Influence of Bio-Fertilizer Type and Amount Jointly on Microbial Community Composition, Crop Production and Soil Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Descriptions and Experimental Design
2.2. Soil Sampling and Analysis of Chemical Properties and Microbial Biomass
2.3. Analysis of Soil Bacterial Community Structure and Diversity
2.4. Real Time Quantitative PCR (RT-qPCR) Analyses [18]
2.5. Bioinformatic Analysis [18]
2.6. Soil Health Index
2.7. Statistical Analysis
3. Results
3.1. Soil Health Indicators
3.1.1. Soil Enzymatic Activities and Microbial Biomass
3.1.2. Bacterial 16S rRNA Gene Copy Numbers
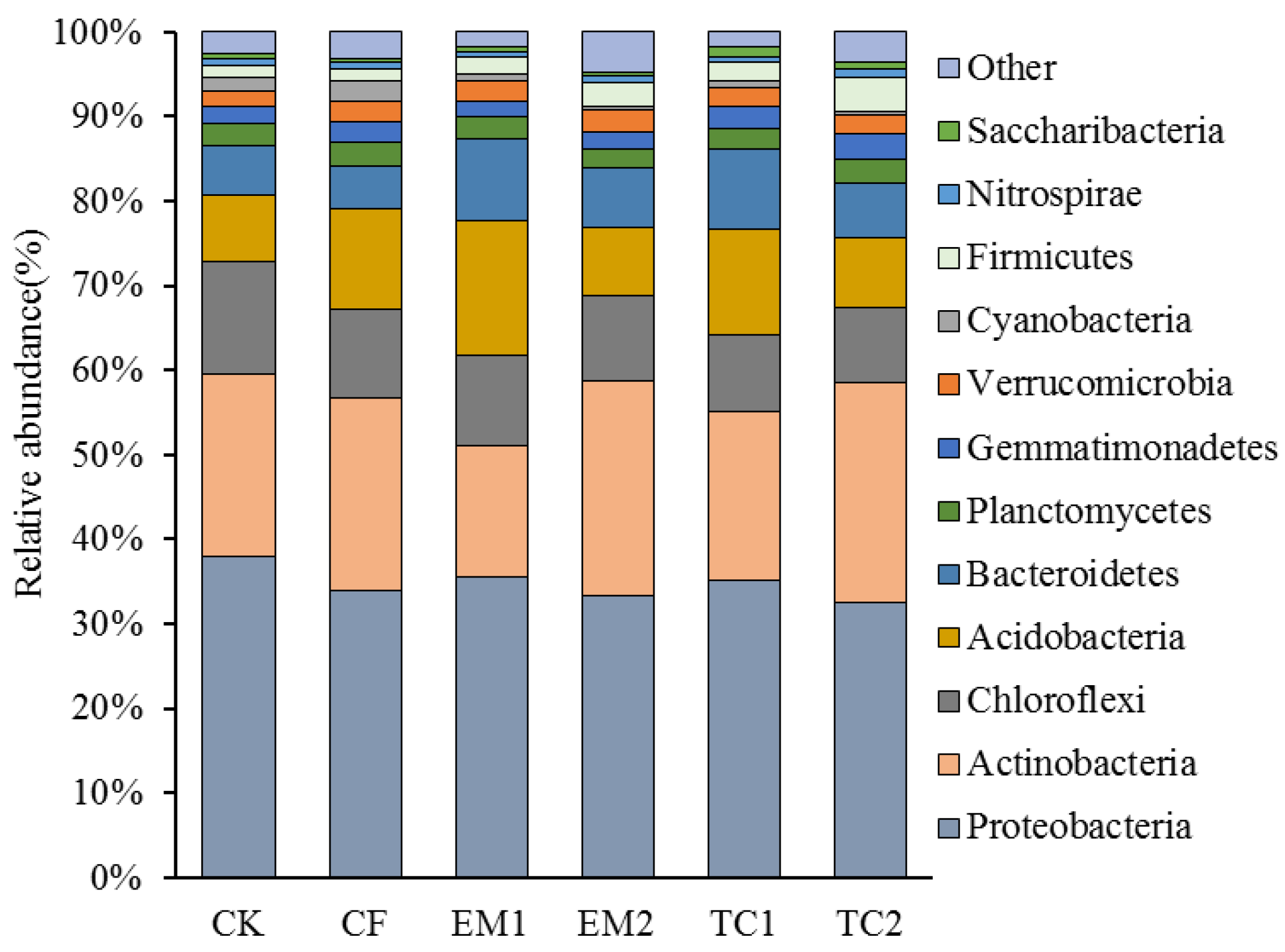
3.1.3. Bacterial Community Composition
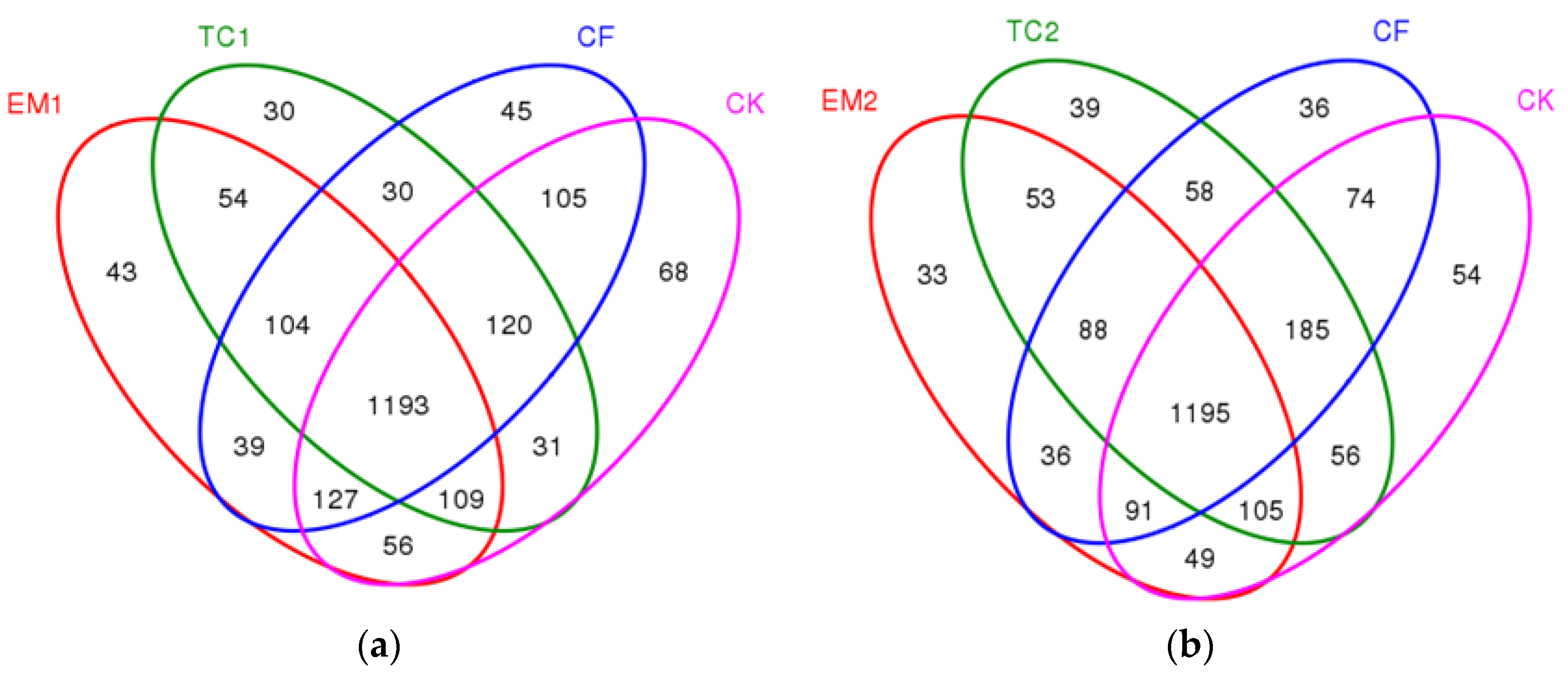
3.1.4. The Venn Diagram of the Different Long-Term Fertilizer Application
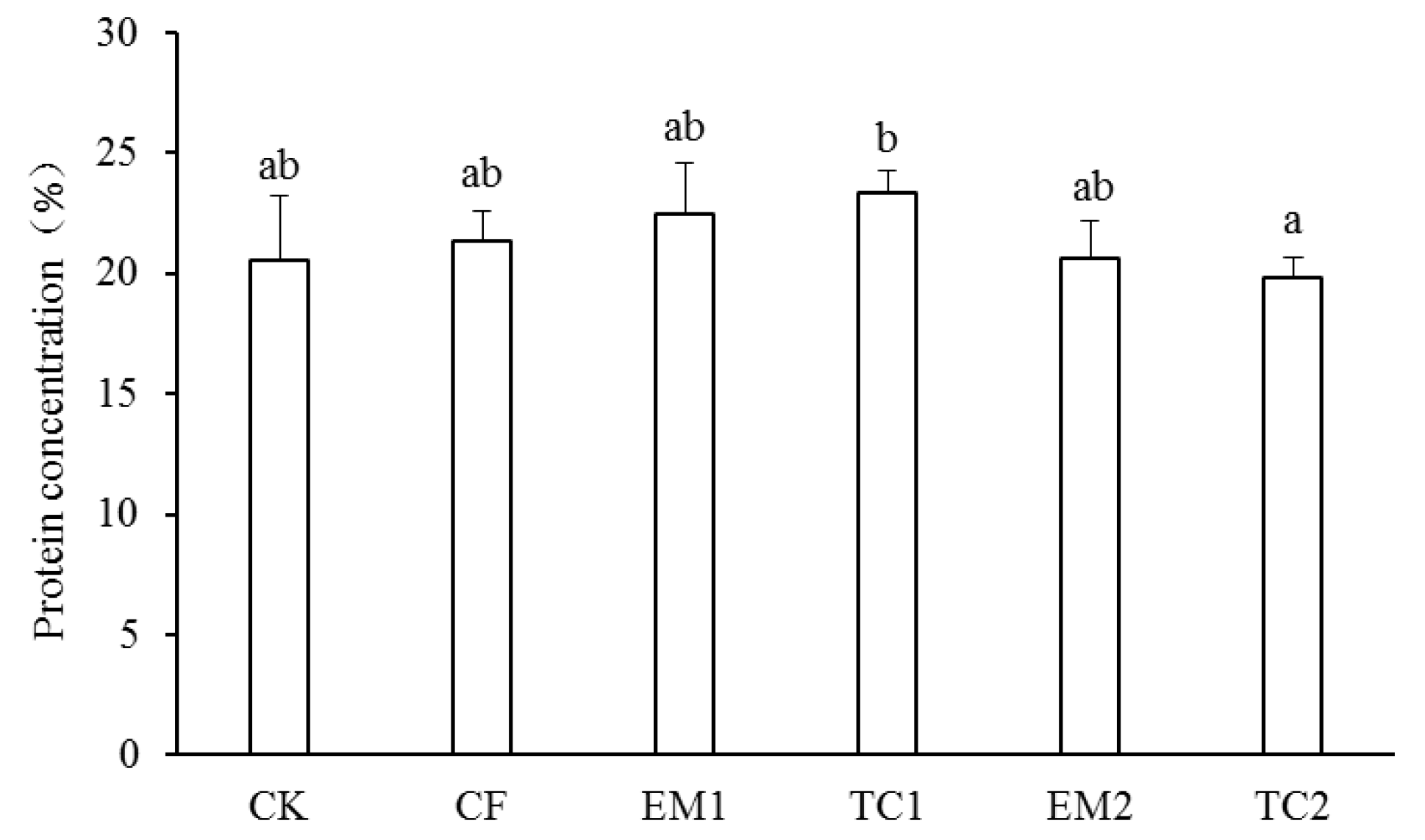
3.2. Grain Quality and Crop Yield
3.3. Soil Health Index (SHI) of Different Fertilizer Treatments
4. Discussion
4.1. Changes in Soil Microbial Biomasses and Enzymatic Activities along the Long-Term Fertilization
4.2. Changes in Bacterial Community Composition along the Long-Term Bio-Fertilizer
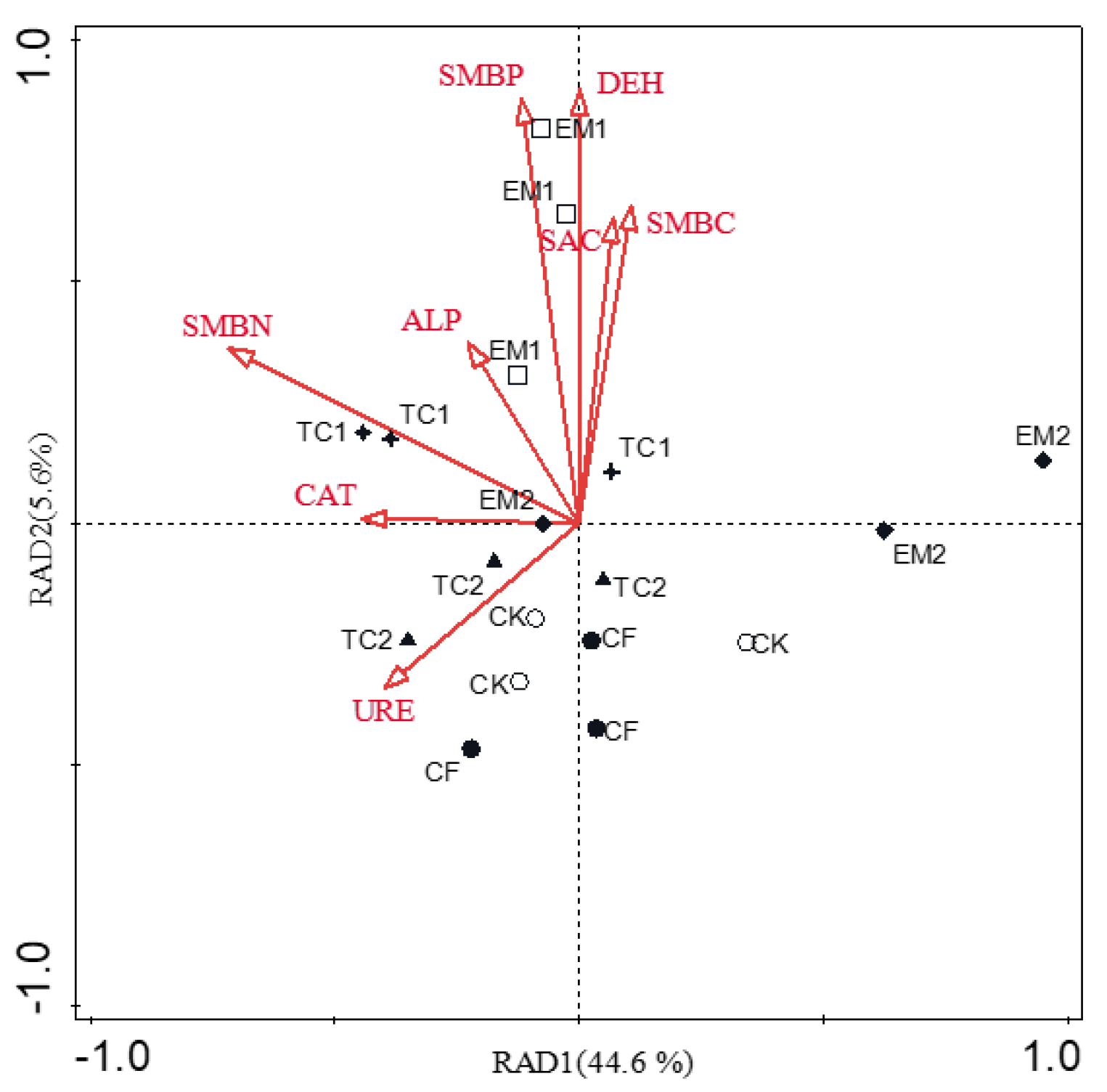
4.3. Relationship between Bacterial Communities and Environmental Variables
4.4. Relationship of the Bio-Fertilizer Application with Soil Health
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Xue, C.; Ryan, P.C.; Zhu, C.; Chen, H.; Duan, Y.; Peng, C.; Guo, S.; Ling, N.; Shen, Q. Alterations in soil fungal community composition and network assemblage structure by different long-term fertilization regimes are correlated to the soil ionome. Biol. Fertil. Soils 2018, 54, 95–106. [Google Scholar] [CrossRef]
- Huang, X.Q.; Liu, L.L.; Wen, T.; Zhu, R.; Zhang, J.B.; Cai, Z.C. Illumina MiSeq investigations on the changes of microbial community in the Fusarium oxysporum f.sp. cubense infected soil during and after reductive soil disinfestation. Microbiol. Res. 2015, 181, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.; El-Hawwary, A.; Kim, S.Y.; Meima-Franke, M.; Bodelier, P. Manure-associated stimulation of soil-borne methanogenic activity in agricultural soils. Biol. Fertil. Soils 2015, 51, 511–516. [Google Scholar] [CrossRef]
- Neufeld, K.R.; Grayston, S.J.; Bittman, S.; Krzic, M.; Hunt, D.E.; Smukler, S.M. Long-term alternative dairy manure management approaches enhance microbial biomass and activity in perennial forage grass. Biol. Fertil. Soils 2017, 53, 613–626. [Google Scholar] [CrossRef]
- Liu, X.Y.; Shi, Y.; Kong, L.; Tong, L.H.; Cao, H.; Zhou, H.; Lv, Y.Z. Long-term application of bio-compost increased soil microbial community diversity and altered its composition and network. Microorganisms 2022, 10, 462. [Google Scholar] [CrossRef]
- Calleja-Cervantes, M.E.; Fernández-González, A.J.; Irigoyen, I.; Fernández-López, M.; Aparicio-Tejo, P.M.; Menéndez, S. Thirteen years of continued application of composted organic wastes in a vineyard modify soil quality characteristics. Soil. Biol. Biochem. 2015, 90, 241–254. [Google Scholar] [CrossRef]
- Francioli, D.; Schulz, E.; Lentendu, G.; Wubet, T.; Buscot, F.; Reitz, T. Mineral vs. organic amendments: Microbial community structure, activity and abundance of agriculturally relevant microbes are driven by long-term fertilization strategies. Front. Microbiol. 2016, 7, 1446. [Google Scholar] [CrossRef]
- Zhong, W.; Gu, T.; Wang, W.; Zhang, B.; Lin, X.; Huang, Q.; Shen, W. The effects of mineral fertilizer and organic manure on soil microbial community and diversity. Plant Soil 2010, 326, 511–522. [Google Scholar] [CrossRef]
- Sinha, R.K.; Valani, D.; Chauhan, K.; Agarwal, S. Embarking on a second green revolution for sustainable agriculture by vermiculture biotechnology using earthworms: Reviving the dreams of Sir Charles Darwin. J. Agric. Saf. Health 2014, 1, 50–64. [Google Scholar] [CrossRef]
- Shi, Y.; Qiu, L.; Guo, L.; Man, J.; Shang, B.; Pu, R.; Ou, X.; Dai, C.; Liu, P.; Yang, Y.; et al. K Fertilizers Reduce the Accumulation of Cd in Panax notoginseng (Burk.) F.H. by improving the quality of the microbial community. Front. Plant Sci. 2020, 11, 888. [Google Scholar] [CrossRef]
- Wang, B.; Li, R.; Ruan, Y.; Ou, Y.; Zhao, Y.; Shen, Q. Pineapple-banana rotation reduced the amount of Fusarium oxysporum more than maize–banana rotation mainly through modulating fungal communities. Soil Biol. Biochem. 2015, 86, 77–86. [Google Scholar] [CrossRef]
- Tian, W.; Wang, L.; Li, Y.; Zhuang, K.M.; Li, G.; Zhang, J.B.; Xiao, X.J.; Xi, Y.G. Responses of microbial activity, abundance, and community in wheat soil after three years of heavy fertilization with manure-based compost and inorganic nitrogen. Agric. Ecosyst. Environ. 2015, 213, 219–227. [Google Scholar] [CrossRef]
- Kracmarova, M.; Uhlik, O.; Strejcek, M.; Szakova, J.; Cerny, J.; Balik, J.; Tlustos, P.; Kohout, P.; Demnerova, K.; Stiborova, H. Soil microbial communities following 20 years of fertilization and crop rotation practices in the Czech Republic. Environ. Microbiome 2022, 17, 13. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Lockwood, P.; Daniel, H.; King, K.; Hulugalle, N.; Kristiansen, P. Short-term effects of organic amendments on properties of a Vertisol. Waste Manag. Res. 2010, 28, 1087–1095. [Google Scholar] [CrossRef]
- Kersey, J.; Myrold, D. Response of soil health indicators to organic matter removal and compaction manipulations at six LTSP sites in the Western US. For. Ecol. Manag. 2021, 490, 119104. [Google Scholar] [CrossRef]
- Purakayastha, T.J.; Pathak, H.; Kumari, S.; Biswas, S.; Chakrabarty, B.; Padaria, R.N.; Kamble, K.; Pandey, M.; Sasmal, S.; Singh, A. Soil health card development for efficient soil management in Haryana, India. Soil Tillage Res. 2019, 191, 294–305. [Google Scholar] [CrossRef]
- Chae, Y.; Cui, R.; Woong Kim, S.; An, G.; Jeong, S.; An, Y. Exoenzyme activity in contaminated soils before and after soil washing: ß-glucosidase activity as a biological indicator of soil health. Ecotoxicol. Environ. Saf. 2017, 135, 368–374. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Xue, R.; Wang, C.; Liu, M.; Zhang, D.; Li, K.; Li, N. A new method for soil health assessment based on Analytic Hierarchy Process and meta-analysis. Sci. Total. Environ. 2019, 650, 2771–2777. [Google Scholar] [CrossRef]
- Jusoh, A.; Che, M.L.; Manaf, L.A.; Latiff, P.A. Composting of rice straw with effective microorganisms (EM) and its influence on compost quality. J. Environ. Health Sci. 2013, 10, 17. [Google Scholar] [CrossRef]
- Khaliq, A.; Abbasi, M.K.; Hussain, T. Effects of integrated use of organic and inorganic nutrient sources with effective microorganisms (EM) on seed cotton yield in Pakistan. Bioresour. Technol. 2006, 97, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yuan, L.; Liu, Y.; Ji, J.; Hou, H. Long-term application of manures plus chemical fertilizers sustained high rice yield and improved soil chemical and bacterial properties. Eur. J. Agron. 2017, 90, 34–42. [Google Scholar] [CrossRef]
- Zhao, J.; Ni, T.; Li, J.; Lu, Q.; Fang, Z.; Huang, Q.; Zhang, R.; Li, R.; Shen, B.; Shen, Q. Effects of organic–inorganic compound fertilizer with reduced chemical fertilizer application on crop yields, soil biological activity and bacterial community structure in a rice–wheat cropping system. Appl. Soil Ecol. 2016, 99, 1–12. [Google Scholar] [CrossRef]
- Islam, M.R.; Singh, C.P.; Kim, Y.; Kim, M.; Sa, T. Community level functional diversity and enzyme activities in paddy soils under different long-term fertilizer management practices. Biol. Fertil. Soils 2011, 47, 599–604. [Google Scholar] [CrossRef]
- Hu, W.; Jiao, Z.; Wu, F.; Liu, Y.; Dong, M.; Ma, X. Long-term effects of fertilizer on soil enzymatic activity of wheat field soil in loess plateau, China. Ecotoxicology 2014, 23, 2069–2080. [Google Scholar] [CrossRef] [PubMed]
- Nayak, D.R.; Babu, Y.J.; Adhya, T.K. Long-term application of compost influences microbial biomass and enzyme activities in a tropical Aeric Endoaquept planted to rice under flooded condition. Soil Biol. Biochem. 2007, 39, 1897–1906. [Google Scholar] [CrossRef]
- Dong, W.Y.; Zhang, X.Y.; Liu, X.Y.; Fu, X.L.; Chen, F.S.; Wang, H.M.; Sun, X.M.; Wen, X.F. Responses of soil microbial communities and enzyme activities to nitrogen and phosphorus additions in Chinese fir plantations of subtropical China. Biogeosciences 2015, 12, 5537–5546. [Google Scholar] [CrossRef]
- Mandal, A.; Patra, A.K.; Singh, D.; Swarup, A.; Masto, R.E. Effect of long-term application of manure and fertilizer on biological and biochemical activities in soil during crop development stages. Bioresour. Technol. 2007, 98, 3585–3592. [Google Scholar] [CrossRef]
- Liang, Q.; Chen, H.; Gong, Y.; Yang, H.; Kuzyakov, Y. Effects of 15 years of manure and mineral fertilizers on enzyme activities in particle-size fractions in a north china plain soil. Eur. J. Soil. Biol. 2014, 60, 112–119. [Google Scholar] [CrossRef]
- Parthasarathi, K.; Ranganathan, L.S. Aging effect on enzyme activities in pressmud vermicasts of Lampito mauritii (kinberg) and Eudrilus eugeniae (kinberg). Biol. Fertil. Soils 2000, 30, 347–350. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms—A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Leff, J.W.; Jones, S.E.; Prober, S.M.; Barberán, A.; Borer, E.T.; Firn, J.L.; Harpole, W.S.; Hobbie, S.E.; Hofmockel, K.S.; Knops, J.M.H. Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proc. Natl. Acad. Sci. USA 2015, 112, 10967–10972. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, G.; De Filippis, F.D.; Cesarano, G.; Storia, A.L.; Ercolini, D.; Scala, F. Organic farming induces changes in soil microbiota that affect agro-ecosystem functions. Soil Biol. Biochem. 2016, 103, 327–336. [Google Scholar] [CrossRef]
- Li, F.; Chen, L.; Zhang, J.; Yin, J.; Huang, S. Bacterial community structure after long-term organic and inorganic fertilization reveals important associations between soil nutrients and specific taxa involved in nutrient transformations. Front. Microbiol. 2017, 8, 187. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, X.; Guo, X.; Wang, D.; Chu, H. Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw. Soil Biol. Biochem. 2015, 88, 9–18. [Google Scholar] [CrossRef]
- Zhou, J.; Guan, D.; Zhou, B.; Zhao, B.; Ma, M.C.; Qin, J.; Jiang, X.; Chen, S.F. Influence of 34-years of fertilization on bacterial communities in an intensively cultivated black soil in northeast China. Soil Biol. Biochem. 2015, 90, 42–51. [Google Scholar] [CrossRef]
- Cai, F.; Pang, G.; Li, R.; Li, R.; Gu, X.; Shen, Q.; Chen, W. Bioorganic fertilizer maintains a more stable soil microbiome than chemical fertilizer for monocropping. Biol. Fertil. Soils 2017, 53, 861–872. [Google Scholar] [CrossRef]
- Ryckeboer, J.R.; Mergaert, J.; Vaes, K.; Klammer, S.; Clercq, D.D.; Coosemans, J.; Insam, H.; Swings, J. A survey of bacteria and fungi occurring during composting and self-heating processes. Ann. Microbiol. 2003, 53, 349–410. [Google Scholar] [CrossRef]
- Hartmann, M.; Frey, B.; Mayer, J.; Mader, P.; Widmer, F. Distinct soil microbial diversity under long-term organic and conventional farming. ISME J. 2015, 9, 1177–1194. [Google Scholar] [CrossRef]
- Zhang, W.; Qiao, W.; Gao, D.; Dai, Y.; Deng, J.; Yang, G.; Han, X.; Ren, G. Relationship between soil nutrient properties and biological activities along a restoration chronosequence of pinus tabulaeformis plantation forests in the Ziwuling mountains, China. Catena 2018, 161, 85–95. [Google Scholar] [CrossRef]
- Wang, R.; Liu, T.; Lu, C.; Zhang, Z.; Guo, P.; Jia, B.; Hao, B.; Wang, Y.; Guo, W. Bioorganic fertilizers improve the adaptability and remediation efficiency of Puccinellia distans in multiple heavy metals-contaminated saline soil by regulating the soil microbial community. J. Hazard. Mater. 2023, 448, 130982. [Google Scholar] [CrossRef] [PubMed]
- Segura, C.; Horrocks, C.; Lopez-Aizpun, M.; Blackwell, M.S.A.; Darch, T.; Hood, J.; Le Cocq, K.; McAuliffe, G.A.; Lee, M.R.F.; Cardenas, L. Response of soil health indicators to dung, urine, and mineral fertilizer application in temperate pastures. J. Environ. Manag. 2023, 330, 117096. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, J.; Manuel, D.; Op De Beeck, M.; Shahbaz, M.; Chen, Y.; Deng, X.; Xu, Z.; Li, J.; Liu, Z. Rotation cropping and organic fertilizer jointly promote soil health and crop production. J. Environ. Manag. 2022, 315, 115190. [Google Scholar] [CrossRef] [PubMed]

| pH | SOC (g/kg) | TN (g/kg) | TP (g/kg) | TK (g/kg) | |
|---|---|---|---|---|---|
| CK | 7.59 | 6.91 ± 0.3 a | 0.48 ± 0.02 ab | 1.03 ± 0.12 b | 228.87 ± 1.5 b |
| EM1 | 7.58 | 13.51 ± 0.6 c | 0.74 ± 0.1 a | 1.74 ± 0.08 a | 247.03 ± 1.8 a |
| TC1 | 7.58 | 10.33 ± 0.5 b | 0.72 ± 0.03 c | 1.65 ± 0.03 c | 253.2 ± 0.7 ab |
| EM2 | 7.54 | 9.73 ± 0.9 ab | 0.56 ± 0.01 c | 1.28 ± 0.07 c | 245.3 ± 4.2 c |
| TC2 | 7.50 | 8.34 ± 1.0 ab | 0.61 ± 0.01 ab | 1.25 ± 0.04 ab | 244.8 ± 7.2 bc |
| CF | 7.52 | 7.97 ± 1.6 ab | 0.54 ± 0.02 b | 1.32 ± 0.12 ab | 240.57 ± 1.0 bc |
| Enzymatic Activity * | EM1 | TC1 | EM2 | TC2 | CF | CK |
|---|---|---|---|---|---|---|
| ALP | 5.95 ± 0.2 ab | 6.41 ± 0.2 b | 5.47 ± 0.3 ab | 6.03 ± 0.1 ab | 5.14 ± 0.7 a | 5.04 ± 0.3 a |
| SAC | 50.99 ± 6.3 b | 52.85 ± 6.5 b | 44.20 ± 6.8 b | 45.09 ± 1.6 b | 35.93 ± 5.4 ab | 23.35 ± 3.6 a |
| DEH | 19.68 ± 0.5 a | 14.77 ± 0.6 ab | 14.98 ± 1.5 ab | 13.79 ± 6.2 ab | 10.51 ± 1.6 b | 14.29 ± 6.3 ab |
| URE | 2.05 ± 0.1 a | 2.10 ± 0.1 a | 2.0 ± 0.1 a | 2.43 ± 0.2 a | 2.42 ± 0.1 a | 1.99 ± 0.1 a |
| SMBC | 518.46 ± 19.4 a | 518.03 ± 4.8 a | 507.29 ± 25.0 a | 497.29 ± 24.7 a | 489.10 ± 8.8 a | 486.11 ± 24.3 a |
| SMBN | 49.41 ± 10.8 ab | 35.01 ± 8.7 a | 25.01 ± 1.9 bc | 28.55 ± 12.8 bc | 27.93 ± 3.2 bc | 18.10 ± 8.3 c |
| SMBP | 268.49 ± 39.5 a | 163.57 ± 8.0 a | 84.02 ± 8.6 b | 24.70 ± 13.2 c | 14.82 ± 3.2 c | 9.80 ± 0.5 c |
| Treatment | Acre Yield (kg) | Growth Rate (%) | Fertilizer Yield Contribution Rate (%) |
|---|---|---|---|
| CK | 92.23 ± 10.4 e | / | / |
| EM1 | 455.3 ± 3.1 a | 393.7 | 79.7 |
| TC1 | 413.37 ± 1.0 b | 348.2 | 77.7 |
| EM2 | 395.03 ± 5.3 b | 328.3 | 76.7 |
| TC2 | 334.47 ± 9.8 c | 262.6 | 72.4 |
| CF | 306.23 ± 5.3 d | 232.0 | 69.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Tong, L.; Lv, Y. Influence of Bio-Fertilizer Type and Amount Jointly on Microbial Community Composition, Crop Production and Soil Health. Agronomy 2023, 13, 1775. https://doi.org/10.3390/agronomy13071775
Li L, Tong L, Lv Y. Influence of Bio-Fertilizer Type and Amount Jointly on Microbial Community Composition, Crop Production and Soil Health. Agronomy. 2023; 13(7):1775. https://doi.org/10.3390/agronomy13071775
Chicago/Turabian StyleLi, Lijun, Lihong Tong, and Yizhong Lv. 2023. "Influence of Bio-Fertilizer Type and Amount Jointly on Microbial Community Composition, Crop Production and Soil Health" Agronomy 13, no. 7: 1775. https://doi.org/10.3390/agronomy13071775
APA StyleLi, L., Tong, L., & Lv, Y. (2023). Influence of Bio-Fertilizer Type and Amount Jointly on Microbial Community Composition, Crop Production and Soil Health. Agronomy, 13(7), 1775. https://doi.org/10.3390/agronomy13071775





