Abstract
Composting is considered an efficient and environmentally friendly alternative for plant waste management, resulting in compost, a high value-added product. During the process, microorganisms play a crucial role as organic matter-degrading agents. However, the highly recalcitrant nature of the lignocellulose present in plant residues sets a challenge to the microorganisms involved in the process. Therefore, the objective of this study was to evaluate the effect of a lignocellulolytic microbial consortium, previously selected in composting processes, to promote and improve the biodegradability of plant residues. For this purpose, a laboratory-scale inoculation strategy was optimized by applying different strains and doses of Bacillus spp., as well as different incubation times. Subsequently, the impact of the application of the consortium on the waste material as a pretreatment of a real composting process was evaluated. Samples from both experiments were subjected to the evaluation of parameters related to the biodegradation of the lignocellulosic fraction, in addition to those related to the stability and maturity of a compost. The inoculum proved to be effective in promoting the bioactivation of the material, favoring a higher respirometric rate and biodegradability during laboratory-scale pretreatment. In this case, inoculation with B. safensis at high doses resulted in AT4 values higher than those observed for the rest of the treatments after ten days of incubation, while B. licheniformis inoculated at lower doses was able to maintain higher AT4 values after ten days, compared to those observed in the rest of the samples analyzed. Additionally, inoculation with both strains resulted in a continuous decrease in the percentage of hemicellulose that could be detected until the end of the incubation period (thirty days), reaching biodegradation rates close to 40%. On the other hand, although the inoculation did not significantly affect the basic conditioning parameters at the beginning of the composting process (organic matter, C/N ratio, and moisture), a change in the dynamics of the lignocellulosic fractions was observed during the process, as well as in the evolution of other stability and maturity parameters, in particular the AT4 index and the lignin/holocellulose ratio, revealing an acceleration of the bio-oxidative phase during the full-scale composting process. Therefore, the treatment of plant waste with lignocellulolytic microorganisms is proposed as an effective alternative to activate the biodegradability of organic waste at the beginning of a composting process, resulting in better-quality products.
1. Introduction
The world population is expected to reach 8.6 billion in 2030, 9.8 billion in 2050, and 11.2 billion in 2100 [1], with this increase concentrated in the most developed countries [2]. As a consequence of this imminent uncontrolled growth, new challenges are being generated on a global scale, where food supply is the most urgent. Attention is currently focused on agricultural ecosystems, such as intensive agriculture, as the main suppliers of food to the world [3]. However, the intensive use of agricultural systems results in the inherent massive generation of plastic and lignocellulosic waste [4]. Therefore, for this agricultural system to be sustainable, it must reflect the principles of the circular economy, which involve proper and efficient waste management [5]. The amount of agricultural waste generated worldwide is increasing. Millati et al. reported that around 2 billion tons of agro-waste are generated worldwide annually, containing cellulose, hemicellulose, and lignin in different quantities [6]. In Spain, one of the main agricultural producing countries, about 744,000 tons of lignocellulosic waste was generated in 2022 [7]. Considering that the generation of waste is inherent to agricultural production, it is mandatory to promote sustainable alternatives for the management of such waste, avoiding the innumerable environmental problems caused by inadequate disposal, as well as revaluing a material with high organic and energetic potential [8]. In addition, following the new circular economic model, there is a need to deviate from the traditional linear model of biofertilizer production and adopt a circular production system from different types of waste-based resources. Although the application of synthetic fertilizers since the 1950s has greatly contributed to food security in many developing countries, it also has drawbacks due to its overuse [9].
Since the valorization of these by-products is an important step in agricultural development and environmental protection, some ecological alternatives have been proposed to meet the objectives of sustainability and circularity, such as the composting process [10]. Composting is not a new alternative, but it is one of the most effective technologies to reuse organic waste as ecological amendments, improve soil fertility, provide nutrients, and promote plant growth [8,11]. Provided the process is properly managed, the final compost will be mature and stable, as well as free of phytotoxic substances. In addition, a correct thermal profile guarantees the elimination of most pathogenic microorganisms [11].
However, at present, the processing conditions of organic wastes in industrial-scale composting plants can be very different from those considered as standard in terms of raw materials, processing temperatures, and maturation times [12]. In addition, the particle size, the presence of contaminants, pesticides, additives, and plastics can be highly variable and greatly affect the degradation process. Specifically, in the case of plastics integrated in composting processes, they can cause contamination of the final product and must be mechanically separated in the final stage [13,14,15].
The standards ASTM D7075-04 [16] and ISO 14001:2015 [17] have been developed to assess the environmental performance of so-called natural biopolymers (among them, one of the most important is lignocellulose) characterized by their compostability and biodegradability [18,19]. Unfortunately, for a polymer to be considered compostable, 90% of its carbon content must be converted to carbon dioxide, according to ASTM International. In addition, there are a number of factors that affect the biodegradation rate of natural biopolymers, such as their chemical structures, functional groups, crystallinities, and polymeric chains [20], in addition to other environmental factors such as temperature, oxygen, and pH [21].
Therefore, it is necessary to optimize and know the characteristics of the raw materials, microbial diversity, as well as to control the basic monitoring parameters of the composting process to guarantee obtaining a high-quality agricultural product [22]. If these conditions are not met, the treated waste may not be suitable for application as an organic amendment [10,11]. In this sense, although lignin can be considered one of the most represented biopolymers in nature, its recalcitrant nature can slow down its own biodegradation and biotransformation, thus affecting the quality of the final product [11,23].
Lignin-carbohydrate complexes act as a protective factor by enveloping cellulose in plant cell walls and slow down microbial decomposition. Thus, degradation of recalcitrant polymeric compounds, such as lignin (10–25% of lignocellulosic biomass), is a crucial step for OM stabilization [24]. Inefficient biodegradation of organic matter compromises the stability of the final product, and the application of immature compost results in nutrient immobilization, inhibition of plant growth, and anaerobic conditions in the soil [10,11,24].
The microorganisms present in the composting process play a crucial role in the degradation and stabilization of organic matter, as well as in its transformation into a stable, mature, and humified final product suitable for agricultural use. This is due to the effect of the selective pressure to which the microbial community present in the composting process is subjected, i.e., the indigenous microbiota will be adapted and will have developed metabolic tools to survive using the material, in this case lignocellulosic, present there [11]. However, an inadequate amount or a low biodegradation capacity of indigenous microbes can lead to a reduced biodegradation capacity of compost and may result in a lower efficiency and an undesirable compost quality [10]. Therefore, the strategy of using specialized microbial cultures is a promising approach to facilitate the biodegradation of recalcitrant waste such as lignocellulosic material, as well as to favor compost maturation and improve its final quality. Since the composting process requires high functional diversity achieved by the action of a large microbial diversity, microbial consortia tend to be more effective compared to the application of a single strain, as the latter inoculants may not meet multiple requirements or could be eliminated by the indigenous microbiota when competing for nutrients [10,11,24]. Abdel-Rahman et al. [25] used pure and mixed cultures based on cellulolytic bacteria for accelerating the composting process, composed of a mixture of rice straw and cow dung. The mixed consortia showed an extended thermophilic phase and accelerated maturation in comparison to pure cultures. However, Duan et al. [26] showed that the efficiency of pure cultures is highly dependent on the inoculum concentration. The best candidates are usually microbes naturally present in the compost pile since they can balance the native microbiota and accelerate the composting process by promoting the growth of the desired microorganisms [10]. In this sense, Martínez-Gallardo et al. [27] have shown that the bioaugmentation strategy in the olive mill sludge composting process with specialized inoculants, previously isolated from the proper sludge, favors the decontamination of this waste, while accelerating the process and improving the quality of the final product.
No previous research works have analyzed in depth the effect of different microbial inoculation strategies in composting processes, as a previous biotechnological tool to promote the early bioactivation of the most biodegradation-resistant waste, such as lignocellulosic waste. In this way, to favor the ideal development of the composting process as well as to improve the quality of the final product is crucially necessary. Therefore, the objective of this study was to evaluate the effect of a lignocellulolytic microbial consortium, previously selected in composting processes, to promote and improve the biodegradability of lignocellulosic waste. To carry out this study, the following objectives were established: (1) optimization of the best inoculation strategy at the laboratory scale by evaluating the application of different doses of pure and consortium cultures, as well as the incubation time of the bioactivated lignocellulosic material, and (2) evaluation of the impact of the application of the consortium on plant waste as a pretreatment of a real composting process.
2. Materials and Methods
The methodology and materials described below refer to the two main experimental blocks developed in this research work. First, a laboratory-scale protocol was designed and evaluated for the bioactivation of plant organic wastes through the application of a potentially lignocellulolytic microbial consortium (Section 2.3). In a second phase, the efficacy of this protocol was tested in a small-scale composting process (Section 2.4).
2.1. Raw Material
The raw material used in this work is described in Table 1. The raw material consisted of two types of plant material, such as fresh plant residues from intensive agriculture, composed mainly of tomato plant remains devoid of fruit, as well as pruning remains from urban gardens, to give consistency to the initial mixture.

Table 1.
Characteristics of the raw materials.
2.2. Microorganisms
The microorganisms used in this study belong to the strain collection of the BIO-175 research group, “Development of Microbiological Techniques for the Improvement of Soils of Agricultural Interest”, of the University of Almeria (Spain). The microorganisms were isolated during previous composting processes and were selected on the basis of their capacity for degrading xylan and lignin [28]. The strains used were Bacillus safensis 190 and Bacillus licheniformis 1974. Growth of both strains is considered optimal at 30–37 °C, although they can grow in the 10–50 °C range. Both strains tolerate a pH range between 4.5 and 8 [29,30]. For the optimal growth of these bacteria, they were grown in Petri dishes in APHA medium (Oxoid, UK) at 30 °C for 24 h. Subsequently, after the incubation time, they were stored at 4 °C in slant agar tubes until use.
2.3. Optimization and Follow-Up of the Bioactivation Assay
In this first experimental phase, inocula were obtained for subsequent inoculation at the laboratory scale in the starting mixture at different doses and incubation conditions. The appropriate parameters were also analyzed to evaluate the effect of microbial inoculation on the biodegradation of the lignocellulosic fractions.
2.3.1. Inoculum Preparation
In order to obtain sufficient microbial biomass to address the bioactivation protocol, pure cultures of both strains, B. safensis and B. licheniformis, were prepared in tubes containing 5 mL of nutrient broth (NB). The tubes were incubated at 30 °C for 24 h under shaking conditions at 100 rpm. Once the liquid pre-inoculum was obtained in each case, both strains were inoculated by mass surface extension, using a sterile swab, onto 120 mm square plates with APHA medium. The plates were incubated under the same previous conditions. Subsequently, the biomass corresponding to each strain was collected under aseptic conditions. A viability analysis of the microbial biomass was carried out by counting the colony-forming units per gram of biomass (CFU g−1) according to Miles and Misra’s method [31]. In this way, inoculum densities of 108 and 107 CFU mL−1 were obtained for B. safensis and B. licheniformis, respectively.
2.3.2. Bioactivation Protocol
The preparation of the starting mixture was carried out in plastic containers of 30 × 60 × 15 cm3 as reactors to carry out the bioactivation treatment of the waste by applying the microbial inoculants described above. All the containers were covered with their respective perforated lids to avoid water condensation problems. Pruning and plant debris were mixed and homogenized in the containers in a 1:1 (v:v) ratio to adjust the C/N ratio around 25. The final weight of the initial mixture was approximately 1 kg per container.
Inoculation with both strains was performed independently, using two inoculum doses: a high dose (106 CFU g−1 of starting mixture) and a low dose (104 CFU g−1 of starting mixture). The inoculum doses selected were based on previous works [32,33]. The inoculum was added to the starting mixture, suspended in the water used for wetting, by spraying. Thus, parallel to inoculation, the moisture of the samples was adjusted to around 65%. Finally, the containers were incubated at room temperature (25 °C) and in the dark, and the materials were manually turned every 48 h, to avoid problems of anaerobiosis. Likewise, three sampling times were established: 0, 10, and 30 days, counting from the day of inoculation. Each inoculation treatment was carried out in triplicate, and a block of non-inoculated samples (control) was also taken into account. Samples were taken to evaluate the efficacy of bioactivation by studying a series of parameters, as described below.
2.3.3. Bioactivation Assay: Monitoring Parameters
The influences of the bacterial inocula were measured in terms of several indicator parameters grouped into two categories: basic control parameters and biodegradability indicators. These parameters, directly or indirectly, made it possible to follow the activation of the biodegradation of the lignocellulosic fraction in the starting mixtures.
- Basic control parameters
The moisture content was determined by drying at 105 °C for 24 h. Organic matter (OM) content was assessed by the determination of loss on ignition at 550 °C to a constant weight. Total carbon (C) and nitrogen (N) were determined in solid samples by dry combustion at 950 °C using a LecoTruSpec C–N Elemental Analyzer (Leco Co., St. Joseph, MI, USA).
- Biodegradability indicators
The biological stability of the samples was evaluated by analyzing the parameters such as biodegradability of lignocellulose fractions and a respirometric test. Lignocellulose fractions (%), such as cellulose (CEL), hemicellulose (HC), and lignin (LIG) fractions, were determined using the fiber analyzer Ankom (Ankom Tech., Macedon, NY, USA), according to the method established by the manufacturer. The biodegradation rate (BR) of cellulose, hemicellulose, and lignin was calculated according to the formula of Wang et al. [34]:
where:
BR = [(mo − mn)/mo] × 100,
BR: the biodegradation rate (%) in a given sampling (n).
mn: the content of the lignocellulosic fraction (cellulose, hemicellulose, or lignin), for a given sampling (n).
mo: the initial content of the lignocellulosic fraction (cellulose, hemicellulose, or lignin).
The respirometric test was carried out by means of applying a modified method described by Barrena et al. [35] and using a respirometer system similar to that previously described by Ponsá et al. [36]. Each sample (100 g) was placed in a reactor after adjusting the moisture to 50%. Each reactor was constituted by a PVC vessel (30 cm × 10 cm), with a 2 mm-diameter porous metal net arranged at the base to support the material and provide an air distribution chamber. This system was continuously immersed in a 37 °C water bath. An air flow controller was used to adjust the airflow in the reactors (Bronkhorst Hitec, Gelderland, NL, USA). The air was passed through a humidifier at the same temperature as the reactor to avoid water losses and humidity changes. The exhaust air from the reactors was sent to an oxygen sensor (Alphasense Ltd., Essex, UK) after dehumidification in a water trap. The biodegradability of the samples was assessed by measuring the total oxygen consumed during 4 days after overcoming the latency phase (AT4) [37]. The results are expressed as g O2 kg−1 OM h−1.
2.4. Real-Scale Composting Process of Bioactivated Material
After optimizing the dose and application time of the microbial inoculum in the laboratory-scale bioactivation test, the impact of its application as a pretreatment of a real composting process was evaluated.
2.4.1. Pretreatment and Start-Up of Composting Process
The starting mixture was constituted by the raw materials’ plant waste and pruning waste in a ratio of 1:1 (v:v) to adjust the C/N ratio around 25. To carry out the bioactivation protocol in the small-scale composting process, the starting mixture was spread homogeneously on an impermeable surface and inoculated with an aqueous suspension of the microbial consortium, until reaching a moisture content of 60%. In this case, the inoculum concentration per gram of starting mixture was selected based on the previously obtained results. In this case, a suspension of 104 CFU of each bacterial strain was applied for each gram of residue. In parallel, another initial mixture was prepared under the same conditions but without microbial inoculum (NI). The inoculated (I) but not piled material was incubated at room temperature for 10 days in the material storage area of the semi-pilot composting plant, to reach the bioactivated state before starting the composting process. During the incubation time (pretreatment phase—PRE), the material was covered with a mesh to allow aeration and prevent moisture condensation.
2.4.2. Control and Monitoring of the Composting Process
To start the composting process 10 days after applying the bioactivation protocol, the inoculated (I) and non-inoculated (NI) mixtures were stacked in two piles, with a final volume of approximately 1 m3 and a weight of approximately 300 kg.
During the bio-oxidative phase, manual turning was applied every 2 weeks, until the temperature inside the pile was below 40 °C (cooling phase). The temperature was monitored daily by means of a Pt 100 temperature probe. Samples of 1 kg were periodically taken from the piles to monitor the composting process and evaluate the effectiveness of the bioactivation strategy. Samplings were named as the Initial Phase (0), Thermophilic Phase (25 days), Cooling Phase (48 days), and Final Product (197 days). Basic control parameters (moisture, MO, C/N ratio) and biodegradability indicators (cellulose, hemicellulose, and lignin fractions, as well as oxygen consumption) were determined as indicated above (Section 2.3.3).
2.5. Statistical Analysis
Analyses were performed in triplicate and data are presented as the mean. A one-way analysis of variance (ANOVA) and multiple-comparison tests of Fisher’s least significant difference (LSD) at a 95% confidence level were used to test for significant differences between factor levels. Normality and homogeneity of the variances were checked using the Shapiro–Wilk and Levene tests, respectively, before the ANOVA. Finally, relations among the main parameters analyzed were evaluated by principal component analysis. All the analyses were carried out using Statgraphics Centurion XVII, version 17.1.1 (Stat-Point, Inc., The Plains, VA, USA).
3. Results and Discussion
3.1. Evaluation of Bioactivation Strategy at the Laboratory Scale
3.1.1. Basic Control Parameters
During the optimization of the bioactivation strategy, the effect of microbial inoculation on the behavior of organic matter, moisture, C/N ratio, fibers (cellulose, hemicellulose, and lignin), and oxygen consumption (AT4 index) was analyzed for 30 days.
The organic matter present in a mixture composed of lignocellulosic residues will undergo numerous biotransformation processes carried out by microorganisms [38]. Shorter carbon chains can be transformed into simpler compounds or, on the contrary, they can regroup and form more complex molecules. After the consumption of labile compounds, other more resistant materials start to slowly degrade in later stages, constituting the core of humic compounds, together with the degradation products of the previous stage [39,40]. Although the pretreatment test performed in this work cannot be fully compared to a composting process, a rapid decrease of carbohydrates is expected, and the reactions taking place in the organic component of the mixtures are very similar to what occurs during the early stages of composting.
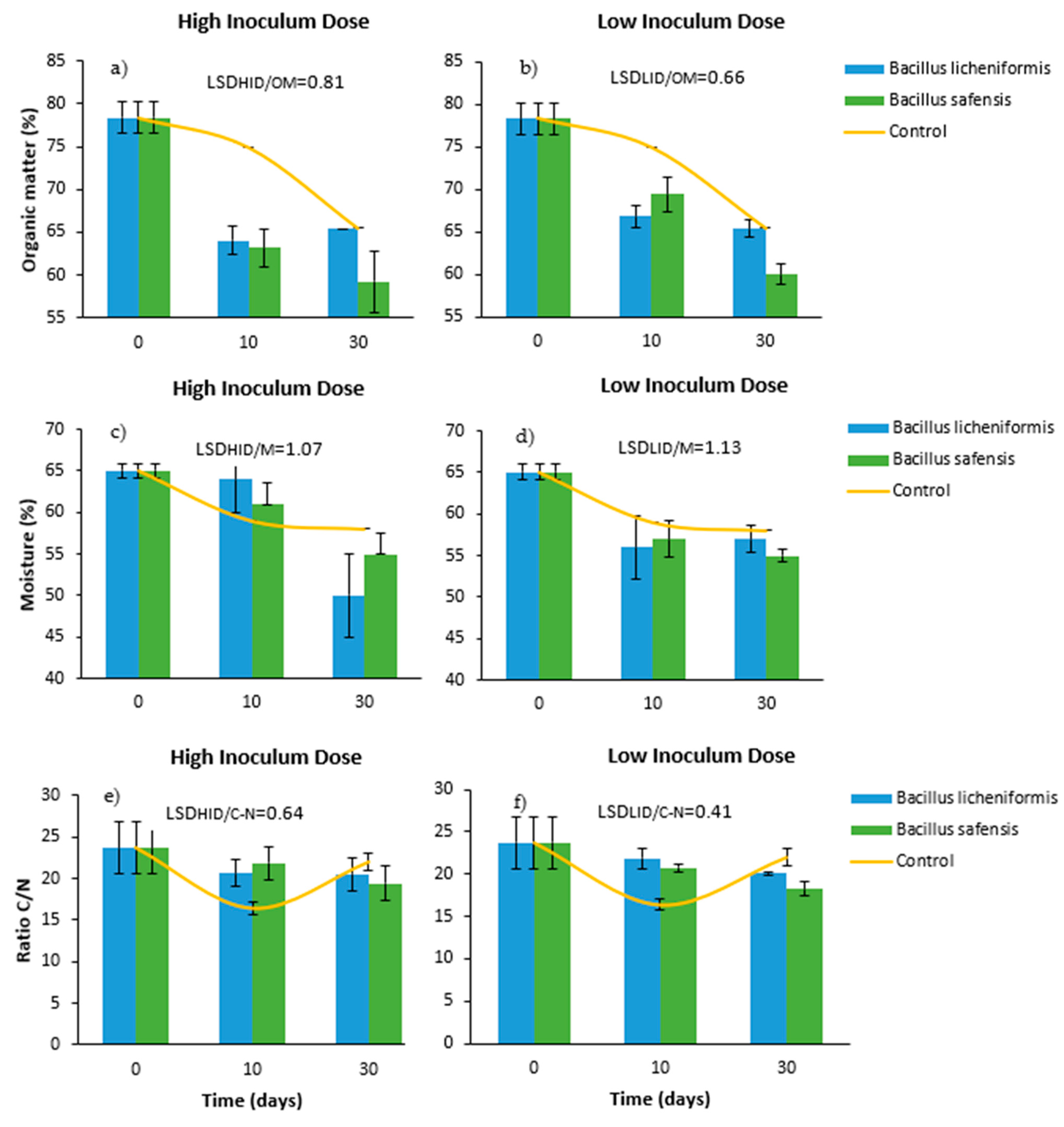
Figure 1a,b show a high organic matter content in the starting mixtures (75–80%). This value gradually decreased to around 60–65% after 30 days of treatment. Overall, the decrease in organic matter content was more pronounced in those mixtures inoculated with both inoculants compared to the control, especially when the inoculants were inoculated at high doses, and after 10 days of incubation (Figure 1a). This fact reveals that the microbial inoculants had a significant effect on the activation of the degradation of the mixtures, a fact that could be verified from the early stages of the trial.
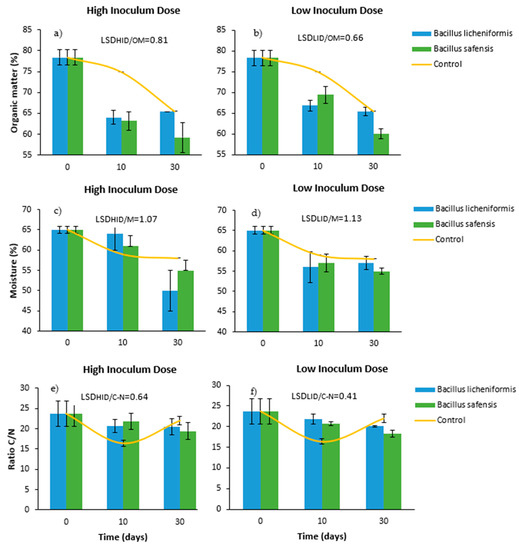
Figure 1.
Effect of treatment with B. licheniformis and B. safensis on the percentage of organic matter (a,b), moisture (c,d), and C/N ratio (e,f) as a function of the incubation time. (a,c,e) High inoculum dose and (b,d,f) low inoculum dose. Control: non-inoculated mixture. Each graph shows the LSD (least significant difference) value as a function of the treatment. Results are means (n = 3) ± SD (vertical bars). LSD: Fisher’s least significant difference, p < 0.05.
Since the aim of this work was to implement an efficient and low-cost biological treatment protocol prior to composting, it did not seem logical to extend the treatment beyond 10 days, nor to apply incubation conditions that make the process more expensive. In any case, according to Antizar-Ladislao et al. [38], at high temperatures, the degradation of organic matter may be reduced because microbial growth may be affected. In this phase, special attention was paid to ensure that the temperature of the mixtures was similar to the room temperature throughout the test.
Initially, the mixtures showed a moisture content of around 65% (Figure 1c,d). According to Liang et al. [41], during the composting process, the optimum humidity for aerobic microorganisms to properly develop ranges around 50–70%. Excessively high humidity can cause anaerobiosis problems, since water displaces air in the free spaces between particles, reducing oxygen transfer. On the other hand, when the percentage is less than 30%, biological activity can significantly drop. As shown in Figure 1c,d, the initial humidity progressively decreased during the incubation time of the samples, although in no case was it below 50%. Therefore, in view of the results related to the moisture content of the samples, it was observed that the moisture content of the pretreated samples remained in a range suitable for the development of aerobic microorganisms until 30 days after inoculation. Although no significant differences were established between doses and treatments, inoculation with lower doses caused a more intense decrease in moisture 10 days after the microbial inoculation (Figure 1d), which could be related to a more effective activation of the initial mixtures.
In this work, the initial C/N ratio values ranged around 24. The results derived from this phase revealed a gradual and gentle decrease of this parameter in the inoculated samples during the 30 days of incubation. This effect was observed in the same terms, regardless of the inoculum doses applied (Figure 1e,f), so that the C/N values detected at the end of the trial ranged from 18 to 20. However, it is worth noting the different behavior of the inoculated mixtures with respect to the non-inoculated controls. In the latter case, a sharp drop in the C/N ratio was observed after 10 days, reaching values below 18, which could be counterproductive. According to Moreno and Moral [42], if the waste to be composted has a C/N ratio lower than 18–19, composting will proceed faster, but nitrogen will be lost in the form of ammonia. These losses can affect the fertilizer quality of the final product, and pose an environmental problem, since ammonia is a gas with a considerable greenhouse effect. Therefore, the implementation of a biological treatment protocol prior to composting must take into account that the reduction of the C/N ratio caused in the treated mixtures does not compromise the implementation of the process a posteriori [43].
It is also worth mentioning that, in the untreated samples, the evolution of the C/N ratio was more irregular, dropping sharply 10 days after the start of the trial, and then increasing again to values similar to those at the beginning (Figure 1d,f). According to Iglesias-Jiménez et al. [44], this phenomenon could be explained by a decrease in the biological activity of the mixtures, which is reflected by an increase in the C/N ratio. This fact did not occur in the samples treated with both microbial strains.
3.1.2. Respirometric Analysis
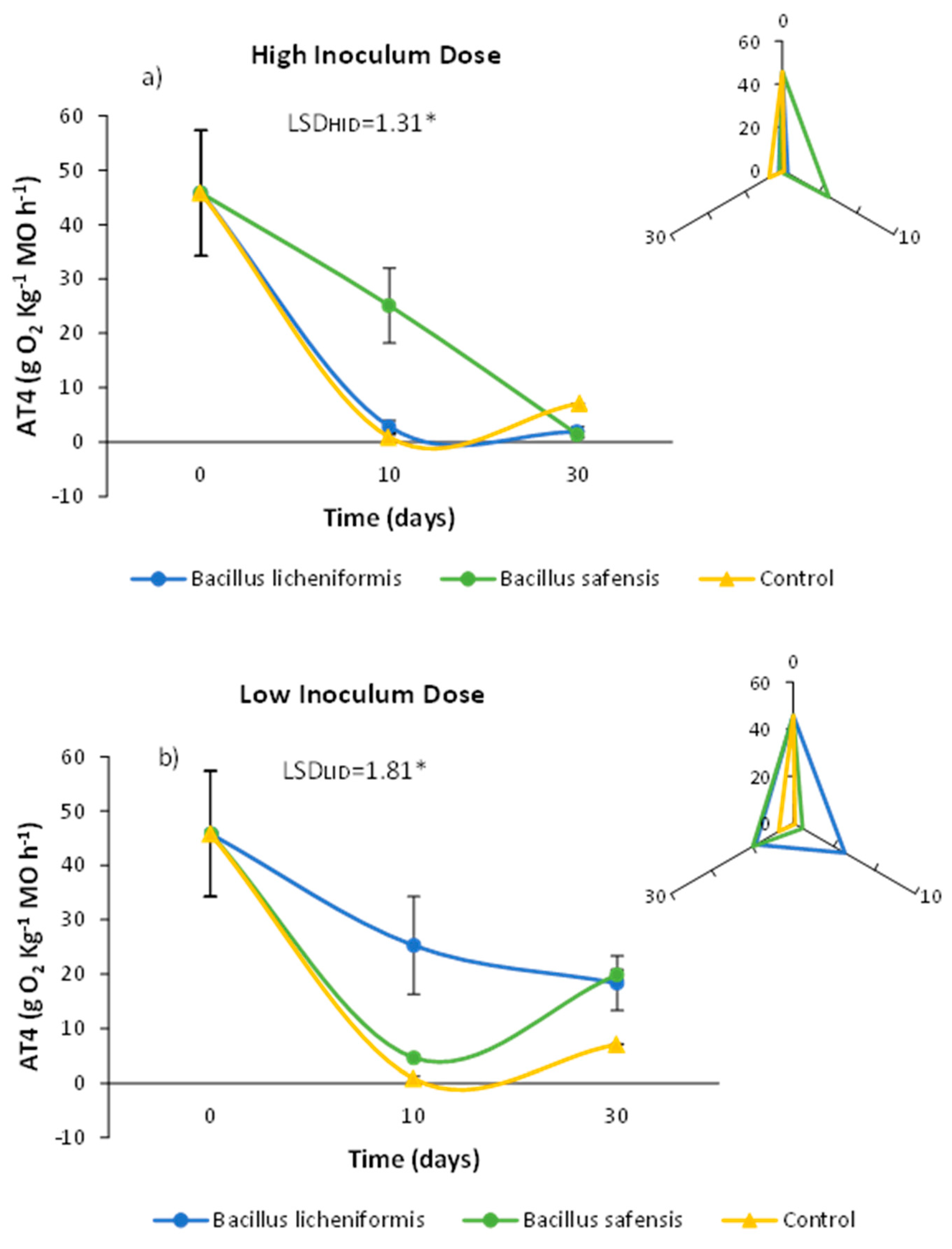
Respirometric measurements are mainly based on the quantification of biological activity on a given substrate. Traditional respirometric studies refer to the level of microbial activity in terms of oxygen consumption as a function of time. When microbial activity intensifies on a substrate considered biodegradable, a strong oxygen demand and high carbon dioxide production rates are generated. For this reason, the rate of O2 consumption during composting could be indicative of the more or less advanced stage of the process [45]. In this sense, starting mixtures in a composting process are characterized by a high rate of O2 demand and CO2 production, compared to what is observed in poorly biodegradable mixtures, or in mature, more stable, and less active composts [43]. In this way, it is conceivable that the biological treatment of mixtures prior to the composting process could serve to increase the biodegradability of the materials in relation to other non-inoculated mixtures. Thus, in Figure 2, it is noteworthy that inoculation with both strains resulted in different AT4 profiles throughout the incubation time.
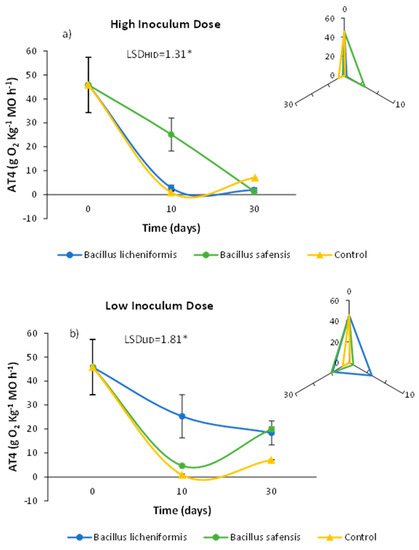
Figure 2.
Effect of treatment with B. licheniformis and B. safensis on the AT4 values as a function of incubation time. (a) High inoculum dose and (b) low inoculum dose. Control: non-inoculated mixture. Each graph shows the LSD (least significant difference) value as a function of the treatment. Results are means (n = 3) ± SD (vertical bars). LSD *: Fisher’s least significant difference, p < 0.05.
Thus, inoculation with B. safensis at high doses resulted in AT4 values higher than those observed for the rest of the treatments after 10 days of incubation (Figure 2a), while B. licheniformis inoculated at lower doses was able to maintain higher AT4 values after 10 days, compared to those observed in the rest of the samples analyzed (Figure 2b). In any case, after 30 days, the samples inoculated in an independent way with both strains at low doses showed AT4 values higher than those observed in the non-inoculated samples (Figure 2b).
3.1.3. Lignocellulosic Fractions and Biodegradation Rates
During the early stages of the composting process, the most easily degradable compounds are depleted. However, many bacteria and fungi, in later stages of the process, are able to break down other polymeric materials, belonging to the lignocellulosic matrix, providing more soluble compounds in the windrow environment. However, although the degradation of the lignocellulosic fractions is described sequentially, it most likely occurs simultaneously throughout the process, as much of the microbiota in the compost pile has a multifunctional character [24].
In the following, the evolution of the different lignocellulosic fractions during the inoculation trial is described. The results are shown both in terms of the percentage of hemicellulose, cellulose, and lignin, and in terms of the degradation rates of the different fractions.
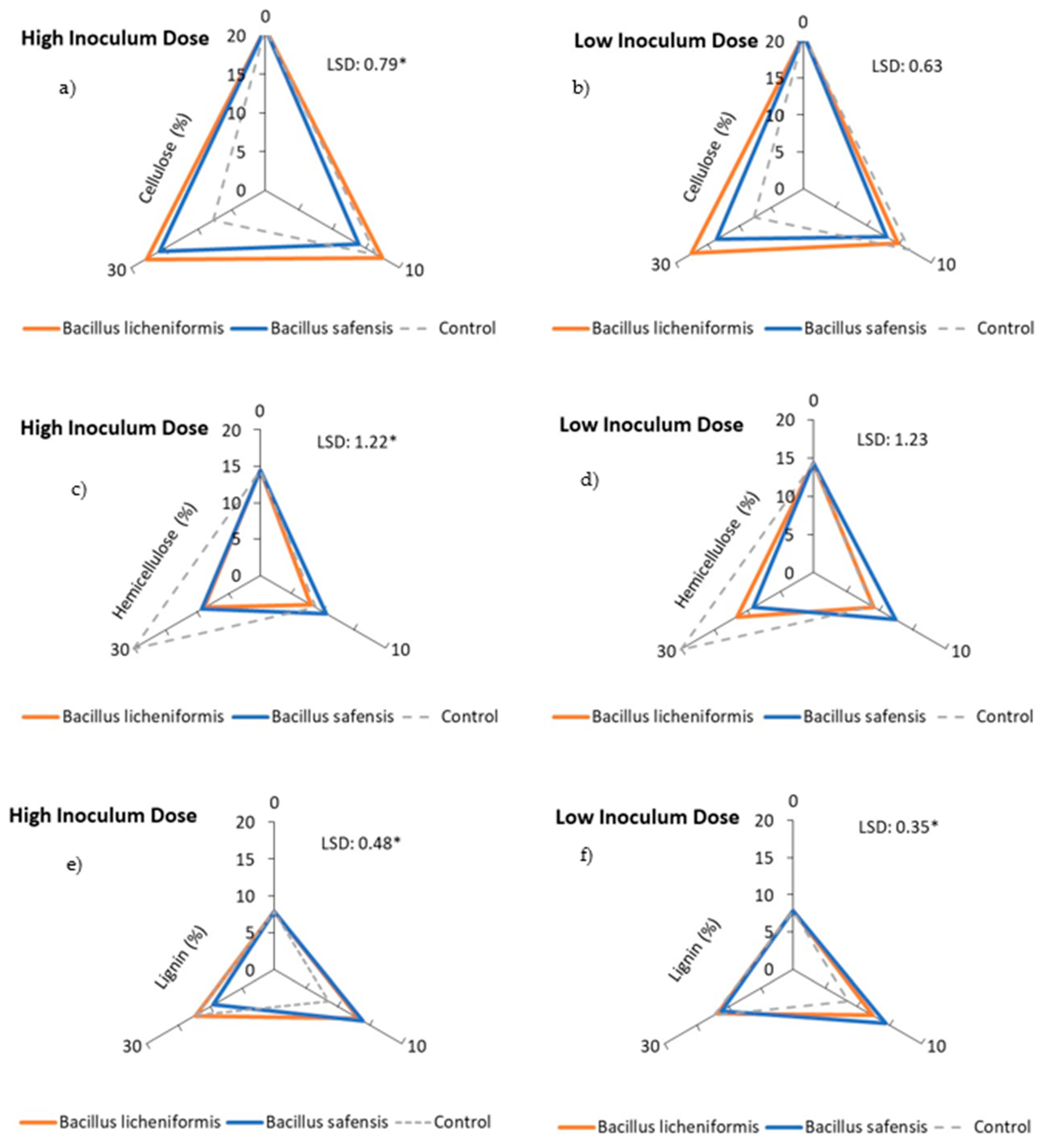
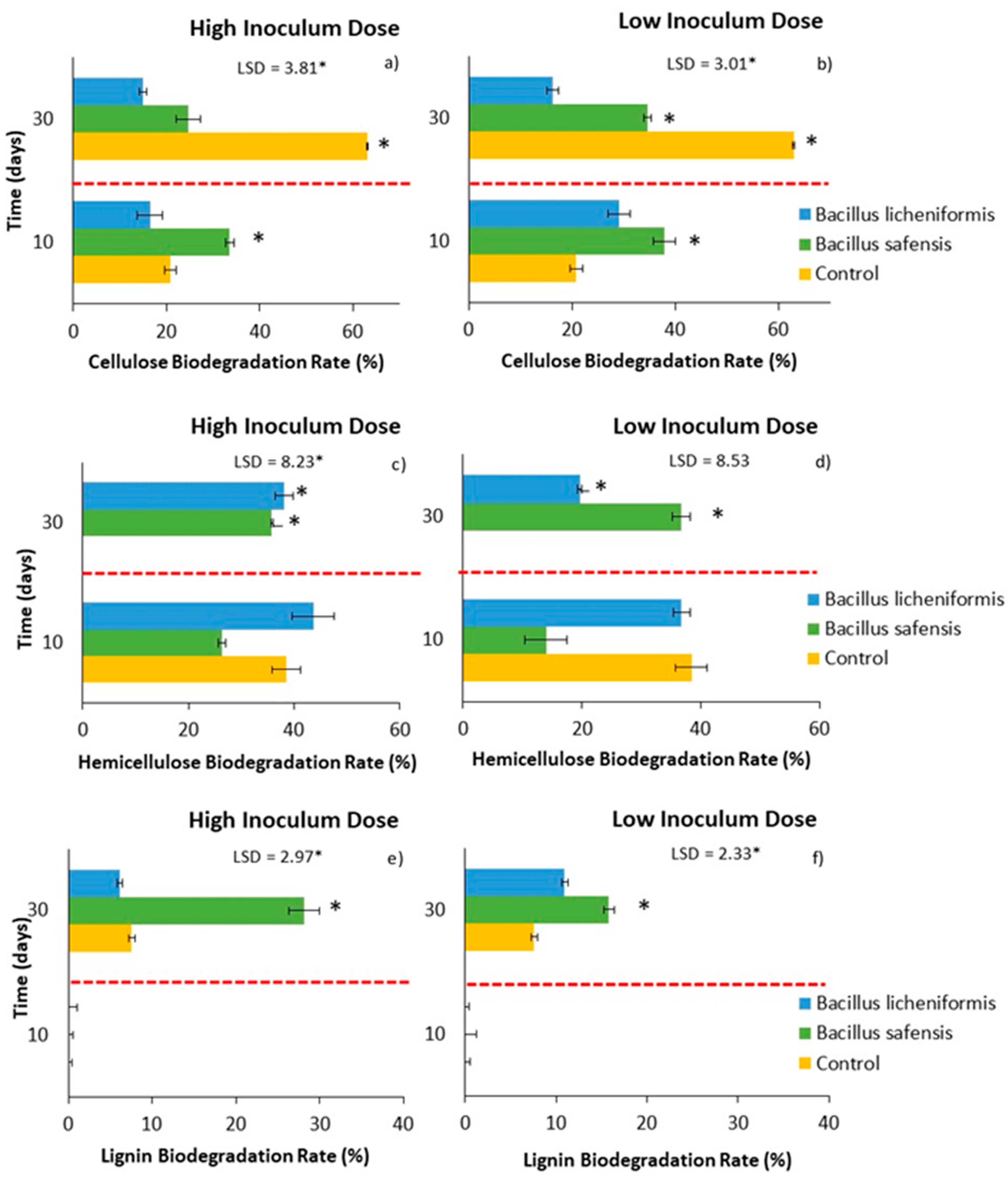
Figure 3a,b show the percentage of cellulose over the incubation time for both inoculated samples and non-inoculated control mixtures. Thus, a decrease in the cellulose content of the inoculated samples could be observed after 10 days of incubation, somewhat more pronounced when the strains were treated with B. safensis at low doses (Figure 3b). In general, cellulose degradation was faster in the mixtures treated with both strains, as degradation rates of around 30–40% were reached after 10 days of incubation, higher than those observed in the control mixtures (Figure 4a,b). However, contrary to expectations, this rate was much higher than that observed after 30 days in the non-inoculated samples, a fact that could be justified on the basis of the higher potential of Bacillus spp. strains to degrade hemicellulose, and the possible competition phenomena that could occur in the inoculated mixtures. In general, the inoculation with B. safensis was the most effective treatment after 10 days of incubation in terms of cellulose degradation (Figure 4a,b). This fact could be related to a more immediate access to this fraction by the microbiota present in the mixtures, favored in part by the hemicellulolytic capacity of B. safensis.
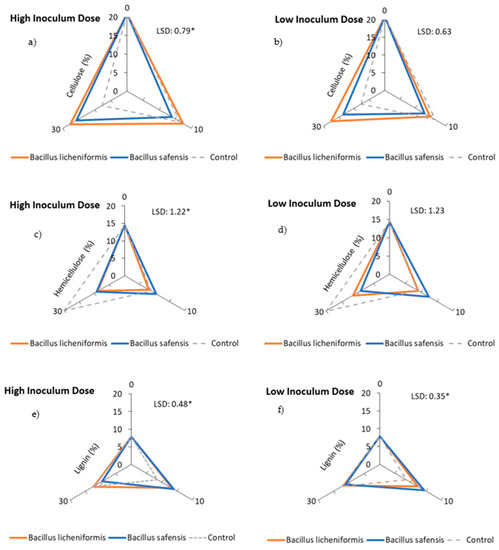
Figure 3.
Effect of treatment with B. licheniformis and B. safensis on the percentage of cellulose (a,b), hemicellulose (c,d), and lignin (e,f) as a function of incubation time. (a,c,e) High inoculum dose and (b,d,f) low inoculum dose. Control: non-inoculated mixture. Each graph shows the LSD (least significant difference) value as a function of the treatment. Results are means (n = 3) ± SD (vertical bars). LSD *: Fisher’s least significant difference, p < 0.05.
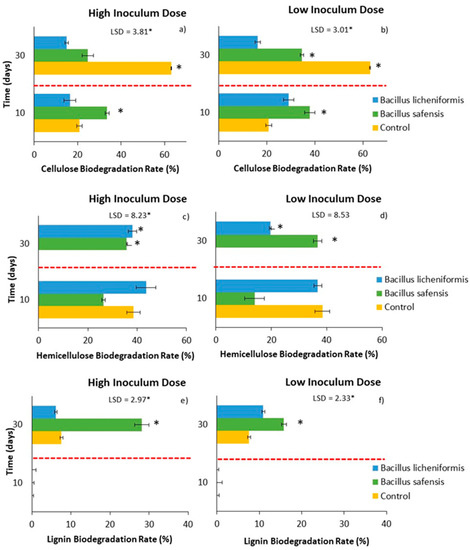
Figure 4.
Effect of treatment with B. licheniformis and B. safensis on the biodegradation rate of cellulose (a,b), hemicellulose, (c,d) and lignin (e,f) as a function of incubation time. (a,c,e) High inoculum dose and (b,d,f) low inoculum dose. Control: non-inoculated mixture. Red dashed lines indicate the separation between the two samplings (10 and 30 days). Each graph shows the LSD (least significant difference) value as a function of the treatment. Results are means (n = 3) ± SD (vertical bars). LSD *: Fisher’s least significant difference, p < 0.05.
On the other hand, inoculation with both strains resulted in a continuous decrease in the percentage of hemicellulose that could be detected until the end of the incubation period (30 days) (Figure 3c,d), reaching biodegradation rates, in some cases, close to 40% (Figure 4c,d). The behavior of the non-inoculated samples was very different, especially during the second part of the incubation period, as the percentage of hemicellulose increased after 10 days, resulting in a negative degradation rate for hemicellulose during this period in the non-inoculated samples.
It is worth noting that the hemicellulose content of the non-inoculated samples followed a similar trend to that observed for the C/N ratio (Figure 1e,f), since, although the C/N ratio decreased after 10 days of incubation, it increased again towards the end of the incubation period. This phenomenon could be related to a decrease in biological activity and a slowing down of the mineralization process. It should also be noted that both Bacillus spp. strains had been previously characterized for their lignocellulolytic capacity, showing an important hemicellulolytic potential, which could justify their behavior in the inoculated mixtures. Although the inoculum dose did not significantly influence the rate of hemicellulose biodegradation, the results showed an overall positive effect of B. safensis on this rate, which increased until the end of the incubation period (Figure 4c,d).
The results reported in the literature on the degradation of lignocellulosic fractions show very diverse and sometimes contradictory information [46,47]. Considering the great diversity of compostable materials and their composition in relation to the lignocellulosic content, it is not surprising that the results sometimes have a complex interpretation [48]. In general, lignin biodegradation occurs in the later stages of composting and a very low decomposition rate is usually detected. Moreover, the content of this polymer determines not only its own decomposition, but also that of the other lignocellulosic components, cellulose and hemicellulose. Lignin, in fact, is able to act as a protective factor for these other fractions. In particular, there is a strong association between lignin and hemicellulose, favored by the presence of phenolic compounds [46]. This complex association makes it difficult to access enzymes involved in biodegradation processes. Moreover, lignin is considered one of the main precursors for the formation of humic substances present in the soil, as it provides the basic structure due to its resistance to soil degradation phenomena [49].
In view of the results observed for the lignin content of the samples, an increase in this fraction 10 days after the application of the treatments, as well as in the control samples (non-inoculated samples), should be noted. This increase may be due not only to the difficulty of lignin to be biodegraded, but also to the heterogeneity of the starting mixtures, and to the possible concentration of the biomass during the time the test lasted. Jurado et al. [11] have been able to verify this same behavior at the start of plant waste composting processes. Even so, after 30 days of incubation, a decrease in the percentage of lignin began to be detected, especially in those mixtures inoculated with B. safensis (Figure 3e,f and Figure 4e,f), which could be related to the notable degradation of this complex polymer, although later than that observed for hemicellulose and cellulose. It is possible that the higher rates of hemicellulose and cellulose biodegradation during the first weeks of treatment in turn favored the release of lignin from the lignocellulosic matrix, so that lignin started to degrade later in the trial. In any case, it was found that the application of both biological treatments, B. licheniformis and B. safensis, on plant residues somehow mobilized the complex lattice of hemicellulose, cellulose, and lignin, contributing to their degradation.
Therefore, based on the results obtained in the laboratory-scale bioactivation assay, inoculation with B. safensis gave rise to a greater degradation of the lignocellulosic fractions. In the case of cellulose, the degradation was especially notable in the short term (10 days), while in the case of hemicellulose and lignin it was later, although it was observed continuously until the 30 days that the test lasted. On the other hand, regarding the biodegradability of the samples (AT4), inoculation with both microorganisms at low doses allowed maintaining the biodegradability of the materials in the longer term, compared to what was observed in non-inoculated materials.
3.2. Real-Scale Composting Process of Bioactivated Material
Temperature is an indicator parameter for the evolution of the composting process, often used to understand how microbial activity develops and to determine the stability of the organic matter [50]. There is an optimum decomposition temperature for each type of organic matter. This is determined by the rate of degradation of the materials, and it coincides with the maximum rates of microbial activity in the pile. This fact justifies the importance of temperature control during the bio-oxidative phase of the process.
In this work, once the effect of the biological treatment on lignocellulose degradation at a lab-scale was proven, the next phase was to investigate the influence of the microbial consortium (B. licheniformis + B. safensis) in mixtures prepared from plant and pruning waste as a treatment strategy prior to the composting process (Section 2.4).
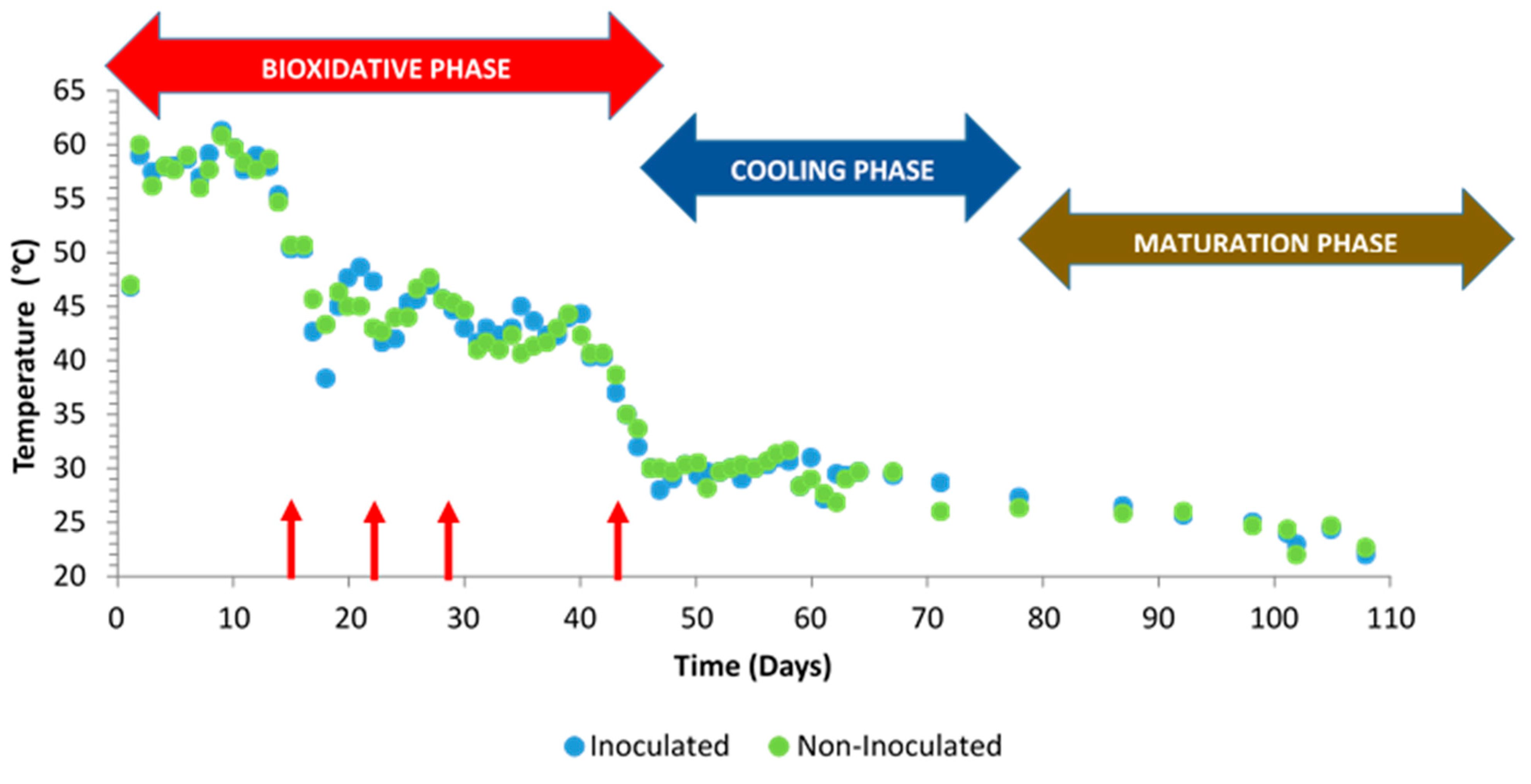
At the beginning of the composting process, an initial temperature of 25 °C was recorded, though this value was increased up to 60 °C during the first week (Figure 5). As expected, a thermal reactivation was promoted after turnings, except for at 45 days, when the piles entered the cooling phase (in Figure 5, turnings are indicated with red arrows). The United Stated Environmental Protection Agency [50] recommends maintaining the compost piles at above 55 °C for 15 days or at least 3 consecutive days. These conditions were maintained in this work during the first two weeks from the beginning of the process.
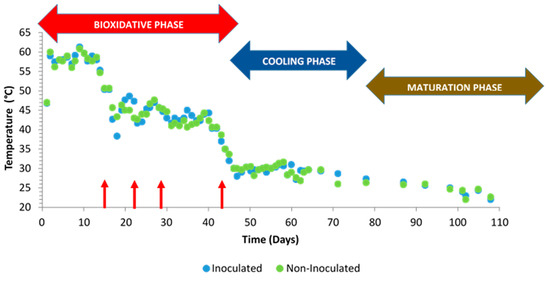
Figure 5.
Temperature evolution in inoculated and non-inoculated compost piles with a microbial consortium 10 days before the start of the process. Turnings are indicated with red arrows.
The temperature into the piles greatly influenced the composting process durability since it directly affects the organic matter degradation rate. Stentiford and Bertoldi [51] suggested that temperatures over 55 °C maximize the sanitation of the materials, those between 45 and 55 °C favor the biodegradation rates, and those between 35 and 45 °C allow for the increase of the biodiversity in the composting process. In this work, temperature evolution was similar both in inoculated and non-inoculated piles.
Although the current European legislation on waste raises a series of recommendations on the management of organic waste throughout the composting process, each country is governed by a series of specific regulations. In the particular case of Spain, this is included in the BOE A-2017-14332 on fertilizer products [52]. In this regulation, reference is made to the basic characteristics that a compost must have in quality terms, with special emphasis on humidity (<40%), organic matter content (35–35%), or the C/N ratio (<15–20).
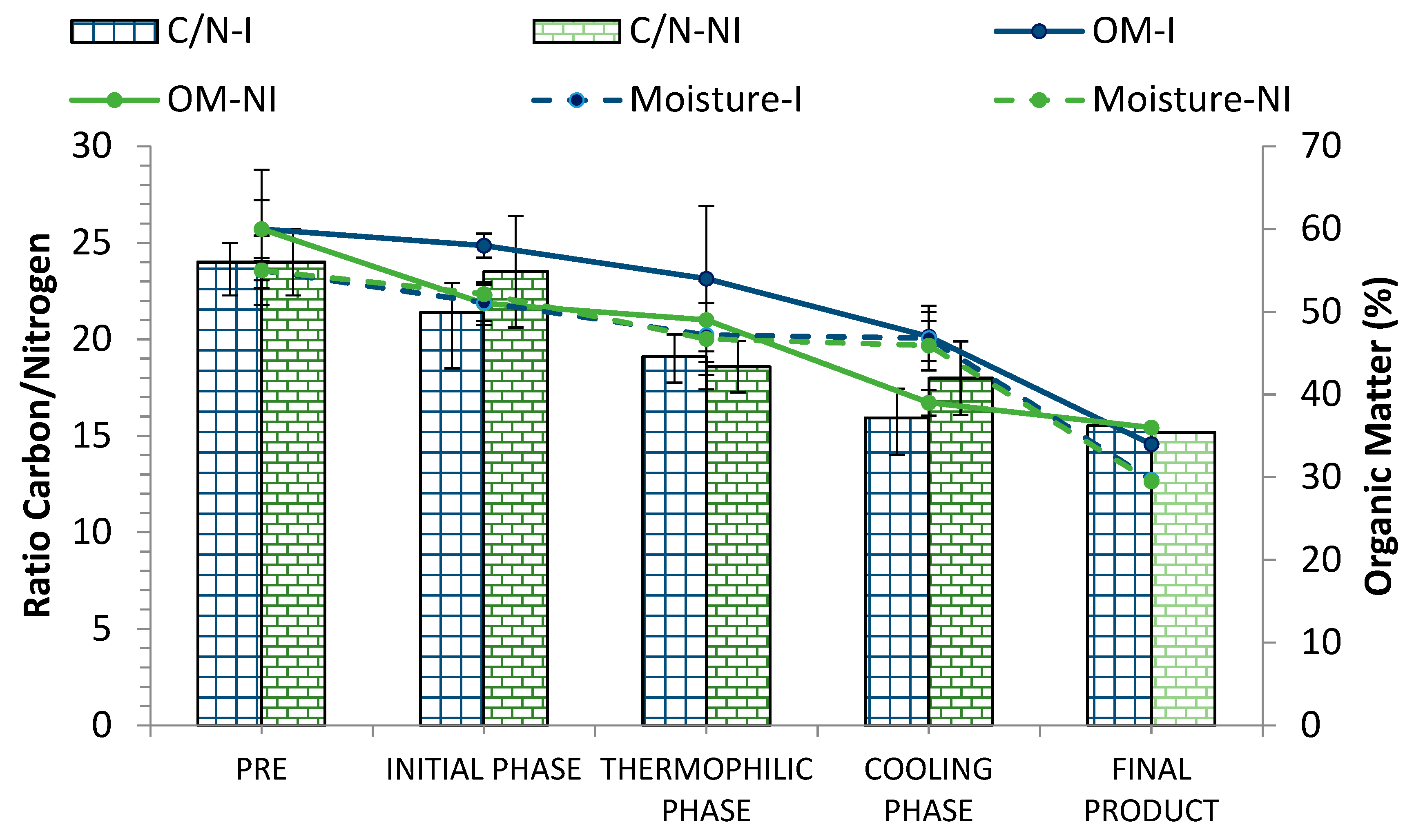
During the process, humidity contributes to a better degradation of organic matter and maintenance of the temperature for a longer period since it favors microbial activity. However, the reduction of the moisture values throughout the process is a positive indicator of compost maturity [33,53]. In this work, the initial moisture in the pretreatment phase (PRE) was around 50–55% and was then maintained above 45% during the whole bio-oxidative phase (Figure 6), decreasing to optimal values in the final product (30%).
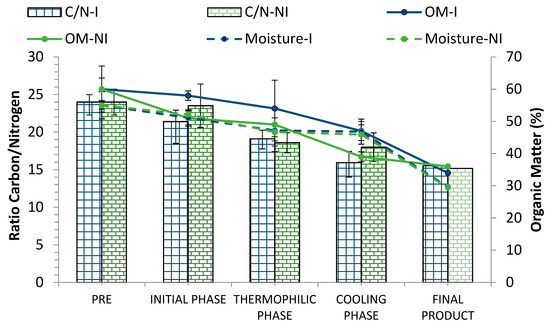
Figure 6.
Evolution of the C/N ratio, organic matter (%), and moisture (%) in inoculated (I) and non-inoculated (NI) compost piles with a microbial consortium 10 days before the start of the process. C/N: C/N ratio, OM: organic matter, M: moisture. Results are means (n = 3) ± SD (vertical bars).
Regarding the percentage of organic matter (OM), only in the non-pretreated materials did a slight decrease occur in this parameter 10 days after the inoculation with the microbial consortium (Figure 6). However, in the case of pretreated materials (PRE), OM remained more or less stable in the early stages of the process; though, as expected, OM gradually decreased throughout the process in both piles, reaching similar values in the final product (around 35%).
On the other hand, the pretreatment of the materials did not significantly affect the C/N ratio values at the beginning of the process. This parameter is of vital importance when conditioning the starting materials, as it affects the optimal evolution of the composting process. In both piles (inoculated and non-inoculated), the initial C/N ratio was within the appropriate range for the activation of the process (Figure 6). Similar results regarding the evolution of the temperature, organic matter, and the C/N ratio were observed in previous works [33].
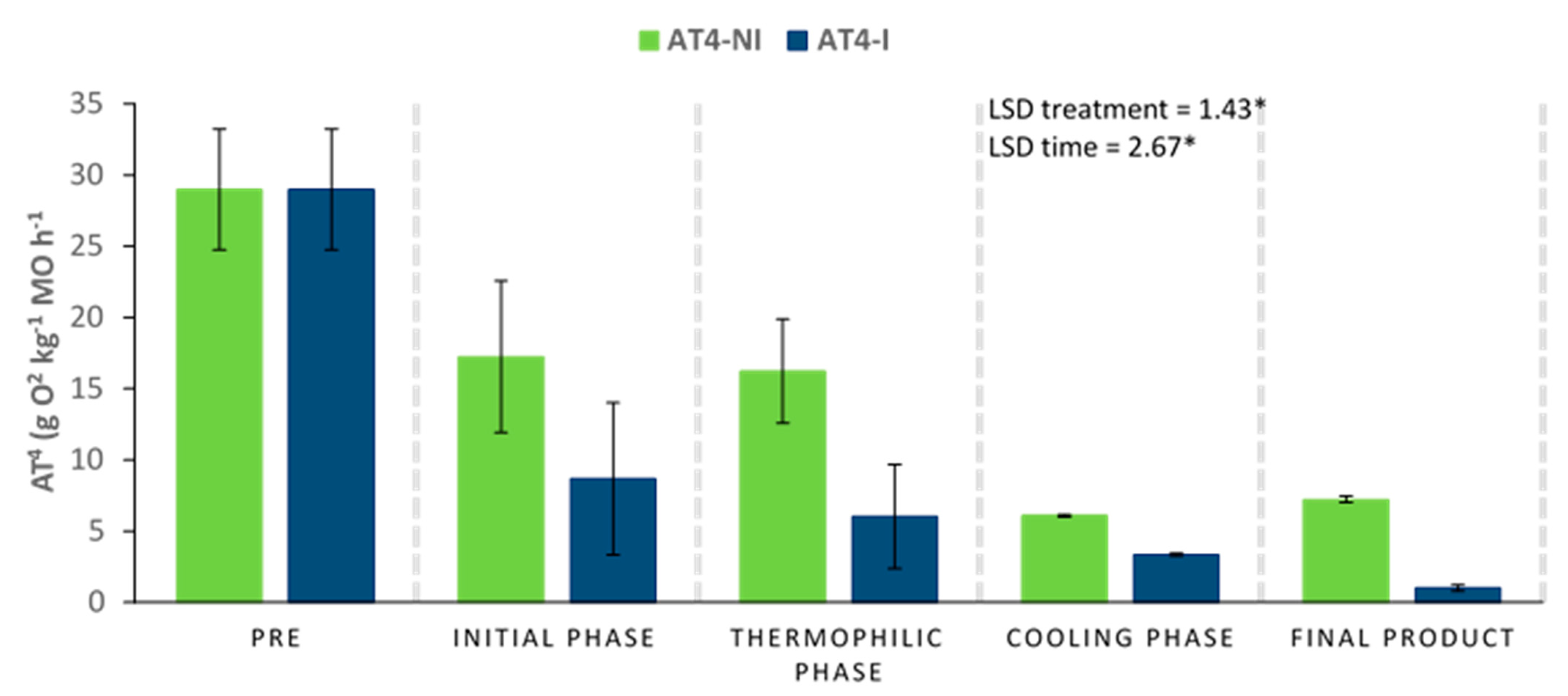
The four-day accumulated oxygen consumption (AT4) could be considered as a suitable stability parameter, directly related to the microbial activity inside the compost piles. Excessively high AT4 values could influence the phytotoxic nature of the samples. This effect has also been described when the content of phenolic compounds is too high [54,55,56]. In this work, the pretreatment of the raw materials with the microbial consortium 10 days before the start of the composting process could induce a faster biodegradation of the materials. The activation of this biodegradation was probably the reason why the AT4 values at time 0 were lower for the inoculated piles compared to the non-inoculated ones (Figure 7). In general terms, the evolution of this parameter was downward in both piles, although the AT4 values reached at the end of the process were lower in the inoculated pile, which could be an indication of higher stability and maturity in the final product.
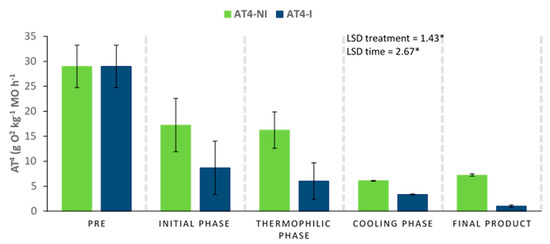
Figure 7.
Evolution of the AT4 parameter (accumulated oxygen consumption) in compost piles bioactivated with a microbial consortium 10 days before the start of the process. Inoculated (I) and non-inoculated (NI) compost piles. Results are means (n = 3) ± SD (vertical bars). LSD *: Fisher’s least significant difference, p < 0.05, regarding treatment and time.
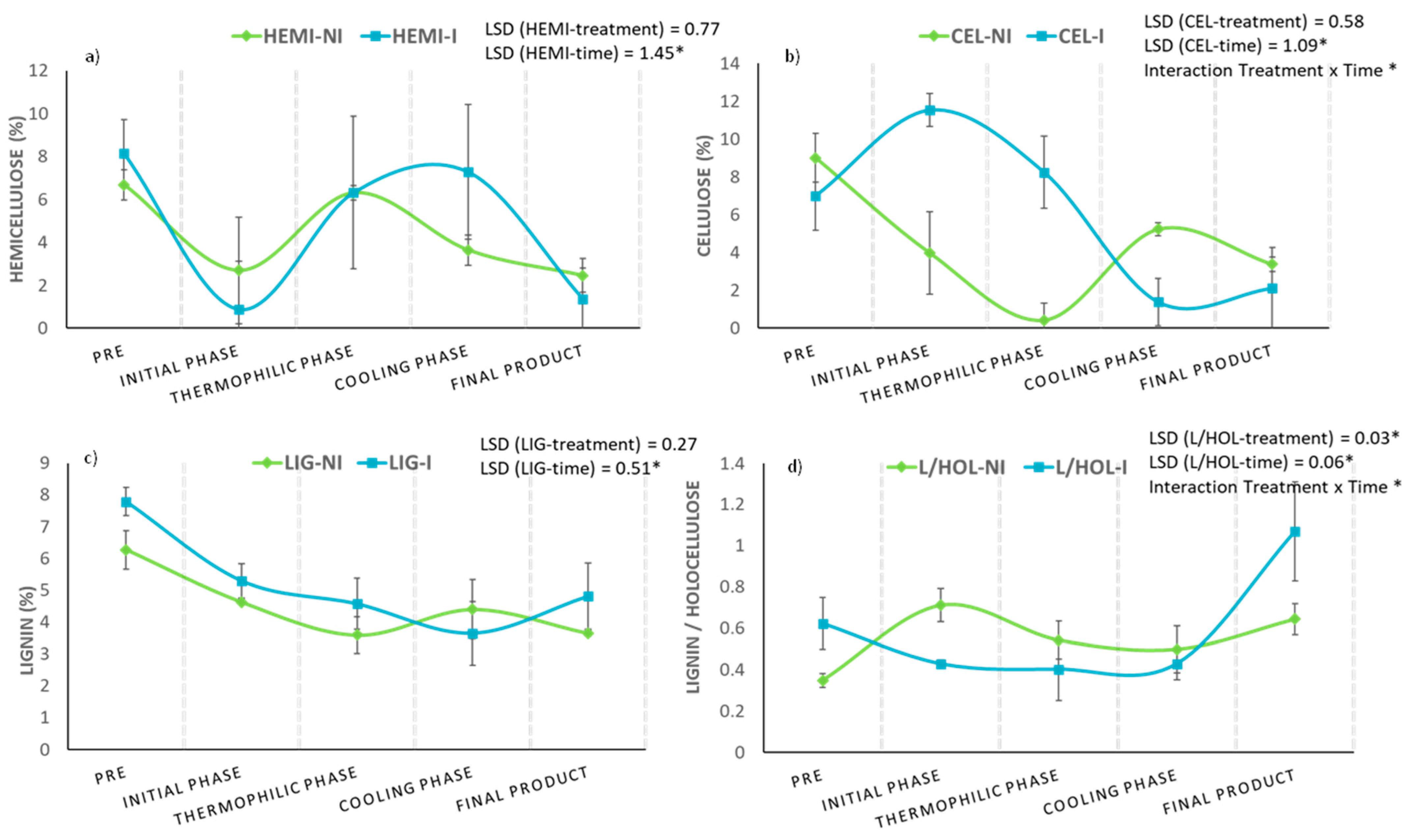
When evaluating the effect of bioactivation on the evolution of lignocellulosic fractions, it must be taken into account that the lignin-hemicellulose complex between the cellulose fibers decreases the available surface of this polymer and prevents easy access by the microorganisms of the process and their enzymes [54,55]. On the contrary, previous works describe that hemicellulose is the fraction of lignocellulose that is easier to degrade and that it generally decomposes to a greater extent, compared to cellulose and lignin [57], being subject to a greater loss during composting.
Figure 8 shows the evolution of hemicellulose, cellulose, and lignin fractions during two parallel composting processes (inoculated and non-inoculated). In the case of hemicellulose and lignin fractions (Figure 8a,c), no significant differences could be detected a priori regarding the “pretreatment” factor, but a more intense decrease for hemicellulose was corroborated in the bioactivated pile at the beginning of the composting process. This fact could be related to the increase in the percentage of cellulose observed during the whole bio-oxidative phase (Figure 8b), as the degradation of hemicellulose in the lignin-hemicellulose matrix could favor the release of the cellulosic component and, consequently, facilitate its biotransformation. On the other hand, although pretreatment with the microbial consortium did not significantly affect the differences in the overall cellulose content of both piles, the interaction between the factors “time × pretreatment” significantly influenced the evolution of this parameter (p < 0.05) (Figure 8b).
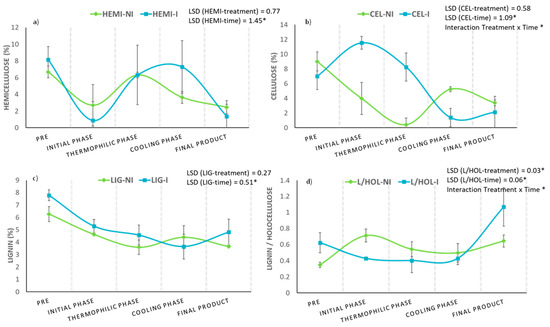
Figure 8.
Evolution of (a) hemicellulose (%), (b) cellulose (%), (c) lignin (%), and (d) lignin/holocellulose ratio in inoculated (I) and non-inoculated (NI) compost piles with a microbial consortium 10 days before the start of the process. HEMI: hemicellulose, CEL: cellulose, LIG: lignin and L/HOL: lignin/holocellulose ratio. Each graph shows the LSD (least significant difference) value as a function of the treatment. Results are means (n = 3) ± SD (vertical bars). LSD *: Fisher’s least significant difference, p < 0.05.
In order to achieve a greater degree of success in the study of the dynamics of lignocellulosic fractions in composting processes, it has been necessary to develop different indices that help to understand their behavior. This is the case of the lignin/holocellulose ratio (L/HOL), whose values tend to increase during the process [11,48,58]. In this work, this parameter almost doubled its value at the end of the process in the bioactivated pile, compared to the non-inoculated one, mainly due to the notable drop in the holocellulose content during the process (Figure 8d).
Recently, Want et al. [59] corroborated that bioaugmentation of a composting process with Bacillus spp. increased the compost temperature, prolonged the duration of the thermophilic phase (1–2 days), and promoted the decomposition of lignocellulosic components (18.1–42.1%). Moreover, inoculation with Bacillus spp. not only affected the succession of bacterial communities by changing the main physico-chemical parameters, but also enhanced the ability to promote plant growth.
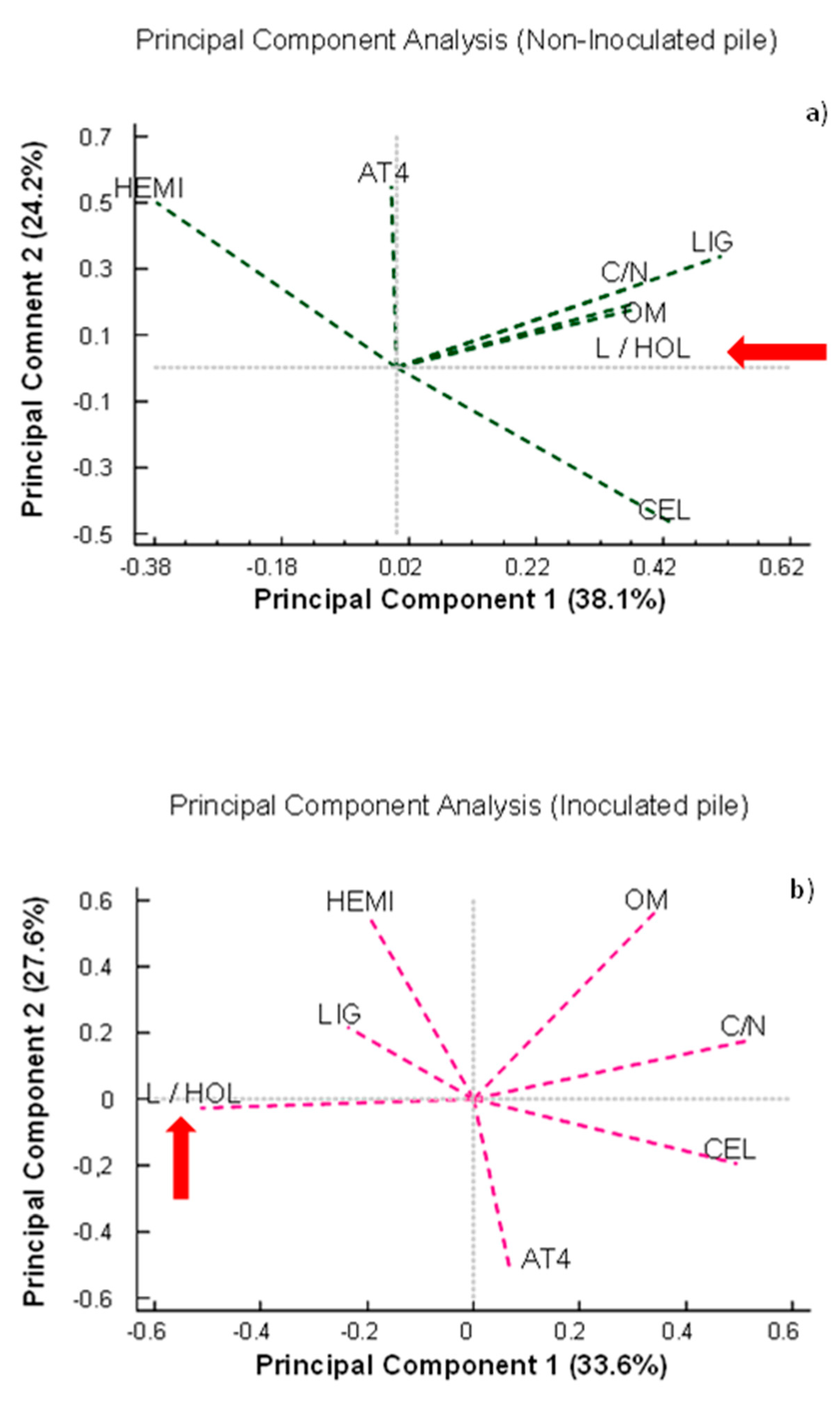
It should be taken into account that the behavior of the lignocellulosic matrix cannot be analyzed independently of the other parameters, as all of them together influence the biodegradability of the samples. Therefore, once the results were objectively described, all the study variables were analyzed by means of a principal component analysis (Figure 9). This analysis revealed that the parameters of monitoring, stability, and maturation of the composting process were differently related to each other depending on the treatment. In the non-inoculated pile (Figure 9a), the L/HOL ratio was positively correlated with OM, the C/N ratio, and the percentages of lignin and cellulose, which was not logical considering that since the L/HOL ratio is an indicator of the stability and maturity of the process (red arrow), it should increase as the C/N ratio and OM decrease. However, a more logical behavior was observed in the case of the pile bioactivated with the microbial consortium. In this case, the L/HOL ratio was negatively correlated with the C/N ratio, OM, cellulose content, and AT4 (Figure 9b). The relationship between the different variables studied therefore confirmed that the bioactivation of the materials prior to the composting process could favor the biodegradation processes, facilitate the access of microorganisms to the lignocellulosic complex, and therefore, improve the dynamics of the composting process in terms of stabilization and maturity.
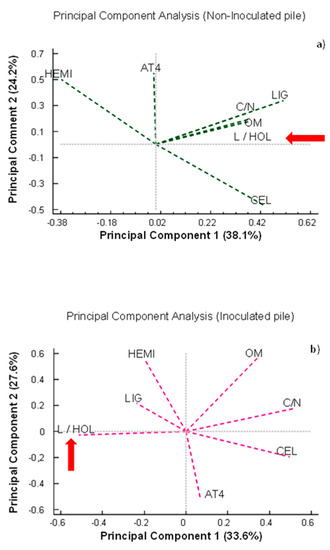
Figure 9.
Principal component analysis (PCA) based on data on monitoring, stability, and maturation parameters. HEMI: hemicellulose; AT4: cumulative oxygen consumption after 4 days; C/N: carbon/nitrogen ratio; LIG: lignin; L/HOL: lignin/holocellulose ratio; OM: organic matter; CEL: cellulose. The red arrow shows the position of the L/HOL ratio in relation to the other parameters analyzed. (a) Non-inoculated pile and (b) inoculated pile. PC: principal component.
4. Conclusions
The results, although preliminary, revealed how inoculation with a microbial formulation based on B. licheniformis and B. safensis served to bioactivate the mixture of lignocellulosic materials in stages prior to the composting process. Based on the results obtained in the laboratory-scale bioactivation assay, inoculation with B. safensis gave rise to a greater degradation of the lignocellulosic fractions. In the case of cellulose, the degradation was especially notable in the short term (10 days), while in the case of hemicellulose and lignin it was later, although it was observed continuously until the 30 days that the test lasted. Regarding the biodegradability of the samples (AT4), inoculation with both microorganisms at low doses allowed maintaining the biodegradability of the materials in the longer term, compared to what was observed in non-inoculated materials.
Although the inoculation did not significantly affect the basic conditioning parameters at the beginning of the process (organic matter, C/N ratio, and moisture), a change in the dynamics of the lignocellulosic fractions was observed during the composting process, as well as in the evolution of other stability and maturity parameters, in particular the AT4 index and the lignin/holocellulose ratio. These results, therefore, revealed the potential of Bacillus safensis and Bacillus licheniformis to degrade the lignocellulosic fraction of plant residues in bioaugmentation strategies prior to the start of the composting process, accelerating the bio-oxidative phase and obtaining products with a higher degree of stability and maturity.
Author Contributions
Conceptualization, M.J.L. and F.S.-E.; methodology, M.R.M.-G., A.J.T. and M.J.E.-G.; investigation, M.R.M.-G., F.S.-E., J.A.L.-G. and M.M.J.; data curation, M.R.M.-G. and M.J.E.-G.; writing—original draft preparation, M.R.M.-G. and F.S.-E.; writing—review and editing, F.S.-E. and M.J.L.; supervision, F.S.-E. and M.J.L.; funding acquisition, F.S.-E. and M.J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been funded thanks to the project UAL2020-BIO-B1964 (Operative Program FEDER Andalucía 2014–2020, Regional Ministry of Economy, Knowledge, Business, and University).
Data Availability Statement
Data will be made available upon request.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationship that could have appeared to influence the work reported in this paper.
References
- World Population Prospects, 2017 Revision. Available online: https://population.un.org/wpp/publications/files/wpp2017_keyfindings.pdf (accessed on 20 February 2023).
- FAO. Food and Agriculture Organization: The State of Food Security and Nutrition in the World 2017. Building Resilience for Peace and Food Security. 2017. Available online: https://www.refworld.org.es/docid/5b0860214.html (accessed on 20 February 2023).
- Aznar-Sánchez, J.A.; Velasco-Muñoz, J.F.; García-Arca, D.; López-Felices, B. Identification of opportunities for applying the circular economy to intensive agriculture in Almería (South-East Spain). Agronomy 2020, 10, 1499. [Google Scholar] [CrossRef]
- Pazienza, P.; De Lucia, C. For a new plastics economy in agriculture: Policy reflections on the EU strategy from a local perspective. J. Cleaner Prod. 2020, 253, 119844. [Google Scholar] [CrossRef]
- Duque-Acevedo, M.; Belmonte-Ureña, L.J.; Plaza-Úbeda, J.A.; Camacho-Ferre, F. The management of agricultural waste biomass in the framework of circular economy and bioeconomy: An opportunity for greenhouse agriculture in Southeast Spain. Agronomy 2020, 10, 489. [Google Scholar] [CrossRef]
- Millati, R.; Cahyono, R.B.; Ariyanto, T.; Azzahrani, I.N.; Putri, R.U.; Taherzadeh, M.J. Agricultural, industrial, municipal, and forest wastes. In Sustainable Resource Recovery and Zero Waste Approaches; Taherzadeh, M.J., Bolton, K., Wong, J., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–22. ISBN 978-04-4464-200-4. [Google Scholar]
- INE National Statistics Institute (Spain). Survey on Waste Generation in the Industrial Sector. 2022. Available online: https://www.ine.es/jaxi/Datos.htm?path=/t26/e068/p02/serie/l0/&file=03001.px (accessed on 15 March 2023).
- De Mendonça Costa, L.A.; de Mendonça Costa, M.S.S.; Damaceno, F.M.; Chiarelotto, M.; Bofinger, J.; Gazzola, W. Bioaugmentation as a strategy to improve the compost quality in the composting process of agro-industrial wastes. Environ. Technol. Innov. 2021, 22, 101478. [Google Scholar] [CrossRef]
- Wu, H.; Ge, Y. Excessive application of fertilizer, agricultural non-point source pollution, and farmers’ policy choice. Sustainability 2019, 11, 1165. [Google Scholar] [CrossRef]
- Greff, B.; Szigeti, J.; Nagy, Á.; Lakatos, E.; Varga, L. Influence of microbial inoculants on co-composting of lignocellulosic crop residues with farm animal manure: A review. J. Environ. Manag. 2022, 302, 114088. [Google Scholar] [CrossRef]
- Jurado, M.; López, M.J.; Suárez-Estrella, F.; Vargas-García, M.C.; López-González, J.A.; Moreno, J. Exploiting composting biodiversity: Study of the persistent and biotechnologically relevant microorganisms from lignocellulose-based composting. Bioresour. Technol. 2014, 162, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Folino, A.; Pangallo, D.; Calabrò, P. Assessing bioplastics biodegradability by standard and research methods: Current trends and open issues. J. Environ. Chem. Eng. 2023, 11, 109424. [Google Scholar] [CrossRef]
- Cucina, M.; De Nisi, P.; Trombino, L.; Tambone, F.; Adani, F. Degradation of bioplastics in organic waste by mesophilic anaerobic digestion, composting and soil incubation. Waste Manag. 2021, 134, 67–77. [Google Scholar] [CrossRef]
- Gadaleta, G.; De Gisi, S.; Todaro, F.; Notarnicola, M. Carbon footprint and total cost evaluation of different bio-plastics waste treatment strategies. Clean Technol. 2022, 4, 570–583. [Google Scholar] [CrossRef]
- Gadaleta, G.; Ferrara, C.; De Gisi, S.; Notarnicola, M.; De Feo, G. Life cycle assessment of end-of-life options for cellulose-based bioplastics when introduced into a municipal solid waste management system. Sci. Total Environ. 2023, 871, 161958. [Google Scholar] [CrossRef]
- ASTM D7075-04; Standard Practice for Evaluating and Reporting Environmental Performance of Biobased Products. ASTM International: West Conshohocken, PA, USA, 2004.
- ISO 14001:2015; Environmental management systems—Requirements with guidance for use. ISO: Geneva, Switzerland, 2015.
- Emadian, S.M.; Onay, T.T.; Demirel, B. Biodegradation of bioplastics in natural environments. Waste Manag. 2017, 59, 526–536. [Google Scholar] [CrossRef]
- Bishop, G.; Styles, D.; Lens, P.N. Environmental performance comparison of bioplastics and petrochemical plastics: A review of life cycle assessment (LCA) methodological decisions. Resour. Conserv. Recycl. 2021, 168, 105451. [Google Scholar] [CrossRef]
- Burgos, N.; Valdés, A.; Jiménez, A. Valorization of agricultural wastes for the production of protein-based biopolymers. J. Renew. Mater. 2016, 4, 165–177. [Google Scholar] [CrossRef]
- Kaur, H.; Banipal, T.S.; Thakur, S.; Bakshi, M.S.; Kaur, G.; Singh, N. Novel biodegradable films with extraordinary tensile strength and flexibility provided by nanoparticles. ACS Sustain. Chem. Eng. 2013, 1, 127–136. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, H.; Wang, B.; Chen, C.; Zou, X.; Cheng, T.; Li, J. Aerobic co-composting of mature compost with cattle manure: Organic matter conversion and microbial community characterization. Bioresour. Technol. 2023, 382, 129187. [Google Scholar] [CrossRef]
- Liu, N.; Zhou, J.; Han, L.; Huang, G. Characterization of lignocellulosic compositions’ degradation during chicken manure composting with added biochar by phospholipid fatty acid (PLFA) and correlation analysis. Sci. Total Environ. 2017, 586, 1003–1011. [Google Scholar] [CrossRef]
- López-González, J.A.; Suárez-Estrella, F.; Vargas-García, M.C.; López, M.J.; Jurado, M.M.; Moreno, J. Dynamics of bacterial microbiota during lignocellulosic waste composting: Studies upon its structure, functionality and biodiversity. Bioresour. Technol. 2015, 175, 406–416. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; El-Din, M.N.; Refaat, B.M.; Abdel-Shakour, E.H.; Ewais, E.E.D.; Alrefaey, H.M. Biotechnological application of thermotolerant cellulose-decomposing bacteria in composting of rice straw. Ann. Agric. Sci. 2016, 61, 135–143. [Google Scholar] [CrossRef]
- Duan, M.; Zhang, Y.; Zhou, B.; Qin, Z.; Wu, J.; Wang, Q.; Yin, Y. Effects of Bacillus subtilis on carbon components and microbial functional metabolism during cow manure–straw composting. Bioresour. Technol. 2020, 303, 122868. [Google Scholar] [CrossRef]
- Martínez-Gallardo, M.R.; López, M.J.; Jurado, M.M.; Suárez-Estrella, F.; López-González, J.A.; Sáez, J.A.; Moral, R.; Moreno, J. Bioremediation of Olive Mill Wastewater sediments in evaporation ponds through in situ composting assisted by bioaugmentation. Sci. Total Environ. 2020, 703, 135537. [Google Scholar] [CrossRef]
- López-González, J.A.; Vargas-García, M.C.; López, M.J.; Suárez-Estrella, F.; Jurado, M.; Moreno, J. Enzymatic characterization of microbial isolates from lignocellulose waste composting: Chronological evolution. J. Environ. Manag. 2014, 145, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Satomi, M.; La Duc, M.T.; Venkateswaran, K. Bacillus safensis sp. nov., isolated from spacecraft and assembly-facility surfaces. Int. J. Syst. Evol. Microbiol. 2006, 56, 1735–1740. [Google Scholar] [CrossRef]
- Kothari, V.V.; Kothari, R.K.; Kothari, C.R.; Bhatt, V.D.; Nathani, N.M.; Koringa, P.G.; Joshi, C.G.; Vyas, B.R. Genome Sequence of Salt-Tolerant Bacillus safensis Strain VK, Isolated from Saline Desert Area of Gujarat, India. Genome Announc. 2013, 1, e00671-13. [Google Scholar] [CrossRef] [PubMed]
- Miles, A.A.; Misra, S.S.; Irwin, J.O. The estimation of the bactericidal power of the blood. Epidemiol. Infect. 1938, 38, 732–749. [Google Scholar] [CrossRef]
- Barrena, R.; Pagans, E.; Faltys, G.; Sánchez, A. Effect of inoculation dosing on the composting of source-selected organic fraction of municipal solid wastes. J. Chem. Technol. Biotechnol. 2006, 81, 420–425. [Google Scholar] [CrossRef]
- Jurado, M.M.; Suárez-Estrella, F.; López, M.J.; Vargas-García, M.C.; López-González, J.A.; Moreno, J. Enhanced turnover of organic matter fractions by microbial stimulation during lignocellulosic waste composting. Biores. Technol. 2015, 186, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fan, B.; Hu, Q.; Yin, Z. Effect of inoculation with Penicillium expansum on the microbial community and maturity of compost. Biores. Technol. 2011, 102, 11189–11193. [Google Scholar] [CrossRef]
- Barrena, R.; d’Imporzano, G.; Ponsá, S.; Gea, T.; Artola, A.; Vázquez, F.; Sánchez, A.; Adani, F. In search of a reliable technique for the determination of the biological stability of the organic matter in the mechanical–biological treated waste. J. Hazar. Mater. 2009, 162, 1065–1072. [Google Scholar] [CrossRef]
- Ponsá, S.; Gea, T.; Sánchez, A. Different indices to express biodegradability in organic solid wastes. J. Environ. Qual. 2010, 39, 706–712. [Google Scholar] [CrossRef]
- Adani, F.; Ubbiali, C.; Generini, P. The determination of biological stability of composts using the Dynamic Respiration Index: The results of experience after two years. Waste Manag. 2006, 26, 41–48. [Google Scholar] [CrossRef]
- Antizar-Ladislao, B.; Lopez-Real, J.; Beck, A.J. Degradation of polycyclic aromatic hydrocarbons (PAHs) in an aged coal tar contaminated soil under in-vessel composting conditions. Environ. Pollut. 2006, 141, 459–468. [Google Scholar] [CrossRef]
- Tomati, U.; Madejon, E.; Galli, E. Evolution of humic acid molecular weight as an index of compost stability. Compost Sci. Util. 2000, 8, 108–115. [Google Scholar] [CrossRef]
- Castaldi, P.; Alberti, G.; Merella, R.; Melis, P. Study of the organic matter evolution during municipal solid waste composting aimed at identifying suitable parameters for the evaluation of compost maturity. Waste Manag. 2005, 25, 209–213. [Google Scholar] [CrossRef]
- Liang, C.; Das, K.C.; McClendon, R.W. The influence of temperature and moisture contents regimes on the aerobic microbial activity of a biosolids composting blend. Biores. Technol. 2003, 86, 131–137. [Google Scholar] [CrossRef]
- Moreno, J.; Moral, R. Compostaje; Mundi-Prensa: Madrid, Spain, 2008; 570p. [Google Scholar]
- Azim, K.; Soudi, B.; Boukhari, S.; Perissol, C.; Roussos, S.; Thami Alami, I. Composting parameters and compost quality: A literature review. Org. Agric. 2018, 8, 141–158. [Google Scholar] [CrossRef]
- Iglesias-Jiménez, E.; Barral-Silva, M.T.; Marhuenda-Egea, F. Indicadores de la Estabilidad y Madurez del Compost. In Compostaje; Moreno Casco, J., Moral Herrero, R., Eds.; Mundi Prensa: Madrid, Spain, 2008; pp. 243–283. [Google Scholar]
- Hue, N.V.; Liu, J. Predicting compost stability. Compost Sci. Util. 1995, 3, 8–15. [Google Scholar] [CrossRef]
- Haddadin, M.S.; Haddadin, J.; Arabiyat, O.I.; Hattar, B. Biological conversion of olive pomace into compost by using Trichoderma harzianum and Phanerochaete chrysosporium. Biores. Technol. 2009, 100, 4773–4782. [Google Scholar] [CrossRef]
- Francou, C.; Linères, M.; Derenne, S.; Le Villio-Poitrenaud, M.; Houot, S. Influence of green waste, biowaste and paper-cardboard initial ratios on organic matter transformations during composting. Biores. Technol. 2008, 99, 8926–8934. [Google Scholar] [CrossRef]
- Malherbe, S.; Cloete, T.E. Lignocellulose biodegradation: Fundamentals and applications. Rev. Environ. Sci. Biotechnol. 2002, 1, 105–114. [Google Scholar] [CrossRef]
- Smidt, E.; Meissl, K.; Schmutzer, M.; Hinterstoisser, B. Co-composting of lignin to build up humic substances—Strategies in waste management to improve compost quality. Ind. Crops Prod. 2008, 27, 196–201. [Google Scholar] [CrossRef]
- EPA (Environmental Protection Agency, USA). Environmental Regulations and Technology. Control of Pathogens and Vector Attraction in Sewage Sludge; EPA625-R-92-013; EPA: Washington, DC, USA, 2023.
- Stentiford, E.; de Bertoldi, M. Composting: Process. In Solid Waste Technology & Management; Christensen, T.H., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2010; Volume 1–2. [Google Scholar]
- BOE-A-2017-14332; Regulation 999/2017 on Fertilizer Products. Ministry of the Presidency and for the Territorial Administrations: Madrid, Spain, 2017; pp. 119396–119450.
- Ameen, A.; Ahmad, J.; Raza, S. Effect of pH and moisture content on composting of Municipal solid waste. Int. J. Sci. Res. Publ. 2016, 6, 35–37. [Google Scholar]
- Komilis, D.P.; Ham, R.K. The effect of lignin and sugars to the aerobic decomposition of solid wastes. Waste Manag. 2003, 23, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Said-Pullicino, D.; Kaiser, K.; Guggenberger, G.; Gigliotti, G. Changes in the chemical composition of water-extractable organic matter during composting: Distribution between stable and labile organic matter pools. Chemosphere 2007, 66, 2166–2176. [Google Scholar] [CrossRef] [PubMed]
- Estrella-González, M.J.; Suárez-Estrella, F.; Jurado, M.M.; López, M.J.; López-González, J.A.; Siles-Castellano, A.B.; Muñoz-Mérida, A.; Moreno, J. Uncovering new indicators to predict stability, maturity and biodiversity of compost on an industrial scale. Bioresour. Technol. 2020, 313, 123557. [Google Scholar] [CrossRef]
- Wei, H.; Tucker, M.P.; Baker, J.P.; Harris, M.; Luo, Y.; Xu, Q.; Himmel, M.E.; Dimg, S.Y. Tracking dynamics of plant biomass composting by changes in substrate structure, microbial community, and enzyme activity. Biotechnol. Biofuels 2012, 5, 20. [Google Scholar] [CrossRef]
- Estrella-González, M.J.; Jurado, M.M.; Suárez-Estrella, F.; López, M.J.; López-González, J.A.; Siles-Castellano, A.; Moreno, J. Enzymatic profiles associated with the evolution of the lignocellulosic fraction during industrial-scale composting of anthropogenic waste: Comparative analysis. J. Environ. Manag. 2019, 248, 109312. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, T.; Xing, Z.; Zhang, Q.; Niu, X.; Yu, Y.; Teng, Z.; Chen, J. Enhanced lignocellulose degradation and composts fertility of cattle manure and wheat straw composting by Bacillus inoculation. J. Environ. Chem. Eng. 2023, 11, 109940. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).