Ecotoxicological Evaluation of Earthworm (Eisenia fetida) Induced by Enrofloxacin and Di-(2-Ethylhexyl) Phthalate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemical Reagents Used in Experiments
2.3. Experimental Design
2.4. Determination of ROS Content and Oxidative Stress Biomarker Activity
2.5. DNA Damage Assessment
2.6. Determination of Digestive Enzyme Activities
2.7. Ecological Risk Assessment
2.8. Data Analysis
3. Results and Discussion
3.1. Survival of Earthworms
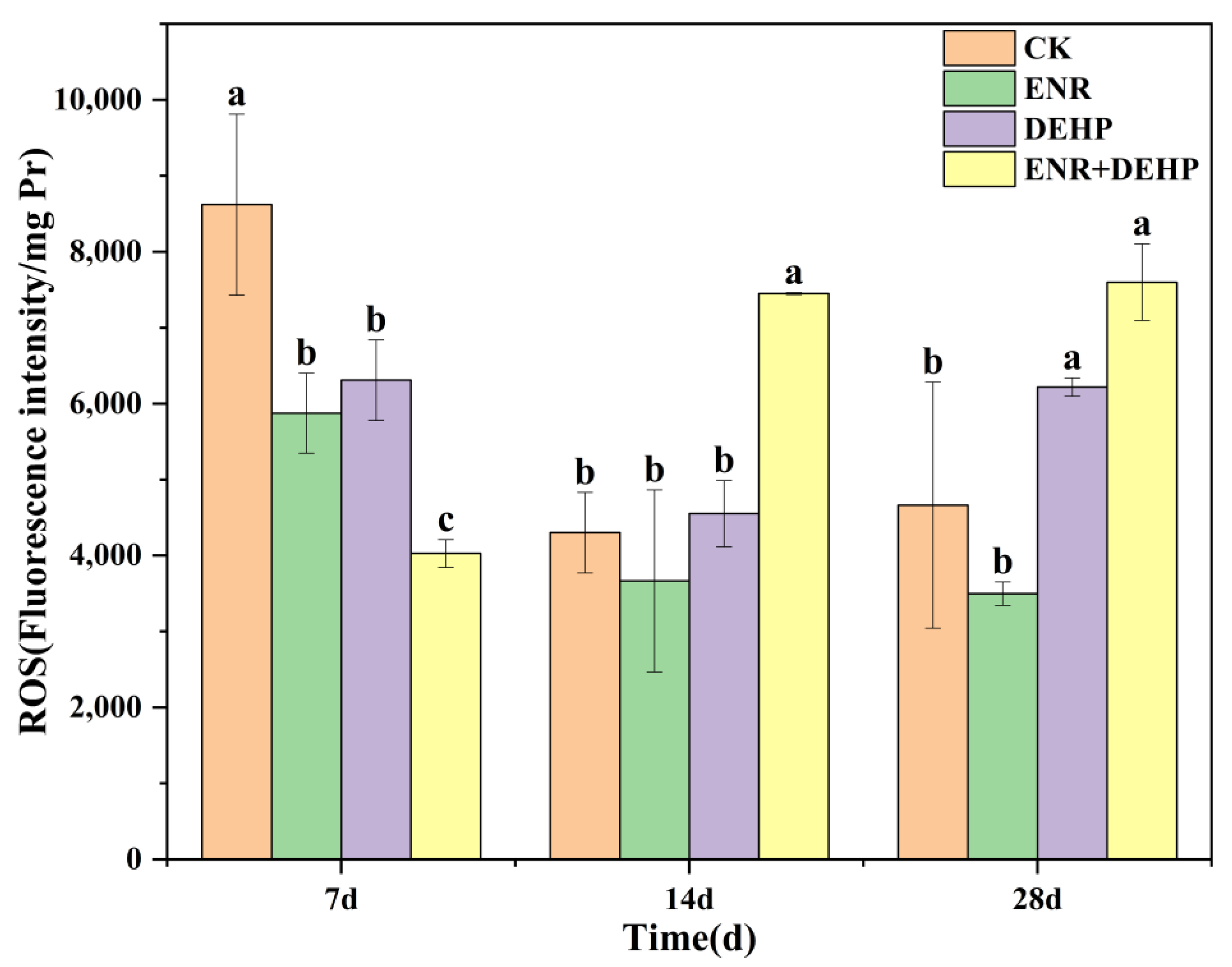
3.2. ROS Levels
3.3. Enzyme Activity Associated with Antioxidant Defense
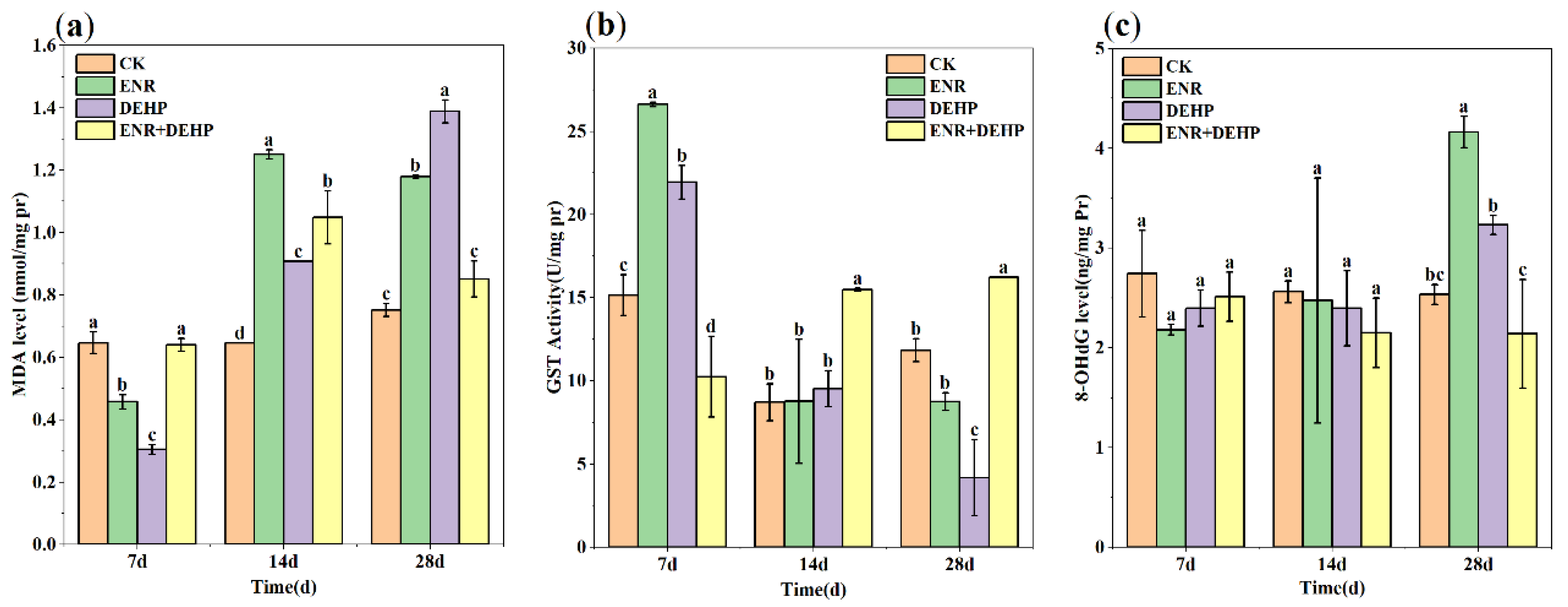
3.4. Lipid Peroxidation (LPO)
3.5. Glutathione S-Transferase (GST)
3.6. Degree of DNA Damage
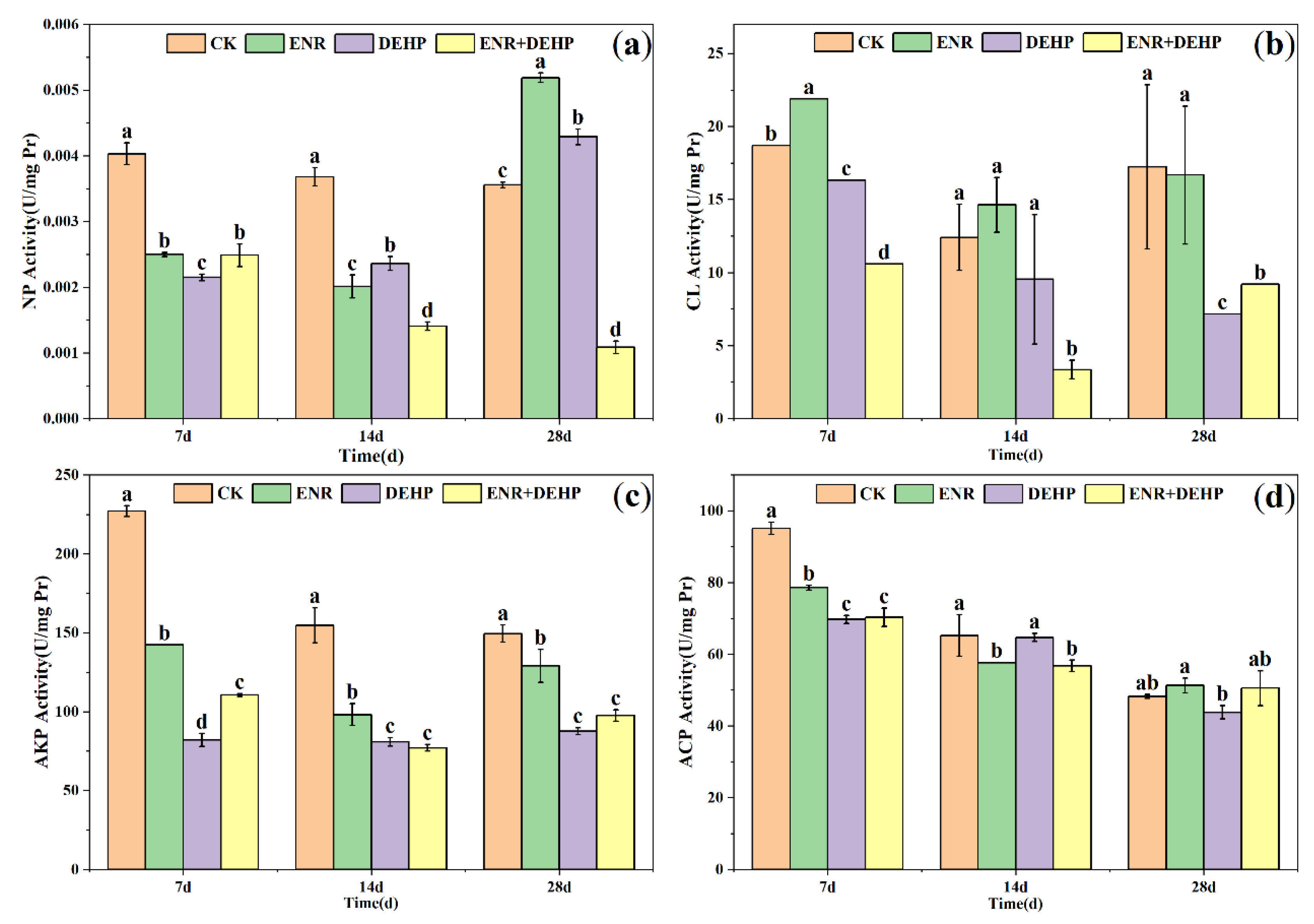
3.7. Earthworm Digestive Enzymes
3.8. IBR Analysis
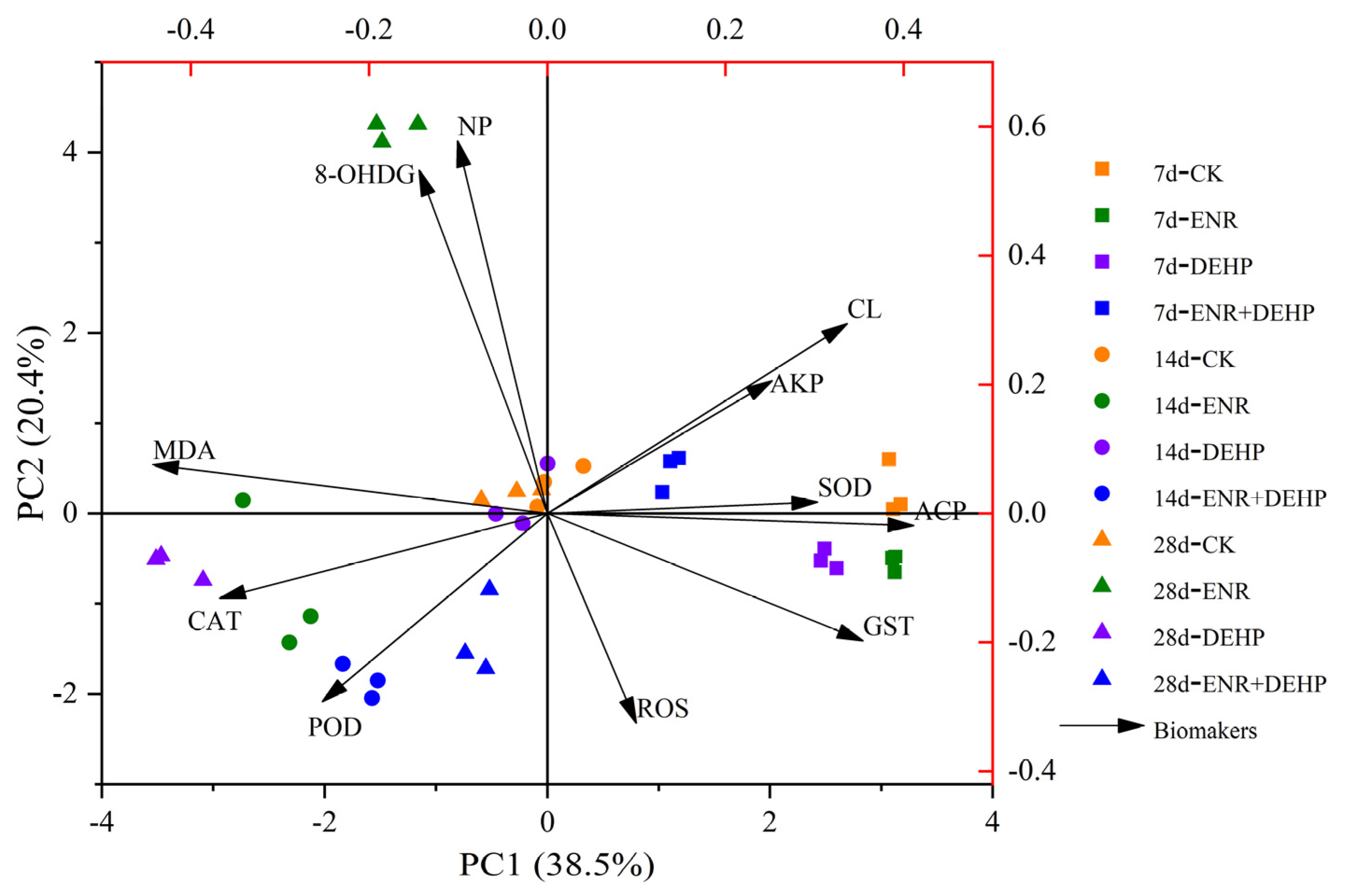
3.9. Correlation Analysis between Indices
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, W.Z.; Zheng, X.W.; Gu, P.; Wang, N.; Lai, Z.N.; He, J.; Zheng, Z. Distribution and risk assessment of phthalates in water and sediment of the Pearl River Delta. Environ. Sci. Pollut. Res. Int. 2020, 27, 12550–12565. [Google Scholar] [CrossRef]
- Adamovsky, O.; Buerger, A.N.; Vespalcova, H.; Sohag, S.R.; Hanlon, A.T.; Ginn, P.E.; Craft, S.L.; Smatana, S.; Budinska, E.; Persico, M.; et al. Evaluation of Microbiome-Host Relationships in the Zebrafish Gastrointestinal System Reveals Adaptive Immunity Is a Target of Bis(2-ethylhexyl) Phthalate (DEHP) Exposure. Environ. Sci. Technol. 2020, 54, 5719–5728. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Gao, J.; Li, X.; Zhang, C.; Zhu, L.; Wang, J.; Wang, J. Phthalate induced oxidative stress and DNA damage in earthworms (Eisenia fetida). Environ. Int. 2019, 129, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, F.; Xiang, L.; Gu, C.; Redmile-Gordon, M.; Sheng, H.; Wang, Z.; Fu, Y.; Bian, Y.; Jiang, X. Risk Assessment of Agricultural Plastic Films Based on Release Kinetics of Phthalate Acid Esters. Environ. Sci. Technol. 2021, 55, 3676–3685. [Google Scholar] [CrossRef]
- Hu, X.Y.; Wen, B.; Shan, X.Q. Survey of phthalate pollution in arable soils in China. J. Environ. Monit. 2003, 5, 649–653. [Google Scholar] [CrossRef]
- Chen, Y.S.; Luo, Y.M.; Zhang, H.B.; Song, J. Preliminary study on PAEs pollution of greenhouse soils. Acta Pedol. Sin. 2011, 48, 516–523. [Google Scholar] [CrossRef]
- Kim, D.; Cui, R.; Moon, J.; Kwak, J.I.; An, Y.J. Soil ecotoxicity study of DEHP with respect to multiple soil species. Chemosphere 2019, 216, 387–395. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Zhu, L.; Wang, J. Individual and combined effects of enrofloxacin and cadmium on soil microbial biomass and the ammonia-oxidizing functional gene. Sci. Total. Environ. 2018, 624, 900–907. [Google Scholar] [CrossRef]
- Yang, R.; Wang, J.; Zhu, L.; Wang, J.; Yang, L.; Mao, S.; Conkle, J.L.; Chen, Y.; Kim, Y.M. Effects of interaction between enrofloxacin and copper on soil enzyme activity and evaluation of comprehensive toxicity. Chemosphere 2021, 268, 129208. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Johnson, T.A.; Su, J.Q.; Qiao, M.; Guo, G.X.; Stedtfeld, R.D.; Hashsham, S.A.; Tiedje, J.M. Diverse and abundant antibiotic resistance genes in Chinese swine farms. Proc. Natl. Acad. Sci. USA 2013, 110, 3435–3440. [Google Scholar] [CrossRef]
- Shawver, S.; Wepking, C.; Ishii, S.; Strickland, M.S.; Badgley, B.D. Application of manure from cattle administered antibiotics has sustained multi-year impacts on soil resistome and microbial community structure. Soil. Biol. Biochem. 2021, 157, 108252. [Google Scholar] [CrossRef]
- Boxall, A.B.A.; Johnson, P.; Smith, E.J.; Sinclair, C.J.; Stutt, E.S.; Levy, L.S. Uptake of veterinary medicines from soils into plants. J. Agric. Food Chem. 2006, 54, 2288–2297. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, Y.; Ai, X.; Qiu, J.; Wang, X. Acute and sub-acute effects of enrofloxacin on the earthworm species Eisenia fetida in an artificial soil substrate. Eur. J. Soil. Biol. 2015, 66, 19–23. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, C.; Lu, X.; Ai, X.; Qiu, J. Identification of a cytochrome P450 gene in the earthworm Eisenia fetida and its mRNA expression under enrofloxacin stress. Ecotoxicol. Environ. Saf. 2018, 150, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, Y.; Thunders, M.; Matthew, C.; Wang, X.; Ai, X.; Zhou, X.; Qiu, J. Effect of enrofloxacin on the proteome of earthworms. Sci. Total. Environ. 2018, 616–617, 531–542. [Google Scholar] [CrossRef]
- Christen, V.; Crettaz, P.; Oberli-Schrammli, A.; Fent, K. Antiandrogenic activity of phthalate mixtures: Validity of concentration addition. Toxicol. Appl. Pharmacol. 2012, 259, 169–176. [Google Scholar] [CrossRef]
- Li, L.; Li, H.; Qu, P.; Xiao, M.; Zhang, G.; Zhang, Q.; Cai, Y.; Jin, C.; Yang, J.; Wu, S.; et al. An Antagonism Joint Action of Lead and Di-2-Ethylhexyl Phthalate Explains an Improved Ability of Learning and Memory after Combined Exposure in Weaning Rats. Biol. Trace Elem. Res. 2019, 191, 126–134. [Google Scholar] [CrossRef]
- Li, Y.; Tang, H.; Hu, Y.; Wang, X.; Ai, X.; Tang, L.; Matthew, C.; Cavanagh, J.; Qiu, J. Enrofloxacin at environmentally relevant concentrations enhances uptake and toxicity of cadmium in the earthworm Eisenia fetida in farm soils. J. Hazard. Mater. 2016, 308, 312–320. [Google Scholar] [CrossRef]
- Wei, S.; Wang, F.; Chen, Y.; Lan, T.; Zhang, S. The joint toxicity effect of five antibiotics and dibutyl phthalate to luminescent bacteria (Vibrio fischeri). Environ. Sci. Pollut. Res. Int. 2018, 25, 26504–26511. [Google Scholar] [CrossRef]
- Li, X.W.; Xie, Y.F.; Li, C.L.; Zhao, H.N.; Zhao, H.; Wang, N.; Wang, J.F. Investigation of residual fluoroquinolones in a soil-vegetable system in an intensive vegetable cultivation area in Northern China. Sci. Total. Environ. 2014, 468–469, 258–264. [Google Scholar] [CrossRef]
- Huang, C.; Ge, Y.; Yue, S.; Qiao, Y.; Liu, L. Impact of soil metals on earthworm communities from the perspectives of earthworm ecotypes and metal bioaccumulation. J. Hazard. Mater. 2021, 406, 124738. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.J.; Bai, J.; Li, W.Y.; Zhao, L.X.; Li, Y.T. Removal of persistent DDT residues from soils by earthworms: A mechanistic study. J. Hazard. Mater. 2019, 365, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.J.; Bai, J.; Li, W.; Murrell, J.C.; Zhang, Y.; Wang, J.; Luo, C.; Li, Y. Mechanisms of the enhanced DDT removal from soils by earthworms: Identification of DDT degraders in drilosphere and non-drilosphere matrices. J. Hazard. Mater. 2021, 404, 124006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, T.; Du, Z.; Juhasz, A.; Zhu, L.; Wang, J.; Wang, J.; Li, B. Applying fungicide on earthworms: Biochemical effects of Eisenia fetida exposed to fluoxastrobin in three natural soils. Environ. Pollut. 2020, 258, 113666. [Google Scholar] [CrossRef]
- Song, P.; Ping, L.; Gao, J.; Li, X.; Zhu, M.; Wang, J. Ecotoxicological effects of fertilizers made from pulping waste liquor on earthworm Eisenia fetida. Ecotoxicol. Environ. Saf. 2018, 166, 237–241. [Google Scholar] [CrossRef]
- Sanchez-Hernandez, J.C.; Rios, J.M.; Attademo, A.M.; Malcevschi, A.; Andrade Cares, X. Assessing biochar impact on earthworms: Implications for soil quality promotion. J. Hazard. Mater. 2019, 366, 582–591. [Google Scholar] [CrossRef]
- Junior, S.F.S.; da Silva, E.O.; de Farias Araujo, G.; Soares, L.O.S.; Parente, C.E.T.; Malm, O.; Saggioro, E.M.; Correia, F.V. Antioxidant system alterations and biological health status of earthworms following long-term exposure to antibiotic-contaminated poultry litter. Environ. Sci. Pollut. Res. Int. 2022, 29, 23607–23618. [Google Scholar] [CrossRef]
- Ma, T.; Zhou, W.; Chen, L.; Wu, L.; Christie, P.; Zhang, H.; Luo, Y. Toxicity effects of di-(2-ethylhexyl) phthalate to Eisenia fetida at enzyme, cellular and genetic levels. PLoS ONE 2017, 12, e0173957. [Google Scholar] [CrossRef]
- USEPA. Ecological Effects Test Guidelines—OCSPP 850.3100: Earthworm Sub Chronic Toxicity Test. EPA 712-C-016. 2012. Available online: https://nepis.epa.gov/Exe/ZyPDF.cgi/P100IREJ.PDF?Dockey=P100IREJ.PDF (accessed on 20 May 2023).
- Zhang, C.; Zhou, T.; Wang, J.; Zhang, S.; Zhu, L.; Du, Z.; Wang, J. Acute and chronic toxic effects of fluoxastrobin on zebrafish (Danio rerio). Sci. Total. Environ. 2018, 610–611, 769–775. [Google Scholar] [CrossRef]
- Villar, I.; Alves, D.; Perez-Diaz, D.; Mato, S. Changes in microbial dynamics during vermicomposting of fresh and composted sewage sludge. Waste Manag. 2016, 48, 409–417. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, Z.; Zhu, T.; Shu, W.; Geng, L. Ecological improvement of antimony and cadmium contaminated soil by earthworm Eisenia fetida: Soil enzyme and microorganism diversity. Chemosphere 2021, 273, 129496. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhou, D.; Wang, J.; Li, Y.; Liu, Y.; Ning, Y. Evaluation of the toxicity effects of microplastics and cadmium on earthworms. Sci. Total. Environ. 2022, 836, 155747. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, W.; Burgeot, T.; Porcher, J.M. A novel “Integrated Biomarker Response” calculation based on reference deviation concept. Environ. Sci. Pollut. Res. Int. 2013, 20, 2721–2725. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.; Pandey, P.; Ogata, A.; Teoh, G.; Krett, N.; Halgren, R.; Anderson, K. Cytochrome c-dependent and -independent induction of apoptosis in multiple myeloma cells. J. Biol. Chem. 1997, 272, 29995–29997. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free. Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef]
- Han, Y.; Liu, T.; Wang, J.; Wang, J.; Zhang, C.; Zhu, L. Genotoxicity and oxidative stress induced by the fungicide azoxystrobin in zebrafish (Danio rerio) livers. Pestic. Biochem. Physiol. 2016, 133, 13–19. [Google Scholar] [CrossRef]
- Liu, T.; Wang, X.; Chen, D.; Li, Y.; Wang, F. Growth, reproduction and biochemical toxicity of chlorantraniliprole in soil on earthworms (Eisenia fetida). Ecotoxicol. Environ. Saf. 2018, 150, 18–25. [Google Scholar] [CrossRef]
- Liang, J.; Xia, X.; Zhang, W.; Zaman, W.Q.; Lin, K.; Hu, S.; Lin, Z. The biochemical and toxicological responses of earthworm (Eisenia fetida) following exposure to nanoscale zerovalent iron in a soil system. Environ. Sci. Pollut. Res. Int. 2017, 24, 2507–2514. [Google Scholar] [CrossRef]
- Liu, W.J.; Gao, J.P.; Wang, G.Y.; Zhu, L.S.; Wang, J.H.; Wang, J. Oxidating Stress and DNA Damage of DEHP to Soil Earthworms. Acta Pedol. Sin. 2017, 54, 1170. [Google Scholar] [CrossRef]
- Baihetiyaer, B.; Jiang, N.; Li, X.; He, B.; Wang, J.; Fan, X.; Sun, H.; Yin, X. Oxidative stress and gene expression induced by biodegradable microplastics and imidacloprid in earthworms (Eisenia fetida) at environmentally relevant concentrations. Environ. Pollut. 2023, 323, 121285. [Google Scholar] [CrossRef]
- He, F.; Li, X.; Huo, C.; Chu, S.; Cui, Z.; Li, Y.; Wan, J.; Liu, R. Evaluation of fluorene-caused ecotoxicological responses and the mechanism underlying its toxicity in Eisenia fetida: Multi-level analysis of biological organization. J. Hazard. Mater. 2022, 437, 129342. [Google Scholar] [CrossRef]
- Hou, K.; Shi, B.; Liu, Y.; Lu, C.; Li, D.; Du, Z.; Li, B.; Zhu, L. Toxicity evaluation of pyraclostrobin exposure in farmland soils and co-exposure with nZnO to Eisenia fetida. J. Hazard. Mater. 2022, 433, 128794. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhang, W.; Li, J.; Lin, K.; Ji, R. Antioxidant and gene expression responses of Eisenia fetida following repeated exposure to BDE209 and Pb in a soil-earthworm system. Sci. Total. Environ. 2016, 556, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Li, P.; Tan, J.; Peng, C.; Zhang, F.; Zhang, W.; Jiang, X. Oxidative stress and detoxification mechanisms of earthworms (Eisenia fetida) after exposure to flupyradifurone in a soil-earthworm system. J. Environ. Manag. 2022, 322, 115989. [Google Scholar] [CrossRef]
- Cao, B.; Lv, H.; Nie, T.; Ma, Y.; Jiang, Z.; Hu, Y.; Yang, C.; Zhang, Y. Combined toxicity of acetochlor and metribuzin on earthworm Eisenia fetida: Survival, oxidative stress responses and joint effect. Appl. Soil. Ecol. 2022, 178, 104583. [Google Scholar] [CrossRef]
- Conesa, A.; Punt, P.J.; van den Hondel, C.A. Fungal peroxidases molecular aspects and applications. J. Biotechnol. 2002, 93, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Kumar, V.; Usmani, Z.; Rani, R.; Chandra, A.; Gupta, V.K. A comparative evaluation towards the potential of Klebsiella sp. and Enterobacter sp. in plant growth promotion, oxidative stress tolerance and chromium uptake in Helianthus annuus (L.). J. Hazard. Mater. 2019, 377, 391–398. [Google Scholar] [CrossRef]
- Song, P.; Jiang, N.; Zhang, K.; Li, X.; Li, N.; Zhang, Y.; Wang, Q.; Wang, J. Ecotoxicological evaluation of zebrafish liver (Danio rerio) induced by dibutyl phthalate. J. Hazard. Mater. 2022, 425, 128027. [Google Scholar] [CrossRef]
- He, F.; Liu, R.; Tian, G.; Qi, Y.; Wang, T. Ecotoxicological evaluation of oxidative stress-mediated neurotoxic effects, genetic toxicity, behavioral disorders, and the corresponding mechanisms induced by fluorene-contaminated soil targeted to earthworm (Eisenia fetida) brain. Sci. Total. Environ. 2023, 871, 162014. [Google Scholar] [CrossRef]
- Hannam, M.L.; Bamber, S.D.; Galloway, T.S.; John Moody, A.; Jones, M.B. Effects of the model PAH phenanthrene on immune function and oxidative stress in the haemolymph of the temperate scallop Pecten maximus. Chemosphere 2010, 78, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Hockaday, W.C.; Masiello, C.A.; Alvarez, P.J.J. Earthworm avoidance of biochar can be mitigated by wetting. Soil. Biol. Biochem. 2011, 43, 1732–1737. [Google Scholar] [CrossRef]
- Hayes, J.D.; Strange, R.C. Glutathione S-Transferase Polymorphisms and Their Biological Consequences. Pharmacology 2000, 61, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Li, X.W.; Xiao, X.L.; Liang, Y.P.; Zhang, C.P.; Zhang, S.L.; Gu, Z.M.; Qi, Z.Q. Inhibition Effect of Alachlor on Glutathione S-transferase of Chironomus riparius (Diptera: Chironomidae). Asian J. Ecotoxicol. 2015, 2, 243–250. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Jiang, N.; Yao, X.; Wang, Q.; Lv, H.; Wang, C.; Wang, J. Evaluation of soil ecological health after exposure to environmentally relevant doses of Di (2-ethylhexyl) phthalate: Insights from toxicological studies of earthworms at different ecological niches. Environ. Pollut. 2023, 322, 121204. [Google Scholar] [CrossRef]
- Enayati, A.A.; Ranson, H.; Hemingway, J. Insect glutathione transferases and insecticide resistance. Insect Mol. Biol. 2005, 14, 3–8. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Z.; Li, X.; Song, P.; Wang, J. Effects of diisononyl phthalate exposure on the oxidative stress and gut microorganisms in earthworms (Eisenia fetida). Sci. Total. Environ. 2022, 822, 153563. [Google Scholar] [CrossRef]
- Chu, L.; Hou, X.; Song, X.; Zhao, X.; Hu, S.; Shen, G. Toxicity of ionic liquids against earthworms (Eisenia fetida). Sci. Total. Environ. 2023, 875, 162411. [Google Scholar] [CrossRef]
- Anjana Vaman, V.S.; Tinu, S.K.; Geetha, C.S.; Lissy, K.K.; Mohanan, P.V. Effect of fibrin glue on antioxidant defense mechanism, oxidative DNA damage and chromosomal aberrations. Toxicol. Mech. Methods 2013, 23, 500–508. [Google Scholar] [CrossRef]
- Di Minno, A.; Turnu, L.; Porro, B.; Squellerio, I.; Cavalca, V.; Tremoli, E.; Di Minno, M.N. 8-Hydroxy-2-deoxyguanosine levels and heart failure: A systematic review and meta-analysis of the literature. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 201–208. [Google Scholar] [CrossRef]
- Wen, S.; Liu, C.; Wang, Y.; Xue, Y.; Wang, X.; Wang, J.; Xia, X.; Kim, Y.M. Oxidative stress and DNA damage in earthworm (Eisenia fetida) induced by triflumezopyrim exposure. Chemosphere 2021, 264, 128499. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Wen, M.; Zhang, J.; Tang, Z.; Wang, C. Effect of phenanthrene on the biological characteristics of earthworm casts and their relationships with digestive and anti-oxidative systems. Ecotoxicol. Environ. Saf. 2020, 193, 110359. [Google Scholar] [CrossRef] [PubMed]
- Rong, H.; Wang, C.; Liu, H.; Zhang, M.; Yuan, Y.; Pu, Y.; Huang, J.; Yu, J. Biochemical Toxicity and Potential Detoxification Mechanisms in Earthworms Eisenia fetida Exposed to Sulfamethazine and Copper. Bull. Environ. Contam. Toxicol. 2020, 105, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Polge, C.; Jaquinod, M.; Holzer, F.; Bourguignon, J.; Walling, L.; Brouquisse, R. Evidence for the Existence in Arabidopsis thaliana of the Proteasome Proteolytic Pathway: Activation in response to cadmium. J. Biol. Chem. 2009, 284, 35412–35424. [Google Scholar] [CrossRef]
- Park, I.Y.; Cha, J.R.; Ok, S.M.; Shin, C.; Kim, J.S.; Kwak, H.J.; Yu, Y.S.; Kim, Y.K.; Medina, B.; Cho, S.J.; et al. A new earthworm cellulase and its possible role in the innate immunity. Dev. Comp. Immunol. 2017, 67, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Khalid, H.; Kashif Zahoor, M.; Riaz, D.; Arshad, M.; Yaqoob, R.; Ranian, K. Sewage Sludge-Induced Effect on Growth, Enzyme Inhibition, and Genotoxicity can be Ameliorated Using Wheat Straw and Biochar in Pheretima posthuma Earthworms. Front. Environ. Sci. 2022, 10, 888394. [Google Scholar] [CrossRef]
- Wang, K.; Pang, S.; Mu, X.; Qi, S.; Li, D.; Cui, F.; Wang, C. Biological response of earthworm, Eisenia fetida, to five neonicotinoid insecticides. Chemosphere 2015, 132, 120–126. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, G.; Yin, P.; Lv, Y.; Yuan, S.; Chen, J.; Wei, B.; Wang, C. Toxicological effects of soil contaminated with spirotetramat to the earthworm Eisenia fetida. Chemosphere 2015, 139, 138–145. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, B.; Wang, C. Ecotoxicological effects on the earthworm Eisenia fetida following exposure to soil contaminated with imidacloprid. Environ. Sci. Pollut. Res. Int. 2014, 21, 12345–12353. [Google Scholar] [CrossRef]
- Xiao, N.; Jing, B.; Ge, F.; Liu, X. The fate of herbicide acetochlor and its toxicity to Eisenia fetida under laboratory conditions. Chemosphere 2006, 62, 1366–1373. [Google Scholar] [CrossRef]
- Luo, Y.R.; Wang, S.H.; Yun, M.X.; Li, X.Y.; Wang, J.J.; Sun, Z.J. The toxic effects of ionic liquids on the activities of acetylcholinesterase and cellulase in earthworms. Chemosphere 2009, 77, 313–318. [Google Scholar] [CrossRef]
- Beaumelle, L.; Hedde, M.; Vandenbulcke, F.; Lamy, I. Relationships between metal compartmentalization and biomarkers in earthworms exposed to field-contaminated soils. Environ. Pollut. 2017, 224, 185–194. [Google Scholar] [CrossRef]
- Jiang, N.; Song, P.; Li, X.; Zhu, L.; Wang, J.; Yin, X.; Wang, J. Dibutyl phthalate induced oxidative stress and genotoxicity on adult zebrafish (Danio rerio) brain. J. Hazard. Mater. 2022, 424, 127749. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Li, G.; Liu, M.; Li, Y.; Yin, S.; Zhao, J.; Zhang, X. Evaluation of DNA damage and antioxidant system induced by di-n-butyl phthalates exposure in earthworms (Eisenia fetida). Ecotoxicol. Environ. Saf. 2015, 115, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Y.; Wen, B.; Zhang, S.; Shan, X.Q. Bioavailability of phthalate congeners to earthworms (Eisenia fetida) in artificially contaminated soils. Ecotoxicol. Environ. Saf. 2005, 62, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Pu, Q.; Wang, H.T.; Pan, T.; Li, H.; Su, J.Q. Enhanced removal of ciprofloxacin and reduction of antibiotic resistance genes by earthworm Metaphire vulgaris in soil. Sci. Total. Environ. 2020, 742, 140409. [Google Scholar] [CrossRef]


| Treatments | Day 7 | Day 14 | Day 28 | |
|---|---|---|---|---|
| CK | CK 1 | 0 | 0 | 1 |
| CK 2 | 0 | 2 | 2 | |
| CK 3 | 1 | 1 | 1 | |
| ENR | ENR 1 | 0 | 2 | 2 |
| ENR 2 | 0 | 2 | 2 | |
| ENR 3 | 0 | 1 | 1 | |
| DEHP | DEHP 1 | 1 | 2 | 2 |
| DEHP 2 | 2 | 2 | 3 | |
| DEHP 3 | 0 | 0 | 1 | |
| ENR + DEHP | ENR + DEHP 1 | 0 | 2 | 2 |
| ENR + DEHP 2 | 0 | 0 | 2 | |
| ENR + DEHP 3 | 0 | 1 | 3 |
| Time | ROS | MDA | GST | SOD | POD | CAT | 8-OHDG | CL | NP | AKP | ACP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | 1 | |||||||||||
| ROS | −0.073 | 1 | ||||||||||
| MDA | 0.682 ** | −0.106 | 1 | |||||||||
| GST | −0.548 ** | 0.344 * | −0.717 ** | 1 | ||||||||
| SOD | −0.553 ** | 0.154 | −0.466 ** | 0.434 ** | 1 | |||||||
| POD | 0.165 | −0.008 | 0.542 ** | −0.162 | −0.115 | 1 | ||||||
| CAT | 0.718 ** | 0.133 | 0.741 ** | −0.479 ** | −0.192 | 0.689 ** | 1 | |||||
| 8-OHDG | 0.354 * | −0.207 | 0.392 * | −0.389 * | −0.029 | −0.111 | 0.188 | 1 | ||||
| CL | −0.361 * | −0.129 | −0.534 ** | 0.405 * | 0.552 ** | −0.343 * | −0.406 * | 0.111 | 1 | |||
| NP | 0.369 * | −0.345 * | 0.321 | −0.215 | −0.123 | −0.262 | −0.047 | 0.721 ** | 0.203 | 1 | ||
| AKP | −0.239 | 0.168 | −0.326 | 0.110 | 0.331 * | −0.200 | −0.162 | 0.148 | 0.566 ** | 0.067 | 1 | |
| ACP | −0.871 ** | 0.219 | −0.645 ** | 0.493 ** | 0.535 ** | −0.257 | −0.663 ** | −0.207 | 0.514 ** | −0.244 | 0.590 ** | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Sun, Q.; Liu, Y.-L.; Xiong, W.-J.; Zeng, S.-H.; Zhang, Y.; Li, Y.; Xu, H.-J. Ecotoxicological Evaluation of Earthworm (Eisenia fetida) Induced by Enrofloxacin and Di-(2-Ethylhexyl) Phthalate. Agronomy 2023, 13, 1777. https://doi.org/10.3390/agronomy13071777
Gao J, Sun Q, Liu Y-L, Xiong W-J, Zeng S-H, Zhang Y, Li Y, Xu H-J. Ecotoxicological Evaluation of Earthworm (Eisenia fetida) Induced by Enrofloxacin and Di-(2-Ethylhexyl) Phthalate. Agronomy. 2023; 13(7):1777. https://doi.org/10.3390/agronomy13071777
Chicago/Turabian StyleGao, Jianpeng, Qinghong Sun, Yuan-Liang Liu, Wei-Jie Xiong, Si-Han Zeng, Yulong Zhang, Yongtao Li, and Hui-Juan Xu. 2023. "Ecotoxicological Evaluation of Earthworm (Eisenia fetida) Induced by Enrofloxacin and Di-(2-Ethylhexyl) Phthalate" Agronomy 13, no. 7: 1777. https://doi.org/10.3390/agronomy13071777
APA StyleGao, J., Sun, Q., Liu, Y.-L., Xiong, W.-J., Zeng, S.-H., Zhang, Y., Li, Y., & Xu, H.-J. (2023). Ecotoxicological Evaluation of Earthworm (Eisenia fetida) Induced by Enrofloxacin and Di-(2-Ethylhexyl) Phthalate. Agronomy, 13(7), 1777. https://doi.org/10.3390/agronomy13071777







