Abstract
Lentil production in arable, Mediterranean-type climates is limited by acute high temperature (HT) commonly occurring during the reproductive stage. With changing climate and greater weather extremes, there is a need to increase the HT tolerance of lentil to sustain production, and global germplasm provides adaptation opportunities. The current study assessed 81 genotypes for HT tolerance from a range of global climatic zones. Field screening of germplasm was undertaken over two consecutive years (2014 and 2015), in southern Australia, using a late-sowing approach, which included a subset of 22 genotypes that were screened in both years. Partially shaded temperature treatments within a split-plot arrangement were used to generate two different HT profiles. Stress indices, i.e., the yield stability index (YSI), the stress tolerance index (STI), and a third proposed high-temperature tolerance index (HTTI), were applied to rank the HT tolerance of germplasm. In 2014, under field conditions associated with natural temperature ranges that were favorable for screening, the following five landraces with increased temperature tolerance were identified: AGG 73838, AGG 70118, AGG 70951, AGG 70156, and AGG 70549. Among the 10 commercial varieties tested, one variety (i.e., cv. Nipper) was observed to have HT tolerance. For the YSI, which had the greatest amount of consistency in response across the 2 years (11 of the 22 genotypes), there were two genotypes (AGG 71457 and Nipper) which maintained their yield stability. These results demonstrate the opportunity that germplasm provides to improve the adaptation of lentil to HT. Ultimately, the late-sowing approach is one possible methodology to integrate into contemporary breeding programs for improving adaptation of lentil within Mediterranean-type environments.
1. Introduction
Lentil (Lens culinaris) is one of the oldest domesticated grain legumes [1] which is grown widely throughout the Indian subcontinent, Middle East, Northern Africa, East Africa, southern Europe, North and South Americas, Australia, and Western Asia. Consequently, lentil has evolved over a broad range of growing environments globally, which infers broad genetic variation in tolerance to a range of abiotic constraints associated with these regions, including acute high-temperature (HT) stress. Within southern Australia, lentil has gained increasing importance as a high-value crop within rainfed cropping systems, including the benefits of fixing nitrogen and providing rotation options to limit disease. Lentil production in southern Australia is progressively expanding into more marginal areas, and the risks of HT, particularly during the reproductive phase, can cause significant yield loss. In many lentil producing regions, HT is managed through early sowing and selecting early maturing genotypes, which limit exposure to HT [2,3]. Increased adaptation of lentil through selecting for traits associated with HT tolerance is also an important component in maximizing production potential and increasing yield stability, particularly amid increasing weather volatility associated with climate change [4].
During the past three decades, lentil breeding strategies have focused on the development of varieties which have higher and stable yields across diverse agroecological zones. Despite this, within Australia the national lentil yield of 1.2 t/ha has remained largely unchanged due to a combination of progressive expansion of lentil into drier, lower yielding environments and a lack of genetic improvement in yield [5]. The concept of breeding is based on selecting material with high yield potential across years, hence, it may inadvertently increase the level of resistance to abiotic stresses. However, reliance on this process is not adequate to increase tolerance to targeted abiotic stresses such as HT, due to the fact that a narrow genetic base within breeding programs restricts the development of improved plant types adapted to specific abiotic stresses [6,7], and therefore, the genetics to increase traits is unlikely to exist within the breeding programs. Consequently, pre-breeding strategies for screening diverse germplasm to identify increased adaptation to specific stresses are routinely performed to identify tolerance within landraces beyond the pedigrees within breeding programs. For lentil, pre-breeding screening strategies in Australia have led to commercial varieties with increased tolerance to a range of edaphic constraints including high boron [8], salinity [9], and Ascochyta blight [10], and such gains have been attributed to the identification of germplasm with increased tolerance. In many cases this approach has also led to the development of high-throughput screening methods, which have subsequently been incorporated into breeding programs [10,11].
Future lentil adaptive strategies for tolerance to acute HT tolerance are highly reliant on genetic solutions to reduce production risk of this crop. If rapid and efficient screening methods could be identified, which reliably identify tolerance to HT, these could be incorporated into contemporary breeding programs. Screening for HT is usually field based and involves growing crops in HT regions or using late-sowing methodologies, thus increasing the probability that the reproductive phase occurs when temperatures are high (>30 °C). For late sown crops, development is accelerated due to more rapid accumulation of thermal time and photoperiod sensitivity compared to the typical winter cropping season [12,13], causing reduced biomass and yield potential. Despite these artefact effects, a late-sowing approach has been used as an effective screening method for chickpea [13,14,15], cowpea [16], and lentil [17,18], since it is an efficient and relatively inexpensive methodology [12].
Previously for HT screening, a combination of visual scoring [14], growth/yield traits [15], and stress indices [13] have been used to identify genotypes with increased yield stability under HT. Stress tolerance indices have been successful used for screening a range of abiotic stresses in chickpea (HT) [13] and common bean (HT) [19]. For chickpea, Krishnamurthy et al. (2011) assessed the temperature tolerance of 277 chickpea genotypes using a heat tolerance index across two locations and identified 18 genotypes with HT tolerance, which were subsequently utilized in Indian breeding programs [13]. Similarly, for common bean, 14 genotypes in both glasshouse and field conditions were screened for HT, and stress indices (geometric mean, stress tolerance index, and stress susceptibility index) [20] were effectively used for the selection of genotypes with high yield potential across high- and low-stress conditions [19]. Other screening methodologies including growth cabinets or laboratory-based approaches such as quantifying membrane stability have been proposed surrogates for HT tolerance [17,21].
Using a late-sowing methodology to screen for HT tolerance in plants is likely to combine the impacts of higher ambient daily temperature (minimum and maximum temperature), acute HT effect causing a non-recoverable reduction in yield potential, and water deficit stress. Irrespective of these factors being co-correlated, such screening methodologies are likely to have value within large screening programs. The objectives of this research are to (1) assess the utility of a late-sowing methodology for screening a broad range of global lentil germplasm under field conditions in southern Australia and (2) to identify lentil germplasm with HT tolerance, particularly during the reproductive phase. Specifically, we hypothesize that (i) a late-sowing methodology including a partial shade comparison (non-limiting to photosynthesis) for imposing differential temperature treatments, can be effectively used to test for HT tolerance in lentil and (ii) to identify lentil genotypes with improved HT tolerance relative to current commercial cultivars.
2. Materials and Methods
2.1. Site Description
Two lentil field trials using a late-sowing approach to screen for HT tolerance were established over consecutive years in 2014 (2014 trial) and 2015 (2015 trial) at Horsham, Victoria, Australia (36°44′38″ S, 142°06′04″ E, a.s.l. 134 m) on grey Vertosol soil [22]. The site is characterized by a Mediterranean-type climate with a mean annual rainfall of 413 mm based on 100 years of data (Bureau of Meteorology, station 79028). The screening trials were both a randomized split-plot complete block design with two replicates in the 2014 trial and four replicates in the 2015 trial. The main plot was heat treatment and the split plot was the genotype. For the 2014 trial, 49 genotypes × 2 HT treatments × 2 replicates equated to 196 observations, and for the 2015 trial, 54 genotypes × 2 HT × 4 replicates equated to 432 observations, and 22 genotypes were tested over both years. Prior to sowing, the lentil seed was treated with inoculum (peat-based group E and F), and fertilizer (N% 10, P% 21.9, S% 1.5, and Ca% 1.6) was pre-drilled below the seed bed at rates of 80 and 70 kg/ha for the 2014 and 2015 trials, respectively. Then, fifty-five seeds were sown at a 5 cm depth in a 1 linear meter row with 0.65 m row spacing.
The sowing of lentil was timed for late spring, so the crop flowering period coincided with naturally hotter growing conditions that occur in November and December in southern Australia. For each trial, two different HT treatments were imposed by having half the trial in full sun and the second half of the trial under partial shade using a split-plot approach. Partial shading was imposed by using 50% white UV stabilized high-density polyethylene fabric (196 g/m2), which was installed 0.65 m above the canopy from early flowering until crop maturity. For both experiments, the contrasting HT treatments are defined as “YS” for the full sun temperature treatment and “YP” for the partially shaded temperature treatment, which is nomenclature that is consistent with other abiotic stress studies [23]. Throughout the growing period, the lentil plots were kept weed and pest free and no disease was observed within these trials.
Across both years, the sites were pre-irrigated using flood irrigation of 150 mm, 3 weeks prior to sowing. During the growing period, supplementary irrigation was also applied. For the 2014 trial, which was sown on the 17 October 2014, 81 mm of water was applied between sowing and first flower and 9 mm of water was applied from first flower to maturity. For the 2015 trial, which was sown on the 29 October 2015, 95 and 65 mm of water were applied for the equivalent time periods. For in-season irrigation, water was applied manually for the 2014 trial and automated in 2015 using an Aqua-Traxx® irrigation lay-flat tube. The rainfall amounts for the 2014 trial in the period of sowing to first flower and first flower to maturity were 19 and 87 mm, respectively, and in the same periods, the rainfall amounts were 24 and 89 mm for the 2015 trial (Figure A1). Including rainfall and irrigation, the total water amounts received during the period from sowing to crop maturity were 196 and 273 mm for the 2014 and 2015 trials, respectively.
2.2. Genotypes
Across both trials, the genotypes constituted a combination of commercial cultivars and landraces selected from a range of climates and geographic global regions (Figure 1) to maximize genetic diversity, in which regions associated with HT were the primary focus. For the 2014 trial, a total of 41 landraces and 8 commercial cultivars (49 genotypes) were screened, and for the 2015 trial, 44 landraces and 10 commercial cultivars (54 genotypes) were assessed. These genotypes were sourced from the Australian Grains Genebank (AGG), Horsham, Victoria, Australia. The genetic background of the genotypes and year of screening(s) are summarized in Table A1. The subset of 22 repeated genotypes across both years enabled the response to be tested over two seasons. The genotypes screened in both years included 9 commercial cultivars and 13 landraces. The ten cultivars included were representative of the current commercial varieties grown in winter rainfed cropping systems within southern Australia. A single row approach was used to test lentil response due to limited seed availability from the Genebank. Throughout this manuscript, the prefix of AGG is not used when referring to landraces. For a listing of all the accession numbers (identity) see Table A1.
Figure 1.
World map showing the origins of the lentil genotypes screened in the 2014 and 2015 trials for high-temperature tolerance using a late-sowing field screening approach. Countries not labelled, a. Algeria, b. Tunisia, c. Italy, d. Cyprus, e. Greece, f. Macedonia, g. Bulgaria, h. Turkey, i. Iran, j. Lebanon, k. Egypt, l. Jordan and Syria, m. Iraq, n. Iraq.
2.3. Data Collection
Air temperature and relative humidity (RH) at the height of the canopy (approximately 0.25 m) and at 1.2 m were recorded across the full sun (YS) and partially shaded (YP) temperature treatments at 5 min intervals using TinyTag Ultra 2 sensors TGU-4500. All the Tiny Tag sensors were in white louvered screens to shield from solar radiation. Crop development including time to first flower (GS R1) and to maturity (GS R8) was assessed 3 times per week, as defined by Erskine et al. (1990) [24]. Days to flower and to maturity were calculated based on days from emergence to first flower (GS R1, open flower on any node of a plant) and days to maturity (GS R8, 90% of pods on the plant are golden-brown), respectively. The reproductive window for each genotype was considered from first flower (GS R1) to maturity (GS R8). At crop maturity, plants from each linear meter were cut at the base and dried at 40 °C; total biomass, grain yield, number, size, and harvest index were determined and reported on a per linear meter basis.
2.4. Heat Load
A calculation of heat load was used to characterize the different HT treatments, which combined the duration and temperature to determine the heat stress during the growing period [25]. Briefly, heat load was calculated as a sum of degrees (°C) above the threshold value (>30 °C) for the logged crop canopy temperature data (5 min intervals), averaged to give degree per hour (°C.h). For lentil, growth is reduced at temperatures above 30 °C [26]; therefore, in this study, heat load was calculated using a threshold of 30 °C, and temperatures beyond this threshold were considered to be acute HT stress. Two duration windows were calculated, i.e., from sowing to pre-flowering and from flowering to maturity, and a cumulative heat load was determined and reported for these intervals.
2.5. High-Temperature Stress Indices
Three sets of indices were calculated for the 2014 and 2015 trials, and they were used for assessing the HT tolerance of the lentil landraces tested. The relative utility of these indices as a ranking tool was also compared. For these indices, YS is the yield of the genotype under high-stress conditions (full sun), YP is the yield of genotype under the low-stress conditions (partially shaded), and is the mean yield of all genotypes under low-stress conditions. The first index is a simple ratio of yield response of low- and high-stress crops. This index has previously been defined as the yield stability index (YSI) in a study which compare the drought tolerance of soybean genotypes [27]. The YSI is calculated as follows:
Secondly, the stress tolerance index (STI) has previously been used to assess mungbean breeding lines across optimal and stressed (suboptimal soil water, nutrients and temperature) environments [20]. This approach compares the absolute yield response of stressed and non-stressed plants to the square of the average yield response of crops under low-stress conditions. The STI is calculated as follows:
The third index is the high-temperature tolerance index (HTTI), which is proposed in the current study. The proposed HTTI was developed to provide an alternative weighting between ratio response and absolute yield compared with the STI. The HTTI uses a combination of the square root of the YSI and multiples this by the absolute yield response of the high-stress plants (YS), and it is designed to capture both relative and absolute response to stress in defining a ranking. The HTTI is calculated as follows:
2.6. Data Analysis
Randomized split-plot complete block designs were applied across both years, in which the main plot was temperature treatment and the split plot was the genotype. A best linear unbiased prediction (BLUP) analysis using REML showed significant differences in temperature treatments across years. Subsequently, the trials were analyzed separately using analysis of variance (ANOVA) in which the main effects and the interaction of temperature treatment and genotype were tested for independent variables. All statistical analysis were performed using GENSTAT version 18 [28]. Stress indices were calculated and the interaction of genotype and temperature treatment was significant (p < 0.05). Linear regression models were applied to determine the relationship between the reproductive heat load and yield and to determine the correlation of the 22 genotypes screened across years using three stress indices, i.e., YSI, STI, and HTTI.
3. Results
3.1. Heat Load
In both years, temperatures >30 °C occurred during the reproductive period and were sufficient to achieve HT stress conditions. In 2014, there was a large difference in heat load across the two temperature treatments, i.e., full sun (YS) and partially shaded (YP). In contrast, in 2015, HT was universally more severe across YS and YP and corresponded with lower yields and harvest indices due to the severe stress occurring across the two temperature treatments (Table 1).

Table 1.
Lentil response to high temperature using a late-sowing methodology across the 2014 and 2015 trials. For each year, contrasting temperature environments were compared using partial shading. For both trials, YS is for plants grown in full sun and YP is for plants grown under partial shade.
For the two field trials conducted over consecutive years, the average air temperature at canopy height was substantially lower for the 2014 trial compared to the 2015 trial. During the period between sowing and when the partial shade was imposed (first flower across genotypes), the heat load was 173 and 353 °C/h (>30 °C) for the 2014 and 2015 trials, respectively. For the subsequent growing period between first flower and maturity, the full sun (Ys) cumulative heat loads were 1378 and 1944 °C.h (>30 °C) in 2014 and 2015, respectively (Figure 2). During this growth period, the average vapor pressure deficit (VPD) values were 1.2 and 1.6 kPa for the 2014 and 2015 trials, respectively, over the experimental period. Within the average 35-day flowering window (26 November 2014–29 December 2014), the accumulation rate of heat load was 18 °C.h (>30 °C) per day in 2014. In contrast, for the 2015 trial, the accumulation rate of heat load was over double (43 °C.h per day) for the mean flowering window of 29 days (4 December 2015–2 January 2016). The partially shaded temperature treatment decreased the ambient air temperature at canopy height of the YP treatment by 2.0 °C in the 2014 trial and by 0.5 °C in the 2015 trial. This translated to a 441 and 209 °C.h (>30 °C) reduction in 2014 and in 2015, respectively, compared with the full sun conditions (YS) (Figure 2).
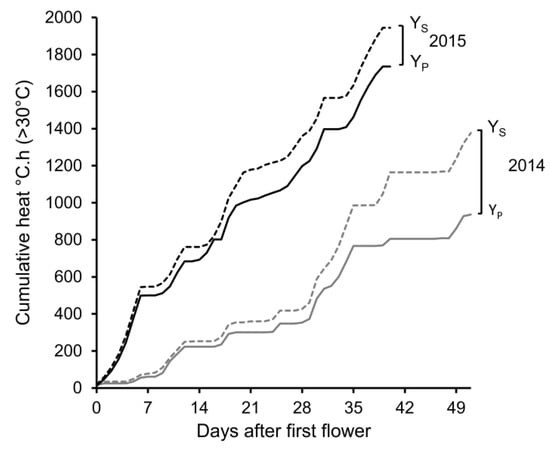
Figure 2.
Cumulative heat load (°C.h > 30 °C) of the full sun, YS (- -) and partially shaded, YP (―) temperature treatments that lentil genotypes were exposed to during the 2014 and 2015 trials at Horsham, Victoria. Heat load was calculated commencing from first flower (GS R1, 0 DAF) through to maturity (GS R8). Days after flowering and maturity were based on the average across genotypes.
For YP, the partially shaded temperature treatments reduced the photosynthetic active radiation (PAR) by 38.5%, which was likely non-limiting to photosynthesis, demonstrated by the significant increase in yield for the YP treatment (Table 1). The clear day average comparison of photosynthetic active radiation (PAR) was 1917 and 2227 µmol/m2/s for partially shaded and full sun, respectively, which exceeded the optimal range for lentil of between 400 and 800 µmol/m2/s [29]. For RH, partial shade had no impact across treatments in either year; for the period between sowing and flowering, the average RH was 57 and 52% for 2014 and 2015, respectively, compared to, during the post flowering period, RH was 56 and 51% across the respective years.
3.2. Lentil Phenology
In both years, the rate of development varied across the genotypes screened, and the partially shaded (YP) temperature treatment extended the days to first flower and to maturity. For the 2014 trial, the first flower (GS R1) occurred between 31 and 65 days after sowing (DAS), with an average of 46 DAS across genotypes. Maturity occurred between 80 and 135 DAS, with a mean of 98 DAS. On average, the YS plants reached physiological maturity 12 days earlier compared to the YP plants. For the 2015 trial, lentil development was faster compared to the 2014 trial. On average across genotypes, flowering occurred 42 DAS, with a range between 32 and 61 DAS. Physiological maturity occurred between 70 and 111 DAS, with an average of 81 DAS across genotypes. For the 2015 trial, the YS temperature treatment accelerated development, i.e., on average, physiological maturity occurred 7 days earlier compared to the YP temperature treatment. The cumulative degree days for the average days between sowing and maturity were relatively consistent across both years, occurring 1854 and 1746 °C.d for 2014 and 2015, respectively.
For the commercial varieties, the average time to first flower (GS R1) occurred 40 and 34 DAS for 2014 and 2015, respectively. For the 2014 trial, CIPAL0901 was the earliest to flower, occurring at 38 DAS. Breeding lines, CIPAL0901 and PBA Bolt were the earliest to reach physiological maturing (82 to 86 DAS) compared to an average of 95 DAS across commercial varieties tested. For the 2015 trial, the commercial varieties flowered within 6 days of each another; CIPAL0901 was the earliest to flower (34 DAS) and Nipper was the latest to flower (40 DAS). For both years, Nipper was the latest commercial variety to commence flowering (48 DAS in 2014 and 40 DAS in 2015) and reach physiological maturity (112 DAS in 2014 and 88 DAS in 2015).
Due to differences in the rate of development across the genotypes assessed, there was variation in the summated heat loads during the reproductive period (Table A2). For the 2014 trial under full sun (YS), genotype 70685 had the shortest duration for the reproductive growth period (33 days), which corresponded to a reproductive heat load of 972 °C.h (>30 °C) compared to 1437 °C.h (>30 °C) for both genotypes 70457 and Nipper, which had the longest reproductive period (68 days) for the YS treatment. Across all genotypes assessed in 2014, the interquartile range in reproductive heat load was 220 °C.h (>30 °C). For the 2015 trial, the genotype with the shortest reproductive duration for YS was 70492 (20 days), which corresponded to a heat load of 818 °C.h (>30 °C) (Table A3). In comparison, the heat load for the genotype (74439) with the longest reproductive window (62 days) for the YS treatment was 2263 °C.h (>30 °C). For the 2015 trial, the interquartile range across all genotypes was 245 °C.h (>30 °C). It was determined, for the 2014 and 2015 trials, that variation in the timing of the reproductive window and associated differences in heat load (consequence of variable phenology) were not correlated with yield response (r = <0.13). Consequently, the analyses using the stress indices, YSI, STI, and HTTI did not need to account for the differences in heat load due to variation in phenology across genotypes.
3.3. Screening—2014
There was a significant interaction between HT (YS and YP) treatment and genotype, and the ratio of yield response across the two temperature treatments varied with genotype (Figure 3a). For lentil grown under the YP temperature treatment, the range in absolute yield response was large across genotypes. For example, the top 19 yielding genotypes could be ranked as significantly greater than the nine lowest yielding genotypes, which constituted largely commercial varieties including CIPAL0901, Boomer, PBA Bolt, PBA Giant, PBA Ace, and PBA Jumbo. Within the high yielding cohort, the only commercial line was Nipper. When the YS temperature treatment was imposed, absolute yields across all genotypes were uniformly lower and statistically equivalent, with the exceptions of Nipper and 73745, which had significantly higher yields than the lowest yielding line, 70596.
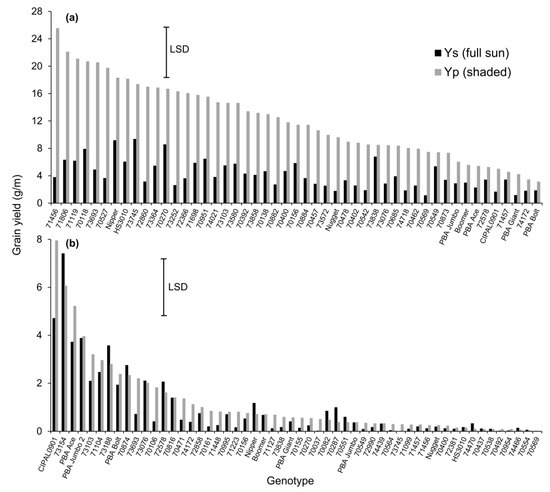
Figure 3.
Grain yield (g/m) for lentil genotypes grown in full sun (YS) and partially shaded (YP) temperature treatments. Lentil landraces were screened for high temperature in (a) 2014 and (b) 2015. Values are ordered from high yielding for YP to lowest. Note the large absolute difference in grain yield across the 2014 and 2015 trial years. The error bar represents least significant difference (LSD) for the interaction between genotype and temperature (p < 0.05).
Across the YS and YP temperature treatments, the average absolute yield was 3.8 and 11.3 g/m, respectively, which corresponded to a 66% reduction in yield due to full sun conditions compared with plants under the partially shaded temperature treatment (Table 1). This yield penalty was due to both the average grain number and the grain size across genotypes being significantly reduced by 13 and 16%, respectively, across the temperature treatments. A comparison of the overall relationship between grain number (no.) and yield (g/m) for lentil genotypes showed that there was a strong linear relationship (yield = 0.02 × grain number, R2 = 0.89), and this relationship was the same across the temperature treatments, with no relationship between grain yield and grain size for crops maturing within the YS temperature treatment. For vegetative growth across all lentil genotypes, there was a significant reduction in biomass of 9% for crops grown under the YS temperature treatment compared to those under the YP temperature treatment.
Stress Tolerance Indices
The yield stability index (YSI), which is the ratio of YS and YP, varied significantly across the genotypes tested (Figure 4a). For example, the genotypes 71457, 73838, 70549, 72578, and PBA Bolt were relatively stable across contrasting temperature treatments (high YSI). In contrast, the genotypes 73572, 71456, 70569, 70527, 72860, and Nugget had a low YSI indicating low yield stability for the YS temperature treatment. The commercial cultivars PBA Bolt, Boomer, PBA Jumbo, and PBA Ace had a moderate YSI, although the absolute yield was relatively low when grown under hotter conditions (Figure 3a).
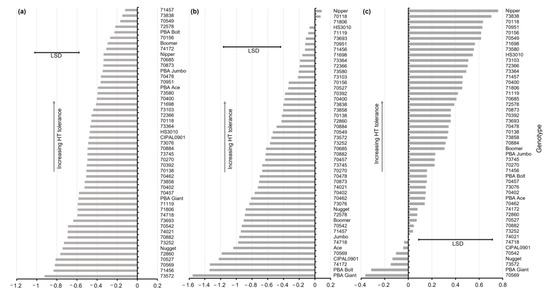
Figure 4.
Ranking of yield stability index (YSI) (a), stress tolerance index (STI) (b) and high-temperature tolerance index (HTTI) (c) based on grain yield for the 49 genotypes screened in 2014. Indices presented are for log10 transformation. Least significant difference (LSD) for genotype comparison (p < 0.05).
The STI was calculated for grain yield (Figure 4b). Across the genotypes screened, there were significant differences in tolerance ranking; 13 lines were identified as having significantly higher STI values compared with the 13 most sensitive genotypes. The top ten ranked lines for STI were 70118, 71806, 71119, 73693, 70951, 71456, 71698, 73364, HS3010, and Nipper. Seven of the commercial cultivars screened, i.e., PBA Giant, PBA Bolt, PBA Ace, PBA Jumbo, Boomer, Nugget, and CIPAL0901, were within the lowest STI ranked genotypes, reflecting both low-to-moderate stability across temperature treatments and low absolute yield.
The HTTI proposed in this paper was calculated for lentil genotype yield response across temperature treatments (Figure 4c). For the genotypes screened, there were significant differences in tolerance ranking, i.e., six genotypes were identified as having significantly higher HTTI values compared with the seven most sensitive genotypes. Similar to the STI, the HTTI ranked genotypes Nipper, 70118, 70951, 71698, and HS3010 within the top ten for tolerance. In contrast, the HTTI ranked the five genotypes 73838, 70156, 70549, 73580, and 73103 within the top ten cohort but excluded 71806, HS3010, 71119, 73693, and 73364, which reflected the HTTI’s greater weighting of relative yield stability (YSI) over absolute yield (YP) compared with the STI. For the commercial cultivars, the STI ranked the cultivars PBA Giant, PBA Bolt, PBA Ace, and PBA Jumbo as low tolerance, whereas the HTTI rated PBA Ace and PBA Jumbo as moderately tolerant and these cultivars had higher YSI values despite their lower absolute yield response. The comparison of YSI, STI, and HTTI values for ranking lentil genotypes showed that the HTTI had better agreement with the YSI (4 out of the top 10 cohort for the HTTI) compared with the agreement between the STI and the YSI (1 out of the top 10 cohort for STI), again because of the HTTI’s bias to relative response across temperature treatments, and so more closely reflected the YSI.
The HTTI was also applied to lentil yield components, grain size, and grain number to assess the agreement between ranking of these and yield. The high correlation between grain number and yield translated to the HTTI ranking of these being similar, for example, the top ten ranking genotypes for yield were also the top seven for grain number, but four out of ten when comparing yield ranking with grain size. For a selection process based on large and stable grain size under HT, the genotypes 74172, 70569, 72578, and 70478 and commercial varieties PBA Giant and CIPAL0901 were also in the top 10 cohort for the HTTI ranking.
3.4. Screening—2015
For the lentil screening in 2015, heat load was substantially higher than in 2014, with a smaller differential between the YS and YP temperature treatments. The average yield pooled across genotypes was 1.4 and 1.1 g/plot for partially shaded and full sun temperature treatments, respectively, compared with 11.3 and 3.8 g/plot in 2014 across the same temperature treatment comparison (Table 1). In 2015, the reduction in yield for the Ys temperature treatment was due to a corresponding reduction in grain number of 13% compared with that of the YP temperature treatment; therefore, grain yield was strongly correlated to grain number (yield = 0.02 × grain number, R2 = 0.93). No correlation was observed between grain yield and size.
There were significant interactions between temperature and the genotypes tested and the relative responses across YS and YP varied with genotype (Figure 3b). For lentil grown under the YP temperature treatment, the top 10 yielding genotypes were made up of six genotypes (73154, 73103, 71104, 73188, 70874, and 73693) and four commercial varieties (CIPAL0901, PBA Ace, PBA Jumbo 2, and PBA Bolt) and collectively ranked as significantly greater than the 37 lowest yielding genotypes. Within the high-ranking cohort, the four genotypes 73103, 73188, 70874, and 73693 had equivalent yields with their respective YS temperature treatments.
Stress Tolerance Indices
The YSI varied across the genotypes tested (Figure 5a); the 10 genotypes with the highest yield stability were statistically more stable compared to the 13 most sensitive genotypes. The genotypes which ranked within the top 10 genotypes based on the YSI were 72990, 70289, 70437, 70551, 74466, 72381, 74470, Nipper, 71457, and HS3010, which demonstrated the yield stability of these across contrasting temperature treatments (high YSI). For this experiment, comparisons of the YSI and absolute yield across the genotypes were generally more varied than those observed in 2014; the genotypes had either high YSI or absolute yield, with few possessing both (Figure 3b).
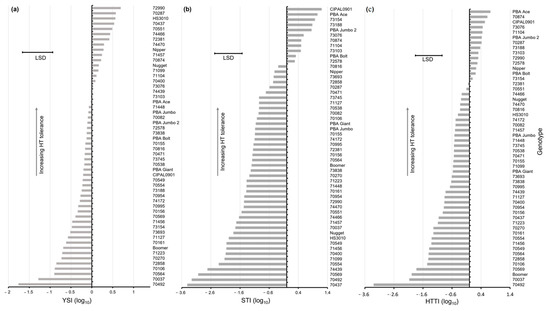
Figure 5.
Ranking of yield stability index (YSI) (a), stress tolerance index (STI) (b), and high-temperature tolerance index (HTTI) (c) based on grain yield for the 54 genotypes screened in 2015. Indices presented are for log10 transformation. Least significant difference (LSD) for genotype comparison (p < 0.05).
For the STI based on grain yield, there was a significant difference in tolerance ranking; 11 lines were identified as having significantly higher STI values compared with 37 of the most sensitive genotypes (Figure 5b). The top ten ranking genotypes for the STI were made up of the six landraces 73154, 73188, 73076, 70874, 71104, and 73103 and the commercial cultivars CIPAL0901, PBA Ace, PBA Jumbo, and PBA Bolt. Four of the commercial cultivars, i.e., Nugget, PBA Boomer, PBA Jumbo 2, and PBA Giant, ranked within the cohort of significantly lower tolerance compared with the top cohort when applying the STI.
The HTTI ranked 13 lentil genotypes as having significantly greater tolerance compared with the 18 most sensitive genotypes (Figure 5c). The top-ranking cohort included eight landraces (70874, 73076, 71104, 70287, 73188, 73103, 72990, and 72578) and five commercial cultivars (PBA Ace, PBA Jumbo 2, PBA Bolt, Nipper, and CIPAL0901). Boomer was the lowest ranking commercial variety for the HTTI. In 2015, the comparison of the ranking using the STI and the HTTI showed stronger agreement compared with that in 2014; among the top ten ranked genotypes, eight matched, which reflected the smaller absolute difference and high correlation between YP and YS responses in 2015.
3.5. Inter-Year Comparison
A subset of twenty-two lentil genotypes were screened across both years (2014 and 2015) to compare the stability of response across two seasons. In assessing the agreement of lentil ranking for HT tolerance across these two independent trials, stress tolerance indices were compared. For the YSI, 11 of the 22 genotypes had similar yield stability across both years, and the YSI values for these genotypes varied by less than 25% between years. These genotypes were 73693, 70400, 72578, 71457, 70156, 70270, 74172, 73103, 74172, PBA Ace, and PBA Bolt. In comparison, for the STI and HTTI, there was less agreement for inter-season comparison. For the STI, 5 of the 22 genotypes which had low STI values were within 25% across the 2 years, which were Nugget, PBA Giant, PBA Jumbo, and 74172. Based on the HTTI, there were six genotypes that had strong agreement across the 2 years (+/− 25%), which were 73076, 72758, 74172, PBA Bolt, PBA Giant, and Nugget. The simple ratio, i.e., the YSI, provided the greatest utility when comparing screening data across years. Conversely, the STI and the HTTI, which encapsulate both relative and absolute plant response, provided valuable intra-year ranking information.
4. Discussion
The high-temperature (HT) tolerance of 81 genotypes was assessed using a late-sowing approach, with the temperature exceeded 30 °C for 30 and 35 days in the 2014 and 2015 trials, respectively, between flowering and physiological maturity. This induced a non-recoverable reduction in grain set and yield due to acute HT effects. Based on the HTTI, which was used for ranking genotypes, in 2014, we identified six lentil lines with HT tolerance, in field conditions that were associated with natural temperature ranges suitable for differentiating variation in HT tolerance. The HT tolerant genotypes were Nipper, 73838, 70118, 70951, 70156, and 70549, which were from Australia, Albania, Lebanon, Bulgaria, Algeria, and Syria, respectively. In 2015, an additional 13 lines were identified, including two genotypes (73103 and Nipper) that were ranked within the ten most stable genotypes in the 2014 trial based on the HTTI. The genotypes tested over the two years were largely from origins with semi-arid and sub-tropical climates, whereas the HT tolerant lines were from climates that are typically characterized by hot conditions and terminal drought during the reproductive period (e.g., Syria and Lebanon). Other studies of pulse crops including chickpea, faba bean, and lentil for drought and heat tolerance have also determined tolerant germplasm was associated with naturally hot environments [2,14,30]. Importantly, this study determined that among the ten commercial varieties that are currently grown in southern Australia, only one variety was identified as having good HT tolerance (i.e., cv. Nipper). The generally poor tolerance of the commercial cultivars may indicate that previous breeding and selection processes for other key traits (e.g., tolerance to boron and salt, herbicide resistance, harvestability, and yield) may have restricted genetics which infer HT tolerance [5,30,31]. The narrow genetic base within breeding programs has been identified globally as a limitation, and strategies are underway to increase the genetic diversity within lentil programs to support adaptation to constraints, including abiotic stresses [32,33]. Despite most commercial varieties having poor relative HT tolerance, the commercial cultivar Nipper ranked as having high-HT tolerance. This may reflect the broader genetic base of Nipper, relative to other Australian commercial cultivars [30,32]. The identification of genotypes with increased heat tolerance relative to most commercial cultivars represents an opportunity to further improve tolerance of future varieties by utilizing global germplasm, for example, significant HT tolerance in lentil was identified in the current study.
For HT studies, the approach of using stress tolerance indices has been successful for screening and ranking a range of pulse crops including chickpea (HT) [13] and common bean (HT) [19]. The current study compared three stress indices, i.e., the YSI, the STI [20], and the proposed HTTI, to define ranking of lentil genotypes. For the YSI, which is a simple ratio of response across contrasting temperature treatments, it had utility when comparing ranking across independent trials over different years. When used to compare the performance of the subset of 22 genotypes which were tested in both years, there was reasonable agreement (11 of 22 genotypes), which indicates that the YSI is less affected by inter-year seasonal variation due to contrasting environmental conditions causing significant variation in absolute yield (i.e., yield was 78% less for YP in 2015 compared to 2014). Among the 22 genotypes, two genotypes consistently ranked within the top 10 genotypes based on the YSI, indicating a high level of genetic tolerance for these genotypes. For the STI and HTTI, these indices integrate both absolute yield and relative stability across HT treatments, and the STI has previously been used to rank drought tolerance in canola [23] and temperature tolerance in lentil [34]. We observed that the STI could also be used to rank HT tolerance in lentil but tended to select genotypes which were high yielding under non-stressed conditions, rather than on comparative yield stability across contrasting temperatures. This concurs with Singh et al. (2015) who assessed multiple indices for evaluating salinity stress, and the STI identified genotypes which produced higher yields under non-stressed conditions but failed to produce high yields in a stressed environment [35]. Subsequently, we proposed the HTTI, which reduced the weighting of absolute yield compared with relative response and had utility in screening lentil for HT in the current study. For inter-year comparison of indices, the STI and the HTTI had poor agreement across years for the subset of 22 genotypes, with five and six genotypes having close agreement for the respective indices. Evidently, these indices are better suited for comparing genotypic responses within a season, rather than across seasons, however, indices such as the YSI provide a better option when inter-year comparison is required.
Late sowing has been recognized as one field option for HT screening with previous studies using this methodology in wheat [36], chickpea [13,14,37,38], cowpea [16], and more recently, lentil [2,17,21]. In the current study, late sowing was combined with imposing partial shading, using 50% polyethylene fabric to generate two different HT profiles during the growing period. Partial shading was applied at the same time across genotypes, and this combined with genetic variation in development rate across genotypes translated to slight differences in summed reproductive heat loads. Despite some observed variation, this was not correlated with yield or ranking order for the stress indices. Furthermore, there was a difference in the reproductive heat load between the six HT sensitive genotypes and six HT tolerant genotypes (1143 vs. 1166 °C.h (>30 °C) for the YS temperature treatment) based on the HTTI ranking. The use of partial shading to create a temperature differential worked effectively in 2014; there were significant differences in growth and response of lentil due to large differences in canopy heat load. Importantly, we measured significantly higher biomass accumulation and grain yields for the partially shaded temperature treatment relative to the full sun temperature treatment. Photosynthetically active radiation (PAR) was also likely to be non-limiting to photosynthesis and plant growth; previous studies have determined that for C3 plants, such as lentil, the light saturation point is approximately 800 µmol/m2/s, which is substantially lower than that measured for the partially shaded temperature treatment (>1917 µmol/m2/s) [29,39]. For screening conducted in 2015, the hotter natural growing environment caused an overwhelming effect of ambient air temperature, and shading did little to create a temperature contrast. The combination of low yields with little differential in temperature treatment inflated the effect of natural plot variance and caused yield stability ratios of genotypes to often exceed unity, and so limited the value of ranking comparative tolerance across genotypes. Evidently, the reliability of using a late-sowing approach is likely to be influenced by inter-season variability in testing conditions. Nonetheless, this methodology provides an economic and efficient way of screening large populations of germplasm. For future pre-breeding application, multi-year and multi-environment trials would be needed to maximize the likelihood of growing conditions conducive of testing HT response. Comparisons of multi-environments would also enable selection for superior genotypes that perform well under diverse environments, and interactions between genetic traits and the environment occur within years which can confound selection processes. Based on the learnings from this study, multi-environment trials may not be practical, and therefore greater replication (e.g., three to four replicates) would be recommended to increase the power of statistical analyses for identifying genotypes with true expression of targeted traits.
Overall, plant response to HT under late sowing would be expected to be an integrated response to non-critical increases in temperature and longer day length limiting biomass accumulation, hastening senescence, potential water deficit, and acute HT stress during the reproductive phase, all of which can reduce grain yield and quality [24,29,40,41,42]. The hastened development of plants in this study compared with a winter sown crop is most likely due to increased temperature rather than the extended comparative day length, and the median cumulative degree day requirement for 50% flowering across 10 Australian cultivars ranges from 1113 to 1282 °C.d (average 1199 °C.d) [43]. Based on the average degree days requirement of 1199 °C.d, this translates to approximately 126 days after sowing (DAS) in southern Australia. In contrast, under late sowing in this study, 50% flowering occurred 63 and 57 DAS for the 2014 and 2015 trials, respectively, which aligns with approximately 1199 °C. DAS. This indicates that temperature was the major factor influencing DAS, and the effects of photoperiod tended to be less on plant development across the genotypes in this study. The average total biomass for the control (YP) of cultivar PBA Bolt in the 2014 and 2015 trials was 2 t/ha, whereas total biomass under winter growing conditions is typically 6 to 7 t/ha. Furthermore, warm night temperatures (28 °C), which are frequently observed for late-sowing screening have adverse effects on plants, reducing pollen production in groundnut and contributing to yield loss [44]. To further develop late-sowing methodologies, staggered time of sowing (TOS) could also be used to generate multiple environments; utilizing TOS treatments could create a balance between limited penalties associated with hastened development and adequate imposition of HT during the reproductive phase.
For late sowing, consideration needs to be given to if these growing conditions penalize yield potential of the commercial cultivars due to the narrow and early flowering window (38 to 42 DAS). For dry bean, by applying a boundary analysis, it was determined that maximum yield was linked with an optimal flowering time [45]. Universally, for crops grown in their normal seasonal window, time of sowing and associated flowering is vital to achieving maximum yield potential [42,43,44,45,46,47]. For screening studies that are conducted using a late-sowing approach, the atypical growing conditions and associated hastened phenology translate to time of flowering being less relevant, particularly when comparative plant growth across genotypes is the focus, rather than absolute response. In the current study, we determined that despite the early and narrow flowering period for the sensitive commercial cultivars, there was only a difference in the time to flowering (GS R1) between these and the HT tolerant genotypes (40 vs. 46 days) and there was no relationship between the time to flower and yield. Taken together, these indicate that the commercial lines were unlikely to have inflated yield penalties due to their slightly earlier flowering window compared with the landraces screened.
For field screening trials with limited material, there is a need to either have small or single row plots for testing genotypes. It is known that there are numerous potential artefact effects of small plots on growth, and large edge effects alter access to water, nutrients, and light [7,48]. Consequently, edge effects can limit the applicability of crop development, biomass, and yield data compared to crops grown in a larger canopy context. Moreover, if there are differences in vigor across genotypes, this may affect growth of adjacent plots due to competition [48]. Other factors that may affect comparison of commercial cultivars that represent the industry benchmark and landraces are that advanced breeding cultivars have traits such as different plant architecture, growth rate, and stature compared to historic cultivars and landraces, and new varieties tend to be shorter and intercept less radiation [7,49]. For the current screening trial, the limited seed available from seed banks (e.g., Australian Grains Genebank) determined the experimental approach of single row; however, because the single rows were evenly spaced at wide intervals (0.65 m), access to resources was likely to be near equivalent across genotypes. The use of replication, with genotypes that were randomized also limited the confounding effects of single observation on potential competition between contrasting genotypes.
5. Conclusions
The genetic variation of a diverse set of lentil genotypes was assessed for HT tolerance using late-sown field screening trials which combined partial shading during the reproductive period as a method to create differential heat treatments. This study identified six lentil genotypes with high yield stability under HT compared with current commercial varieties by using multiple heat tolerance indices, i.e., the YSI, STI, and HTTI, for ranking purposes. The genotypes identified were 73838, 70118, 70951, 70156, 70549, and Nipper, which were from Albania, Lebanon, Bulgaria, Algeria, Syria, and Australia, respectively. The HTTI index provided utility for comparing both the relative and absolute response of lentil genotypes screened. Ultimately, screening for HT tolerance requires a multi-level approach, and late sowing with partial shading provides an inexpensive preliminary methodology that can be coupled with further assessment of shortlisted genotypes within field heat chambers under winter growing conditions and controlled environment bioassays. Importantly, this work provides cause for optimism for increasing the adaptation of lentil to hot and more marginal growing environments, through establishing reliable screening methods and subsequent breeding for HT tolerance within lentil.
Author Contributions
Conceptualization, J.D.B. and J.G.N.; methodology, A.J.D., J.D.B. and J.G.N.; formal analysis, A.J.D., J.D.B. and J.G.N.; investigation, A.J.D.; data curation, A.J.D.; writing—original draft preparation, A.J.D.; writing—review and editing, A.J.D., J.D.B. and J.G.N.; supervision, J.D.B. and J.G.N.; funding acquisition, J.D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Agriculture Victoria and the Grains Research and Development Corporation within Australia (Project “Increasing lentil tolerance to heat waves using genetic solutions”, DAV00142). The PhD studies were supported through the Centre for Agricultural Innovation, a partnership between the University of Melbourne and Agriculture Victoria.
Data Availability Statement
Not Applicable.
Acknowledgments
Lentil germplasm for the experiments were supplied by the Australian Grains Genebank, Horsham, Victoria. We thank Sue Finch (Statistical Consulting Centre, The University of Melbourne) and Debra Partington (Agriculture Victoria) for statistical advice and Ashley Purdue, Russel Argall, and Keisha Savage for their technical support within field experiments.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Lentil genotypes screened for high temperature tolerance using late sowing field conditions in 2014 (49 genotypes), 2015 (54 genotypes), and both years (22 genotypes). The genotypes screened constitute landraces sourced from the Australian Grains Genebank (AGG) and Australian commercial cultivars. Described within the table: year of the trial the genotype was tested, identity is the unique name used by AGG, region/country signifies collection site, accession name (ACC), donor no. is the global identifier.
Table A1.
Lentil genotypes screened for high temperature tolerance using late sowing field conditions in 2014 (49 genotypes), 2015 (54 genotypes), and both years (22 genotypes). The genotypes screened constitute landraces sourced from the Australian Grains Genebank (AGG) and Australian commercial cultivars. Described within the table: year of the trial the genotype was tested, identity is the unique name used by AGG, region/country signifies collection site, accession name (ACC), donor no. is the global identifier.
| Identity | Region/Country | ACC Name | Donor No. | Trial |
|---|---|---|---|---|
| AGG 70156 | Constatine/Algeria | ILL 857 | IG 857 | 2014 & 2015 |
| AGG 70270 | Hudeiba/Sudan | ILL 1861 | IG 1861 | 2014 & 2015 |
| AGG 70400 | Al Hasakah/Syria | ILL 4532 | IG 4532 | 2014 & 2015 |
| AGG 70549 | Santa Fe/Argentina | ILL 6109 | IG 70164 | 2014 & 2015 |
| AGG 70569 | Al Hasakah/Syria | ILL 6525 | IG 71476 | 2014 & 2015 |
| AGG 70882 | /Bangladesh | 9920308 | 2014 & 2015 | |
| AGG 71456 | Taybi village/Lebanon | ILL 485 | PI 300565 | 2014 & 2015 |
| AGG 71457 | Iribid/Jordan | ILL 486 | PI 302398 | 2014 & 2015 |
| AGG 72578 | Uttar Pradesh/India | PI 472290 | 2014 & 2015 | |
| AGG 73693 | /Libya | LENS 157 | 2014 & 2015 | |
| AGG 73745 | /Italy | ITA-828752 | B 1045 | 2014 & 2015 |
| AGG 73838 | Lushnje/Albania | PI 606693 | BERATI | 2014 & 2015 |
| AGG 74172 | Merida/Venezuela | JCMMF4-1 | 2014 & 2015 | |
| Boomer | Australia | 2014 & 2015 | ||
| CIPAL0901 | Australia | 2014 & 2015 | ||
| HS3010 | Australia | 2014 & 2015 | ||
| Nipper | Australia | 2014 & 2015 | ||
| Nugget | Australia | 2014 & 2015 | ||
| PBA Ace | Australia | 2014 & 2015 | ||
| PBA Bolt | Australia | 2014 & 2015 | ||
| PBA Giant | Australia | 2014 & 2015 | ||
| PBA Jumbo | Australia | 2014 & 2015 | ||
| AGG 70003 * | Saskatchewan/Canada | LAIRD | PI 607915 | 2014 |
| AGG 70118 | Doures/Lebanon | ILL 484 | PI 300564 | 2014 |
| AGG 70138 | Manisa/Turkey | ILL 647 | IG 647 | 2014 |
| AGG 70392 | Aleppo/Syria | ILL 4497 | IG 4497 | 2014 |
| AGG 70402 | Al Hasakah/Syria | ILL 4542 | IG 4542 | 2014 |
| AGG 70457 | /Morocco | ILL 4788 | 11/03/1962 | 2014 |
| AGG 70462 | Bavaria/Germany | ILL 4879 | IG 4879 | 2014 |
| AGG 70478 | /Jordan | ILL 5223 | IG 5223 | 2014 |
| AGG 70527 | Bale/Ethiopia | ILL 5912 | OITA 703 | 2014 |
| AGG 70542 | Hyderabad/Pakistan | ILL 6066 | IG 70121 | 2014 |
| AGG 70884 | Bangladesh | 09920303 | 2014 | |
| AGG 70685 | Diyarbakir/Turkey | PI 509377 | 2014 | |
| AGG 70873 | /Bangladesh | 9920291 | 2014 | |
| AGG 70951 | Lovech/Bulgaria | ILL 512 | IG 512 | 2014 |
| AGG 71119 | /Syria | ILL 240 | PI 254553 | 2014 |
| AGG 71698 | /Cyprus | RPIP 33-039-10124 | PI 431610 | 2014 |
| AGG 71806 | Voronezh/Russia | IG 123615 | Borinskaja | 2014 |
| AGG 72366 | /Turkey | RPIP 33-153-11133 | PI 432278 | 2014 |
| AGG 72860 | Tehran/Iran | PI 472573 | 2014 | |
| AGG 73076 | Punjab/Pakistan | PI 513259 | 2014 | |
| AGG 73103 | Punjab/Pakistan | PI 513286 | MASSAR | 2014 |
| AGG 73252 | Urfa/Turkey | PI 568219 | W6 11532 | 2014 |
| AGG 73364 | Alexandria/Egypt | PI 606595 | W6 10474 | 2014 |
| AGG 73572 | /Libya | ILL 4804 | IG 4804 | 2014 |
| AGG 73580 | /Greece | LENS 30 | LENS 30 | 2014 |
| AGG 73858 | Delhi/India | ILL 221 | 13.3 | 2014 |
| AGG 74021 | /Jordan | ILL 5586 | IG 5586 | 2014 |
| AGG 70037 | Amzibegovo/Macedonia | ILL 623B | IG 623 | 2015 |
| AGG 70082 | Mosul/Iraq | ILL 53 | IG 53 | 2015 |
| AGG 70106 | Cairo/Egypt | ILL 337 | GIZA | 2015 |
| AGG 70155 | Oran/Algeria | ILL 856 | IG 856 | 2015 |
| AGG 70161 | Esfahan/Iran | ILL 901 | IG 901 | 2015 |
| AGG 70287 | Dahuk/Iraq | ILL 2153 | IG 2153 | 2015 |
| AGG 70437 | /Algeria | ILL 4658 | IG 4658 | 2015 |
| AGG 70471 | Irbid/Jordan | ILL 5065 | IG 5065 | 2015 |
| AGG 70492 | Cartago/Costa Rica | ILL 210 | 74TA-209 | 2015 |
| AGG 70538 | Larnaca/Cyprus | ILL 5984 | IG 69593 | 2015 |
| AGG 70551 | La Rioja/Argentina | ILL 6111 | IG 70166 | 2015 |
| AGG 70554 | /Tunisia | ILL 6268 | IG 71200 | 2015 |
| AGG 70564 | Baluchistan/Pakistan | ILL 6385 | IG 71317 | 2015 |
| AGG 70816 | /Bangladesh | ILXB 87099 | 2015 | |
| AGG 70874 | /Bangladesh | 9920343 | 2015 | |
| AGG 70954 | Cordoba/Spain | ILL 915 | IG 915 | 2015 |
| AGG 70995 | Diyarbakir/Turkey | ILL 122 | PI 172938 | 2015 |
| AGG 71099 | Gujarat/India | ILL 220 | PI 212904 | 2015 |
| AGG 71104 | /Yemen | ILL225 | PI 2440046 | 2015 |
| AGG 71127 | /Ethiopia | ILL 247 | PI 273664 | 2015 |
| AGG 71223 | Cairo/Egypt | ILL 337 | PI 298357 | 2015 |
| AGG 71448 | Aleppo/Syria | ILL 477 | PI 300255 | 2015 |
| AGG 72381 | Tehran/Iran | ILL 1040; ILL 882; ILL 749 | PI 435956 | 2015 |
| AGG 72858 | Hamadan/Iran | PI 472571 | 2015 | |
| AGG 72990 | Adiyaman/Turkey | PI 509356 | 2015 | |
| AGG 73154 | Hyderabad/Pakistan | PI 513337 | PI 513337 | 2015 |
| AGG 73188 | Punjab/Pakistan | PI 513372 | MASSAR | 2015 |
| AGG 74439 | Xinjiang/China | ILL 8288 | IG 123539 | 2015 |
| AGG 74466 | /Libya | ILL 8411 | IG 123662 | 2015 |
| AGG 74470 | /Libya | ILL 8457 | IG 123708 | 2015 |
| AGG 74718 | /Tajikistan | ILL 8321 | IG 123572 | 2015 |
| PBA Jumbo2 | Australia | 2015 |
* AGG 70003 remained in the vegetative period for the duration of the experiment, and therefore was not described in the Section 3.

Table A2.
Days after sowing (DAS) to flowering, maturity, and the corresponding reproductive heat load (°C.h > 30 °C) for lentil genotypes screened in the 2014 trial across two contrasting environments (YS and YP). Genotypes are ordered from earliest to latest to flower based on YS.
Table A2.
Days after sowing (DAS) to flowering, maturity, and the corresponding reproductive heat load (°C.h > 30 °C) for lentil genotypes screened in the 2014 trial across two contrasting environments (YS and YP). Genotypes are ordered from earliest to latest to flower based on YS.
| Full Sun (YS) | Partially Shaded (YP) | |||||
|---|---|---|---|---|---|---|
| Genotype | Flowering (DAS) | Maturity (DAS) | Heat Load (°C.h > 30 °C) | Flowering (DAS) | Maturity (DAS) | Heat Load (°C.h > 30 °C) |
| 70478 | 31 | 87 | 1251 | 46 | 96 | 856 |
| 70882 | 38 | 80 | 990 | 38 | 82 | 885 |
| 70884 | 38 | 82 | 1145 | 38 | 105 | 1148 |
| 72578 | 38 | 83 | 1236 | 38 | 83 | 971 |
| 74172 | 38 | 81 | 1053 | 38 | 81 | 805 |
| CIPAL0901 | 38 | 80 | 962 | 38 | 84 | 971 |
| 70457 | 39 | 107 | 1437 | 46 | 129 | 1649 |
| 70873 | 39 | 80 | 990 | 38 | 81 | 805 |
| PBA Giant | 39 | 91 | 1285 | 39 | 100 | 1147 |
| 70527 | 40 | 102 | 1432 | 41 | 135 | 1841 |
| 70542 | 40 | 86 | 1236 | 39 | 87 | 971 |
| 73076 | 40 | 80 | 990 | 41 | 90 | 1009 |
| 73103 | 40 | 84 | 1236 | 42 | 82 | 885 |
| PBA Bolt | 40 | 87 | 1236 | 38 | 85 | 971 |
| Boomer | 41 | 82 | 1145 | 40 | 107 | 1148 |
| Nugget | 41 | 91 | 1285 | 42 | 130 | 1806 |
| 72860 | 42 | 91 | 1285 | 47 | 102 | 968 |
| Nipper | 42 | 110 | 1437 | 46 | 115 | 1137 |
| PBA Ace | 42 | 87 | 1236 | 38 | 105 | 1148 |
| 70549 | 43 | 87 | 1237 | 45 | 90 | 853 |
| 71457 | 43 | 86 | 1236 | 45 | 97 | 939 |
| 72366 | 44 | 105 | 1431 | 40 | 115 | 1293 |
| 73745 | 44 | 112 | 1356 | 62 | 128 | 1325 |
| HS3010 | 44 | 85 | 1230 | 42 | 95 | 1009 |
| 70156 | 45 | 86 | 1147 | 42 | 114 | 1251 |
| 73838 | 45 | 84 | 1147 | 45 | 86 | 814 |
| 73858 | 45 | 87 | 1063 | 45 | 91 | 936 |
| 73693 | 48 | 87 | 1010 | 44 | 106 | 1142 |
| 70569 | 49 | 101 | 1183 | 48 | 131 | 1628 |
| 71698 | 49 | 91 | 1036 | 56 | 110 | 903 |
| 73252 | 49 | 101 | 1183 | 47 | 133 | 1642 |
| 70402 | 50 | 91 | 1035 | 46 | 88 | 853 |
| 70951 | 50 | 86 | 987 | 48 | 114 | 1056 |
| 70392 | 51 | 94 | 1035 | 46 | 113 | 1022 |
| 70462 | 52 | 89 | 1035 | 48 | 91 | 814 |
| 70118 | 53 | 96 | 1040 | 50 | 96 | 809 |
| 70138 | 53 | 100 | 1165 | 52 | 117 | 1106 |
| 71456 | 53 | 92 | 1035 | 49 | 130 | 1610 |
| 71806 | 54 | 92 | 1018 | 49 | 105 | 945 |
| 71119 | 55 | 94 | 972 | 55 | 108 | 908 |
| 73364 | 55 | 107 | 1134 | 51 | 112 | 975 |
| 74021 | 55 | 89 | 982 | 53 | 106 | 945 |
| 70270 | 56 | 109 | 1125 | 44 | 108 | 1142 |
| 70685 | 56 | 89 | 972 | 52 | 96 | 809 |
| 73580 | 56 | 107 | 1125 | 56 | 94 | 764 |
| 74718 | 56 | 94 | 968 | 49 | 127 | 1453 |
| 70400 | 56 | 88 | 968 | 46 | 94 | 853 |
| 73572 | 62 | 108 | 909 | 65 | 128 | 1325 |

Table A3.
Days after sowing (DAS) to flower, maturity, and the reproductive heat load (°C.h > 30 °C) for 54 lentil genotypes screened in the 2015 trial.
Table A3.
Days after sowing (DAS) to flower, maturity, and the reproductive heat load (°C.h > 30 °C) for 54 lentil genotypes screened in the 2015 trial.
| Full Sun (YS) | Partially Shaded (YP) | |||||
|---|---|---|---|---|---|---|
| Genotype | Flowering (DAS) | Maturity (DAS) | Heat Load (°C.h > 30 °C) | Flowering (DAS) | Maturity (DAS) | Heat Load (°C.h > 30 °C) |
| 70874 | 32 | 70 | 1484 | 44 | 72 | 1090 |
| 74439 | 33 | 95 | 2263 | 46 | 111 | 2037 |
| PBA Ace | 34 | 74 | 1463 | 35 | 83 | 1785 |
| PBA Jumbo2 | 34 | 73 | 1403 | 34 | 79 | 1554 |
| 71104 | 35 | 79 | 1669 | 34 | 80 | 1565 |
| 73154 | 35 | 71 | 1329 | 35 | 83 | 1785 |
| 74172 | 35 | 72 | 1350 | 38 | 70 | 1210 |
| CIPAL0901 | 36 | 72 | 1350 | 36 | 77 | 1449 |
| 73188 | 37 | 74 | 1463 | 37 | 91 | 1945 |
| PBA Giant | 37 | 73 | 1403 | 38 | 77 | 1523 |
| Nugget | 37 | 76 | 1556 | 35 | 79 | 1554 |
| PBA Bolt | 37 | 77 | 1669 | 37 | 83 | 1709 |
| 72578 | 38 | 92 | 2076 | 38 | 99 | 1914 |
| Boomer | 38 | 75 | 1460 | 38 | 80 | 1523 |
| 70471 | 39 | 72 | 1320 | 40 | 78 | 1398 |
| 71457 | 39 | 74 | 1363 | 39 | 81 | 1464 |
| 72858 | 39 | 83 | 1814 | 39 | 93 | 1844 |
| 73076 | 39 | 80 | 1568 | 38 | 91 | 1914 |
| PBA Jumbo | 39 | 81 | 1579 | 38 | 82 | 1679 |
| 70156 | 40 | 80 | 1512 | 41 | 83 | 1628 |
| 70551 | 40 | 73 | 1247 | 40 | 79 | 1453 |
| 70554 | 40 | 81 | 1580 | 44 | 87 | 1735 |
| 71127 | 40 | 82 | 1580 | 39 | 83 | 1609 |
| 71223 | 40 | 80 | 1568 | 46 | 91 | 1788 |
| 73103 | 40 | 85 | 1831 | 38 | 89 | 1892 |
| HS3010 | 40 | 70 | 1219 | 41 | 77 | 1398 |
| 70082 | 41 | 74 | 1307 | 40 | 86 | 1791 |
| 70287 | 41 | 75 | 1335 | 42 | 81 | 1408 |
| 71456 | 41 | 82 | 1580 | 44 | 89 | 1739 |
| 74466 | 41 | 73 | 1247 | 40 | 77 | 1293 |
| Nipper | 41 | 87 | 1890 | 40 | 89 | 1791 |
| 73693 | 42 | 80 | 1512 | 43 | 90 | 1759 |
| 70816 | 43 | 75 | 1335 | 38 | 79 | 1523 |
| 74470 | 43 | 72 | 1194 | 47 | 81 | 1396 |
| 70400 | 44 | 77 | 1400 | 53 | 82 | 964 |
| 70995 | 44 | 81 | 1580 | 44 | 93 | 1788 |
| 70037 | 45 | 75 | 2021 | 41 | 82 | 1553 |
| 71448 | 45 | 82 | 1580 | 45 | 90 | 1759 |
| 70437 | 46 | 80 | 1504 | 45 | 79 | 1398 |
| 70549 | 46 | 78 | 1494 | 45 | 84 | 1628 |
| 70569 | 46 | 83 | 1740 | 46 | 83 | 1616 |
| 73838 | 46 | 75 | 1335 | 47 | 79 | 1386 |
| 70538 | 47 | 78 | 1494 | 48 | 90 | 1667 |
| 70564 | 47 | 86 | 1832 | 44 | 95 | 1788 |
| 71099 | 47 | 77 | 1342 | 40 | 85 | 1691 |
| 70155 | 48 | 80 | 1401 | 49 | 84 | 1536 |
| 72990 | 50 | 87 | 1610 | 44 | 95 | 1788 |
| 73745 | 50 | 80 | 1331 | 48 | 83 | 1581 |
| 70161 | 51 | 81 | 1299 | 54 | 104 | 1417 |
| 70954 | 53 | 81 | 976 | 55 | 85 | 1223 |
| 70106 | 54 | 88 | 1343 | 42 | 98 | 1788 |
| 70270 | 57 | 83 | 1063 | 45 | 103 | 1917 |
| 70492 | 61 | 81 | 818 | 48 | 85 | 1644 |
| 72381 | 61 | 79 | 739 | 47 | 78 | 1386 |
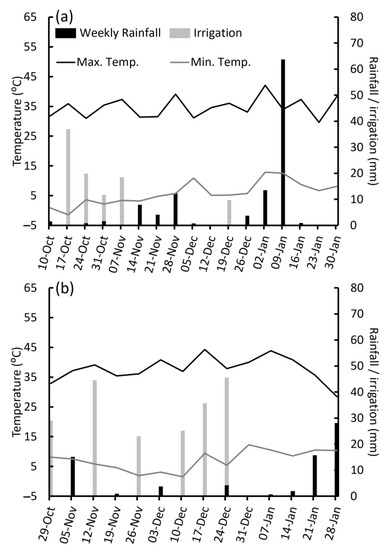
Figure A1.
Rainfall and irrigation as weekly totals and minimum and maximum temperatures as weekly averages for the (a) 2014 and (b) 2015 trials at Horsham, Victoria. Rainfall and temperature data were obtained through the Queensland Government database SILO climate data base. (Bureau of Meteorology station 79028, Longerenong).
References
- Zohary, D. Monophyletic vs. polyphyletic origin of the crops on which agriculture was founded in the Near East. Genet. Resour. Crop. Evol. 1999, 46, 133–142. [Google Scholar] [CrossRef]
- Gaur, P.M.; Samineni, S.; Krishnamurthy, L.; Kumar, S.; Ghanem, M.E.; Beebe, S.; Rao, I.; Chaturvedi, S.K.; Basu, P.S.; Nayyar, H.; et al. High Temperature Tolerance in Grain Legumes. In Proceedings of the Legume Perspectives, Saskatoon, SK, Canada, 7–11 July 2015; pp. 23–24. [Google Scholar]
- Siddique, K.H.M.; Erskine, W.; Hobson, K.; Knights, E.J.; Leonforte, A.; Khan, T.N.; Paull, J.G.; Redden, R.; Materne, M. Cool-season grain legume improvement in Australia-use of genetic resources. Crop. Pasture Sci. 2013, 64, 347–360. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assesement Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014.
- Sadras, V.O.; Rosewarne, G.M.; Lake, L. Australian lentil breeding between 1988 and 2019 has delivered greater yield gain under stress than under high-yield conditions. Front. Plant Sci. 2021, 12, 674327. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Kant, R.; Kumar, S.; Basu, P.S.; Sarker, A.; Singh, N.P. Heat Tolerance in Lentil under Field Conditions. Legume Genom. Genet. 2016, 7, 1–11. [Google Scholar] [CrossRef]
- Cossani, C.M.; Sadras, V.O. Symmetric response to competition in binary mixtures of cultivars associates with genetic gain in wheat yield. Evol. Appl. 2021, 14, 2064–2078. [Google Scholar] [CrossRef] [PubMed]
- Hobson, K.; Armstrong, R.; Nicolas, M.; Connor, D.; Materne, M. Response of lentil (Lens culinaris) germplasm to high concentrations of soil boron. Euphytica 2006, 151, 371–382. [Google Scholar] [CrossRef]
- Maher, L.; Armstrong, R.; Connor, D. Salt Tolerant Lentils–A Possibility for the Future? In Proceedings of the 11th Australian Agronomy Conference, Geelong, VIC, Australia, 2–6 February 2003.
- Rodda, M.S.; Davidson, J.; Javid, M.; Sudheesh, S.; Blake, S.; Forster, J.W.; Kaur, S. Molecular breeding for Ascochyta blight resistance in lentil: Current progress and future directions. Front. Plant Sci. 2017, 8, 1136. [Google Scholar] [CrossRef]
- Materne, M.; McNeil, D.L. Breeding Methods and Achievements. In Lentil; Yadav, S.S., Stevenson, P.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 241–253. [Google Scholar]
- Sadras, V.O.; Vadez, V.; Purushothaman, R.; Lake, L.; Marrou, H. Unscrambling confounded effects of sowing date trials to screen for crop adaptation to high temperature. Field Crops Res. 2015, 177, 1–8. [Google Scholar] [CrossRef]
- Krishnamurthy, L.; Gaur, P.M.; Basu, P.S.; Chaturvedi, S.K.; Tripathi, S.; Vadez, V.; Rathore, A.; Varshney, R.K.; Gowda, C.L.L. Large genetic variation for heat tolerance in the reference collection of chickpea (Cicer arietinum L.) germplasm. Plant Genet. Resour. Characterisation Util. 2011, 9, 59–69. [Google Scholar] [CrossRef]
- Canci, H.; Toker, C. Evaluation of Yield Criteria for Drought and Heat Resistance in Chickpea (Cicer arietinum L.). J. Agron. Crop Sci. 2009, 195, 47–54. [Google Scholar] [CrossRef]
- Devasirvatham, V.; Gaur, P.M.; Raju, T.N.; Trethowan, R.M.; Tan, D.K.Y. Field response of chickpea (Cicer arietinum L.) to high temperature. Field Crops Res. 2015, 172, 59–71. [Google Scholar] [CrossRef]
- Ismail, A.M.; Hall, A.E. Reproductive-stage heat tolerance, leaf membrane thermostability and plant morphology in cowpea. Crop. Sci. 1999, 39, 1762–1768. [Google Scholar] [CrossRef]
- Sehgal, A.; Sita, K.; Kumar, J.; Kumar, S.; Singh, S.; Siddique, K.H.M.; Nayyar, H. Effects of drought, heat and their interaction on the growth, yield and photosynthetic function of lentil (Lens culinaris Medikus) genotypes varying in heat and drought sensitivity. Front. Plant Sci. 2017, 8, 1776. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, D.R.; Tarafdar, S.; Das, M.; Kundagrami, S. Screening lentil (Lens culinaris Medik.) germplasms for heat tolerance. Trends Biosci. 2012, 5, 143. [Google Scholar]
- Porch, T.G. Application of stress indices for heat tolerance screening of common bean. J. Agron. Crop Sci. 2006, 192, 390–394. [Google Scholar] [CrossRef]
- Fernandez, G.C.J. Effective Selection Criteria for Assessing Plant Stress Tolerance. In Proceedings of the International Symposium on Adaptation of Vegetables and other Food Crops in Temperature and Water Stress, AVRDC, Shanhua, Taiwan, 13–16 August 1992; pp. 257–270. [Google Scholar]
- Sita, K.; Sehgal, A.; Kumar, J.; Kumar, S.; Singh, S.; Siddique, K.; Nayyar, H. Identification of high-temperature tolerant lentil (Lens culinaris Medik.) Genotypes through leaf and pollen traits. Front. Plant Sci. 2017, 8, 744. [Google Scholar] [CrossRef] [PubMed]
- Isbell, R. The Australian Soil Classification; CSIRO Australia: Collingwood, VI, Australia, 1996; Volume 4, p. 143.
- Rad, A.H.S.; Abbasian, A. Evaluation of drought tolerance in winter rapeseed cultivars based on tolerance and sensitivity indices. Žemdirbystė 2011, 98, 41–48. [Google Scholar]
- Erskine, W.; Ellis, R.H.; Summerfield, R.J.; Roberts, E.H.; Hussain, A. Characterization of responses to temperature and photoperiod for time to flowering in a world lentil collection. Theor. Appl. Genet. 1990, 80, 193–199. [Google Scholar] [CrossRef]
- Nuttall, J.; Barlow, K.; Christy, B.; O‘Leary, G.; Fitzgerald, G. Heat Shock Response in Wheat under Free Air CO2 Enrichment. In Proceedings of the Building Productive, Diverse and Sustainable Landscapes. In Proceedings of the 17th Australian Agronomy Conference, Hobart, Australia, 20–24 September 2015; pp. 543–546. [Google Scholar]
- Bhandari, K.; Siddique, K.H.M.; Turner, N.C.; Kaur, J.; Singh, S.; Agrawal, S.K.; Nayyar, H. Heat stress at reproductive stage disrupts leaf carbohydrate metabolism, impairs reproductive function, and severely reduces seed yield in lentil. J. Crop Improv. 2016, 30, 118–151. [Google Scholar] [CrossRef]
- Bouslama, M.; Schapaugh, W.T., Jr. Stress Tolerance in Soybeans. I. Evaluation of three screening techniques for heat and drought tolerance. Crop. Sci. 1984, 24, 933–937. [Google Scholar] [CrossRef]
- VSN International. Genstat for Windows, 18th ed.; VSN International Ltd.: Hemel Hempstead, UK, 2015. [Google Scholar]
- Yuan, H.Y.; Saha, S.; Vandenberg, A.; Bett, K.E. Flowering and growth responses of cultivated lentil and wild lens germplasm toward the differences in red to far-red ratio and photosynthetically active radiation. Front. Plant Sci. 2017, 8, 386. [Google Scholar] [CrossRef]
- Khazaei, H.; Street, K.; Bari, A.; Mackay, M.; Stoddard, F.L. The FIGS (Focused Identification of Germplasm Strategy) approach identifies traits related to drought ddaptation in vicia faba genetic resources. PLoS ONE 2013, 8, e63107. [Google Scholar] [CrossRef]
- Lombardi, M.; Materne, M.; Cogan, N.O.; Rodda, M.; Daetwyler, H.D.; Slater, A.T.; Forster, J.W.; Kaur, S. Assesment of genetic variation within a global collection of lentil (Lens culinaris Medik.) culitvars and lardraces using SNP markers. BMC Genet. 2014, 15, 150. [Google Scholar] [CrossRef]
- Tullu, A.; Diederichsen, A.; Suvorova, G.; Vandenberg, A. Genetic and genomic resources of lentil: Status, use and prospects. Plant Genet. Resour. 2011, 9, 19–29. [Google Scholar] [CrossRef]
- Haile, T.A.; Stonehouse, R.; Weller, J.L.; Bett, K.E. Genetic basis for lentil adaptation to summer cropping in northern temperate environments. bioRxiv 2021. [Google Scholar] [CrossRef]
- El Haddad, N.; Rajendran, K.; Smouni, A.; Es-Safi, N.E.; Benbrahim, N.; Mentag, R.; Nayyar, H.; Maalouf, F.; Kumar, S. Screening the FIGS set of lentil (Lens Culinaris Medikus) germplasm for tolerance to terminal heat and combined drought-heat stress. Agronomy 2020, 10, 1036. [Google Scholar] [CrossRef]
- Singh, S.; Sengar, R.; Kulshreshtha, N.; Datta, D.; Tomar, R.; Rao, V.; Garg, D.; Ojha, A. Assessment of multiple tolerance indices for salinity stress in bread wheat (Triticum aestivum L.). J. Agric. Sci. 2015, 7, 49. [Google Scholar] [CrossRef]
- Sial, M.A.; Arain, M.A.; Khanzada, S.; Naqvi, M.H.; Dahot, M.U.; Nizamani, N.A. Yield and quality parameters of wheat genotypes as affected by sowing dates and high temperature stress. Pak. J. Bot. 2005, 37, 575. [Google Scholar]
- Gaur, P.M.; Samineni, S.; Varshney, R.K. Drought and heat tolerance in chickpea. Legume Perspetives 2014, 9, 15–17. [Google Scholar]
- Upadhyaya, H.; Dronavalli, N.; Laxmipathi Gowda, C.; Sube, S. Identification and evaluation of chickpea germplasm for tolerance to heat stress. Crop Sci. 2011, 51, 2079. [Google Scholar] [CrossRef]
- Santos, M.V.; Ferreira, E.A.; Valadão, D.; Ramos de Oliveira, F.L.; Machado, V.D.; Silveria, R.R.; de Freitas Souza, M. Brachiaria physiological parameters in agroforestry systems. Anim. Prod. 2017, 47, 5. [Google Scholar] [CrossRef]
- Sadras, V.; Dreccer, M.F. Adaptation of wheat, barley, canola, field pea and chickpea to the thermal environments of Australia. Crop. Pasture Sci. 2015, 66, 1137–1150. [Google Scholar] [CrossRef]
- Bonada, M.; Sadras, V. Critical appraisal of methods to investigate the effect of temperature on grapevine berry composition. Aust. J. Grape Wine Res. 2015, 21, 1–17. [Google Scholar] [CrossRef]
- Erskine, W.; Hussain, A.; Tahir, M.; Bahksh, A. Field evaluation of a model of photothermal flowering responses in a world lentil collection. Theor. Appl. Genet. 1994, 88, 423. [Google Scholar] [CrossRef] [PubMed]
- Lake, L.; Chauhan, Y.S.; Ojeda, J.; Cossani, C.; Thomas, D.; Hayman, P.; Sadras, V. Modelling phenology to probe for trade-offs between frost and heat risk in lentil and faba bean. Eur. J. Agron. 2021, 122, 126154. [Google Scholar] [CrossRef]
- Prasad, P.V.; Craufurd, P.; Summerfield, R. Fruit number in relation to pollen production and viability in groundnut exposed to short episodes of heat stress. Ann. Bot. 1999, 84, 381–386. [Google Scholar] [CrossRef]
- White, J.W.; Beaver, J.; Burridge, J.D.; Jochua, C.; Miguel, M.; Porch, T.G.; Rosas, J.C.; Lynch, J. Boundary Line Analysis of "Noisy" Common Bean Yield Data. In Proceedings of the ASA, CSSA, and CSA International Annual Meeting, Baltimore, MD, USA, 4–7 November 2018. [Google Scholar]
- Flohr, B.M.; Hunt, J.R.; Kirkegaard, J.A.; Evans, J.R. Water and temperature stress define the optimal flowering period for wheat in south-eastern Australia. Field Crops Res. 2017, 209, 108–119. [Google Scholar] [CrossRef]
- Lilley, J.M.; Flohr, B.M.; Whish, J.P.M.; Farre, I.; Kirkegaard, J.A. Defining optimal sowing and flowering periods for canola in Australia. Field Crops Res. 2019, 235, 118–128. [Google Scholar] [CrossRef]
- Rebetzke, G.J.; Fischer, R.A.; van Herwaarden, A.F.; Bonnett, D.G.; Chenu, K.; Rattey, A.R.; Fettell, N.A. Plot size matters: Interference from intergenotypic competition in plant phenotyping studies. Funct. Plant Biol. 2014, 41, 107–118. [Google Scholar] [CrossRef]
- Gent, M. Crop Evolution, Adaptation and Yield; Evans, L.T., Ed.; Cambridge University Press: Cambridge, UK, 1996; p. 500. ISBN 0-521-29588-0. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).