Effect of Biosynthesized Nanoselenium on Controlling Tomato Root-Knot Nematode Meloidogyne incognita
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Bacterial and Nematode Strains, and Culture Conditions
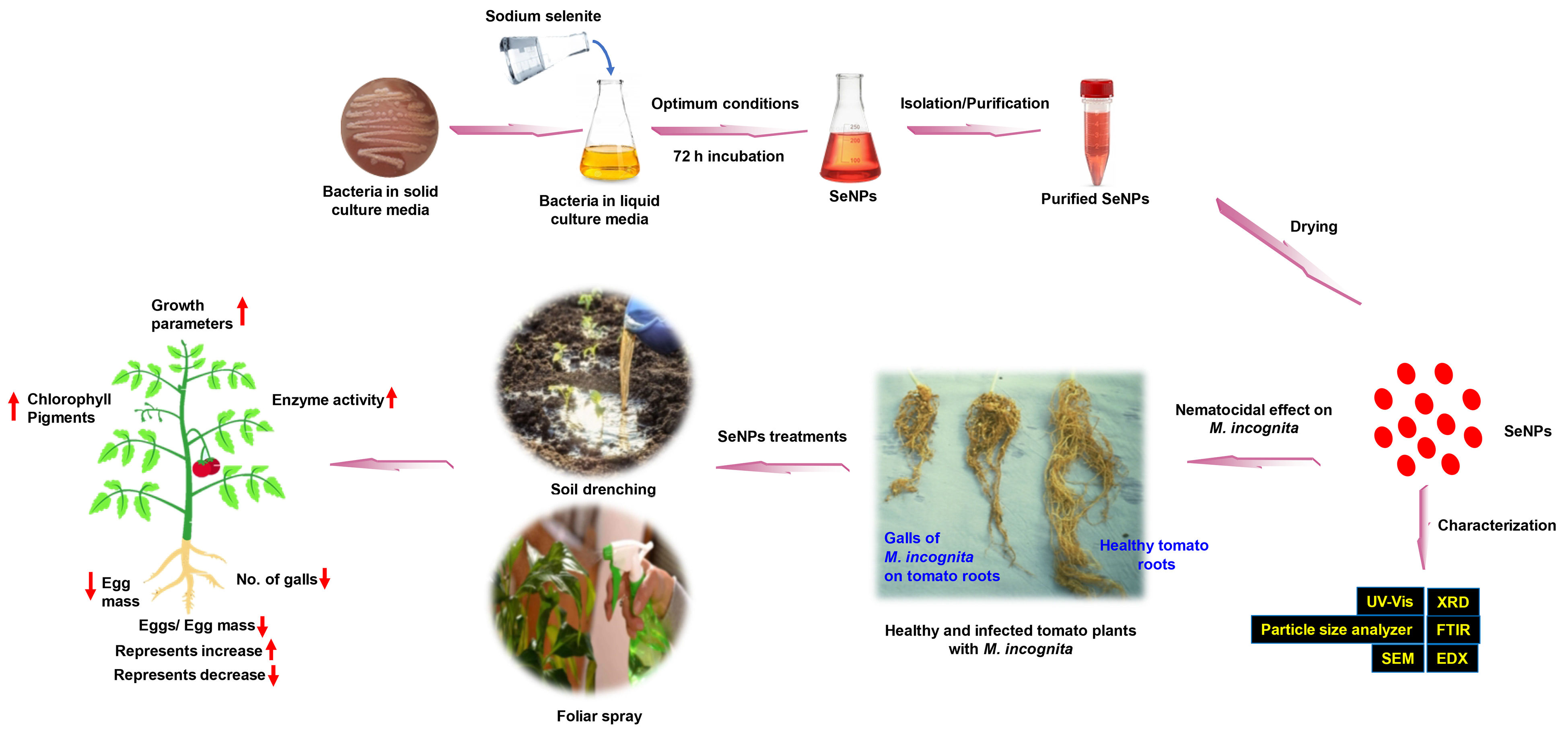
2.2. Biosynthesis and Purification of Selenium Nanoparticles Using P. stutzeri BB19
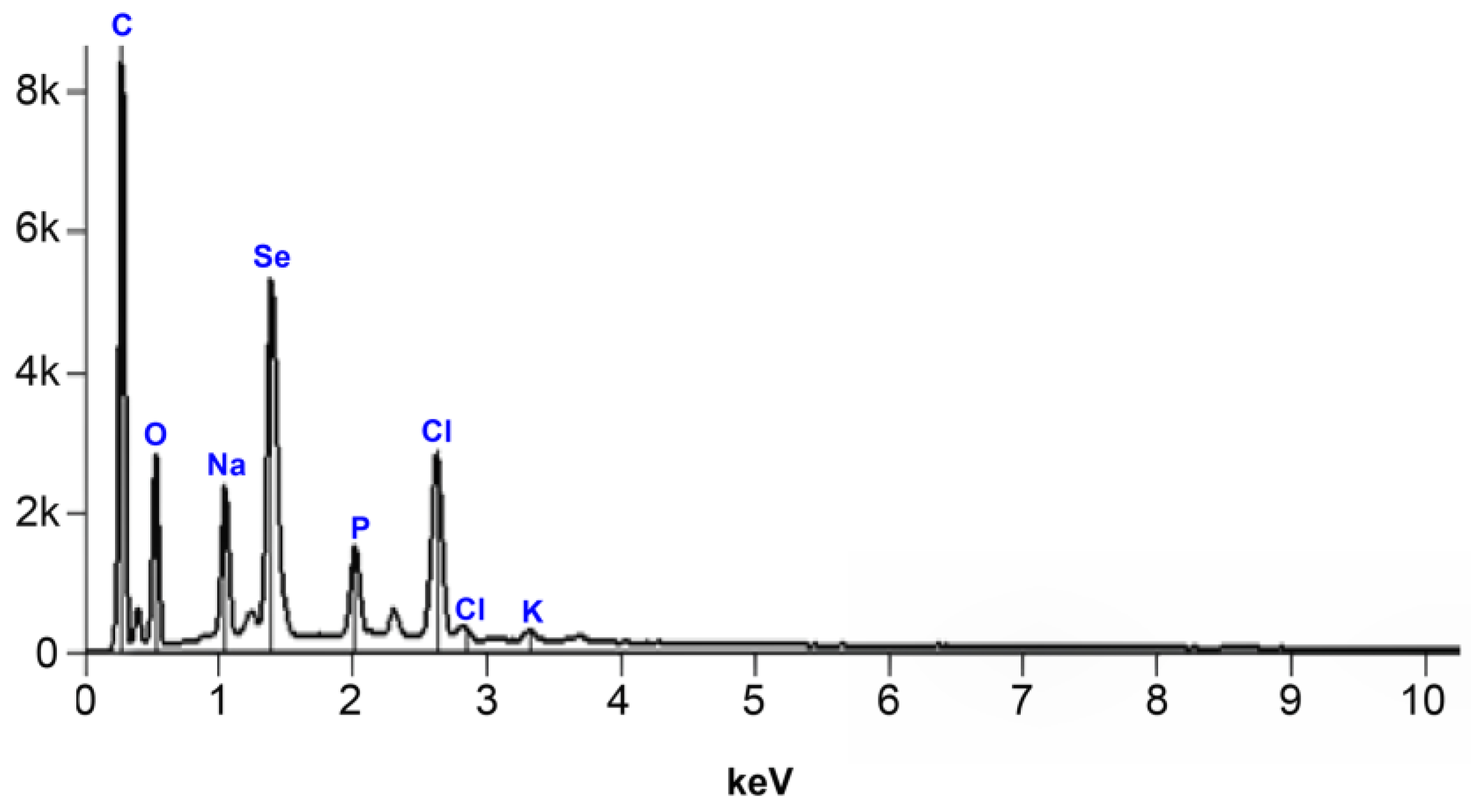
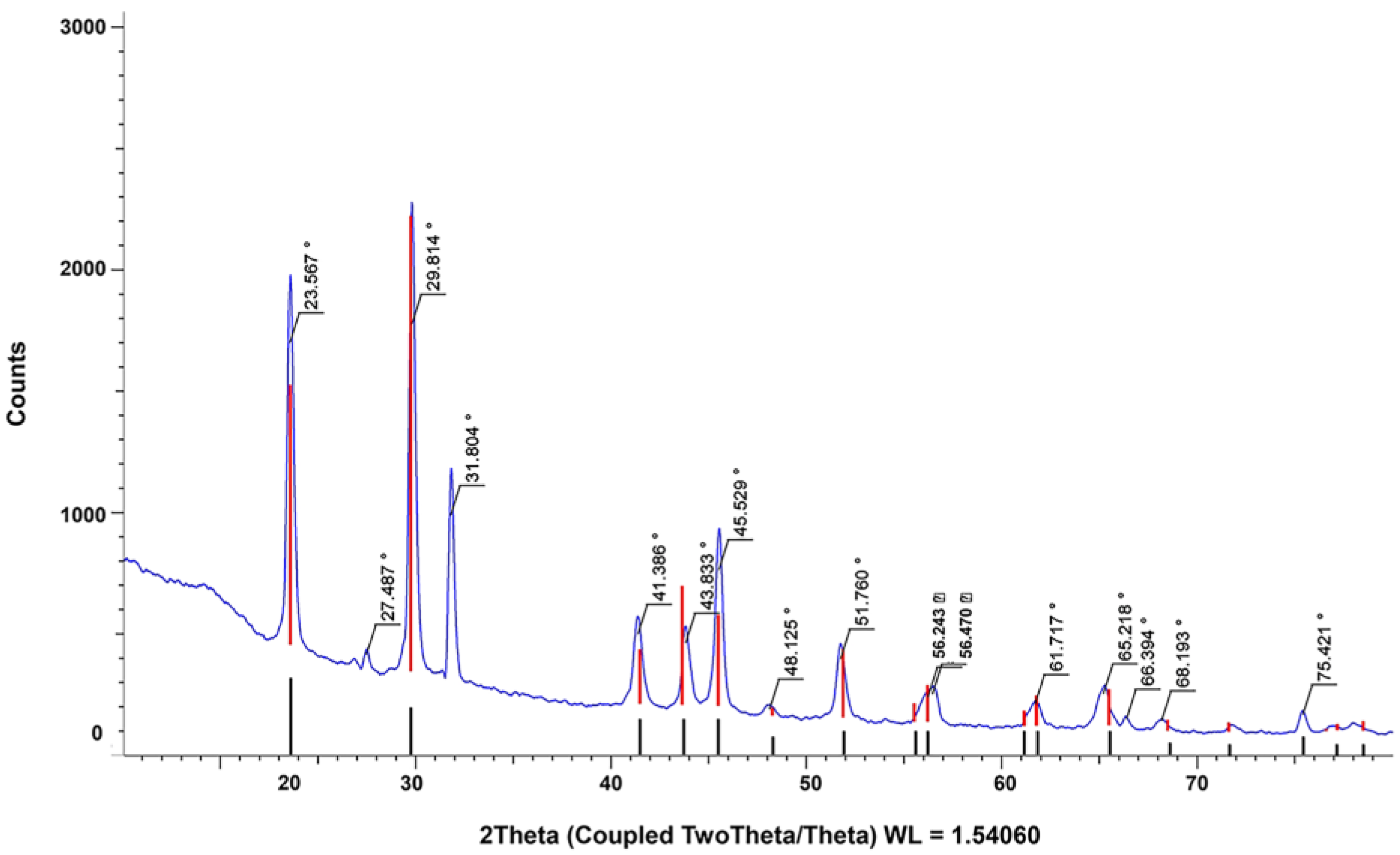
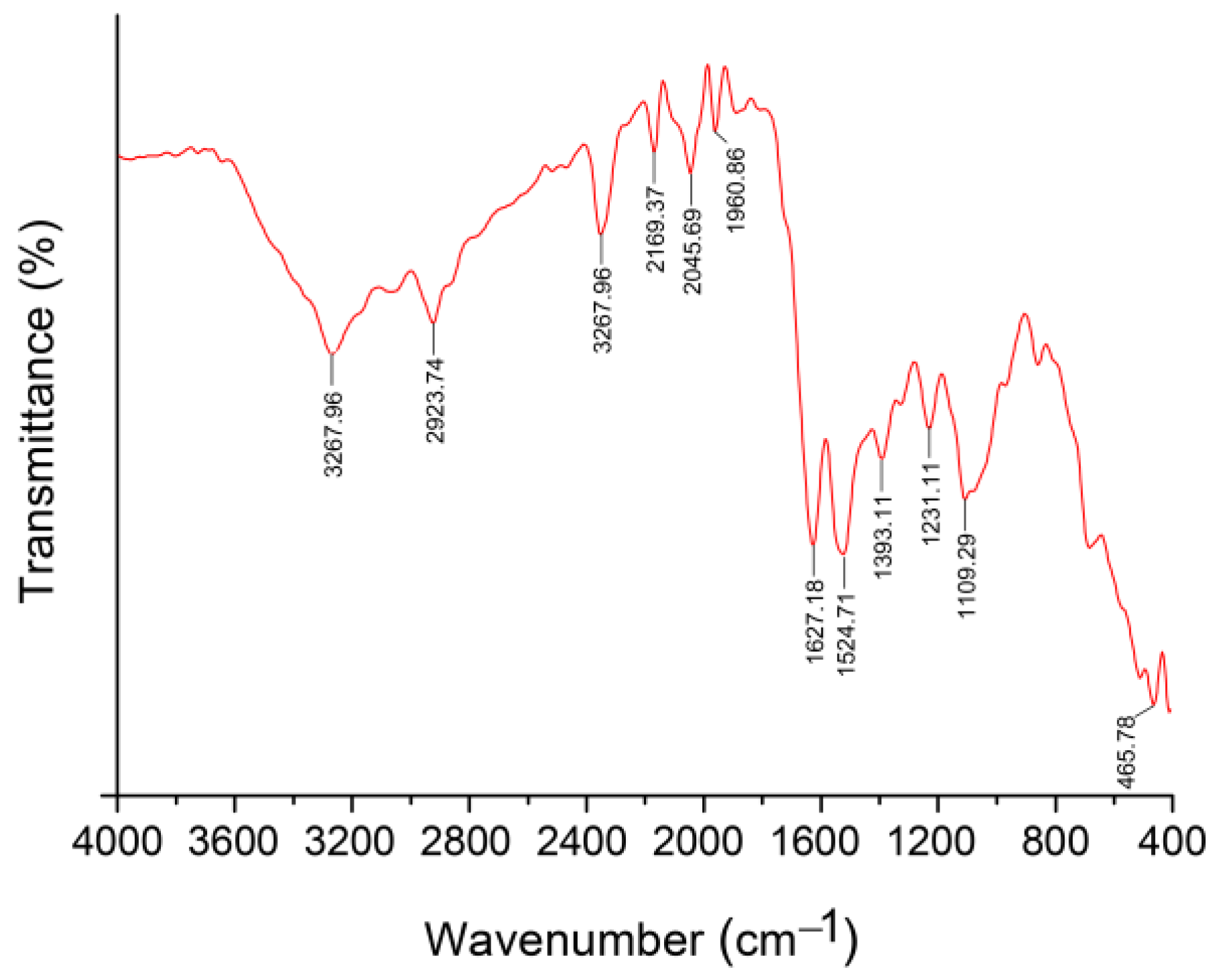
2.3. Characterization of Biosynthesized Selenium Nanoparticles
2.4. Effect of BioSeNPs on Tomato Plants Infected with M. incognita, Root Galling, and Egg Hatching of M. incognita (Greenhouse Experiments)
2.5. Estimation of Growth Parameters and Photosynthetic Pigments
2.6. Assay of Antioxidant Enzymes Activity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Biosynthesis and Characterization of Selenium Nanoparticles Using P. stutzeri BB19
3.2. Effect of BioSeNPs Treatments on Tomato Plants Infected with M. incognita under Greenhouse Conditions
3.2.1. Effect of BioSeNPs on Growth Parameters of Tomato Plants
3.2.2. Effect of BioSeNPs on Photosynthetic Pigments of Leaves of Tomato Plants
3.2.3. Effect of BioSeNPs on Enzyme Activity of Leaves of Tomato Plants
3.2.4. Effect of BioSeNPs on Number of Galls and Egg Hatching of M. incognita-infected Tomato Plants
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dorais, M.; Ehret, D.L.; Papadopoulos, A.P. Tomato (Solanum lycopersicum) health components: From the seed to the consumer. Phytochem. Rev. 2008, 7, 231–250. [Google Scholar] [CrossRef]
- Blok, V.C.; Jones, J.T.; Phillips, M.S.; Trudgill, D.L. Parasitism genes and host range disparities in biotrophic nematodes: The conundrum of polyphagy versus specialisation. Bioessays 2008, 30, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Eves-van den Akker, S. Plant-nematode interactions. Curr. Opin. Plant Biol. 2021, 62, 102035. [Google Scholar] [CrossRef] [PubMed]
- Garita, S.A.; Bernardo, V.F.; Gonzalez, M.; Ripodas, J.I.; Arango, M.C.; Ruscitti, M. The false root-knot nematode: Modification of the root anatomy and alteration of the physiological performance in tomato plants. Rhizosphere 2021, 20, 100424. [Google Scholar] [CrossRef]
- Park, E.-J.; Jang, H.-J.; Park, J.-Y.; Yang, Y.K.; Kim, M.J.; Park, C.S.; Lee, S.; Yun, B.-S.; Lee, S.-J.; Lee, S.W.; et al. Efficacy evaluation of Streptomyces nigrescens KA-1 against the root-knot nematode Meloidogyne incognita. Biol. Control 2023, 179, 105150. [Google Scholar] [CrossRef]
- Qiao, K.; Liu, Q.; Zhang, S. Evaluation of fluazaindolizine, a new nematicide for management of Meloidogyne incognita in squash in calcareous soils. Crop Prot. 2021, 143, 105469. [Google Scholar] [CrossRef]
- Talavera, M.; Verdejo-Lucas, S.; Ornat, C.; Torres, J.; Vela, M.D.; Macias, F.J.; Cortada, L.; Arias, D.J.; Valero, J.; Sorribas, F.J. Crop rotations with Mi gene resistant and susceptible tomato cultivars for management of root-knot nematodes in plastic houses. Crop Prot. 2009, 28, 662–667. [Google Scholar] [CrossRef]
- D’Errico, G.; Giacometti, R.; Roversi, P.F.; d’Errico, F.P.; Woo, S.L. Mode of action and efficacy of iprodione against the root-knot nematode Meloidogyne incognita. Ann. Appl. Biol. 2017, 171, 506–510. [Google Scholar] [CrossRef]
- An, G.; Hong, T.; Park, H.; Lim, W.; Song, G. Oxamyl exerts developmental toxic effects in zebrafish by disrupting the mitochondrial electron transport chain and modulating PI3K/Akt and p38 Mapk signaling. Sci. Total Environ. 2023, 859, 160458. [Google Scholar] [CrossRef]
- Hanafi, A.; Dasenaki, M.; Bletsou, A.; Thomaidis, N.S. Dissipation rate study and pre-harvest intervals calculation of imidacloprid and oxamyl in exported Egyptian green beans and chili peppers after pestigation treatment. Food Chem. 2018, 240, 1047–1054. [Google Scholar] [CrossRef]
- Regmi, H.; Desaeger, J. Integrated management of root-knot nematode (Meloidogyne spp.) in Florida tomatoes combining host resistance and nematicides. Crop Prot. 2020, 134, 105170. [Google Scholar] [CrossRef]
- Wang, W.; Ling, Y.; Deng, L.; Yao, S.; Zeng, K. Effect of L-cysteine treatment to induce postharvest disease resistance of Monilinia fructicola in plum fruits and the possible mechanisms involved. Pestic. Biochem. Physiol. 2023, 191, 105367. [Google Scholar] [CrossRef]
- Tryfon, P.; Antonoglou, O.; Vourlias, G.; Mourdikoudis, S.; Menkissoglu-Spiroudi, U.; Dendrinou-Samara, C. Tailoring Ca-based nanoparticles by polyol process for use as nematicidals and pH adjusters in agriculture. ACS Appl. Nano Mater. 2019, 2, 3870–3881. [Google Scholar] [CrossRef]
- Salwan, R.; Sharma, M.; Sharma, A.; Sharma, V. Insights into plant beneficial microorganism-triggered induced systemic resistance. Plant Stress 2023, 7, 100140. [Google Scholar] [CrossRef]
- ElMekawy, A.; Hegab, H.M.; Alsafar, H.; Yousef, A.F.; Banat, F.; Hasan, S.W. Bacterial nanotechnology: The intersection impact of bacteriology and nanotechnology on the wastewater treatment sector. J. Environ. Chem. Eng. 2023, 11, 109212. [Google Scholar] [CrossRef]
- Huang, T.; Holden, J.A.; Heath, D.E.; O’Brien-Simpson, N.M.; O’Connor, A.J. Engineering highly effective antimicrobial selenium nanoparticles through control of particle size. Nanoscale 2019, 11, 14937–14951. [Google Scholar] [CrossRef]
- Luo, H.; Wang, F.; Bai, Y.; Chen, T.; Zheng, W. Selenium nanoparticles inhibit the growth of HeLa and MDA-MB-231 cells through induction of S phase arrest. Colloids Surf. B Biointerfaces 2012, 94, 304–308. [Google Scholar] [CrossRef]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Peng, Q.; Baron, M.; Melcova, M.; Opatrilova, R.; Zidkova, J.; et al. Nano-selenium and its nanomedicine applications: A critical review. Int. J. Nanomed. 2018, 13, 2107–2128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoeibi, S.; Mozdziak, P.; Golkar-Narenji, A. Biogenesis of selenium nanoparticles using green chemistry. Top. Curr. Chem. 2017, 375, 88. [Google Scholar] [CrossRef] [PubMed]
- Zare, B.; Babaie, S.; Setayesh, N.; Shahverdi, A.; Shahverdi, A. Isolation and characterization of a fungus for extracellular synthesis of small selenium nanoparticles extracellular synthesis of selenium nanoparticles using fungi. Nanomed. J. 2013, 1, 13–19. [Google Scholar]
- Oremland, R.S.; Herbel, M.J.; Blum, J.S.; Langley, S.; Beveridge, T.J.; Ajayan, P.M.; Sutto, T.; Ellis, A.V.; Curran, S. Structural and spectral features of selenium nanospheres produced by Se-respiring bacteria. Appl. Environ. Microbiol. 2004, 70, 52–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AbdelRazek, G.M.; Yaseen, R. Effect of some rhizosphere bacteria on root-knot nematodes. Egypt. J. Biol. Pest Control 2020, 30, 140. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Taylor, A.L.; Sasser, J.N. Biology, Identification and Control Ofroot-Knot Nematodes (Meloidogyne Species); North Carolina State University: Raleigh, NC, USA, 1978; p. 111, vii. [Google Scholar]
- Hussey, R.S.; Barker, K.R. A comparison of methods of collecting inocula of Meloidogyne species, including a new technique. Plant Dis. Rep. 1973, 42, 865–872. [Google Scholar]
- Borah, S.N.; Goswami, L.; Sen, S.; Sachan, D.; Sarma, H.; Montes, M.; Peralta-Videa, J.R.; Pakshirajan, K.; Narayan, M. Selenite bioreduction and biosynthesis of selenium nanoparticles by Bacillus paramycoides SP3 isolated from coal mine overburden leachate. Environ. Pollut. 2021, 285, 117519. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, C.C.; Knauft, D.A.; Dickson, D.W. A technique for screening peanut for resistance to Meloidogyne arenaria. Plant Dis. 1983, 67, 957–958. [Google Scholar] [CrossRef] [Green Version]
- Henderson, C.F.; Tilton, E.W. Tests with acaricides against the brown wheat mite12. J. Econ. Entomol. 1955, 48, 157–161. [Google Scholar] [CrossRef]
- Mackinney, G. Absorption of light by chlorophyll solutions. J. Biol. Chem. 1941, 140, 315–322. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Hegedüs, A.; Erdei, S.; Horváth, G. Comparative studies of H2O2 detoxifying enzymes in green and greening barley seedlings under cadmium stress. Plant Sci. 2001, 160, 1085–1093. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; González, L.; Tablada, M.; Robledo, Y.C. InfoStat Versión Estudiantil; Grupo Infostat, FCA, Universidad Nacional de Córdoba: Cordoba, Argentina, 2015. [Google Scholar]
- Ramamurthy, C.; Sampath, K.S.; Arunkumar, P.; Kumar, M.S.; Sujatha, V.; Premkumar, K.; Thirunavukkarasu, C. Green synthesis and characterization of selenium nanoparticles and its augmented cytotoxicity with doxorubicin on cancer cells. Bioprocess Biosyst. Eng. 2013, 36, 1131–1139. [Google Scholar] [CrossRef]
- Mulvaney, P. Surface plasmon spectroscopy of nanosized metal particles. Langmuir 1996, 12, 788–800. [Google Scholar] [CrossRef]
- Avendaño, R.; Chaves, N.; Fuentes, P.; Sánchez, E.; Jiménez, J.I.; Chavarría, M. Production of selenium nanoparticles in Pseudomonas putida KT2440. Sci. Rep. 2016, 6, 37155. [Google Scholar] [CrossRef] [Green Version]
- Kora, A.J.; Rastogi, L. Biomimetic synthesis of selenium nanoparticles by Pseudomonas aeruginosa ATCC 27853: An approach for conversion of selenite. J. Environ. Manag. 2016, 181, 231–236. [Google Scholar] [CrossRef]
- Rajkumar, K.; Mvs, S.; Koganti, S.; Burgula, S. Selenium nanoparticles synthesized using Pseudomonas stutzeri (MH191156) show antiproliferative and anti-angiogenic activity against cervical cancer cells. Int. J. Nanomed. 2020, 15, 4523–4540. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Z.; Liu, H.; Zhang, L.; Gao, P.; Li, D. Biosynthesis and structural characteristics of selenium nanoparticles by Pseudomonas alcaliphila. Colloids Surf. B Biointerfaces 2011, 88, 196–201. [Google Scholar] [CrossRef]
- Alizadeh, S.R.; Abbastabar, M.; Nosratabadi, M.; Ebrahimzadeh, M.A. High antimicrobial, cytotoxicity, and catalytic activities of biosynthesized selenium nanoparticles using Crocus caspius extract. Arab. J. Chem. 2023, 16, 104705. [Google Scholar] [CrossRef]
- Alam, H.; Khatoon, N.; Raza, M.; Ghosh, P.C.; Sardar, M. Synthesis and characterization of nano selenium using plant biomolecules and their potential applications. BioNanoScience 2019, 9, 96–104. [Google Scholar] [CrossRef]
- Visha, P.; Nanjappan, K.; Selvaraj, P.; Jayachandran, S.; Elango, A.; Kumaresan, G. Biosynthesis and structural characteristics of selenium nanoparticles using Lactobacillus acidophilus bacteria by wet sterilization process. Int. J. Adv. Vet. Sci. Technol. 2015, 4, 178–183. [Google Scholar]
- Tugarova, A.V.; Mamchenkova, P.V.; Dyatlova, Y.A.; Kamnev, A.A. FTIR and Raman spectroscopic studies of selenium nanoparticles synthesised by the bacterium Azospirillum thiophilum. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 192, 458–463. [Google Scholar] [CrossRef]
- Kannan, S.; Mohanraj, K.; Prabhu, K.; Barathan, S.; Sivakumar, G. Synthesis of selenium nanorods with assistance of biomolecule. Bull. Mater. Sci. 2014, 37, 1631–1635. [Google Scholar] [CrossRef]
- Udalova, Z.V.; Folmanis, G.E.; Khasanov, F.K.; Zinovieva, S.V. Selenium nanoparticles-an inducer of tomato resistance to the root-knot nematode Meloidogyne incognita (Kofoid et White, 1919) Chitwood 1949. Dokl. Biochem. Biophys. 2018, 482, 264–267. [Google Scholar] [CrossRef]
- Domokos-Szabolcsy, E.; Marton, L.; Sztrik, A.; Babka, B.; Prokisch, J.; Fari, M. Accumulation of red elemental selenium nanoparticles and their biological effects in Nicotiniatabacum. Plant Growth Regul. 2012, 68, 525–531. [Google Scholar] [CrossRef]
- Hu, T.; Li, H.; Li, J.; Zhao, G.; Wu, W.; Liu, L.; Wang, Q.; Guo, Y. Absorption and bio-transformation of selenium nanoparticles by wheat seedlings (Triticum aestivum L.). Front. Plant Sci. 2018, 9, 597. [Google Scholar] [CrossRef] [Green Version]
- Husen, A.; Siddiqi, K.S. Plants and microbes assisted selenium nanoparticles: Characterization and application. J. Nanobiotechnol. 2014, 12, 28. [Google Scholar] [CrossRef] [Green Version]
- Hussein, H.A.A.; Darwesh, O.M.; Mekki, B.B. Environmentally friendly nano-selenium to improve antioxidant system and growth of groundnut cultivars under sandy soil conditions. Biocatal. Agric. Biotechnol. 2019, 18, 101080. [Google Scholar] [CrossRef]
- Ragavan, P.; Ananth, A.; Rajan, M.R. Impact of selenium nanoparticles on growth, biochemical characteristics and yield of cluster bean Cyamopsis tetragonoloba. Int. J. Environ. Agric. Biotechnol. 2017, 2, 2917–2926. [Google Scholar] [CrossRef]
- Reddy Pullagurala, V.L.; Adisa, I.O.; Rawat, S.; Kalagara, S.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. ZnO nanoparticles increase photosynthetic pigments and decrease lipid peroxidation in soil grown cilantro (Coriandrum sativum). Plant Physiol. Biochem. 2018, 132, 120–127. [Google Scholar] [CrossRef]
- Juárez-Maldonado, A.; Ortega-Ortíz, H.; Morales-Díaz, A.B.; González-Morales, S.; Morelos-Moreno, Á.; Cabrera-De la Fuente, M.; Sandoval-Rangel, A.; Cadenas-Pliego, G.; Benavides-Mendoza, A. Nanoparticles and nanomaterials as plant biostimulants. Int. J. Mol. Sci. 2019, 20, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Y.; Zhao, T.; Mao, G.; Zhang, M.; Li, F.; Zou, Y.; Yang, L.; Wu, X. Antitumor activity of hyaluronic acid-selenium nanoparticles in Heps tumor mice models. Int. J. Biol. Macromol. 2013, 57, 57–62. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Abdelrahman, M.; Hosseini, M.S.; Hoveizeh, N.F.; Tran, L.P. Alleviation of the effect of salinity on growth and yield of strawberry by foliar spray of selenium-nanoparticles. Environ. Pollut. 2019, 253, 246–258. [Google Scholar] [CrossRef]
- Huang, C.; Qin, N.; Sun, L.; Yu, M.; Hu, W.; Qi, Z. Selenium improves physiological parameters and alleviates oxidative stress in srawberry seedlings under low-temperature stress. Int. J. Mol. Sci. 2018, 19, 1913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debona, D.; Rodrigues, F.; Rios, J.A.; Nascimento, K.J. Biochemical changes in the leaves of wheat plants infected by Pyricularia oryzae. Phytopathology 2012, 102, 1121–1129. [Google Scholar] [CrossRef] [Green Version]
- Bai, K.; Hong, B.; He, J.; Huang, W. Antioxidant capacity and hepatoprotective role of chitosan-stabilized selenium nanoparticles in concanavalin A-induced liver injury in mice. Nutrients 2020, 12, 857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baycheva, O.; Samaliev, H.; Udalova, Z.; Trayanov, K.; Zinovieva, S.; Folman, G. Selenium and it’s effect on plant-parasite system Meloidogyne Arenaria-Tiny Tim tomatoes. Bulgar. J. Agric. Sci. 2018, 24, 252–258. [Google Scholar]

| Factor | Plant Length (cm) | Root Length (cm) | Shoot Length (cm) | Root Fresh Weight (g) | Shoot Fresh Weight (g) |
|---|---|---|---|---|---|
| Treatment time (TT) | NS | NS | NS | NS | ** |
| Pre-infection (Pre) | 70.8 a ± 1.6 | 25.8 a ± 1.2 | 44.9 a ± 1.0 | 5.92 a ± 0.4 | 11.7 b ± 0.4 |
| Post-infection (Post) | 71.7 a ± 2.5 | 24.7 a ± 0.8 | 47.0 a ± 1.8 | 6.31 a ± 0.4 | 13.7 a ± 0.7 |
| Application method (AM) | NS | * | ** | NS | ** |
| Foliar spray (FS) | 72.0 a ± 1.7 | 24.0 b ± 0.9 | 48.0 a ± 1.3 | 6.05 a ± 0.4 | 13.5 a ± 0.7 |
| Soil drench (SD) | 70.4 a ± 2.4 | 26.5 a ± 1.1 | 43.9 b ± 1.5 | 6.18 a ± 0.4 | 11.9 b ± 0.4 |
| BioSeNPs (ppm) | ** | ** | ** | ** | ** |
| NC | 61.0 d ± 2.3 | 21.7 b ± 0.9 | 39.3 d ± 1.4 | 4.80 c ± 0.1 | 11.0 c ± 0.2 |
| BioSe2 | 69.3 c ± 3.5 | 24.3 ab ± 2.2 | 44.9 c ± 2.3 | 4.73 c ± 0.6 | 12.7 ab ± 1.1 |
| BioSe6 | 75.3 b ± 2.4 | 26.8 a ± 2.0 | 48.5 b ± 1.7 | 5.64 c ± 0.4 | 13.7 ab ± 1.1 |
| BioSe10 | 80.6 a ± 3.7 | 27.6 a ± 1.4 | 53.0 a ± 3.0 | 6.68 b ± 0.5 | 13.9 a ± 1.3 |
| PC (Ethoprophos Smart-N) | 70.0 c ± 1.3 | 26.0 a ± 0.9 | 44.0 c ± 0.4 | 8.73 a ± 0.5 | 12.2 bc ± 0.6 |
| TT ×AM × BioSeNPs | ** | ** | ** | * | ** |
| NC | 61.0 fg ± 5.3 | 21.7 d–g ± 2.2 | 39.3 fg ± 3.2 | 4.80 c–e ± 0.2 | 11.0 c–e ± 0.6 |
| Pre × FS × BioSe2 | 68.0 d–f ± 1.2 | 18.7 fg ± 1.8 | 49.3 c–e ± 2.9 | 2.30 f ± 0.8 | 8.67 e ± 1.5 |
| Pre × FS× BioSe6 | 78.3 b–d ± 6.2 | 27.7 b–e ± 6.9 | 50.7 b–d ± 3.5 | 5.33 b–e ± 0.1 | 13.8 bc ± 0.9 |
| Pre × FS × BioSe10 | 71.3 c–f ± 3.2 | 20.7 e–g ± 1.8 | 50.7 b–d ± 3.5 | 5.13 b–e ± 0.6 | 12.1 c–e ± 0.6 |
| Pre × SD × BioSe2 | 81.7 bc ± 1.2 | 35.7 a ± 0.3 | 46.0 d–f ± 1.2 | 6.40 b–d ± 0.6 | 13.5 bc ± 1.0 |
| Pre × SD × BioSe6 | 78.0 b–d ± 3.5 | 31.3 a–c ± 3.2 | 46.7 d–f ± 0.3 | 6.03 b–e ± 1.0 | 13.0 cd ± 1.7 |
| Pre × SD × BioSe10 | 68.3 d–f ± 0.7 | 29.0 a–d ± 0.0 | 39.3 fg ± 0.7 | 6.96 a–c ± 0.8 | 9.47 de ± 0.9 |
| Post × FS × BioSe2 | 75.7 b–e ± 3.5 | 25.0 b–g ± 0.0 | 50.7 b–d ± 3.5 | 6.07 b–e ± 1.1 | 17.7 a ± 1.2 |
| Post × FS× BioSe6 | 79.7 b–d ± 1.5 | 24.7 c–g ± 2.6 | 55.0 bc ± 1.2 | 7.30 ab ± 0.4 | 18.3 a ± 1.9 |
| Post × FS × BioSe10 | 85.3 b ± 4.4 | 28.0 b–e ± 0.6 | 57.3 b ± 3.8 | 7.27 ab ± 1.4 | 17.6 a ± 3.4 |
| Post × SD × BioSe2 | 51.7 g ± 2.0 | 18.0 g ± 0.0 | 33.7 g ± 2.0 | 4.13 d–e ± 0.6 | 10.9 c–e ± 0.3 |
| Post × SD × BioSe6 | 65.0 ef ± 2.1 | 23.3 d–g ± 1.2 | 41.7 ef ± 0.9 | 3.90 ef ± 0.3 | 9.43 de ± 0.5 |
| Post × SD × BioSe10 | 97.3 a ± 2.9 | 32.7 ab ± 1.5 | 64.7 a ± 1.5 | 7.35 ab ± 0.8 | 16.3 ab ± 0.3 |
| PC (Ethoprophos Smart-N) | 70.0 d–f ± 3.1 | 26.0 b–f ± 2.1 | 44.0 d–f ± 1.0 | 8.73 a ± 1.2 | 12.2 c–e ± 1.3 |
| Factors | Chlorophyll a (mg/g FW) | Chlorophyll b (mg/g FW) | Carotenoids (mg/g FW) |
|---|---|---|---|
| Treatment time (TT) | ** | ** | ** |
| Pre-infection (Pre) | 2.90 b ± 0.29 | 2.04 b ± 0.25 | 1.21 b ± 0.22 |
| Post-infection (Post) | 3.89 a ± 0.31 | 2.50 a ± 0.39 | 1.37 a ± 0.17 |
| Application method (AM) | ** | * | ** |
| Foliar spray (FS) | 3.47 a ± 0.29 | 2.07b ± 0.24 | 1.48 a ± 0.22 |
| Soil drench (SD) | 3.32 b ± 0.34 | 2.47 a ± 0.40 | 1.10 b ± 0.16 |
| BioSeNPs (ppm) | ** | ** | ** |
| NC | 1.98 e ± 0.02 | 1.58 b ± 0.02 | 0.51 e ± 0.03 |
| BioSe2 | 3.66 b ± 0.13 | 2.57 a ± 0.40 | 2.11 a ± 0.43 |
| BioSe6 | 2.51 d ± 0.16 | 1.78 b ± 0.17 | 0.61 d ± 0.14 |
| BioSe10 | 3.45 c ± 0.78 | 2.87 a ± 1.01 | 1.87 b ± 0.34 |
| PC (Ethoprophos Smart-N) | 5.40 a ± 0.04 | 2.54 a ± 0.38 | 1.35 c ± 0.02 |
| TT × AM × BioSeNPs | ** | ** | ** |
| NC | 1.98 g ± 0.04 | 1.58 c–e ± 0.04 | 0.51 ij ± 0.06 |
| Pre × FS × BioSe2 | 4.20 c ± 0.06 | 4.77 b ± 0.02 | 4.55 a ± 0.04 |
| Pre × FS× BioSe6 | 2.90 e ± 0.05 | 2.54 c ± 0.02 | 0.75 h ± 0.03 |
| Pre × FS × BioSe10 | 0.75 j ± 0.03 | 0.04 f ± 0.01 | 0.65 hi ± 0.03 |
| Pre × SD × BioSe2 | 3.05 e ± 0.01 | 2.25 cd ± 0.03 | 1.08 f ± 0.11 |
| Pre × SD × BioSe6 | 2.30 f ± 0.15 | 2.05 cd ± 0.08 | 0.42 j ± 0.01 |
| Pre × SD × BioSe10 | 1.05 i ± 0.01 | 0.50 ef ± 0.01 | 0.93 g ± 0.01 |
| Post × FS × BioSe2 | 3.76 d ± 0.02 | 1.37 c–e ± 0.02 | 1.36 de ± 0.08 |
| Post × FS× BioSe6 | 3.09 e ± 0.05 | 1.17 de ± 0.02 | 1.26 e ± 0.02 |
| Post × FS × BioSe10 | 5.26 b ± 0.03 | 2.54 c ± 0.03 | 2.54 c ± 0.03 |
| Post × SD × BioSe2 | 3.60 d ± 0.08 | 1.88 cd ± 0.06 | 1.43 d ± 0.02 |
| Post × SD × BioSe6 | 1.75 h ± 0.01 | 1.35 c–e ± 0.03 | 0.03 k ± 0.01 |
| Post × SD × BioSe10 | 6.73 a ± 0.02 | 8.42 a ± 0.02 | 3.36 b ± 0.03 |
| PC (Ethoprophos Smart-N) | 5.40 b ± 0.09 | 2.54 c ± 0.9 | 1.35 de ± 0.04 |
| Factor | CAT Activity (ΔAbs 240 mg−1 Protein min−1) | POD Activity (ΔAbs 460 mg−1 Protein min−1) |
|---|---|---|
| Treatment time (TT) | ** | ** |
| Pre-infection (Pre) | 81.4 b ± 2.7 | 21.6 b ± 1.1 |
| Post-infection (Post) | 84.1 a ± 3.3 | 23.8 a ± 2.1 |
| Application method (AM) | ** | ** |
| Foliar spray (FS) | 86.5 a ± 2.2 | 25.7 a ± 2.1 |
| Soil drench (SD) | 79.0 b ± 3.5 | 19.6 b ± 0.8 |
| BioSeNPs (ppm) | ** | ** |
| NC | 89.0 c ± 0.1 | 19.9 d ± 0.2 |
| BioSe2 | 54.9 e ± 3.2 | 21.6 c ± 2.8 |
| BioSe6 | 82.3 d ± 3.1 | 17.6 e ± 1.2 |
| BioSe10 | 91.7 b ± 2.1 | 29.9 a ± 4.5 |
| PC (Ethoprophos Smart-N) | 95.8 a ± 0.1 | 24.4 b ± 0.1 |
| TT ×AM × BioSeNPs | ** | ** |
| NC | 89.0 e ± 0.3 | 19.9 e ± 0.4 |
| Pre × FS × BioSe2 | 68.7 k ± 0.4 | 36.7 b ± 0.4 |
| Pre × FS× BioSe6 | 84.7 g ± 0.4 | 13.8 h ± 0.1 |
| Pre × FS × BioSe10 | 82.7 h ± 0.4 | 20.8 de ± 0.2 |
| Pre × SD × BioSe2 | 44.5 m ± 0.3 | 15.2 g ± 0.1 |
| Pre × SD × BioSe6 | 75.9 i ± 0.2 | 21.9 d ± 0.2 |
| Pre × SD × BioSe10 | 87.8 f ± 0.1 | 18.6 f ± 0.3 |
| Post × FS × BioSe2 | 61.8 l ± 0.1 | 20.8 de ± 0.4 |
| Post × FS× BioSe6 | 97.8 b ±0.3 | 20.9 de ± 0.5 |
| Post × FS × BioSe10 | 99.8 a ± 0.03 | 55.4 a ± 0.2 |
| Post × SD × BioSe2 | 44.6 m ± 0.2 | 13.7 h ± 0.4 |
| Post × SD × BioSe6 | 70.8 j ± 0.4 | 13.8 h ± 0.5 |
| Post × SD × BioSe10 | 96.6 c ± 0.4 | 24.7 c ± 0.5 |
| PC (Ethoprophos Smart-N) | 95.8 d ± 0.1 | 24.4 c ± 0.3 |
| Factor | No. of Galls (g root−1) | Reduction (%) | Egg Mass (g root−1) | Reduction (%) | Eggs/Egg Mass (g root−1) | Reduction (%) |
|---|---|---|---|---|---|---|
| Treatment time (TT) | * | ** | NS | |||
| Pre-infection (Pre) | 846.1 a ± 165 | - | 232.9 b ± 41 | - | 345.2 a ± 16 | - |
| Post-infection (Post) | 799.0 b ± 163 | - | 263.5 a ± 39 | - | 341.4 a ± 14 | - |
| Application method (AM) | ** | ** | NS | |||
| Foliar spray (FS) | 716.1 b ± 166 | - | 225.5 b ± 41 | - | 349.9 a ± 15 | - |
| Soil drench (SD) | 929.0 a ± 160 | - | 270.9 a ± 38 | - | 336.6 a ± 14 | - |
| BioSeNPs (ppm) | ** | ** | ** | |||
| NC | 2484.3 a ± 50 | - | 651.0 a ± 10 | - | 456.6 a ± 18 | - |
| BioSe2 | 491.6 bc ± 94 | 80.2 | 160.7 c ± 28 | 75.3 | 364.2 a ± 21 | 6.37 |
| BioSe6 | 454.3 c ± 59 | 81.7 | 189.4 b ± 12 | 70.9 | 357.1 a ± 8 | 8.20 |
| BioSe10 | 538.4 b ± 134 | 78.3 | 192.9 b ± 14 | 70.3 | 377.2 a± 23 | 3.03 |
| PC (Ethoprophos Smart-N) | 144.0 d ± 8 | 94.2 | 47.0 d ± 1 | 92.7 | 229.0 b ± 7 | 41.1 |
| TT ×AM × BioSeNPs | ** | ** | ** | |||
| NC | 2484.3 a ± 117 | - | 651.0 a ± 24 | - | 456.6 a ± 41 | - |
| Pre × FS × BioSe2 | 212.3 g ± 8 | 91.4 | 59.0 g ± 3 | 90.9 | 378.3 c ± 6 | 17.1 |
| Pre × FS× BioSe6 | 213.0 g ± 9 | 91.4 | 129.7 f ± 3 | 80.6 | 383.3 c ± 3 | 16.0 |
| Pre × FS × BioSe10 | 443.7 e ± 4 | 82.1 | 196.7 de ±4 | 69.8 | 446.6 a ± 9 | 2.19 |
| Pre × SD × BioSe2 | 290.0 fg ± 13 | 88.3 | 132.0 f ± 3 | 79.7 | 259.0 ef ± 4 | 43.3 |
| Pre × SD × BioSe6 | 755.0 d ± 16 | 69.6 | 181.0 e ± 6 | 72.2 | 330.0 cd ± 12 | 27.7 |
| Pre × SD × BioSe10 | 1290.0 b ± 3 | 48.0 | 235.0 c ± 5 | 63.9 | 366.7 c ± 21 | 19.7 |
| Post × FS × BioSe2 | 451.7 e ± 4 | 81.8 | 141.7 f ± 7 | 78.2 | 363.3 cd ± 3 | 20.4 |
| Post × FS× BioSe6 | 383.3 ef ± 12 | 84.5 | 211.7 c–e ± 6 | 67.5 | 341.7 cd ± 13 | 25.2 |
| Post × FS × BioSe10 | 200.0 g ± 10 | 91.9 | 120.0 f ± 6 | 81.6 | 289.3 de ± 9 | 36.6 |
| Post × SD × BioSe2 | 1012.3 c ± 7 | 59.2 | 310.0 b ± 6 | 52.4 | 386.3 c ± 4 | 15.4 |
| Post × SD × BioSe6 | 466.0 e ± 5 | 81.2 | 235.3 c ± 4 | 63.9 | 373.3 c ± 4 | 18.2 |
| Post × SD × BioSe10 | 220.0 g ± 5 | 91.1 | 220.0 cd ± 8 | 66.2 | 345.3 cd ± 4 | 24.4 |
| PC (Ethoprophos Smart-N) | 144.0 g ± 19 | 94.2 | 47.0 g ± 3 | 92.8 | 229.0 f ± 16 | 49.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daoush, A.S.M.; Hendawey, M.H.; Yaseen, R.; El-Nuby, A.S.M.; Bedair, T.M.; Alwutayd, K.M.; Al-Hoshani, N.; Shaaban, A.; Bashir, A.; Li, L. Effect of Biosynthesized Nanoselenium on Controlling Tomato Root-Knot Nematode Meloidogyne incognita. Agronomy 2023, 13, 1668. https://doi.org/10.3390/agronomy13071668
Daoush ASM, Hendawey MH, Yaseen R, El-Nuby ASM, Bedair TM, Alwutayd KM, Al-Hoshani N, Shaaban A, Bashir A, Li L. Effect of Biosynthesized Nanoselenium on Controlling Tomato Root-Knot Nematode Meloidogyne incognita. Agronomy. 2023; 13(7):1668. https://doi.org/10.3390/agronomy13071668
Chicago/Turabian StyleDaoush, Asmaa Sh. M., Mohamed H. Hendawey, Rabaa Yaseen, Ahmed S. M. El-Nuby, Tarek M. Bedair, Khairiah Mubarak Alwutayd, Nawal Al-Hoshani, Ahmed Shaaban, Anum Bashir, and Lin Li. 2023. "Effect of Biosynthesized Nanoselenium on Controlling Tomato Root-Knot Nematode Meloidogyne incognita" Agronomy 13, no. 7: 1668. https://doi.org/10.3390/agronomy13071668
APA StyleDaoush, A. S. M., Hendawey, M. H., Yaseen, R., El-Nuby, A. S. M., Bedair, T. M., Alwutayd, K. M., Al-Hoshani, N., Shaaban, A., Bashir, A., & Li, L. (2023). Effect of Biosynthesized Nanoselenium on Controlling Tomato Root-Knot Nematode Meloidogyne incognita. Agronomy, 13(7), 1668. https://doi.org/10.3390/agronomy13071668








