Biochar Amendment Suppressed Fusarium Wilt and Altered the Rhizosphere Microbial Composition of Tomatoes
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Collection and Preparation of Biochar and FOL Conidia
2.2. Pot Experiment
2.3. Survey of Plant Growth and Collection of Rhizosphere Soil
2.4. Soil DNA Extraction and Quantitative Real-Time PCR
2.5. Illumina Miseq Sequencing Analysis
2.6. Statistical Analysis
3. Results
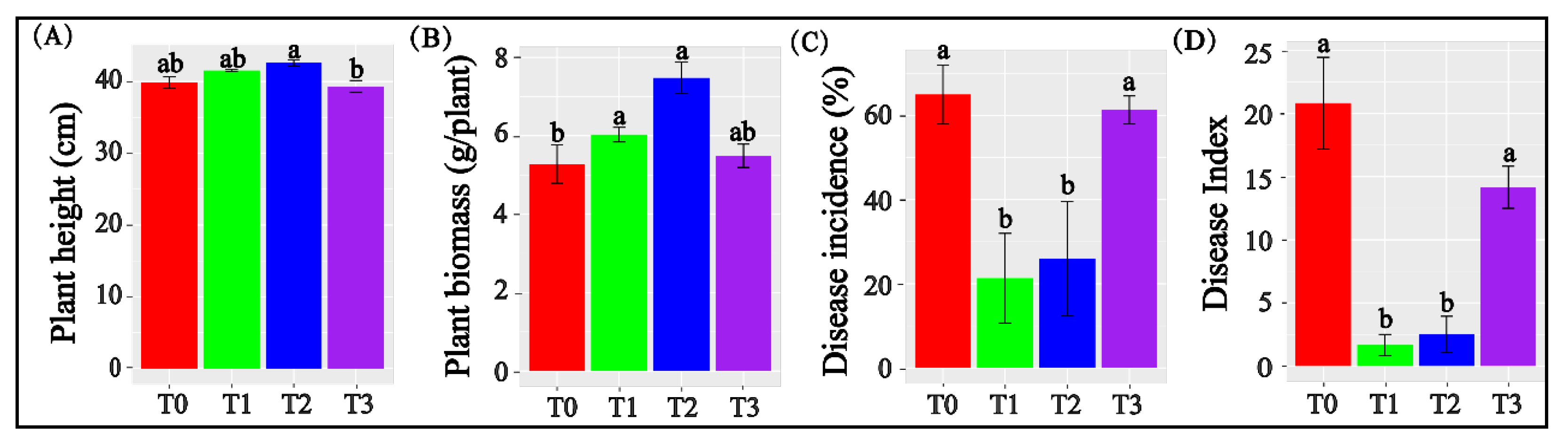
3.1. Biochar Applied at 1% and 2% Promoted Tomato Growth and Inhibited Fusarium Wilt
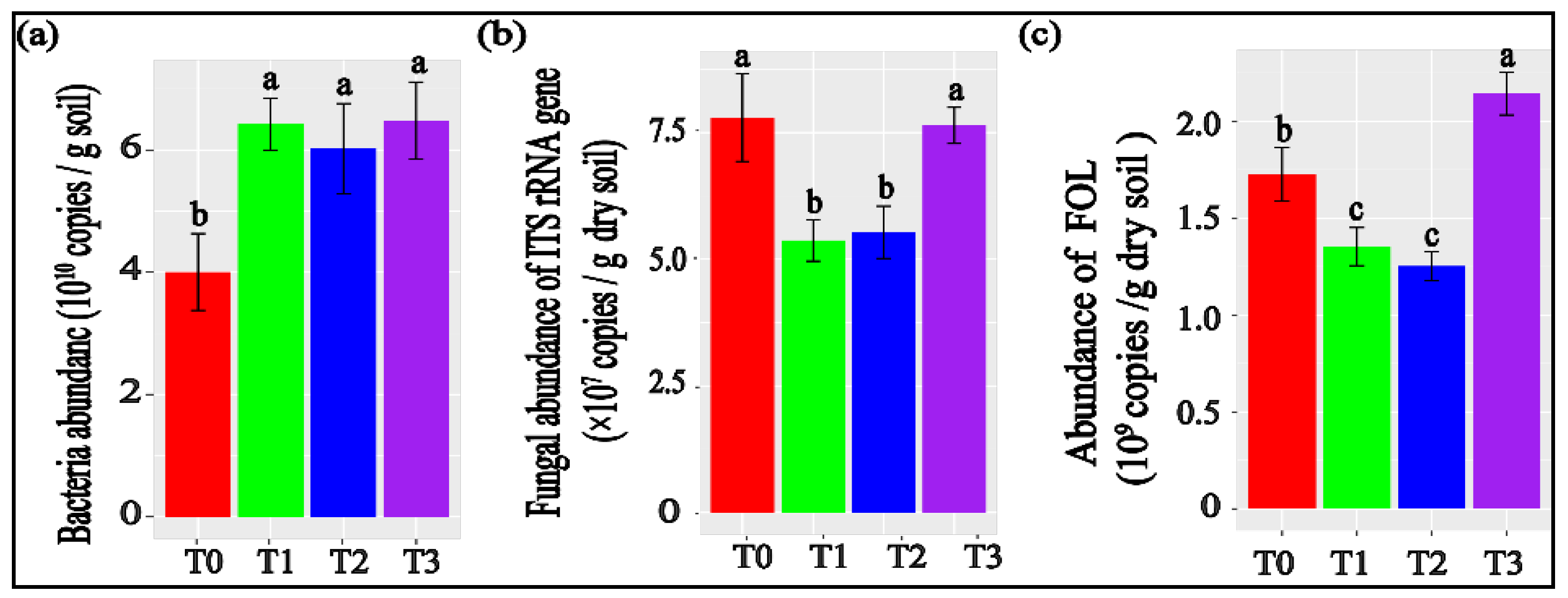
3.2. Tomato Rhizosphere Microbial Abundances Measured with qPCR
3.3. Tomato Rhizosphere Bacterial and Fungal Community ɑ and β Diversities
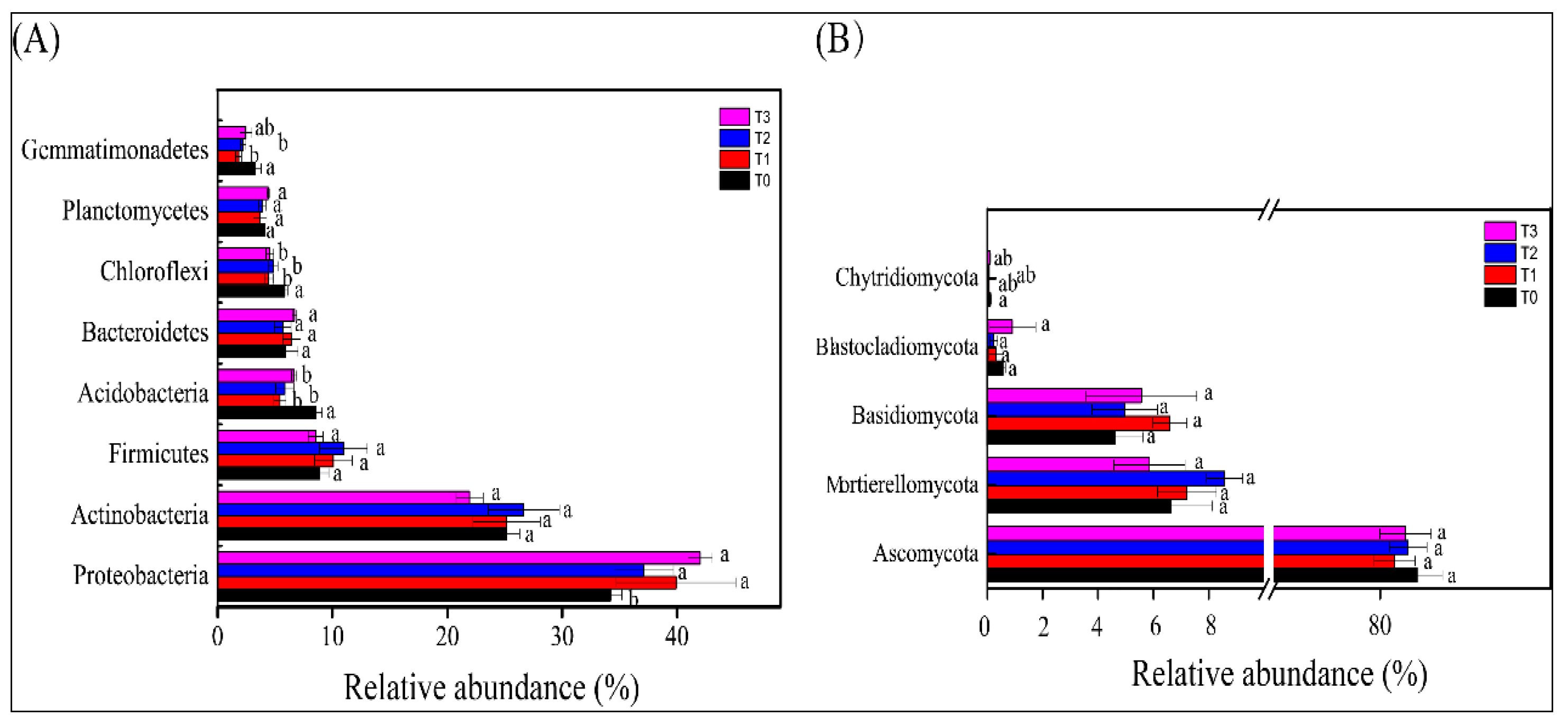
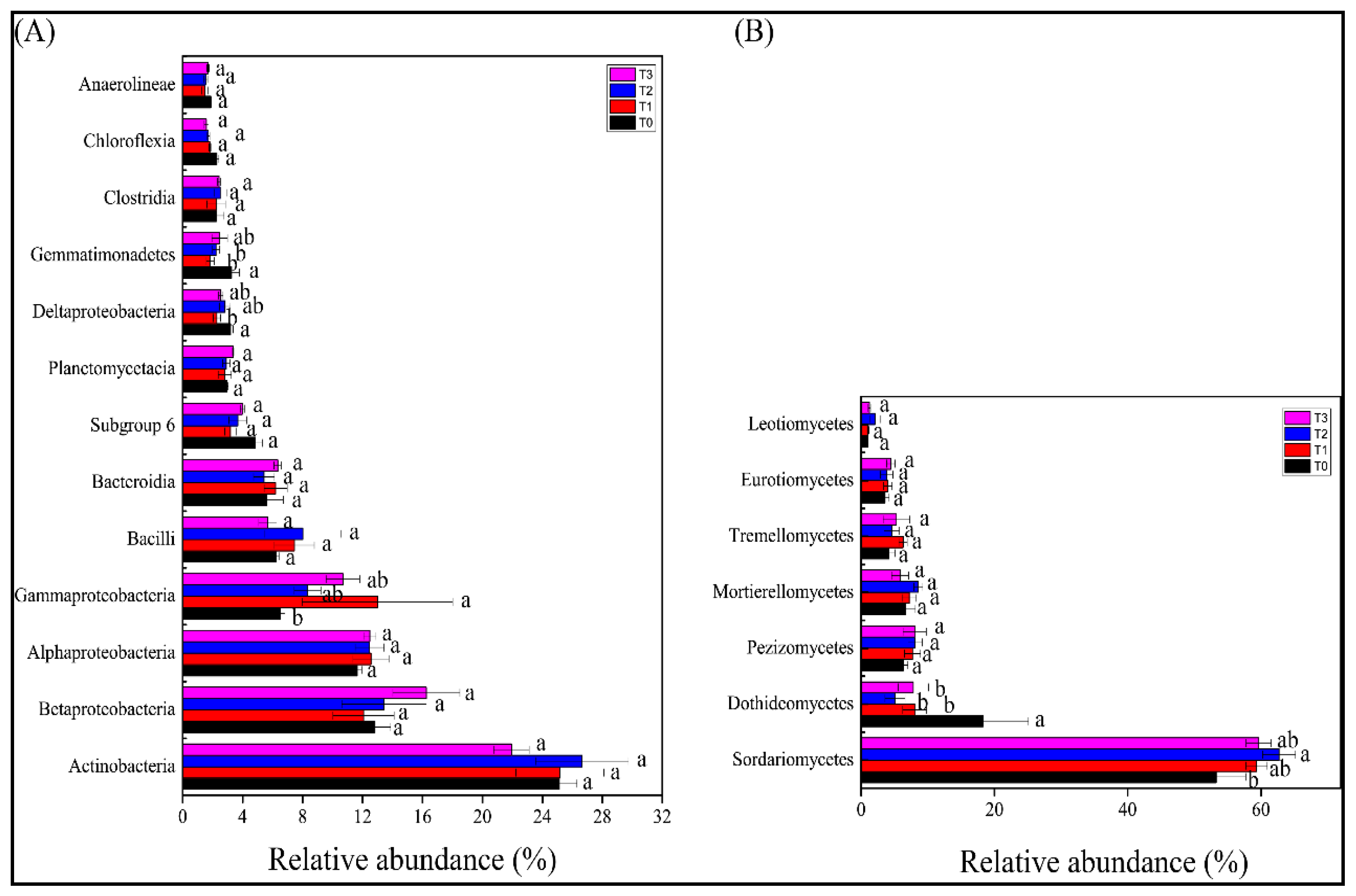
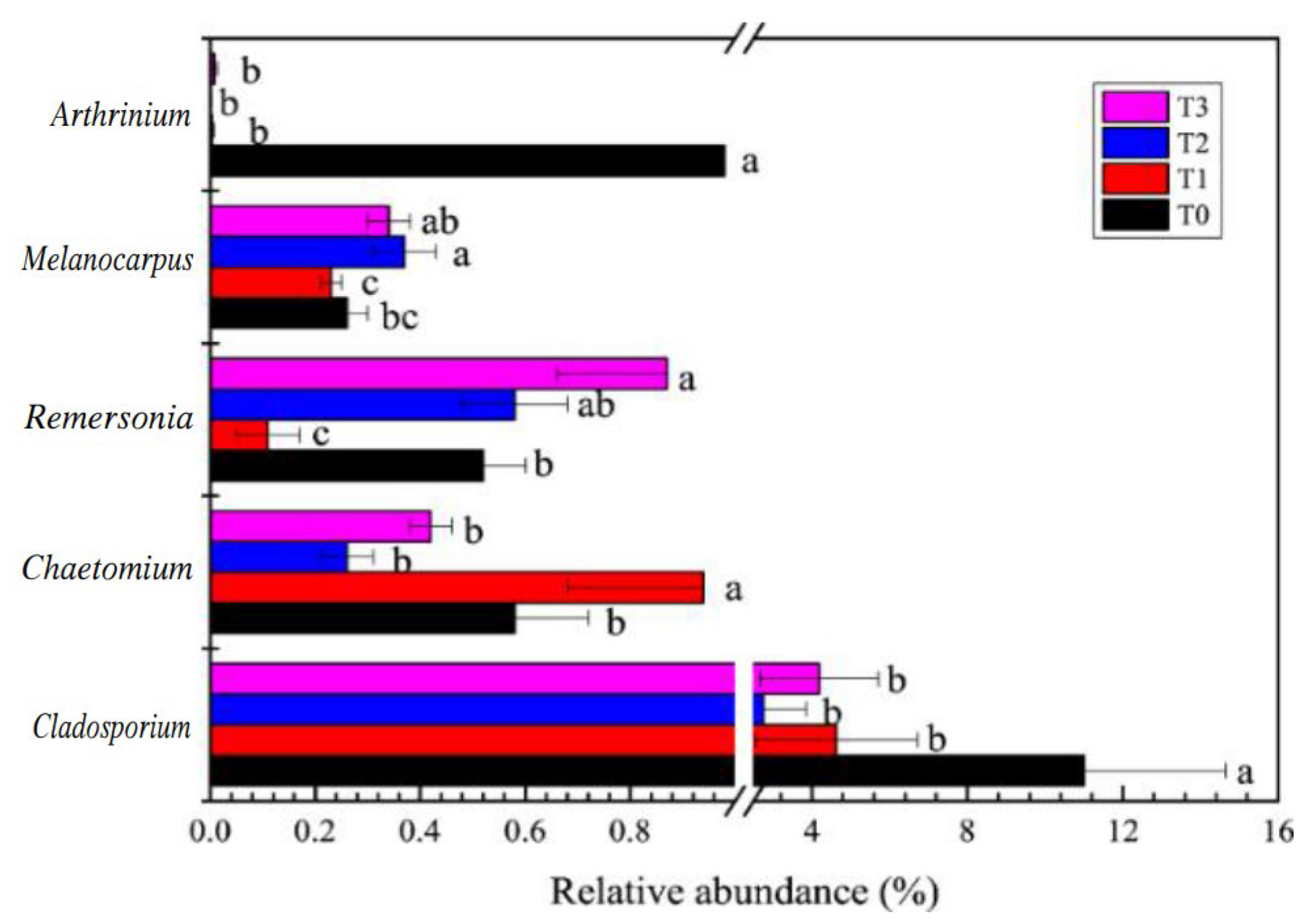
3.4. Bacterial and Fungal Community Composition in the Rhizosphere of Tomato Plants
3.5. Correlations between Tomato Performance and Rhizosphere Microbial Genera
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed]
- Koike, S.; Subbarao, K.; Davis, R.M.; Turini, T. Vegetable Diseases Caused by Soilborne Pathogens; UCANR Publications: Oakland, CA, USA, 2003. [Google Scholar]
- McGovern, R.J. Management of tomato diseases caused by Fusarium oxysporum. Crop Prot. 2015, 73, 78–92. [Google Scholar] [CrossRef]
- Panth, M.; Hassler, S.C.; Baysal-Gurel, F. Methods for management of soilborne diseases in crop production. Agriculture 2020, 10, 16. [Google Scholar] [CrossRef]
- Silva, L.G.; de Andrade, C.A.; Bettiol, W. Biochar amendment increase siol microbial biomass and plant growth and supresses Fusarium wilt in tomato. Trop. Plant Pathol. 2020, 45, 73–83. [Google Scholar] [CrossRef]
- Akhter, A.; Hage-Ahmed, K.; Soja, G.; Steinkellner, S. Potential of Fusarium wilt-inducing chlamydospores, in vitro behaviour in root exudates and physiology of tomato in biochar and compost amended soil. Plant Soil 2016, 406, 425–440. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Frenkel, O.; Elad, Y.; Lew, B.; Graber, E.R. Non-monotonic influence of biochar dose on bean seedling growth and susceptibility to Rhizoctonia solani: The “Shifted R max-Effect”. Plant Soil 2015, 395, 125–140. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation; Routledge: London, UK, 2015. [Google Scholar]
- Rajkovich, S.; Enders, A.; Hanley, K.; Hyland, C.; Zimmerman, A.R.; Lehmann, J. Corn growth and nitrogen nutrition after additions of biochars with varying properties to a temperate soil. Biol. Fertil. Soils 2012, 48, 271–284. [Google Scholar] [CrossRef]
- Spokas, K.A.; Cantrell, K.B.; Novak, J.M.; Archer, D.W.; Ippolito, J.A.; Collins, H.P.; Lentz, R.D. Biochar: A synthesis of its agronomic impact beyond carbon sequestration. J. Environ. Qual. 2012, 41, 973–989. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Elad, Y.; Graber, E.R.; Frenkel, O. Rhizoctonia solani suppression and plant growth promotion in cucumber as affected by biochar pyrolysis temperature, feedstock and concentration. Soil Biol. Biochem. 2014, 69, 110–118. [Google Scholar] [CrossRef]
- Copley, T.R.; Aliferis, K.A.; Jabaji, S. Maple bark biochar affects Rhizoctonia solani metabolism and increases damping-off severity. Phytopathology 2015, 105, 1334–1346. [Google Scholar] [CrossRef]
- Huang, W.K.; Ji, H.L.; Gheysen, G.; Debode, J.; Kyndt, T. Biochar-amended potting medium reduces the susceptibility of rice to root-knot nematode infections. BMC Plant Biol. 2015, 15, 267. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, O.; Jaiswal, A.K.; Elad, Y.; Lew, B.; Kammann, C.; Graber, E.R. The effect of biochar on plant diseases: What should we learn while designing biochar substrates? J. Environ. Qual. 2017, 25, 105–113. [Google Scholar] [CrossRef]
- Viger, M.; Hancock, R.D.; Miglietta, F.; Taylor, G. More plant growth but less plant defence? First global gene expression data for plants grown in soil amended with biochar. GCB Bioenergy 2015, 7, 658–672. [Google Scholar] [CrossRef]
- Gu, Y.; Hou, Y.; Huang, D.; Hao, Z.; Wang, X.; Wei, Z.; Xu, Y. Application of biochar reduces Ralstonia solanacearum infection via effects on pathogen chemotaxis, swarming motility, and root exudate adsorption. Plant Soil 2017, 415, 269–281. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Frenkel, O.; Tsechansky, L.; Elad, Y.; Graber, E.R. Immobilization and deactivation of pathogenic enzymes and toxic metabolites by biochar: A possible mechanism involved in soilborne disease suppression. Soil Biol. Biochem. 2018, 121, 59–66. [Google Scholar] [CrossRef]
- Elad, Y.; David, D.R.; Harel, Y.M.; Borenshtein, M.; Kalifa, H.B.; Silber, A.; Graber, E.R. Induction of systemic resistance in plants by biochar, a soil-applied carbon sequestering agent. Phytopathology 2010, 100, 913–921. [Google Scholar] [CrossRef]
- Van Wees, S.C.; Van der Ent, S.; Pieterse, C.M. Plant immune responses triggered by beneficial microbes. Curr. Opin. Plant Biol. 2008, 11, 443–448. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant-microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2021, 19, 72. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; Rahman, M.K.U.; Gao, D.; Wei, Z.; Wu, F.; Dini-Andreote, F. Interspecific plant interaction via root exudates structures the disease suppressiveness of rhizosphere microbiomes. Mol. Plant 2023, 16, 849–864. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Elad, Y.; Paudel, I.; Graber, E.R.; Cytryn, E.; Frenkel, O. Linking the belowground microbial composition, diversity and activity to soilborne disease suppression and growth promotion of tomato amended with biochar. Sci. Rep. 2017, 7, 44382. [Google Scholar] [CrossRef]
- Jin, X.; u Rahman, M.K.; Ma, C.; Zheng, X.; Wu, F.; Zhou, X. Silicon modification improves biochar’s ability to mitigate cadmium toxicity in tomato by enhancing root colonization of plant-beneficial bacteria. Ecotoxicol. Environ. Saf. 2023, 249, 114407. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Bai, Y.; Rahman, M.K.U.; Kang, X.J.; Pan, K.; Wu, F.Z.; Pommier, T.; Zhou, X.G.; Wei, Z. Biochar stimulates tomato roots to recruit a bacterial assemblage contributing to disease resistance against Fusarium wilt. iMeta 2022, 2, 37. [Google Scholar] [CrossRef]
- Gayoso, C.; Pomar, F.; Novo-Uzal, E.; Merino, F.; de Ilárduya, Ó.M. The Ve-mediated resistance response of the tomato to Verticillium dahliae involves H2O2, peroxidase and lignins and drives PALgene expression. BMC Plant Biol. 2010, 10, 232. [Google Scholar] [CrossRef]
- Xu, X.L.; Ko, W.H. A quantitative confined inoculation method for studies of pathogenicity of fungi on plants. Bot. Bull. Acad. Sin. 1998, 39, 187–190. [Google Scholar]
- Akhter, A.; Hage-Ahmed, K.; Soja, G.; Steinkellner, S. Compost and biochar alter mycorrhization, tomato root exudation, and development of Fusarium oxysporum f. sp. lycoperdici. Front. Plant Sci. 2015, 6, 529. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Shen, Y.; Fu, X.; Wu, F. Application of sodium silicate enhances cucumber resistance to Fusarium wilt and alters soil microbial communities. Front. Plant Sci. 2018, 9, 624. [Google Scholar] [CrossRef]
- Muyzer, G.; De Waal, E.C.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Lievens, B.; Houterman, P.M.; Rep, M. Effector gene screening allows unambiguous identification of Fusarium oxysporum f. sp. lycopersici races and discrimination from other formae speciales. FEMS Microbiol. Lett. 2009, 300, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wang, Z.L.; Wu, F.Z.; Li, X.G.; Zhou, X.G. Litter Mixing Alters Microbial Decomposer Community to Accelerate Tomato Root Litter Decomposition. Microbiol. Spectr. 2022, 10, e00186-22. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; Pan, D.; Ge, X.; Jin, X.; Chen, S.; Wu, F. p-Coumaric can alter the composition of cucumber rhizosphere microbial communities and induce negative plant-microbial interactions. Biol. Fertil. Soils 2018, 54, 363–372. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, J.; Wu, F. Soil microbial communities in cucumber monoculture and rotation systems and their feedback effects on cucumber seedling growth. Plant Soil 2017, 415, 507–520. [Google Scholar] [CrossRef]
- Schöler, A.; Jacquiod, S.; Vestergaard, G.; Schulz, S.; Schloter, M. Analysis of soil microbial communities based on amplicon sequencing of marker genes. Biol. Fertil. Soils 2017, 53, 485–489. [Google Scholar] [CrossRef]
- Jin, X.; Wang, J.; Li, D.L.; Wu, F.Z.; Zhou, X.G. Rotations with Indian mustard and wild rocket suppressed cucumber Fusarium wilt disease and changed rhizosphere bacterial communities. Microorganisms 2019, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, G.; Antignani, V.; Pane, C.; Scala, F. Suppression of soilborne fungal diseases with organic amendments. J. Plant Pathol. 2007, 89, 311–324. [Google Scholar]
- Harel, Y.M.; Elad, Y.; Rav-David, D.; Borenstein, M.; Shulchani, R.; Lew, B.; Graber, E.R. Biochar mediates systemic response of strawberry to foliar fungal pathogens. Plant Soil 2012, 357, 245–257. [Google Scholar] [CrossRef]
- Yao, Q.; Liu, J.; Yu, Z.; Li, Y.; Jin, J.; Liu, X.; Wang, G. Changes of bacterial community compositions after three years of biochar application in a black soil of northeast China. Appl. Soil Ecol. 2017, 113, 11–21. [Google Scholar] [CrossRef]
- Gong, X.; Huang, D.; Liu, Y.; Zeng, G.; Chen, S.; Wang, R.; Xu, P.; Cheng, M.; Zhang, C.; Xue, W. Biochar facilitated the phytoremediation of cadmium contaminated sediments: Metal behavior, plant toxicity, and microbial activity. Sci. Total Environ. 2019, 666, 1126–1133. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Zheng, J.; Zhang, B.; Lu, H.; Chi, Z.; Pan, G.; Li, L.; Zheng, J.; Zhang, X.; et al. Biochar soil amendment increased bacterial but decreased fungal gene abundance with shifts in community structure in a slightly acid rice paddy from Southwest China. Appl. Soil Ecol. 2013, 71, 33–44. [Google Scholar] [CrossRef]
- Kim, J.S.; Sparovek, G.; Longo, R.M.; De Melo, W.J. Bacterial diversity of terra preta and pristine forest soil from the Western Amazon. Soil Biol. Biochem. 2007, 39, 684–690. [Google Scholar] [CrossRef]
- Lee, B.; Lee, S.; Ryu, C.M. Foliar aphid feeding recruits rhizosphere bacteria and primes plant immunity against pathogenic and non-pathogenic bacteria in pepper. Ann. Bot. 2012, 110, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Gao, H.; Zhang, X.; Rahman, M.K.U.; Mazzoleni, S.; Du, M.; Wu, F. Plant extracellular self-DNA inhibits growth and induces immunity via the jasmonate signaling pathway. Plant Physiol. 2023, 192, kiad195. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Brettell, L.E.; Qiu, Z.; Singh, B.K. Microbiome-mediated stress resistance in plants. Trends Plant Sci. 2020, 25, 734–743. [Google Scholar] [CrossRef]
- Peiffer, J.A.; Spor, A.; Koren, O.; Jin, Z.; Tringe, S.G.; Dangl, J.L.; Ley, R.E. Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 6548–6553. [Google Scholar] [CrossRef]
- Goldfarb, K.C.; Karaoz, U.; Hanson, C.A.; Santee, C.A.; Bradford, M.A.; Treseder, K.K.; Brodie, E.L. Differential growth responses of soil bacterial taxa to carbon substrates of varying chemical recalcitrance. Front. Microbiol. 2011, 2, 94. [Google Scholar] [CrossRef]
- Sheng, Y.; Zhu, L. Biochar alters microbial community and carbon sequestration potential across different soil pH. Sci. Total Environ. 2018, 622, 1391–1399. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.; Bakker, P.A. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.A.; Lennon, J.T. Rapid responses of soil microorganisms improve plant fitness in novel environments. Proc. Natl. Acad. Sci. USA 2012, 109, 14058–14062. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Yang, N.; Wen, Z.; Sun, X.; Chai, Y.; Ma, Z. Wheat microbiome bacteria can reduce virulence of a plant pathogenic fungus by altering histone acetylation. Nat. Commun. 2018, 9, 3429. [Google Scholar] [CrossRef]
- Dudenhöffer, J.H.; Scheu, S.; Jousset, A. Systemic enrichment of antifungal traits in the rhizosphere microbiome after pathogen attack. J. Ecol. 2016, 104, 1566–1575. [Google Scholar] [CrossRef]

| T0 | T1 | T2 | T3 | |
|---|---|---|---|---|
| Marmocricola sp. | 0.91 ± 0.08 a | 0.77 ± 0.04 ab | 0.65 ± 0.01 b | 0.64 ± 0.05 b |
| Polycyclovorans sp. | 0.10 ± 0.01 a | 0.01 ± 0.01 c | 0.03 ± 0.01 bc | 0.05 ± 0.01 b |
| Pajaroellobacter sp. | 0.11 ± 0.02 a | 0.06 ± 0.00 b | 0.09 ± 0.01 ab | 0.07 ± 0.01 b |
| Pseudomonas sp. | 0.65 ± 0.03 c | 7.08 ± 1.01 a | 3.11 ± 0.81 bc | 2.08 ± 0.78 bc |
| Microbacterium sp. | 0.45 ± 0.04 c | 0.88 ± 0.04 a | 0.67 ± 0.01 ab | 0.57 ± 0.04 b |
| Neorhizobium sp. | 0.16 ± 0.01 b | 0.43 ± 0.08 a | 0.25 ± 0.01 b | 0.20 ± 0.040 b |
| Amaricoccus sp. | 0.35 ± 0.02 ab | 0.29 ± 0.01 b | 0.40 ± 0.02 a | 0.31 ± 0.02 b |
| Adhaeribacter sp. | 0.14 ± 0.02 b | 0.21 ± 0.02 a | 0.24 ± 0.02 a | 0.19 ± 0.01 ab |
| Altereythrobacter sp. | 0.20 ± 0.02 bc | 0.16 ± 0.02 c | 0.24 ± 0.01 ab | 0.27 ± 0.02 a |
| Novosphingobium sp. | 0.14 ± 0.00 b | 0.09 ± 0.02 c | 0.11 ± 0.02 bc | 0.19 ± 0.01 a |
| Ammoniphilus sp. | 0.05 ± 0.01 b | 0.08 ± 0.02 b | 0.16 ± 0.03 b | 0.27 ± 0.05 a |
| Arenimonas sp. | 0.27 ± 0.03 b | 0.17 ± 0.03 c | 0.31 ± 0.01 b | 0.46 ± 0.01 a |
| Pedobacter sp. | 0.10 ± 0.01 c | 0.33 ± 0.06 b | 0.48 ± 0.04 b | 0.29 ± 0.01 a |
| Opitutus sp. | 0.12 ± 0.01 b | 0.21 ± 0.00 a | 0.19 ± 0.01 a | 0.11 ± 0.01 b |
| Plant Height | Dry Biomass | Disease Incidence | Disease Index | |
|---|---|---|---|---|
| Cladosporium sp. | −0.40 | −0.80 | 0.40 | 0.40 |
| Chaetomium sp. | −0.80 | 1.00 * | −0.80 | −0.80 |
| Remersonia sp. | −0.40 | 0.20 | −0.40 | −0.40 |
| Melanocarpus sp. | 0.80 | 1.00 * | −0.80 | −0.80 |
| Arthrinium sp. | −0.80 | −1.00 * | 0.80 | 0.80 |
| Pedobacter sp. | 0.80 | 1.00 * | −0.80 | −0.80 |
| Polycyclovorans sp. | −0.60 | −0.80 | 1.00 * | 1.00 * |
| Pajaroellobacter sp. | 0.00 | −0.40 | 0.80 | 0.80 |
| Microbacterium sp. | 0.00 | 0.40 | −0.80 * | −0.80 |
| Neorhizobium sp. | 0.60 | 0.80 | −1.00 * | −1.00 * |
| Adhaeribacter sp. | 0.80 | 1.00 * | −0.80 | −0.80 |
| Novosphingobium sp. | −0.80 | −0.60 | 0.80 | 0.80 |
| Arenimonas sp. | −0.40 | 0.00 | 0.400 | 0.40 |
| Opitutus sp. | 0.40 | 0.00 | −0.40 | −0.40 |
| Altererythrobacter sp. | −0.11 | 0.21 | 0.32 | 0.32 |
| Pseudomonas sp. | 0.00 | 0.40 | −0.80 | −0.88 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, X.; Zhou, X.; Wu, F.; Xiang, W.; Pan, K. Biochar Amendment Suppressed Fusarium Wilt and Altered the Rhizosphere Microbial Composition of Tomatoes. Agronomy 2023, 13, 1811. https://doi.org/10.3390/agronomy13071811
Jin X, Zhou X, Wu F, Xiang W, Pan K. Biochar Amendment Suppressed Fusarium Wilt and Altered the Rhizosphere Microbial Composition of Tomatoes. Agronomy. 2023; 13(7):1811. https://doi.org/10.3390/agronomy13071811
Chicago/Turabian StyleJin, Xue, Xingang Zhou, Fengzhi Wu, Wensheng Xiang, and Kai Pan. 2023. "Biochar Amendment Suppressed Fusarium Wilt and Altered the Rhizosphere Microbial Composition of Tomatoes" Agronomy 13, no. 7: 1811. https://doi.org/10.3390/agronomy13071811
APA StyleJin, X., Zhou, X., Wu, F., Xiang, W., & Pan, K. (2023). Biochar Amendment Suppressed Fusarium Wilt and Altered the Rhizosphere Microbial Composition of Tomatoes. Agronomy, 13(7), 1811. https://doi.org/10.3390/agronomy13071811






