Study of Raw Material Pretreatment and the Microbiota Selection Effect on the Composting Process Efficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Trials
2.2. Temperature and Humidity Monitoring
2.3. Sampling and Testing
2.4. Final Compost Quality Testing
2.5. Statistical Analysis
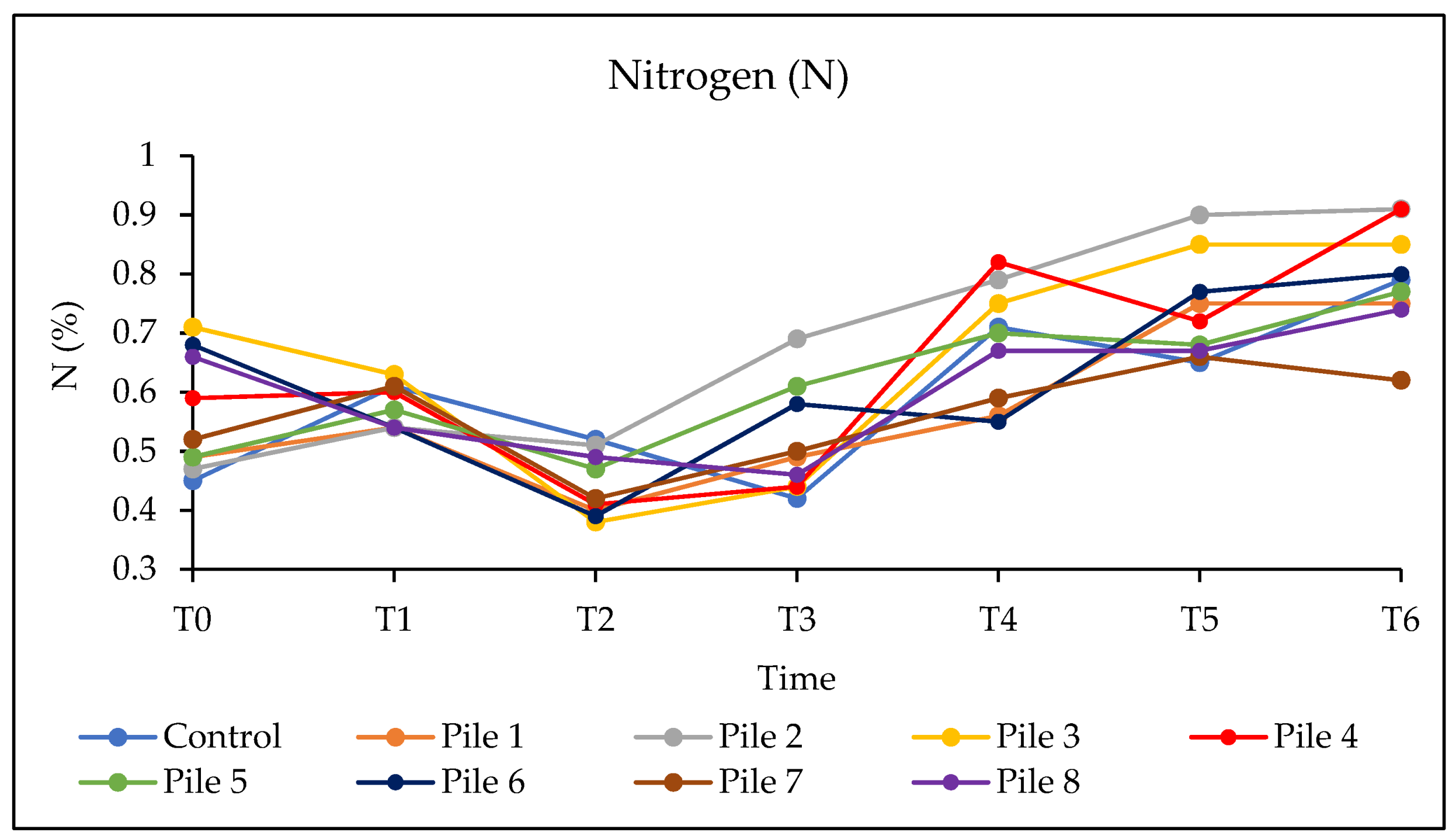
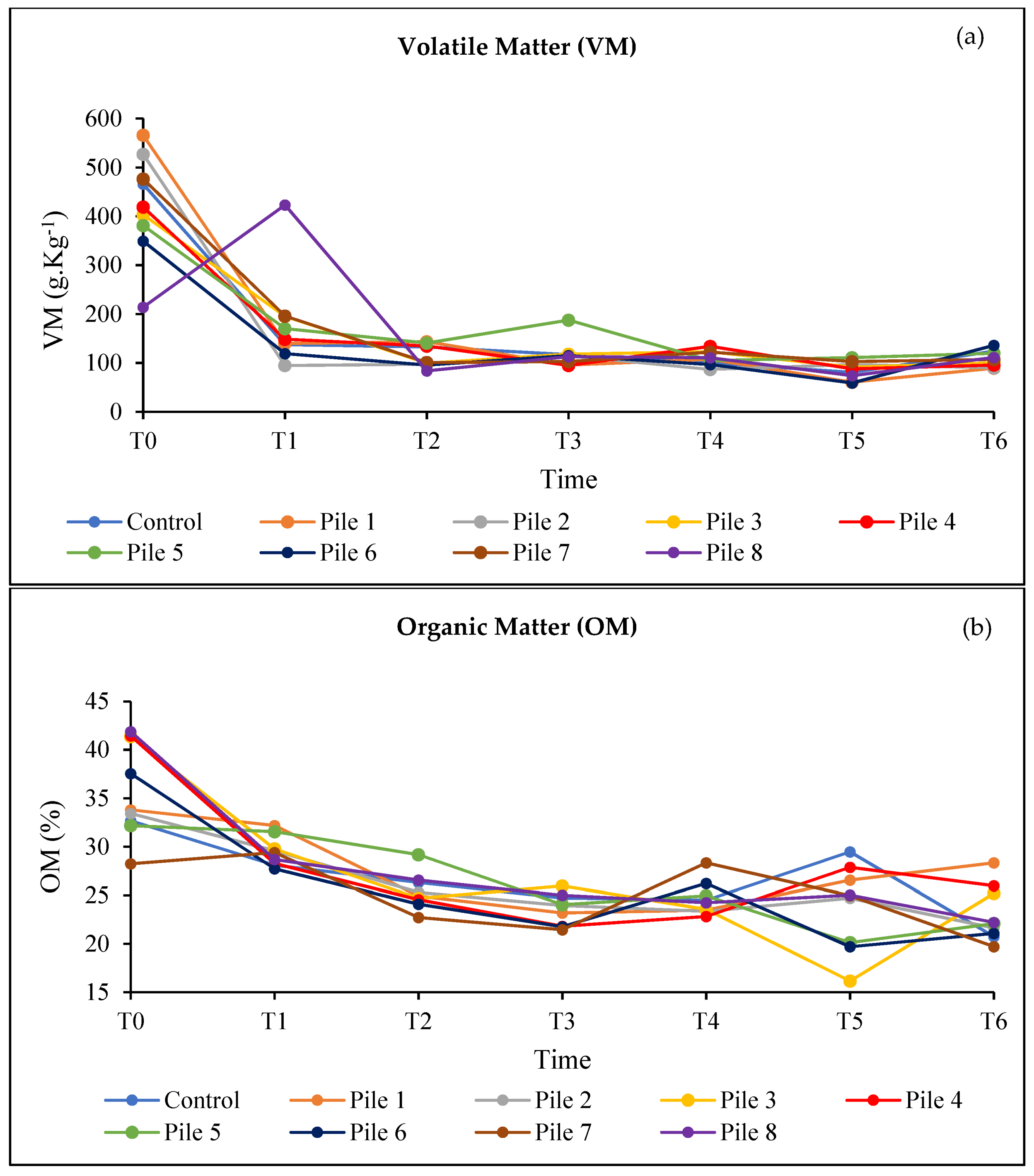
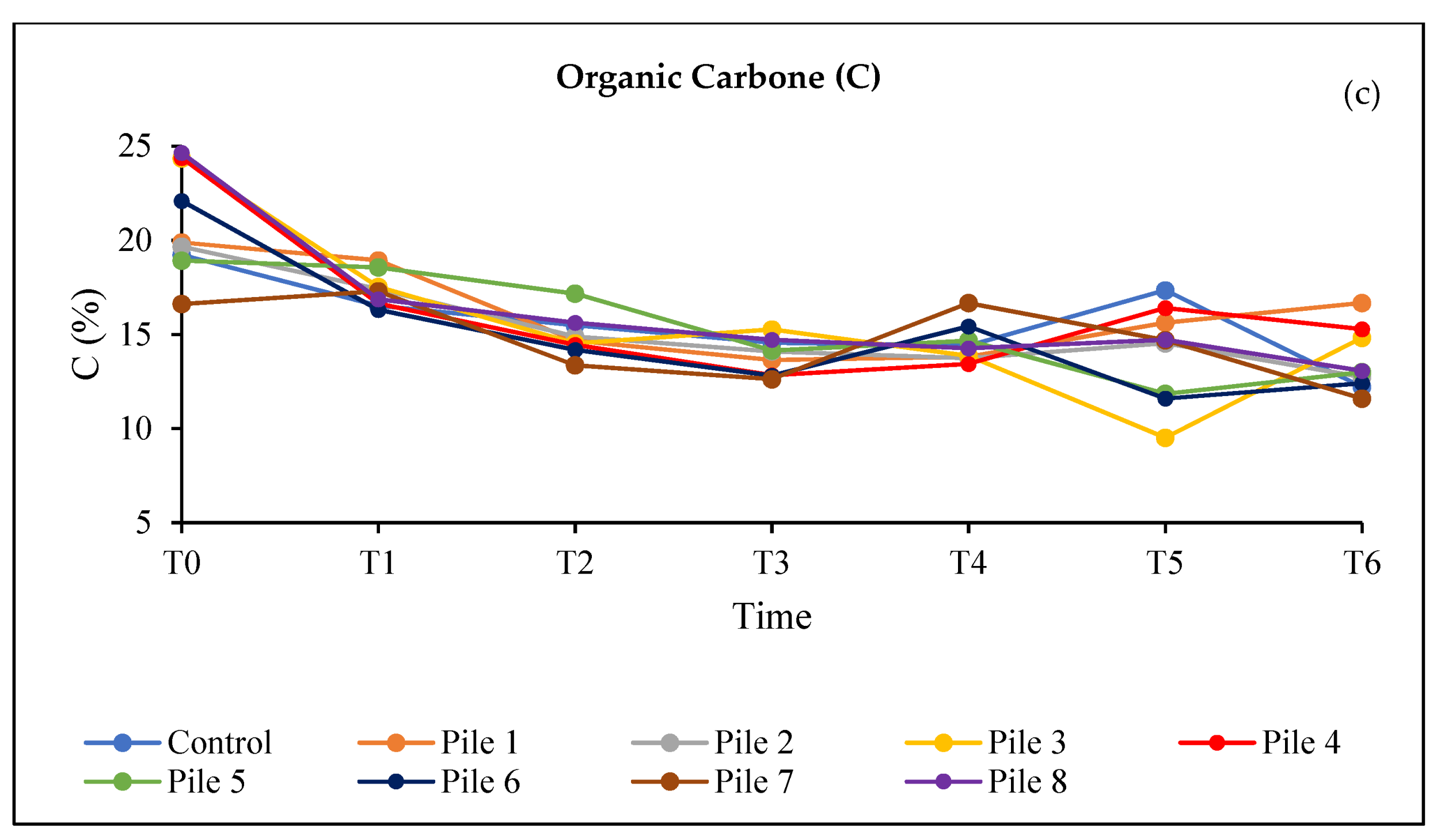
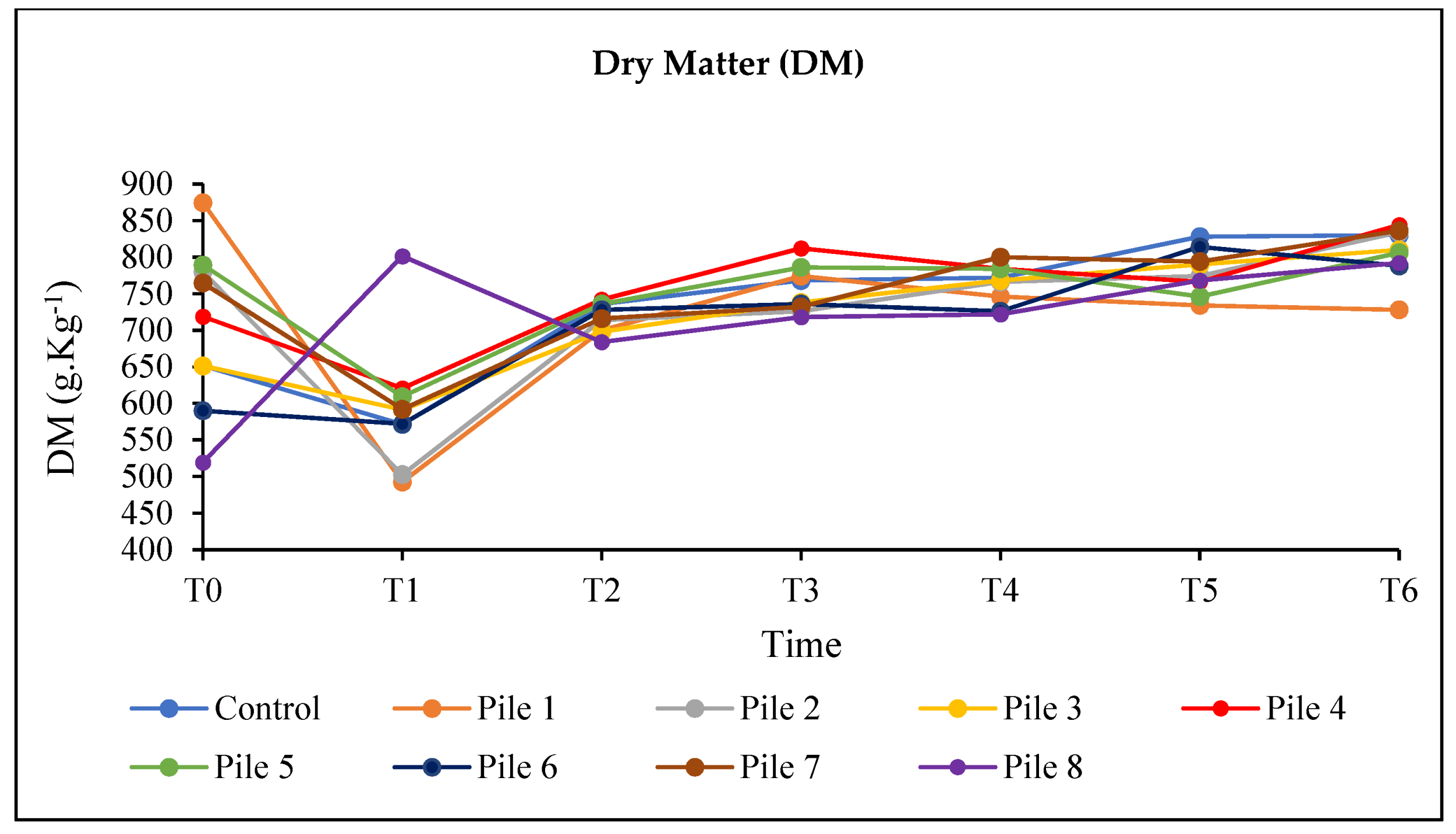
3. Results and Discussion
3.1. Temperature Variation
3.2. pH Variation
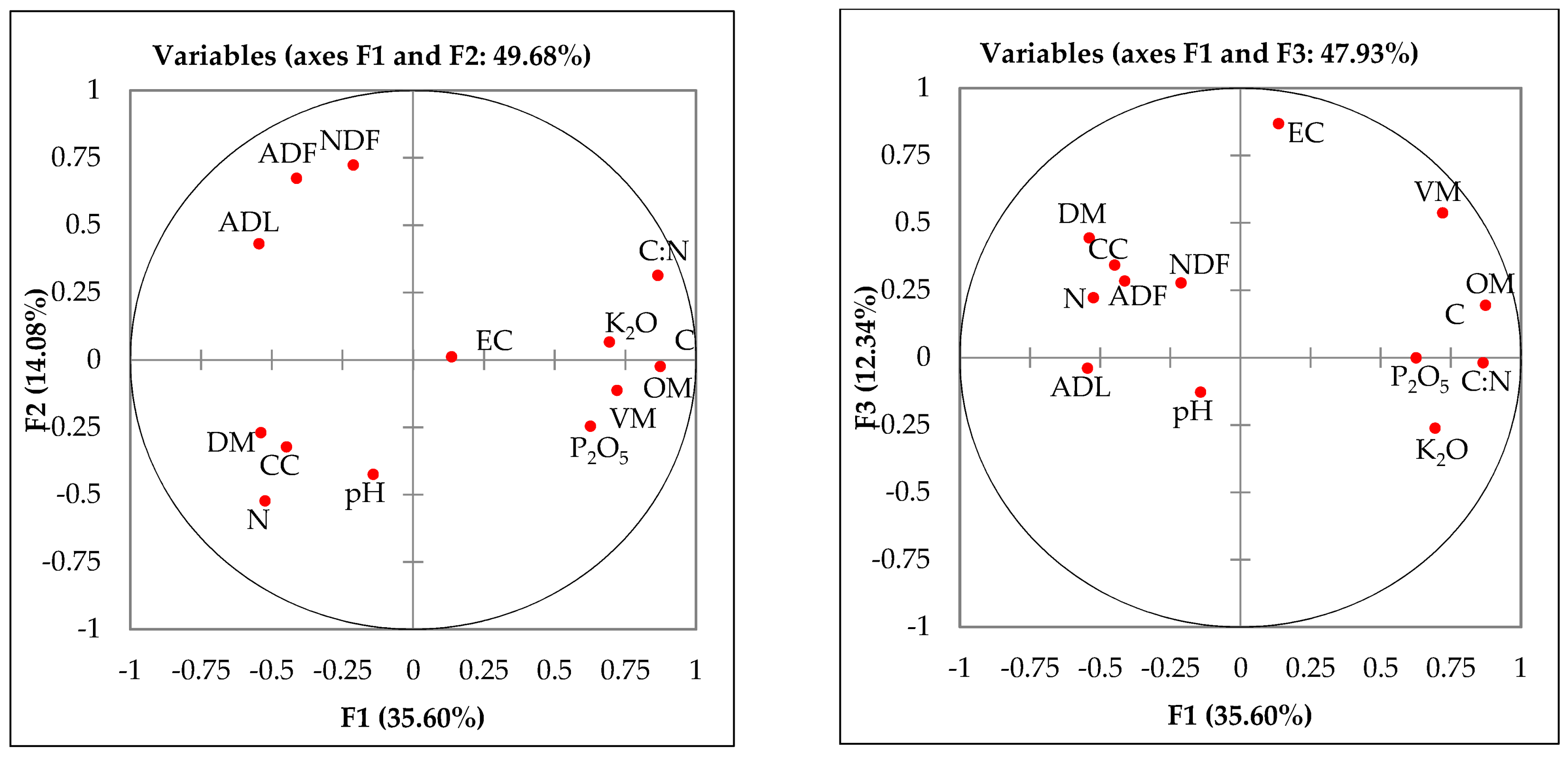
3.3. Principal Component Data Analysis (PCA) of All the Parameters
3.4. Evaluation of the Quality of the Final Compost
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polprasert, C. Organic waste recycling: Technology and management. In Composting-Part 3, 3rd ed.; IWA Publishing: London, UK, 2007; p. 89. [Google Scholar]
- Ryckeboer, J.; Mergaert, J.; Vaes, K.; Klammer, S.; De Clercq, D.; Coosemans, J.; Insam, H.; Swings, J. A survey of bacteria and fungi occurring during composting and self-heating processes. Ann. Microbiol. 2003, 53, 349–410. [Google Scholar]
- Golueke, C.G. Principles of composting. In The Staff of BioCycle Journal of Waste Recycling. The Art and Science of Composting; The JG Press Inc: Emmaus, PL, USA, 1991; pp. 14–27. [Google Scholar]
- M’barek, H.N.; Taidi, B.; Smaoui, T.; Aziz, M.B.; Mansouri, A.; Hajjaj, H. Isolation, screening and identification of ligno-cellulolytic fungi from northern central Morocco. Biotechnol. Agron. Soc. Environ. 2019, 23, 207–217. [Google Scholar]
- Wichuk, K.; Mccartney, D. A review of the effectiveness of current time-temperature regulations on pathogen inactivation during composting. J. Environ. Eng. Sci. 2007, 6, 573–586. [Google Scholar] [CrossRef]
- Tiquia, S.M.; Richard, T.L.; Honeyman, M.S. Effect of windrow turning and seasonal temperatures on composting of hog manure from hoop structures. Environ. Technol. 2000, 21, 1037–1046. [Google Scholar] [CrossRef]
- Bertoldi, M.; Sequi, P.; Lemmes, B.; Papi, T. The Science of Composting; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Mason, I.G. Mathematical modelling of the composting process: A review. Waste Manag. 2006, 26, 3–21. [Google Scholar] [CrossRef]
- Canet, R.; Pomares, F. Changes in physical, chemical and physico-chemical parameters during the composting of municipal solid wastes in two plants in Valencia. Bioresour. Technol. 1995, 51, 259–264. [Google Scholar] [CrossRef]
- Stoffella, P.J.; Kahn, B.A. Compost Utilization in Horticultural Cropping Systems; Lewis: Boca Raton, FL, USA, 2001. [Google Scholar]
- Navarro, A.F.; Cegarra, J.; Roig, A.; Garcia, D. Relationships between organic matter and carbon contents of organic wastes. Bioresour. Technol. 1993, 44, 203–207. [Google Scholar] [CrossRef]
- Brinton, W. Compost Quality Standards and Guidelines; Woods End Research Laboratory: Augusta, ME, USA, 2000. [Google Scholar]
- Larsen, K.L.; McCartney, D.M. Effect of C:N ratio on microbial activity and N- retention: Bench-scale study using pulp and paper biosolids. Compost Sci. Util. 2000, 8, 147–159. [Google Scholar] [CrossRef]
- Bertoldi, M.; Ferranti, M.P.; L’Hermite, P.; Zucconi, F. Compost: Production, Quality, and Use; Commission of the European Communities: London, UK; Elsevier Science Pub. Co.: New York, NY, USA, 1987. [Google Scholar]
- Gobat, J.M.; Aragno, M.; Matthey, W. Le Sol Vivant: Bases De Pédologie, Biologie Des Sols; Collection Gérer L’environnement; Presses Polytechniques et Universitaires Romandes: Lausanne, Switzerland, 1998. [Google Scholar]
- Perner, H.; Schwarz, D.; George, E. Effect of mycorrhizal inoculation and compost supply on growth and nutrient uptake of young leek plants grown on peat-based substrates. Hort. Sci. 2006, 41, 628–632. [Google Scholar] [CrossRef]
- Saharinen, M.H. Evaluation of changes of CEC during composting. Compost Sci. Util. 1998, 6, 29–37. [Google Scholar] [CrossRef]
- Leclerc, B. Guide Des Matières Organiques; Institut Technique de L’agriculture Biologique: Paris, France, 2001. [Google Scholar]
- Iwegbue, C.M.; Egun, A.C.; Emuh, F.N.; Isirimah, N.O. Compost Maturity Evaluation and its Significance to Agriculture. Pak. J. Biol. Sci. 2006, 9, 2933–2944. [Google Scholar] [CrossRef][Green Version]
- Guo, R.; Li, G.; Jiang, T.; Schuchardt, F.; Chen, T.; Zhao, Y.; Shen, Y. Effect of aeration rate, C/N ratio and moisture content on the stability and maturity of compost. Bioresour. Technol. 2012, 112, 171–178. [Google Scholar] [CrossRef]
- Yang, B.; Wyman, C.E. Pretreatment: The key to unlocking low-cost cellulosic ethanol. Biofuels Bioprod. Biorefining 2008, 2, 26–40. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef]
- Laureano-Perez, L.; Teymouri, F.; Alizadeh, H.; Dale, B.E. Understanding factors that limit enzymatic hydrolysis of biomass: Characterization of pretreated corn stover. Appl. Biochem. Biotechnol. 2005, 124, 1081–1099. [Google Scholar] [CrossRef]
- Bobleter, O. Hydrothermal degradation of polymers derived from plants. Prog. Polym. Sci. 1994, 19, 797–841. [Google Scholar] [CrossRef]
- Grabber, J.H. How do lignin composition, structure, and cross-linking affect degradability. A review of cell wall model studies. Crop. Sci. 2005, 45, 820. [Google Scholar] [CrossRef]
- Kayembe, K. Inhibitory Effects of Phenolic Monomers on Methanogenesis in Anaerobic Digestion. Br. Microbiol. Res. J. 2013, 3, 32–41. [Google Scholar] [CrossRef]
- Carrere, H.; Antonopoulou, G.; Affes, R.; Passos, F.; Battimelli, A.; Lyberatos, G.; Ferrer, I. Review of feedstock pretreatment strategies for improved anaerobic digestion: From lab-scale research to full-scale application. Bioresour. Technol. 2016, 199, 386–397. [Google Scholar] [CrossRef]
- Zhang, Y.H.P.; Ding, S.Y.; Mielenz, J.R.; Cui, J.B.; Laser, M.; Himmel, M.E.; McMillan, J.R.; Lynd, L.R. Fractionating recalcitrant lignocellulose at modest reaction conditions. Biotechnol. Bioeng. 2007, 97, 214–223. [Google Scholar] [CrossRef]
- Ariunbaatar, J.; Panico, A.; Esposito, G.; Pirozzi, F.; Lens, P.N.L. Pretreatment methods to enhance anaerobic digestion of organic solid waste. Appl. Energy 2014, 123, 143–156. [Google Scholar] [CrossRef]
- Modenbach, A.A.; Nokes, S.E. The use of high-solids loadings in biomass pretreatment-a review. Biotechnol. Bioeng. 2012, 109, 1430–1442. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Wyman, C.E. Effects of cellulase and xylanase enzymes on the deconstruction of solids from pretreatment of poplar by leading technologies. Biotechnol. Prog. 2009, 25, 302–314. [Google Scholar] [CrossRef]
- Sills, D.L.; Gossett, J.M. Assessment of commercial hemicellulases for saccharification of alkaline pretreated perennial biomass. Bioresour. Technol. 2011, 102, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.B.; Ramakrishnan, S. Chemical and Physicochemical Pretreatment of Lignocellulosic Biomass: A Review. Enzym. Res. 2011, 2011, 787532. [Google Scholar] [CrossRef]
- Sharma, N.; Acharya, S.; Kumar, K.; Singh, N.; Chaurasia, O.P. Hydroponics as an advanced technique for vegetable production: An overview. J. Soil. Water Conserv. 2018, 17, 364–371. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Ai, B.; Liao, Q. A review on extrusion-based lignocellulosic biomass pretreatment for biogas production. Renew. Sustain. Energy Rev. 2019, 114, 109329. [Google Scholar]
- Tannouri, A.; Rizk, Z.; Al Daccache, M.; Ghanem, C.; Azzi, V.; Haddad, R.; Maroun, R.G.; Hobaika, Z.; Badra, R.; Salameh, D. Characterization of Antagonist Potential of Selected Compost Bacterial Isolates (CBI) against Plant and Human Pathogens. Agronomy 2022, 12, 2977. [Google Scholar] [CrossRef]
- Vats, S.; Maurya, D.P.; Shaimoon, M.; Agarwal, A.; Negi, S. Development of a microbial consortium for production of blend of enzymes for hydrolysis of agricultural wastes into sugars. J. Sci. Ind. Res. 2013, 72, 585–590. [Google Scholar]
- Sanchez, C. Lignocellulosic Residues: Biodegradation and Bioconversion by Fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef]
- Achkar, J.E. Méthanisation De Marc De Raisin. Caractérisation et Optimisation Du Procédé et Des Prétraitements. Ph.D. Thesis, Université de Bretagne Sud, Lorient, France, 2017; pp. 30–31. [Google Scholar]
- Tannoury, A.; Salameh, D.; Rizk, Z. Adapted Bacterial Formulation with Fastening Ability of Compost. Patent No: 122234, 8 June 2021. [Google Scholar]
- Zhao, H.-M.; Du, H.; Feng, N.-X.; Mo, C.M. Biodegradation of di-n-butylphthalate and phthalic acid by a novel Providencia sp. 2D and its stimulation in a compost-amended soil. Biol. Fertil. Soils 2016, 52, 65–76. [Google Scholar] [CrossRef]
- Shirling, E.B.; Gottlieb, D. Methods for characterization of Streptomyces species. Int. J. Syst. Bacteriol. 1966, 16, 313–340. [Google Scholar] [CrossRef]
- Rynk, R. Monitoring Moisture in Composting Systems. Biocycle 2008, 41, 53–57. [Google Scholar]
- ISO 10390:2021; Soil, Treated Biowaste and Sludge–Determination of pH. ISO: Geneva, Switzerland, 2021.
- Amery, F.; Vandaele, E.; Körner, I.; Loades, K.; Viaene, J.; Vandecasteele, B.; Willekens, K. Compost Quality Indicators; SOILCOM report number 5.1; European Union: Maastricht, The Netherlands, 2020; 23p. [Google Scholar]
- NF EN 13654-1; Amendements du sol et Supports de Culture-Détermination de l’azote-Partie 1: Méthode de Kjeldahl Modifiée. AFNOR: Saint Denis la Plaine, France, 2002.
- Blakemore, L.; Searle, P.; Daly, B. Methods for Chemical Analysis of Soils; New Zealand Soil Bureau Scientific Report; Department of Scientific and Industrial Research: Wellington, New Zealand, 1987; p. 103. [Google Scholar]
- Sadak, M.Y.; Aymen, E.M.; El Kamel, R. Evaluation du comportement chimique des composts sylvicoles, des tamisats et des mélanges pour la conception des substrats de culture. Rev. Nat. Technol. C- Sci. L’environnement 2013, 8, 54–60. [Google Scholar]
- Afilal, M.E.; Elasri, O.; Merzak, Z. Caractérisations des déchets organiques et évaluation du potentiel Biogaz. J. Mater. Environ. Sci. 2014, 5, 1160–1169. [Google Scholar]
- ISO 15959:2016; Fertilizers–Determination of Extracted Phosphorus. ISO: Geneva, Switzerland, 2016.
- Samara, E.; Matsi, T.; Balidakis, A. Soil application of sewage sludge stabilized with steelmaking slag and its effect on soil properties and wheat growth. Watse Manag. 2017, 68, 378–387. [Google Scholar] [CrossRef]
- ISO 6579-1:2017; Microbiology of the Food Chain-Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp. ISO: Geneva, Switzerland, 2017.
- ISO 9308-1:2014/AMD 1: 2016; Water Quality-Enumeration of Echerchia coli and Coliform Bacteria–Part 1: Membrane Filtration Method for Waters with Low Bacterial Background Flora—Amendment 1. ISO: Geneva, Switzerland, 2016.
- ISO 6888-3:2003; Microbiology of Food and Animal Feeding Atuffs—Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus aureus and Other Species)—Part 3: Detection and MPN technique for low numbers. ISO: Geneva, Switzerland, 2003.
- Zhao, K.; Xu, R.; Zhang, Y.; Tang, H.; Zhou, C.; Cao, A.; Zhao, G.; Guo, H. Development of a novel compound microbial agent for degradation of kitchen waste. Braz. J. Microbiol. 2017, 48, 442–450. [Google Scholar] [CrossRef]
- ISO 7899-2:2000; Water Quality–Detection and Enumeration of Intestinal Enterococci—Part 2: Membrane Filtration method. ISO: Geneva, Switzerland, 2000.
- US EPA. Method 7473 (SW-846): Mercury in Solids and Solutions by Thermal Decomposition, Amalgamation, and Atomic Absorption Spectrophotometry, Revision 0; US EPA: Washington, DC, USA, 1998.
- Sierra, J.; Desfontaines, L.; Faverial, J.; Loranger-Merciris, G.; Boval, M. Composting and vermicomposting of cattle manure and green wastes under tropical conditions: Carbon and nutrient balances and end product quality. Soil Res. 2013, 51, 142–151. [Google Scholar] [CrossRef]
- Bach, P.D.; Nakasaki, K.; Shoda, M.; Kubota, H. Thermal balance in composting operations. Ferment. Technol. 1987, 65, 199–209. [Google Scholar] [CrossRef]
- Yulipriyanto, H. Emission D’effluents Gazeux Lors du Compostage de Substrats Organiques en Relation Avec l’activité Microbiologique (Nitrification/Denitrification). Ph.D. Thesis, Université de Rennes, Rennes, France, 2001. [Google Scholar]
- Zbytniewski, R.A.; Buszewski, B. Characterization of natural organic matter (NOM) derived from sewage sludge compost. Part 2: Multivariate techniques in the study of compost maturation. Biores. Technol. 2005, 96, 479–484. [Google Scholar] [CrossRef]
- Karnchanawong, S.; Mongkontep, T.; Praphunsri, K. Effect of green waste pretreatment by sodium hydroxide and biomass fly ash on composting process. J. Clean Prod. 2016, 146, 14–19. [Google Scholar] [CrossRef]
- Mustin, M. Le Compost, Gestion De La Matière Organique; Editions Francois Dubusc: Paris, France, 1987. [Google Scholar]
- Komilis, D.P.; Ham, K.R.; Park, J. Emission of volatile organic compounds during composting of municipal solid wastes. Water Res. 2004, 38, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Bertoldi, M.; Vallini, G.; Pera, A. The Biology of Composting: A Review. Waste Manag. Res. 1983, 1, 157. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, J.; Zhang, H.; Shi, H.; Liu, G.; Che, J.; Liu, B. Microbial community and function in nitrogen transformation of ectopic fermentation bed system for pig manure composting. Bioresour. Technol. 2020, 319, 124–155. [Google Scholar] [CrossRef]
- Hanc, A.; Száková, J.; Svehla, P. Effect of composting on the mobility of arsenic, chromium and nickel contained in kitchen and garden waste. Bioresour. Technol. 2012, 126, 444–452. [Google Scholar] [CrossRef]
- Atkinson, C.F.; Jones, D.D.; Gauthier, J.J. Biodegradabilities and microbial activities during composting of municipal solid waste in bench-scale reactors. Compost Sci. Util. 1996, 4, 14–23. [Google Scholar] [CrossRef]
- Beck-Friis, B.; Smars, S.; Jonsson, H.; Eklind, Y.; Kirchmann, H. Composting of source-separated household organics at different oxygen levels: Gaining and undestanding of the emission dynamics. Compost Sci. Util. 2003, 11, 41–50. [Google Scholar] [CrossRef]
- Adamou, Z.; Tchicama, M.M.; Saidou, A.K.; Garba, O. Development of compost from agro-food residues: Analysis of the crop nutrients and trace elements. Am. Sci. Res. J. Eng. Technol. Sci. 2018, 43, 1–12. [Google Scholar]
- Ofosu-Budu, G.K.; Hogarh, J.N.; Fobil, J.N.; Quaye, A.; Danso, S.K.A.; Carboo, D. Harmonizing procedures for the evaluation of compost maturity in two compost types in Ghana. Resour. Conserv. Recy. 2010, 54, 205–209. [Google Scholar] [CrossRef]
- Hamelers, B.V.M.; Richard, T.L. The Effect of Dry Matter on the Composting Rate: Theoretical Analysis and Practical Implications. In Proceedings of the Conference 2001 Sacramento ASAE Annual Meeting, Sacramento, CA, USA, 30 July–1 August 2001. [Google Scholar]
- Lasaridi, K.; Protopapa, I.; Kotsou, M.; Pilidis, G.; Manios, T.; Kyriacou, A. Quality assessment of compost in the Greek market: The need for standards and quality assurance. J. Environ. Manag. 2006, 80, 58–65. [Google Scholar] [CrossRef]
- Avnimelech, Y.; Bruner, M.; Ezrony, I.; Sela, R.; Kochba, M. Stability indexes for municipal solid waste compost. Compost Sci. Util. 1996, 4, 13–20. [Google Scholar] [CrossRef]
- Aoun, J.; Bouaoun, D. Etude des caractéristiques physico-chimiques et contribution à la valorisation agronomique du compost des ordures ménagères. Environ. Ing. Dev. 2015, 50, 7932. [Google Scholar] [CrossRef]
- Martin, H. Le Compostage Du Fumier: Une Stratégie Pour Réduire Les Populations D’agents Pathogènes; Ministère de l’Agriculture el de l’Alimentation: Paris, France, 2015. [Google Scholar]


| Treatments | ||||
|---|---|---|---|---|
| Pile Number | NaOH 10% (0.1 M) | HCl 10% (3.2 M) | Inoculum 1% [36] | Inoculum 2.5% [36] |
| Control | − | − | − | − |
| Pile 1 | + | − | + | − |
| Pile 2 | + | − | − | + |
| Pile 3 | + | − | − | − |
| Pile 4 | − | − | + | − |
| Pile 5 | − | − | − | + |
| Pile 6 | − | + | + | − |
| Pile 7 | − | + | − | + |
| Pile 8 | − | + | − | − |
| Shredded Wood Remnants | |||||||
| Timing | Lignin | Hemicellulose | Cellulose | ||||
| Pile Initiation | 46 | 17 | 37 | ||||
| End of composting | 19 | 6 | 25 | ||||
| Cow manure profiling | |||||||
| Organic Matter (OM %) | Nitrogen % | P2O5 % | K2O % | pH | EC | C % | C:N |
| 86.06 | 1.73 | 0.98 | 0.73 | 7.2 | 0.65 | 44.46 | 25.7 |
| CBI * | Nutritional profile and growth characteristics | ||||||
| CBI 1 | Sugars, amino acids, and starch (Thermophile T° range 40–55 °C), secondary metabolites inhibited: Salmonella typhimurium and Escherichia coli | ||||||
| CBI 2 | Amino acids, organic compounds, and starch (Mesophile T° range 30–45 °C) | ||||||
| CBI 3 | Organic compounds and proteins (casein) (Thermophile T° range 40–50 °C) | ||||||
| CBI 4 | Amino acids (serine), organic compounds, and proteins (Mesophile T° range 30–45 °C) | ||||||
| CBI 5 | Sugars, proteins, amino acids, organic compounds, and starch (Mesophilic T° range 37–45 °C), secondary metabolites inhibited: Salmonella typhimurium and Escherichia coli | ||||||
| CBI 6 | Sugars (melezitose), amino acids (proline and serine), proteins (casein), and starch (Thermophile T° range 40–55 °C), secondary metabolites inhibited: Salmonella typhimurium | ||||||
| CBI 7 | Amino acids, starch, proteins, and organic compounds (Mesophile T° range 37–45 °C) | ||||||
| CBI 8 | Sugars, amino acids, starch, organic compounds, and proteins (Mesophile T° range 37–45 °C) | ||||||
| CBI 9 | Sugars, amino acids, organic compounds, and starch (Thermophile T° range 40–50 °C), secondary metabolites inhibited: Salmonella typhimurium and Escherichia coli | ||||||
| CBI 10 | Amino acids, organic compounds, starch, and proteins (Mesophile T° range 30–45 °C) | ||||||
| CBI 11 | Amino acids, starch, organic compounds, and proteins (Thermophile T° range 40–50 °C) | ||||||
| Variables | DM | VM | OM | C | pH | EC | P2O5 | K2O | N | C/N | CC | NDF | ADF | ADL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DM | 1 | |||||||||||||
| VM | −0.0385 | 1 | ||||||||||||
| OM | −0.5055 | 0.6701 | 1 | |||||||||||
| C | −0.5057 | 0.6700 | 1.0000 | 1 | ||||||||||
| pH | 0.2879 | −0.0644 | −0.0654 | −0.0654 | 1 | |||||||||
| EC | 0.2431 | 0.4758 | 0.2299 | 0.2297 | −0.2771 | 1 | ||||||||
| P2O5 | −0.0944 | 0.4797 | 0.4427 | 0.4427 | 0.2216 | 0.0254 | 1 | |||||||
| K2O | −0.5090 | 0.3721 | 0.5519 | 0.5519 | −0.0002 | −0.1008 | 0.4627 | 1 | ||||||
| N | 0.2702 | −0.2460 | −0.1990 | −0.1989 | 0.2713 | 0.1045 | −0.2923 | −0.3278 | 1 | |||||
| C/N | −0.4255 | 0.5837 | 0.6839 | 0.6838 | −0.2134 | 0.0648 | 0.4925 | 0.4792 | −0.8252 | 1 | ||||
| CC | 0.3214 | −0.1463 | −0.2798 | −0.2800 | 0.0939 | 0.0967 | −0.1787 | −0.3541 | 0.4102 | −0.4306 | 1 | |||
| NDF | 0.0727 | −0.0713 | −0.1416 | −0.1416 | −0.0551 | 0.0874 | −0.1185 | −0.1004 | −0.1650 | 0.0261 | 0.0651 | 1 | ||
| ADF | 0.1422 | −0.2062 | −0.2558 | −0.2559 | −0.0072 | 0.1198 | −0.3737 | −0.2394 | 0.0209 | −0.1895 | 0.0530 | 0.6410 | 1 | |
| ADL | 0.1613 | −0.4549 | −0.3539 | −0.3538 | −0.0083 | −0.0960 | −0.3557 | −0.2116 | 0.2023 | −0.4076 | 0.0678 | 0.3596 | 0.4669 | 1 |
| Piles | T0 | T1 | T2 | T3 | T4 | T5 | T6 |
| Control | 43.133 ± 0.017 d | 26.930 ± 0.049 d | 29.833 ± 0.061 d | 34.970 ± 0.016 c | 20.323 ± 0.053 d | 26.770 ± 0.033 d | 16.500 ± 0.049 d |
| P1 | 40.533 ± 0.233 a | 35.150 ± 0.033 a | 36.750 ± 0.008 a | 27.600 ± 0.163 a | 24.543 ± 0.029 a | 20.750 ± 0.024 a | 22.300 ± 0.049 a |
| P2 | 42.013 ± 0.005 b | 32.147 ± 0.033 b | 29.027 ± 0.025 b | 20.417 ± 0.061 b | 17.300 ± 0.049 b | 15.147 ± 0.041 b | 14.033 ± 0.020 b |
| P3 | 34.233 ± 0.069 c | 27.743 ± 0.028 c | 37.813 ± 0.061 c | 35.017 ± 0.017 c | 19.550 ± 0.033 c | 18.250 ± 0.024 c | 17.540 ± 0.024 c |
| P4 | 41.040 ± 0.024 e | 27.580 ± 0.073 c | 35.017 ± 0.017 e | 29.433 ± 0.037 d | 20.350 ± 0.016 e | 22.940 ± 0.041 e | 16.750 ± 0.033 e |
| P5 | 38.583 ± 0.037 f | 32.823 ± 0.061 e | 36.310 ± 0.057 f | 23.250 ± 0.033 e | 21.100 ± 0.033 f | 17.480 ± 0.033 f | 16.823 ± 0.029 e |
| P6 | 32.297 ± 0.033 g | 30.100 ± 0.065 f | 36.063 ± 0.020 g | 22.153 ± 0.045 f | 27.973 ± 0.012 g | 17.050 ± 0.033 g | 15.450 ± 0.049 d |
| P7 | 31.820 ± 0.024 h | 28.530 ± 0.082 g | 31.820 ± 0.033 h | 25.137 ± 0.041 g | 28.163 ± 0.037 h | 22.170 ± 0.041 h | 18.633 ± 0.045 f |
| P8 | 37.330 ± 0.057 i | 31.417 ± 0.057 h | 32.047 ± 0.025 i | 31.833 ± 0.037 h | 21.193 ± 0.053 f | 21.960 ± 0.024 i | 17.710 ± 0.057 g |
| CC | |||||||
| Control | 3.57 × 105 ± 2.69 × 103 d | 8.17 × 105 ± 2.29 × 103 d | 1.39 × 106 ± 1.51 × 103 d | 1.64 × 106 ± 2.74 × 103 d | 4.73 × 1011 ± 4.05 × 108 d | 6.80 × 1012 ± 2.74 × 109 d | 2.31 × 1014 ± 9.42 × 1011 d |
| P1 | 3.43 × 105 ± 2.74 × 103 a | 1.97 × 106 ± 2.74 × 103 a | 8.63 × 105 ± 2.74 × 103 a | 5.43 × 105 ± 2.74 × 103 a | 4.05 × 1011 ± 4.92 × 107 a | 6.48 × 1012 ± 1.28 × 109 a | 1.51 × 1014 ± 7.85 × 1011 a |
| P2 | 1.63 × 105 ± 2.74 × 103 b | 1.90 × 105 ± 8.16 × 101 b | 8.81 × 105 ± 8.16 × 102 b | 3.50 × 105 ± 3.40 × 102 b | 3.51 × 1011 ± 2.74 × 108 b | 8.51 × 1012 ± 1.06 × 109 b | 1.07 × 1014 ± 2.37 × 1012 b |
| P3 | 1.03 × 105 ± 2.74 × 103 c | 2.70 × 105 ± 1.25 × 102 c | 5.41 × 105 ± 7.13 × 102 c | 5.07 × 105 ± 1.51 × 103 c | 4.34 × 1011 ± 4.5 × 108 c | 7.09 × 1012 ± 2.74 × 109 c | 6.97 × 1014 ± 2.12 × 1012 c |
| P4 | 1.01 × 105 ± 8.16 × 103 c | 8.07 × 105 ± 1.88 × 103 e | 7.61 × 105 ± 4.55 × 102 e | 7.27 × 105 ± 1.39 × 103 e | 4.63 × 1011 ± 4.05 × 108 e | 6.90 × 1012 ± 4.08 × 108 e | 3.00 × 1014 ± 3.86 × 1011 e |
| P5 | 1.33 × 105 ± 8.16 × 103 e | 2.60 × 106 ± 5.25 × 102 f | 1.71 × 106 ± 2.69 × 103 f | 1.65 × 106 ± 6.60 × 102 f | 3.95 × 1011 ± 2.37 × 108 f | 5.88 × 1012 ± 6.68 × 108 f | 5.27 × 1014 ± 2.69 × 1012 f |
| P6 | 1.01 × 106 ± 2.45 × 103 f | 7.47 × 105 ± 1.59 × 103 g | 4.77 × 105 ± 5.72 × 102 g | 7.31 × 105 ± 4.11 × 102 e | 4.48 × 1011 ± 4.32 × 107 g | 6.45 × 1012 ± 2.74 × 109 g | 7.83 × 1014 ± 2.74 × 1012 g |
| P7 | 6.70 × 105 ± 8.16 × 103 g | 6.07 × 105 ± 1.14 × 103 h | 1.06 × 106 ± 1.39 × 103 h | 7.47 × 105 ± 1.51 × 103 g | 4.56 × 1011 ± 2.45 × 108 h | 5.46 × 1012 ± 8.38 × 108 h | 6.43 × 1014 ± 2.74 × 1012 h |
| P8 | 1.34 × 106 ± 2.74 × 103 h | 3.87 × 105 ± 2.37 × 103 i | 1.50 × 106 ± 8.29 × 102 i | 1.22 × 106 ± 5.10 × 102 h | 3.37 × 1011 ± 2.74 × 108 i | 6.56 × 1012 ± 2.74 × 109 i | 4.87 × 1014 ± 7.35 × 1011 i |
| Compost Parameters | Pile Number | Standard [75] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| pH | 9.21 | 9.95 | 9.71 | 9.15 | 9.31 | 9.12 | 8.94 | 9.8 | 9.11 | 7–8 |
| EC mS.cm−1 | 0.8841 | 0.7545 | 0.7769 | 1.06 | 0.8981 | 1.128 | 1.016 | 0.7547 | 1.105 | <4 |
| O.M. (%) | 23.7 | 36.12 | 25.62 | 29 | 28.02 | 31.38 | 34.8 | 27.1 | 31.92 | - |
| N (%) | 0.64 | 0.63 | 0.79 | 0.73 | 0.67 | 0.78 | 0.82 | 0.6 | 0.75 | <2 |
| C:N | 21.78 | 33.73 | 19.07 | 23.37 | 24.59 | 23.66 | 24.96 | 26.56 | 25.02 | <20 |
| K2O (%) | 0.484 | 0.639 | 0.359 | 0.387 | 0.399 | 0.415 | 0.452 | 0.545 | 0.358 | 0.25–0.5 |
| P2O5 (%) | 0.4 | 0.53 | 0.49 | 0.44 | 0.52 | 0.4 | 0.45 | 0.49 | 0.4 | 0.7–0.9 |
| Hg (ppm) | 0.0538 | 0.0633 | 0.0677 | 0.0656 | 0.0510 | 0.0227 | 0.0697 | 0.0212 | 0.0517 | ≤8 ppm |
| Detectable Microorganisms | Salmonella sp. | Staphylococcus aureus | Enterococci spp. | Escherichia coli | Bacillus spp. |
|---|---|---|---|---|---|
| Microbiological limits (EPA 2008) | Absence in 25 g | 102–104 CFU/g | 5000 CFU/g | 1000 CFU/g | 103–105 CFU/g |
| Control | Absence | 6 × 103 | 4 × 103 | 2 × 103 ** | 2 × 105 ** |
| Pile 1 | Absence | 103 | 103 | 3 × 102 | 104 |
| Pile 2 | Absence | 2 × 102 | 102 | 102 | 103 |
| Pile 3 | Absence | 2 × 103 | 102 | 2 × 102 | 104 |
| Pile 4 | Absence | 4 × 103 | 103 | 5 × 102 | 2 × 104 |
| Pile 5 | Absence | 2 × 103 | 103 | 2 × 102 | 2 × 103 |
| Pile 6 | Absence | 3 × 103 | 2 × 103 | 6 × 102 | 2 × 104 |
| Pile 7 | Absence | 5 × 102 | 3 × 103 | 2 × 102 | 105 ** |
| Pile 8 | Absence | 4 × 103 | 2 × 103 | 9 × 102 | 105 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tannouri, A.; Rizk, Z.; Daccache, M.; Ghanem, C.; Azzi, V.; Maroun, R.G.; Hobaika, Z.; Salameh, D. Study of Raw Material Pretreatment and the Microbiota Selection Effect on the Composting Process Efficiency. Agronomy 2023, 13, 2048. https://doi.org/10.3390/agronomy13082048
Tannouri A, Rizk Z, Daccache M, Ghanem C, Azzi V, Maroun RG, Hobaika Z, Salameh D. Study of Raw Material Pretreatment and the Microbiota Selection Effect on the Composting Process Efficiency. Agronomy. 2023; 13(8):2048. https://doi.org/10.3390/agronomy13082048
Chicago/Turabian StyleTannouri, Abdo, Ziad Rizk, Marina Daccache, Chantal Ghanem, Valérie Azzi, Richard G. Maroun, Zeina Hobaika, and Dominique Salameh. 2023. "Study of Raw Material Pretreatment and the Microbiota Selection Effect on the Composting Process Efficiency" Agronomy 13, no. 8: 2048. https://doi.org/10.3390/agronomy13082048
APA StyleTannouri, A., Rizk, Z., Daccache, M., Ghanem, C., Azzi, V., Maroun, R. G., Hobaika, Z., & Salameh, D. (2023). Study of Raw Material Pretreatment and the Microbiota Selection Effect on the Composting Process Efficiency. Agronomy, 13(8), 2048. https://doi.org/10.3390/agronomy13082048







