, 529–536. [Google Scholar] [CrossRef]
- Moran, J.F.; Becana, M.; Iturbe-Ormaetxe, I.; Frechilla, S.; Klucas, R.V.; Aparicio-Tejo, P. Drought induces oxidative stress in pea plants. Planta 1994, 194, 346–352. [Google Scholar] [CrossRef]
- Campos, P.S.; Ramalho, J.C.; Lauriano, J.A.; Silva, M.J.; do Ceu Matos, M. Effects of drought on photosynthetic performance and water relations of four Vigna genotypes. Photosynthetica 1999, 36, 79–87. [Google Scholar]
- Egert, M.; Tevini, M. Influence of drought on some physiological parameters symptomatic for oxidative stress in leaves of chives (Allium schoenoprasum). Environ. Exp. Bot. 2002, 48, 43–49. [Google Scholar] [CrossRef]
- Mahdavi Mashaki, K.; Garg, V.; Nasrollahnezhad Ghomi, A.A.; Kudapa, H.; Chitikineni, A.; Zaynali Nezhad, K.; Yamchi, A.; Soltanloo, H.; Varshney, R.K.; Thudi, M. RNA-Seq analysis revealed genes associated with drought stress response in kabuli chickpea (Cicer arietinum L.). PLoS ONE 2018, 13, e0199774. [Google Scholar] [CrossRef]
- Bhuiyan, T.F.; Ahamed, K.U.; Nahar, K.; Al Mahmud, J.; Bhuyan, M.B.; Anee, T.I.; Fujita, M.; Hasanuzzaman, M. Mitigation of PEG-induced drought stress in rapeseed (Brassica rapa L.) by exogenous application of osmolytes. Biocatal. Agric. Biotechnol. 2019, 20, 101197. [Google Scholar] [CrossRef]
- Nyachiro, J.M.; Briggs, K.G.; Hoddinott, J.; Johnson-Flanagan, A.M. Chlorophyll content, chlorophyll fluorescence and water deficit in spring wheat. Cereal Res. Commun. 2001, 29, 135–142. [Google Scholar] [CrossRef]
- Massacci, A.; Nabiev, S.M.; Pietrosanti, L.; Nematov, S.K.; Chernikova, T.N.; Thor, K.; Leipner, J. Response of the photosynthetic apparatus of cotton (Gossypium hirsutum) to the onset of drought stress under field conditions studied by gas-exchange analysis and chlorophyll fluorescence imaging. Plant Physiol. Biochem. 2008, 46, 189–195. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Lakshmanan, G.M.A.; Gomathinayagam, M.; Panneerselvam, R. Alterations in morphological parameters and photosynthetic pigment responses of Catharanthus roseus under soil water deficits. Colloids Surf. B Biointerfaces 2008, 61, 298–303. [Google Scholar] [CrossRef]
- Rahbarian, R.; Khavari-Nejad, R.; Ganjeali, A.; Bagheri, A.; Najafi, F. Drought stress effects on photosynthesis, chlorophyll fluorescence and water relations in tolerant and susceptible chickpea (Cicer arietinum L.) genotypes. Acta Biol. Crac. Ser. Bot. 2011, 53, 47–56. [Google Scholar] [CrossRef]
- Parvaiz, A.; Khalid, U.R.H.; Ashwani, K.; Muhammad, A.; Nudrat, A.A. Salt-induced changes in photosynthetic activity and oxidative defense system of three cultivars of mustard (Brassica juncea L.). Afr. J. Biotechnol. 2012, 11, 2694–2703. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Ashraf, M.Y.; Parvaiz, A. Evaluation of salicylic acid (SA) application on growth, osmotic solutes and antioxidant enzyme activities on broad bean seedlings grown under diluted seawater. Int. J. Plant Physiol. Biochem. 2011, 3, 253–264. [Google Scholar]
- Rasool, S.; Ahmad, A.; Siddiqi, T.O.; Ahmad, P. Changes in growth, lipid peroxidation and some key antioxidant enzymes in chickpea genotypes under salt stress. Acta Physiol. Plant. 2013, 35, 1039–1050. [Google Scholar] [CrossRef]
- Lata, C.; Muthamilarasan, M.; Prasad, M. Drought stress responses and signal transduction in plants. In Elucidation of Abiotic Stress Signaling in Plants; Springer: New York, NY, USA, 2015; pp. 195–225. [Google Scholar]
- Dapanage, M.; Bhat, S. Physiological responses of commercial sugarcane (Saccharum spp. hybrids) varieties to moisture deficit stress tolerance. Indian J. Plant Physiol. 2018, 23, 40–47. [Google Scholar] [CrossRef]
- Takahashi, F.; Kuromori, T.; Sato, H.; Shinozaki, K. Regulatory gene networks in drought stress responses and resistance in plants. Surviv. Strategies Extrem. Cold Desiccation 2018, 1081, 189–214. [Google Scholar]
- Mwadzingeni, L.; Shimelis, H.; Tesfay, S.; Tsilo, T.J. Screening of bread wheat genotypes for drought tolerance using phenotypic and proline analyses. Front. Plant Sci. 2016, 7, 1276. [Google Scholar] [CrossRef] [PubMed]
- Hassanvand, F.; Nejad, A.R.; Fanourakis, D. Morphological and physiological components mediating the silicon-induced enhancement of geranium essential oil yield under saline conditions. Ind. Crops Prod. 2019, 134, 19–25. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Vendruscolo, E.C.G.; Schuster, I.; Pileggi, M.; Scapim, C.A.; Molinari, H.B.C.; Marur, C.J.; Vieira, L.G.E. Stress-induced synthesis of proline confers tolerance to water deficit in transgenic wheat. J. Plant Physiol. 2007, 164, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Lum, M.S.; Hanafi, M.M.; Rafii, Y.M.; Akmar, A.S.N. Effect of drought stress on growth, proline and antioxidant enzyme activities of upland rice. JAPS J. Anim. Plant Sci. 2014, 24, 1487–1493. [Google Scholar]
- Ain-Lhout, F.; Zunzunegui, M.; Barradas, D.; Tirado, R.; Clavijo, A.; Garcia Novo, F. Comparison of proline accumulation in two Mediterranean shrubs subjected to natural and experimental water deficit. Plant Soil 2001, 230, 175–183. [Google Scholar] [CrossRef]
- Seifikalhor, M.; Niknam, V.; Aliniaeifard, S.; Didaran, F.; Tsaniklidis, G.; Fanourakis, D.; Teymoorzadeh, M.; Mousavi, S.H.; Bosacchi, M.; Li, T. The regulatory role of γ-Aminobutyric acid in chickpea plants depends on drought tolerance and water scarcity level. Sci. Rep. 2022, 12, 7034. [Google Scholar] [CrossRef]
- Sánchez, F.J.; Manzanares, M.; de Andres, E.F.; Tenorio, J.L.; Ayerbe, L. Turgor maintenance, osmotic adjustment and soluble sugar and proline accumulation in 49 pea cultivars in response to water stress. Field Crops Res. 1998, 59, 225–235. [Google Scholar] [CrossRef]
- Hoekstra, F.A.; Golovina, E.A.; Buitink, J. Mechanisms of plant desiccation tolerance. Trends Plant Sci. 2001, 6, 431–438. [Google Scholar] [CrossRef]
- Wang, L.; Ruan, Y.L. Regulation of cell division and expansion by sugar and auxin signaling. Front. Plant Sci. 2013, 4, 163. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Bano, A.; Rahman, M.A.; Guo, J.; Kang, Z.; Babar, M. Comparative physiological and metabolic analysis reveals a complex mechanism involved in drought tolerance in chickpea (Cicer arietinum L.) induced by PGPR and PGRs. Sci. Rep. 2019, 9, 2097. [Google Scholar] [CrossRef]
- Ashraf, M.F.M.R.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Yaqoob, H.; Akram, N.A.; Iftikhar, S.; Ashraf, M.; Khalid, N.; Sadiq, M.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Seed pretreatment and foliar application of proline regulate morphological, physio-biochemical processes and activity of antioxidant enzymes in plants of two cultivars of quinoa (Chenopodium quinoa Willd.). Plants 2019, 8, 588. [Google Scholar] [CrossRef] [PubMed]
- Nazar, Z.; Akram, N.A.; Saleem, M.H.; Ashraf, M.; Ahmed, S.; Ali, S.; Abdullah Alsahli, A.; Alyemeni, M.N. Glycinebetaine-induced alteration in gaseous exchange capacity and osmoprotective phenomena in safflower (Carthamus tinctorius L.) under water deficit conditions. Sustainability 2020, 12, 10649. [Google Scholar] [CrossRef]
- Shah Mahmud, T.; Imran, M.; Atta, B.M.; Ashraf, M.Y.; Hameed, A.; Waqar, I.; Maqbool, M.A. Selection and screening of drought tolerant high yielding chickpea genotypes based on physio-biochemical indices and multi-environmental yield trials. BMC Plant Biol. 2020, 20, 171. [Google Scholar]
- Lv, S.; Yang, A.; Zhang, K.; Wang, L.; Zhang, J. Increase of glycine betaine synthesis improves drought tolerance in cotton. Mol. Breed. 2007, 20, 233–248. [Google Scholar] [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef]
- Loggini, B.; Scartazza, A.; Brugnoli, E.; Navari-Izzo, F. Antioxidative defense system, pigment composition, and photosynthetic efficiency in two wheat cultivars subjected to drought. Plant Physiol. 1999, 119, 1091–1100. [Google Scholar] [CrossRef]
- Amman, A. Drought Effect of Drought Conditions at Yield, Yield Components and Physiological Characteristics in Different Varieties of Sunflowers. Master’s Thesis, Faculty of Agriculture and Natural Resources Karaj-Branch Islamic Azad University, Karaj, Iran, 2004. [Google Scholar]
- Saei, M. Study on Relationship Morph-Physiological Traits with Drought Tolerance in Different Genotypes of Sorghum M. Master’s Thesis, Faculty of Agriculture and Natural Resources Karaj Branch Islamic Azad University, Karaj, Iran, 2004. [Google Scholar]
- Shafei, S. Study on Water Shortage Effect at Some of Physiological Traits, Yield and Yield Components in Some Genotypes of Soybean. Master’s Thesis, Islamic Azad University, Tehran, Iran, 2005. [Google Scholar]
- Mafakheri, A.; Siosemardeh, A.F.; Bahramnejad, B.; Struik, P.C.; Sohrabi, Y. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust. J. Crop Sci. 2011, 4, 580–585. [Google Scholar]
- Nounjan, N.; Nghia, P.T.; Theerakulpisut, P. Exogenous proline and trehalose promote recovery of rice seedlings from salt-stress and differentially modulate antioxidant enzymes and expression of related genes. J. Plant Physiol. 2012, 169, 596–604. [Google Scholar] [CrossRef]
- Eyidogan, F.; Öz, M.T. Effect of salinity on antioxidant responses of chickpea seedlings. Acta Physiol. Plant. 2007, 29, 485–493. [Google Scholar] [CrossRef]
- Ploenlap, P.; Pattanagul, W. Effects of exogenous abscisic acid on foliar anthocyanin accumulation and drought tolerance in purple rice. Biologia 2015, 70, 915–921. [Google Scholar] [CrossRef]
- Steyn, W.J.; Wand, S.J.E.; Holcroft, D.M.; Jacobs, G.J.N.P. Anthocyanins in vegetative tissues: A proposed unified function in photoprotection. New Phytol. 2002, 155, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Deeba, F.; Pandey, A.K.; Ranjan, S.; Mishra, A.; Singh, R.; Sharma, Y.K.; Shirke, P.A.; Pandey, V. Physiological and proteomic responses of cotton (Gossypium herbaceum L.) to drought stress. Plant Physiol. Biochem. 2012, 53, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Kalefetoğlu Macar, T.; Ekmekci, Y. Alterations in photochemical and physiological activities of chickpea (Cicer arietinum L.) cultivars under drought stress. J. Agron. Crop Sci. 2009, 195, 335–346. [Google Scholar] [CrossRef]
- Bandurska, H.; Pietrowska-Borek, M.; Cieślak, M. Response of barley seedlings to water deficit and enhanced UV-B irradiation acting alone and in combination. Acta Physiol. Plant. 2012, 34, 161–171. [Google Scholar] [CrossRef]
- Quan, N.T.; Anh, L.H.; Khang, D.T.; Tuyen, P.T.; Toan, N.P.; Minh, T.N.; Minh, L.T.; Bach, D.T.; Ha, P.T.T.; Elzaawely, A.A.; et al. Involvement of secondary metabolites in response to drought stress of rice (Oryza sativa L.). Agriculture 2016, 6, 23. [Google Scholar] [CrossRef]
- Cos, P.; Ying, L.; Calomme, M.; Hu, J.P.; Cimanga, K.; Van Poel, B.; Pieters, L.; Vlietinck, A.J.; Berghe, D.V. Structure—Activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J. Nat. Prod. 1998, 61, 71–76. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Pawlowski, M.; Lasica, A.M.; Jagusztyn-Krynicka, E.K.; Bujnicki, J.M. AAN82231 protein from uropathogenic E. coli CFT073 is a close paralog of DsbB enzymes and does not belong to the DsbI family. Pol. J. Microbiol. 2009, 58, 181–184. [Google Scholar]
- Vessal, S.; Arefian, M.; Siddique, K.H. Proteomic responses to progressive dehydration stress in leaves of chickpea seedlings. BMC Genom. 2020, 21, 523. [Google Scholar] [CrossRef]
- Faghani, E.; Gharechahi, J.; Komatsu, S.; Mirzaei, M.; Khavarinejad, R.A.; Najafi, F.; Farsad, L.K.; Salekdeh, G.H. Comparative physiology and proteomic analysis of two wheat genotypes contrasting in drought tolerance. J. Proteom. 2015, 114, 1–15. [Google Scholar] [CrossRef]
- Seel, W.E.; Hendry, G.A.F.; Lee, J.A. The combined effects of desiccation and irradiance on mosses from xeric and hydric habitats. J. Exp. Bot. 1992, 43, 1023–1030. [Google Scholar] [CrossRef]
- Arefian, M.; Vessal, S.; Bagheri, A. Biochemical changes and SDS-PAGE analyses of chickpea (Cicer arietinum L.) genotypes in response to salinity during the early stages of seedling growth. J. Biol. Environ. Sci. 2014, 8, 99–109. [Google Scholar]
- Lande, N.V.; Subba, P.; Barua, P.; Gayen, D.; Prasad, T.K.; Chakraborty, S.; Chakraborty, N. Dissecting the chloroplast proteome of chickpea (Cicer arietinum L.) provides new insights into classical and non-classical functions. J. Proteom. 2017, 165, 11–20. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).