Combination of Graphene Oxide and Rhizobium Improved Soybean Tolerance in Saline-Alkali Stress
Abstract
1. Introduction
2. Material and Methods
2.1. Experimental Materials
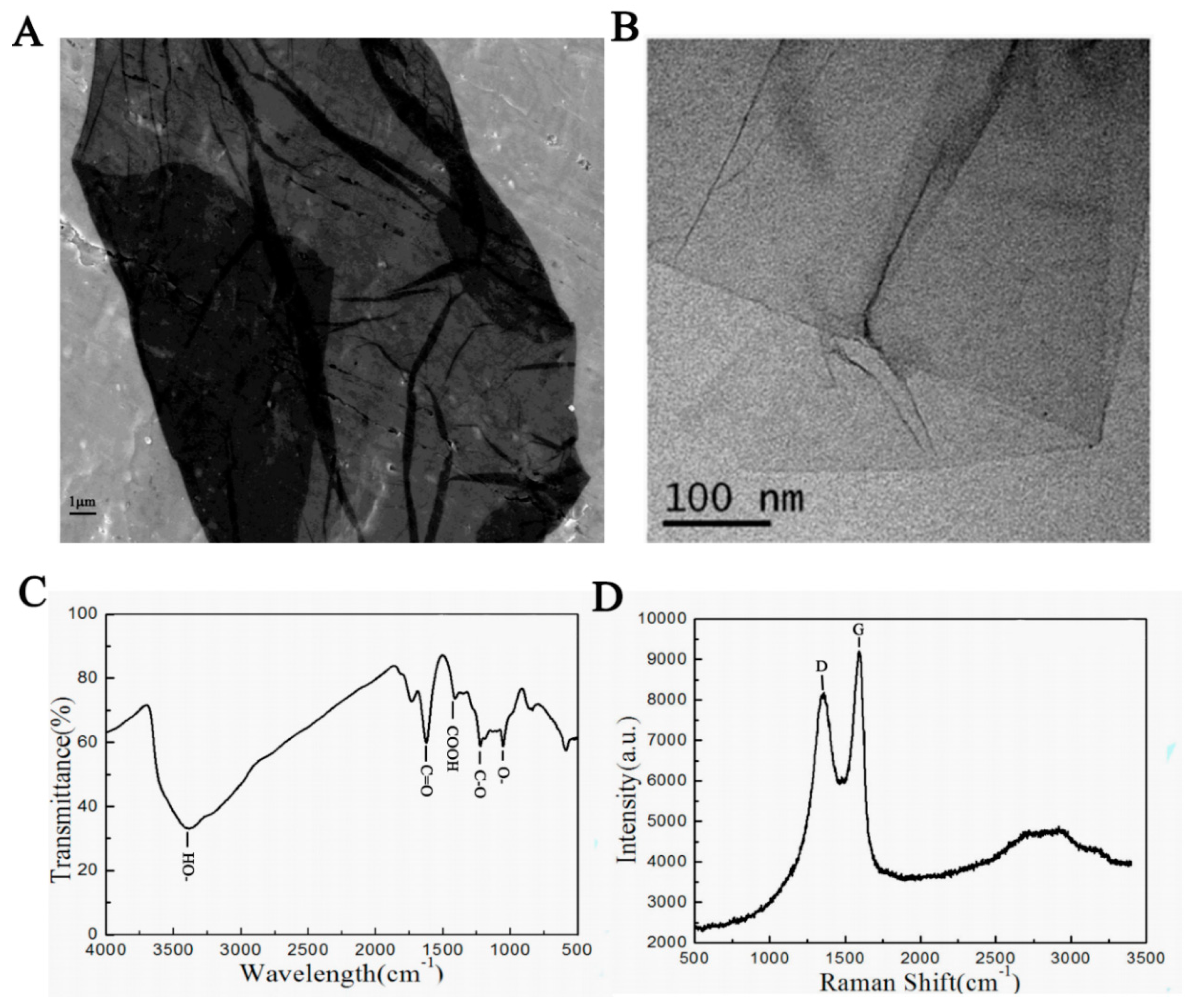
2.2. GO Characteristics
2.3. Determination of Morphological Index
2.4. Determination of Physiological Indexes Related to Saline-Alkali Tolerance
2.5. Determination of Enzymatic Activities
2.6. Determination of Total Nitrogen Content
2.7. Determination of Hormone Levels
2.8. Determination of Gene Expression
2.9. Statistical Analysis
3. Results
3.1. GO Characteristics
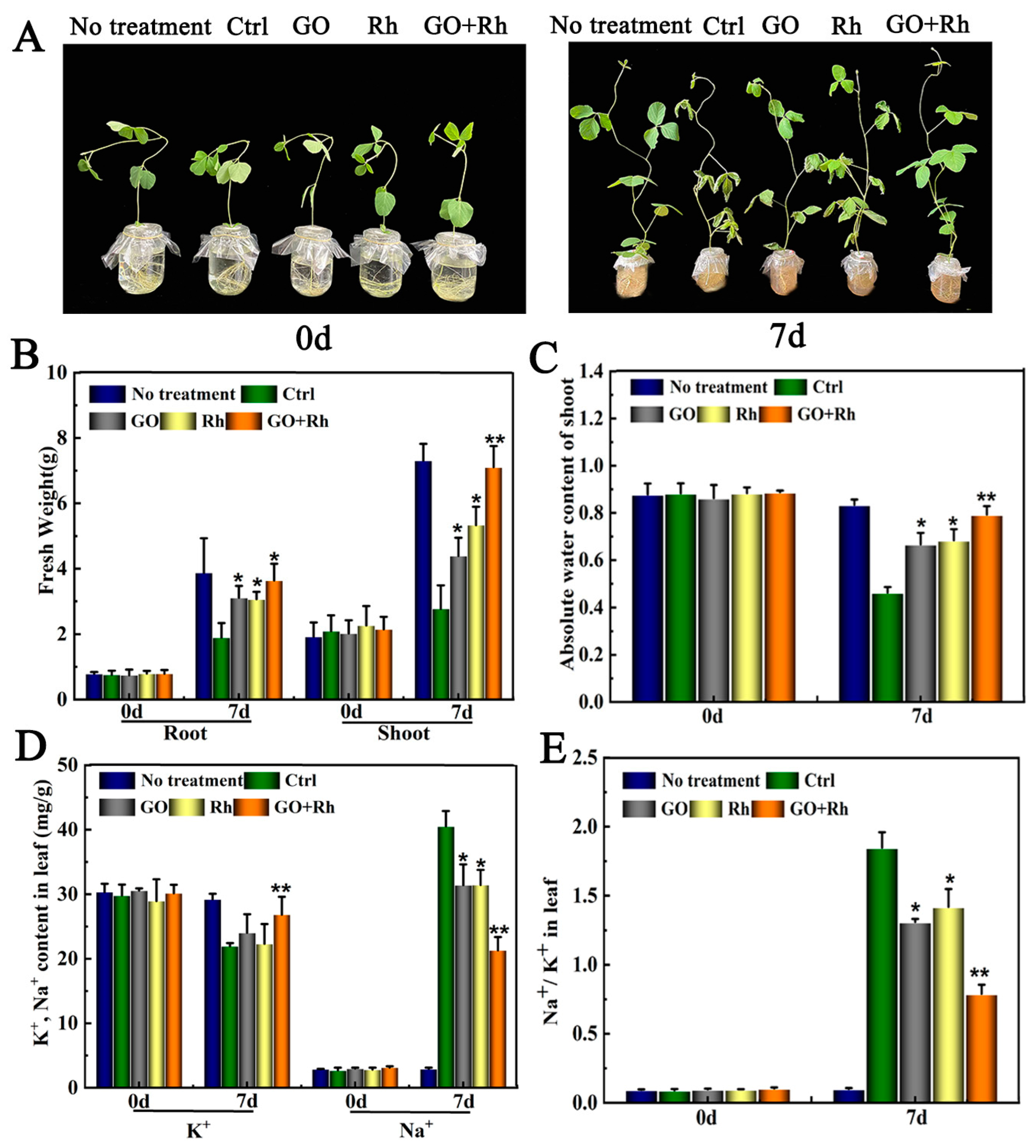
3.2. GO + Rh Enhanced Saline-Alkali Tolerance of Soybean Seedlings
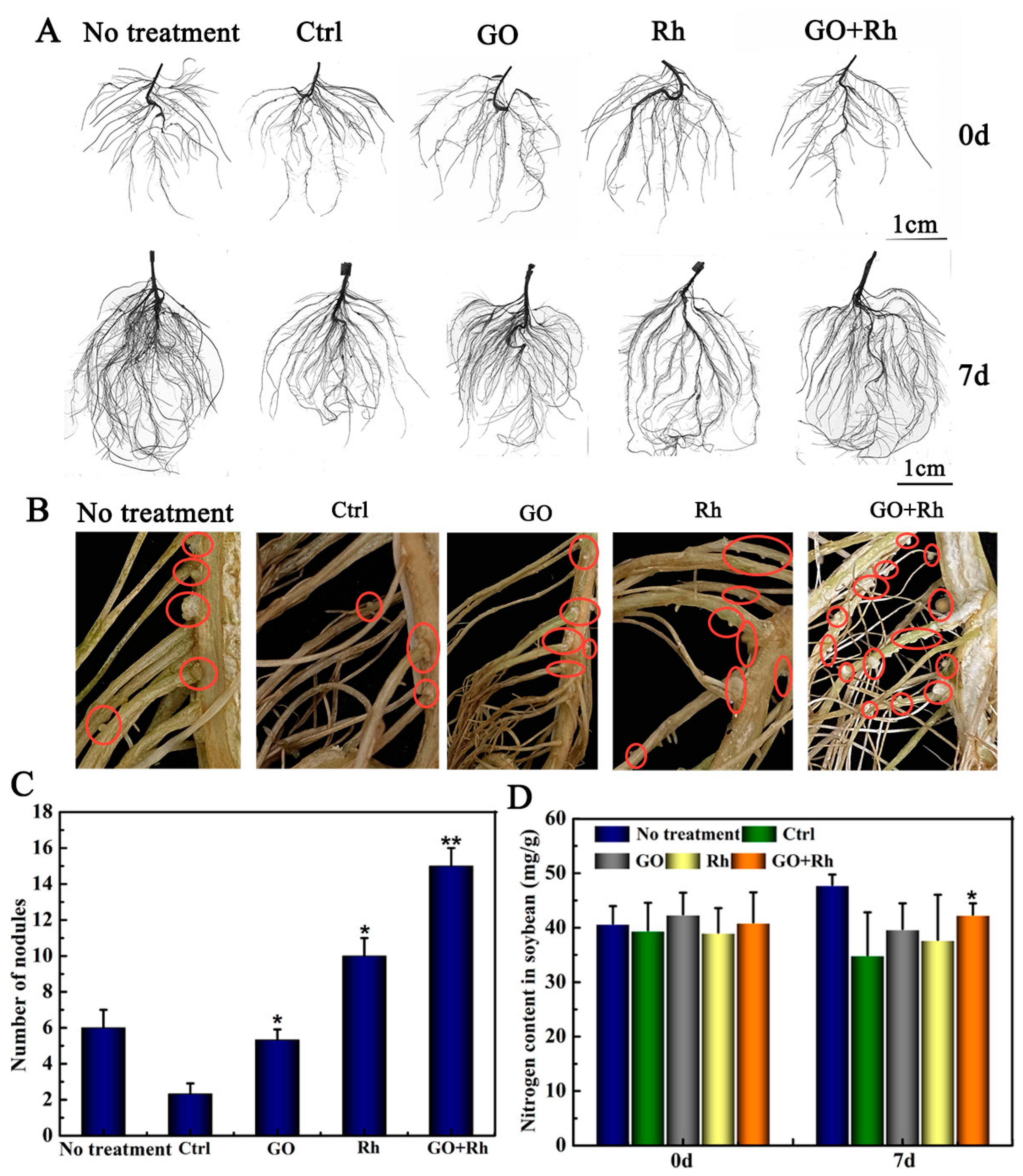
3.3. GO + Rh Promoted N Uptake and Improved Soybean Seedlings Root Parameters
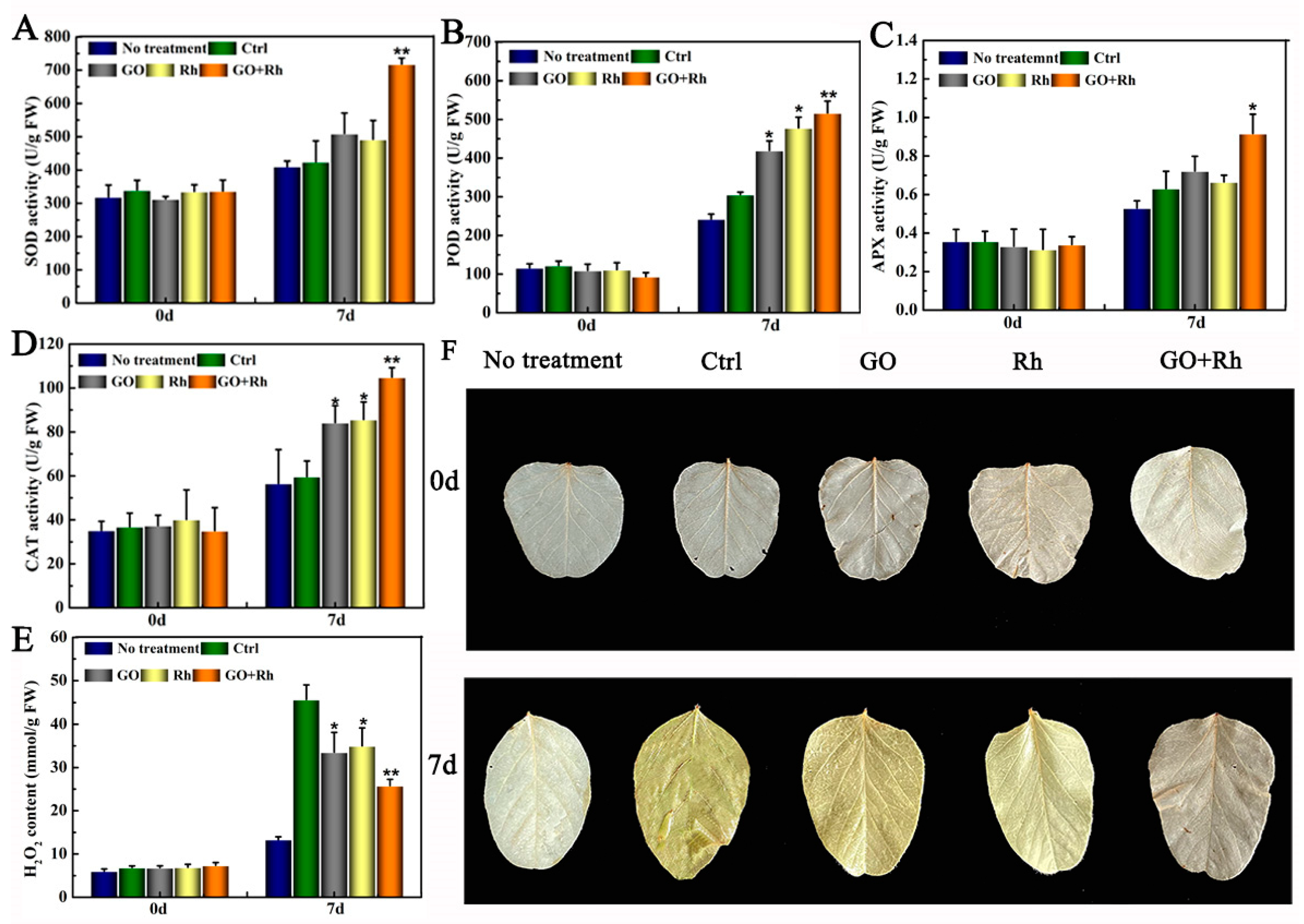
3.4. GO + Rh Increased Antioxidant Enzyme System Activity and Decreased Hydrogen Peroxide Content
3.5. GO + Rh Responds to Soybean’s Osmotic Substances System
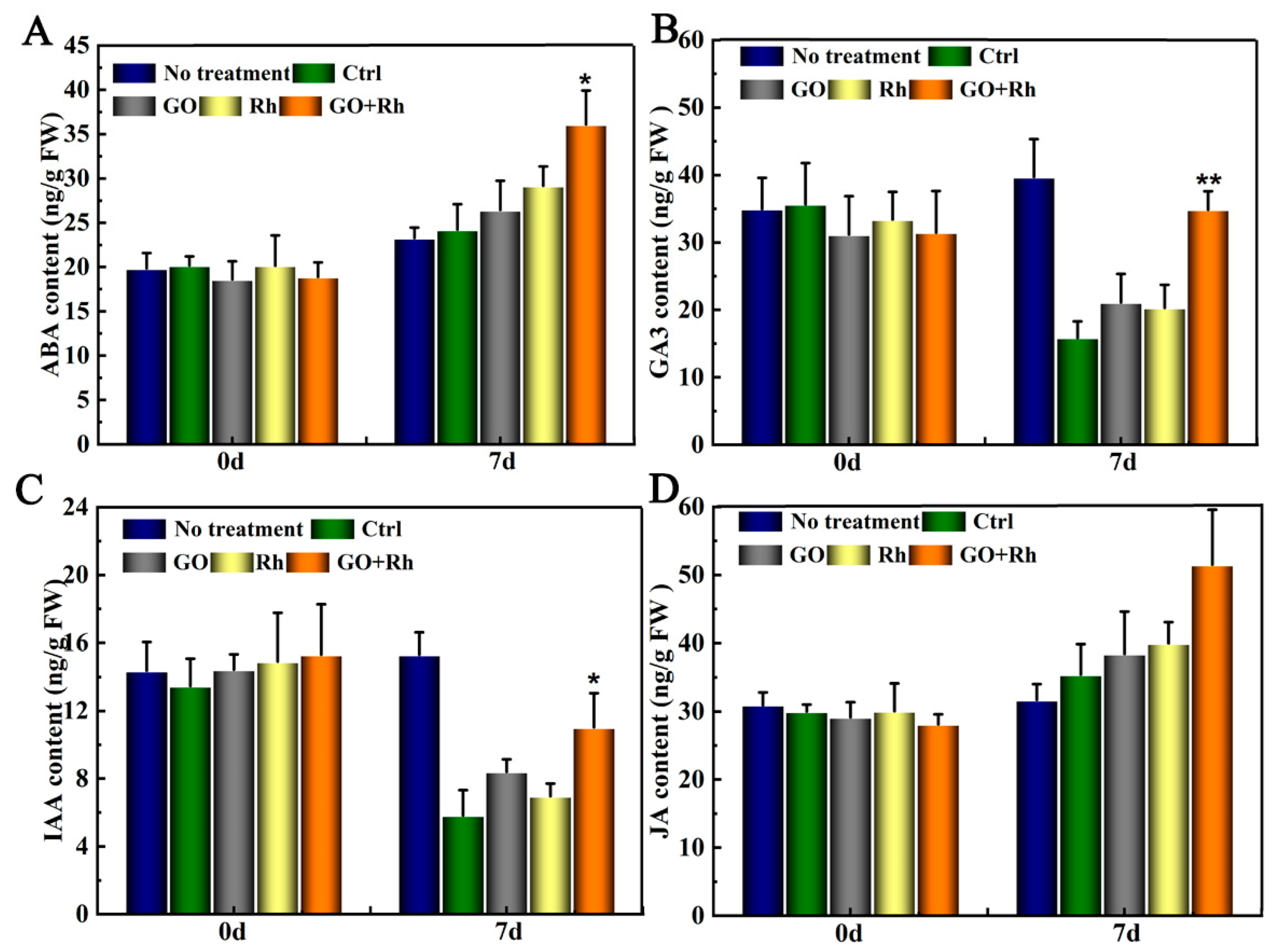
3.6. GO + Rh Specifically Activates GA3 Hormones
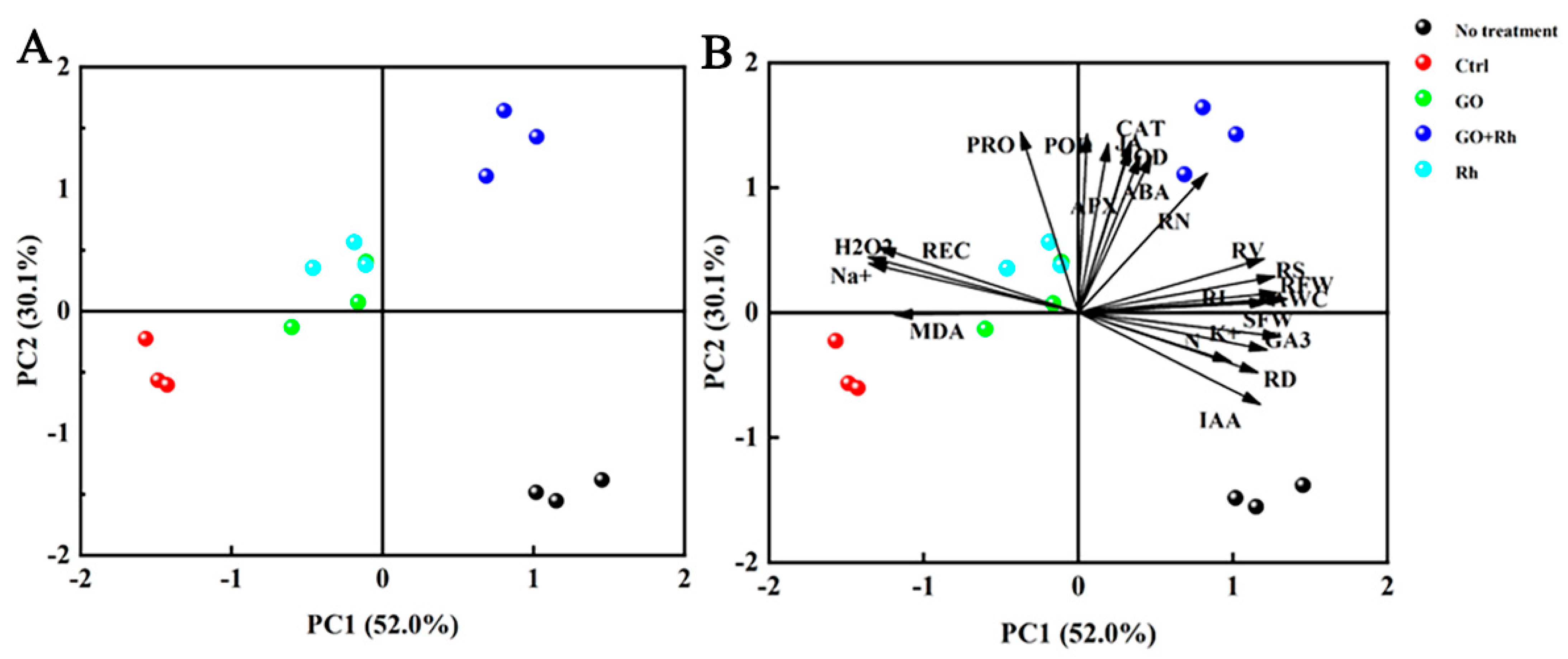
3.7. Relationship between Traits under Saline-Alkali Stress
3.8. GO + Rh Enhanced the Expression of Related to GA3 Hormone Genes
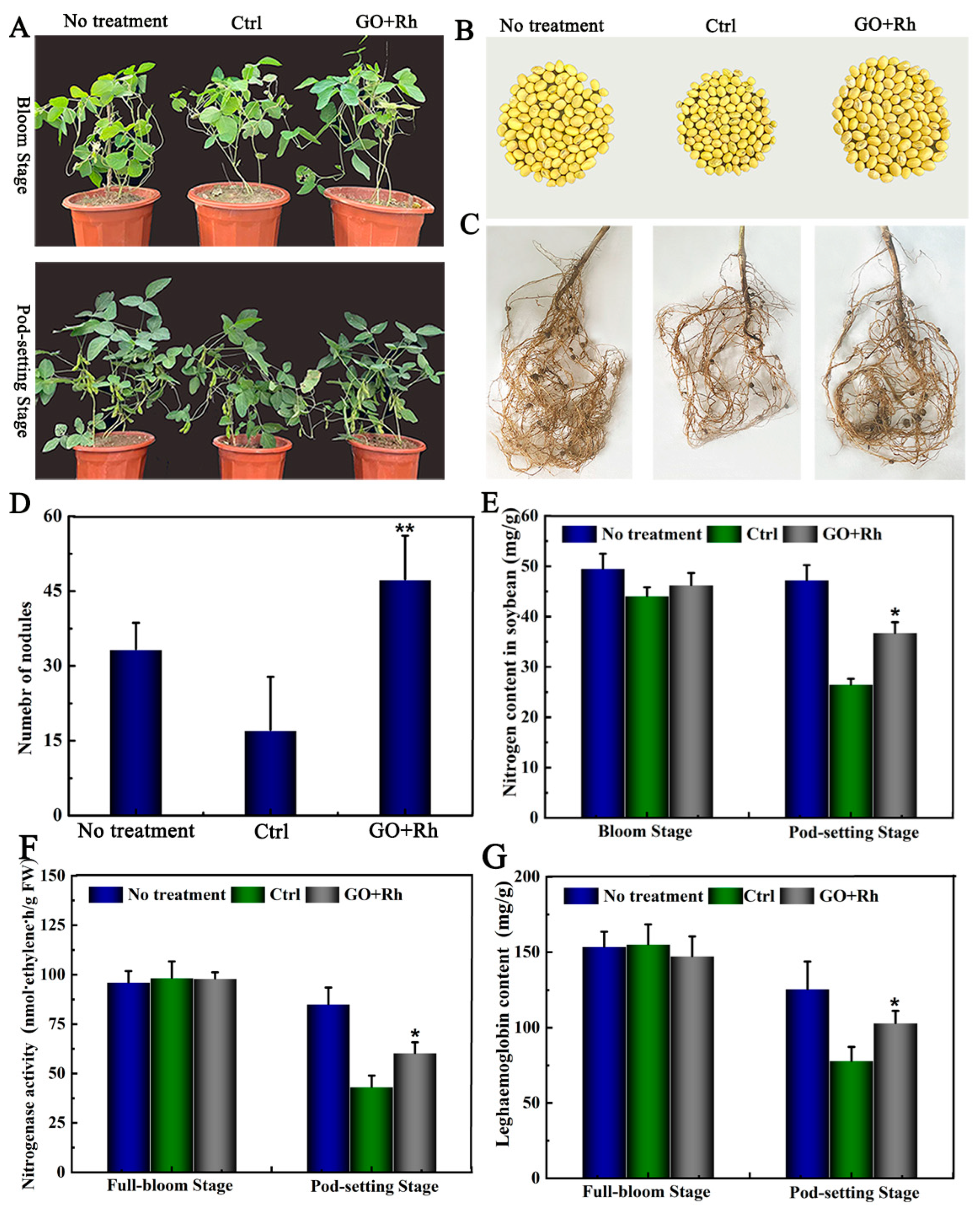
3.9. Effects of GO + Rh on N Uptake, Number of Nodules at Bloom and Pod-Setting Stage, and Yield Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ghassmi-Golezani, K.; Farhangi-Abriz, S. Foliar sprays of salicylic acid and jasmonic acid stimulate H+-ATPase activity of tonoplast, nutrient uptake and salt tolerance of soybean. Ecotoxicol. Environ. Saf. 2018, 166, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Alkharabsheh, H.M.; Seleiman, M.F.; Hewedy, O.A.; Battaglia, M.F.; Jalal, R.S.; Alhammad, B.A.; Schillaci, G.; Ali, N.; Al-Doss, A. Field crop responses and management strategies to mitigate soil salinity in modern agriculture: A Review. Agronomy 2021, 11, 2299. [Google Scholar] [CrossRef]
- Gai, Y.H.; Niu, L.; Dong, B.C.; Wei, J. Effects of different concentrations of salt and alkali stress on photosynthetic characteristics and physiological and biochemical characteristics of wild soybean. Jiangsu Agric. Sci. 2014, 42, 89–93. [Google Scholar]
- Mao, S.; Zhou, W.L.; Yang, F.; Di, X.D.; Lian, J.X.; Yang, Q.J. Research progress on mechanism of plant root response to salt-alkali stress. Acta Agric. Zhejiangensis 2021, 33, 1991–2000. [Google Scholar]
- Ahmad, A.; Tola, E.; Alshahrani, T.S.; Seleiman, M.F. Enhancement of morphological and physiological performance of Zea mays L. under saline stress using ZnO nanoparticles and 24-Epibrassinolide seed priming. Agronomy 2023, 13, 771. [Google Scholar] [CrossRef]
- Taha, R.S.; Seleiman, M.F.; Alotaibi, M.; Alhammad, B.A.; Rady, M.M.; Mahdi, A.H.A. Exogenous potassium treatments elevate salt tolerance and performances of Glycine max L. by boosting antioxidant defense system under actual saline field conditions. Agronomy 2020, 10, 1741. [Google Scholar] [CrossRef]
- Liu, C.; Mao, B.G.; Yuan, D.Y.; Chu, C.C.; Duan, M.J. Salt tolerance in rice: Physiological responses and molecular mechanisms. Crop J. 2020, 10, 13–25. [Google Scholar] [CrossRef]
- Al-Ashkar, I.; Alderfasi, A.; Romdhane, W.B.; Seleiman, M.F.; El-Said, R.A.; Al-Doss, A. Morphological and genetic diversity within salt tolerance detection in eighteen wheat genotypes. Plants 2020, 9, 287. [Google Scholar] [CrossRef]
- Chong, Y.; Ma, Y.; Shen, H.; Tu, X.; Zhou, X.; Xu, J. The in vitro and in vivo toxicity of graphene quantum dots. Biomaterials 2014, 35, 5041–5048. [Google Scholar] [CrossRef]
- González-García, Y.; López-Vargas, E.R.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; González-Morales, S.; Robledo-Olivo, A.; Alpuche-Solís, A.G.; Juárez-Maldonado, A. Impact of carbon nanomaterials on the antioxidant system of tomato seedlings. Int. J. Mol. Sci. 2019, 20, 5858. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Chang, H.; Teng, Y. Sulfonated graphene-induced hormesis is mediated through oxidative stress in the roots of maize seedlings. Sci. Total Environ. 2016, 572, 926–934. [Google Scholar] [CrossRef]
- He, Y.; Qian, L.; Liu, X.; Hu, R.; Huang, M.; Liu, Y. Graphene oxide as an antimicrobial agent can extendthe vase life of cut flowers. Nanotechnol. Res. 2018, 11, 6010–6022. [Google Scholar] [CrossRef]
- Vochita, G.; Oprica, L.; Gherghel, D.; Mihai, C.T.; Boukherroub, R.; Lobiuc, A. Graphene oxide effects in early ontogenetic stages of Triticum aestivum L. seedlings. Ecotoxicol. Environ. Saf. 2019, 181, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.J.; Mens, C.; Hastwell, A.H.; Zhang, M.; Su, H.; Jones, C.H.; Chu, X.; Gresshof, P.M. Legume nodulation: The host controls the party. Plant Cell Environ. 2019, 42, 41–51. [Google Scholar] [CrossRef]
- Meng, N.; Guo, J.S.; Yu, B.J. Ameliorative effects of inoculation with Bradyrhizobium japonicum on Glycine max and Glycine soja seedlings under salt stress. Plant Growth Regul. 2016, 80, 137–147. [Google Scholar] [CrossRef]
- Lou, C. The Remediation of Cadmium Contaminated soil by Nanomaterials, Alfalfa and Rhizobia Composite System. Master’s Thesis, Northwest A&F University, Yangling, China, 2016. [Google Scholar]
- He, Y.J.; Li, D.Q.; Deng, B.; Yang, J. The effect of exogenous Gibberellin(GA3) on the symbiotic of Alfalfa rhizobium. Acta Agrestia Sin. 2022, 30, 385–393. [Google Scholar]
- Mortimer, M.; Dong, L.; Wang, Y.; Patricia, A.H. Physical properties of carbon nanomaterials and nanoceria affect pathways important to the nodulation competitiveness of the symbiotic N2-fixing bacterium Bradyrhizobium diazoefficiens. Nano-Micro Small 2020, 16, 1906055. [Google Scholar] [CrossRef]
- Watanabe, N.; Lam, E. BAX inhibitor-1modulates endoplasmic reticulum stress-mediated programmed cell death in Arabidopsis. J. Biol. Chem. 2008, 283, 3200–3210. [Google Scholar] [CrossRef]
- Zhang, S.N.; Gao, P.Y.; Xie, Q.E.; Zhao, X.H.; Li, X. Cadmiuminduced root growth inhibition is mediated by hydrogen peroxide production in root tip of Arabidopsis. Chin. J. Eco-Agric. 2010, 18, 136–140. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.Y.; Zhou, J.M.; Chen, X.Q.; Du, C.W. Potassium content in ryegrass extracted by HCl at flame photometry. Acta Pedol. Sin. 2013, 44, 624–627. [Google Scholar]
- Wang, Y.J.; Zhao, Y.M.; Tao, G.L. Determination of nitrogen content in flax-semi-micro Kjeldahl nitrogen determination method. J. Qiqihar Light Ind. Inst. 1995, 1, 58–61. [Google Scholar]
- Zhang, Y.M. Effect of Salt Stress on the Longhuang Variety Soybean-Rhizobia Symbiosis System and Its Microbial Community. Master’s Thesis, Northwest A&F University, Yangling, China, 2022. [Google Scholar]
- Singh, S.; Prasad, S.M.; Sharma, S.; Dubey, N.K.; Ramawat, N.; Prasad, R.; Singh, V.P.; Tripathi, D.K.; Chauhan, D.K. Silicon and nitric oxide-mediated mechanisms of cadmium toxicity alleviation in wheat seedlings. Physiol. Plant. 2020, 174, e13065. [Google Scholar] [CrossRef]
- Fang, S.; Gao, K.; Hu, W.; Wang, S.; Chen, B.; Zhou, Z. Foliar and seed application of plant growth regulators affects cotton yield by altering leaf physiology and floral bud carbohydrate accumulation. Field Crop Res. 2019, 231, 105–114. [Google Scholar] [CrossRef]
- Jiao, J.; Yuan, C.; Wang, J.; Xia, Z.; Xie, L.; Chen, F. The role of graphene oxide on tobacco root growth and its preliminary mechanism. J. Nanosci. Nanotechnol. 2016, 16, 12449–12454. [Google Scholar] [CrossRef]
- Ganjavi, L.S.; Mehdi, O.; Gholamreza, G.; Ali, A.; Ali, F. Glycine betaine functionalized graphene oxide as a new engineering nanoparticle lessens salt stress impacts in sweet basil (Ocimum basilicum L.). Plant Physiol. Biochem. 2021, 162, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Dupont, L.; Hérouart, D.; Alloing, G.; Hopkins, J.; Pierre, O.; Frendo, P.; El-Msehli, S. The Legume Root Nodule: From Symbiotic Nitrogen Fixation to Senescence; InTech: London, UK, 2012. [Google Scholar]
- Zhao, L.; Wang, W.; Fu, X.H.; Liu, A.; Cao, J.F.; Jian, F.L. Graphene oxide, a novel nanomaterial as soil water retention agent, dramatically enhances drought stress tolerance in soybean plants. Front. Plant Sci. 2022, 13, 810905. [Google Scholar] [CrossRef]
- Zahir, Z.A.; Shah, M.K.; Naveed, M.; Akhter, M.J. Substrate-dependent auxin production by Rhizobium phaseoli improves the growth and yield of Vigna radiata L. under salt stress conditions. J. Microbiol. Biotechnol. 2010, 20, 1288–1294. [Google Scholar] [CrossRef]
- Rezayian, M.; Niknam, V.; Ebrahimzadeh, H. Oxidative damage and antioxidative system in algae. Toxicol. Rep. 2019, 6, 1309–1313. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative Stress, Antioxidants and Stress Tolerance; Elsevier Ltd.: Oxford, UK, 2002; pp. 405–410. [Google Scholar]
- Noreen, S.; Sultan, M.; Akhter, M.S.; Shah, K.H.; Ummara, U.; Manzoor, H. Foliar fertigation of ascorbic acid and zinc improves growth, antioxidant enzyme activity and harvest index in barley (Hordeum vulgare L.) grown under salt stress. Plant Physiol. Biochem. 2020, 158, 244–254. [Google Scholar] [CrossRef]
- Feng, N.; Yu, M.; Li, Y.; Jin, D.; Zheng, D. Prohexadione-calcium alleviates saline-alkali stress in soybean seedlings by improving the photosynthesis and up-regulating antioxidant defense. Ecotoxicol. Environ. Saf. 2021, 220, 112369. [Google Scholar] [CrossRef]
- Gohari, G.; Alavi, Z.; Esfandiari, E.; Panahirad, S.; Hajihoseinlou, S.; Fotopoulos, V. Interaction between hydrogen peroxide and sodium nitroprusside following chemical priming of Ocimum basilicum L. against salt stress. Physiol. Plant. 2020, 168, 361–373. [Google Scholar] [CrossRef]
- Rady, M.M.; Taha, S.S.; Kusvuran, S. Integrative application of cyanobacteria and antioxidants improves common bean performance under saline conditions. Sci. Hortic. 2018, 233, 61–69. [Google Scholar] [CrossRef]
- Rady, M.M. Effect of 24-epibrassinolide on growth yield, antioxidant system and cadmium content of bean (Phaseolus vulgaris L.) plants under salinity and cadmium stress. Sci. Hortic. 2011, 129, 232–237. [Google Scholar] [CrossRef]
- Ghadakchiasl, A.; Mozafari, A.; Ghaderi, N. Mitigation by sodium nitroprusside of the effects of salinity on the morphophysiological and biochemical characteristics of Rubu sidaeus under in vitro conditions. Physiol. Mol. Biol. Plant. 2017, 23, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Mutava, R.N.; Prince, K.S.J.; Syed, N.H.; Song, L.; Valliyodan, B.; Chen, W.; Nguyen, H.T. Understanding abiotic stress tolerance mechanisms in soybean: A comparative evaluation of soybean response to drought and flooding stress. Plant Physiol. Biochem. 2015, 86, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Wu, N.; Chang, Y.; Li, X.; Xiao, J.; Xiong, L. Carotenoid deficiency impairs ABA and IAA biosynthesis and differentially affects drought and cold tolerance in rice. Plant Mol. Biol. 2013, 83, 475–488. [Google Scholar] [CrossRef]
- Ye, M.R.; Zhu, C.H.; Gan, L.J.; Xia, K. Hormonal interactions in the control of plant stem elongation. Chin. Agric. Sci. Bull. 2007, 23, 228–231. [Google Scholar]
- Lim, G.; Zhang, X.; Chung, M.; Lee, D.J.; Woo, Y.; Cheong, H.; Kim, C. A putative novel transcription factor, AtSKIP, is involved in abscisic acid signalling and confers salt and osmotic tolerance in Arabidopsis. New Phytol. 2010, 185, 103–113. [Google Scholar] [CrossRef]
- Zhao, W.; Zhou, Q.; Tian, Z.Z.; Cui, Y.T.; Liang, Y.; Wang, H.Y. Apply biochar to ameliorate soda saline-alkali land, improve soil function and increase corn nutrient availability in the Songnen Plain. Sci. Total Environ. 2020, 722, 137428. [Google Scholar] [CrossRef]
- Negrão, S.; Schmockel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef]
- Kumar, V.; Rawat, A.K.; Rao, D.L.N. Improving the performance of Bradyrhizobium japonicum by double inoculation in non-fertilized and fertilized wheat- soybean rotation. Agric. Res. 2021, 11, 683–693. [Google Scholar] [CrossRef]
- Li, Z.H. Evaluation of Soybean Drought Tolerance and Root Characteristics of Drought Tolerant Soybean Varieties. Ph.D. Thesis, Shenyang Agricultural University, Shenyang, China, 2020. [Google Scholar]
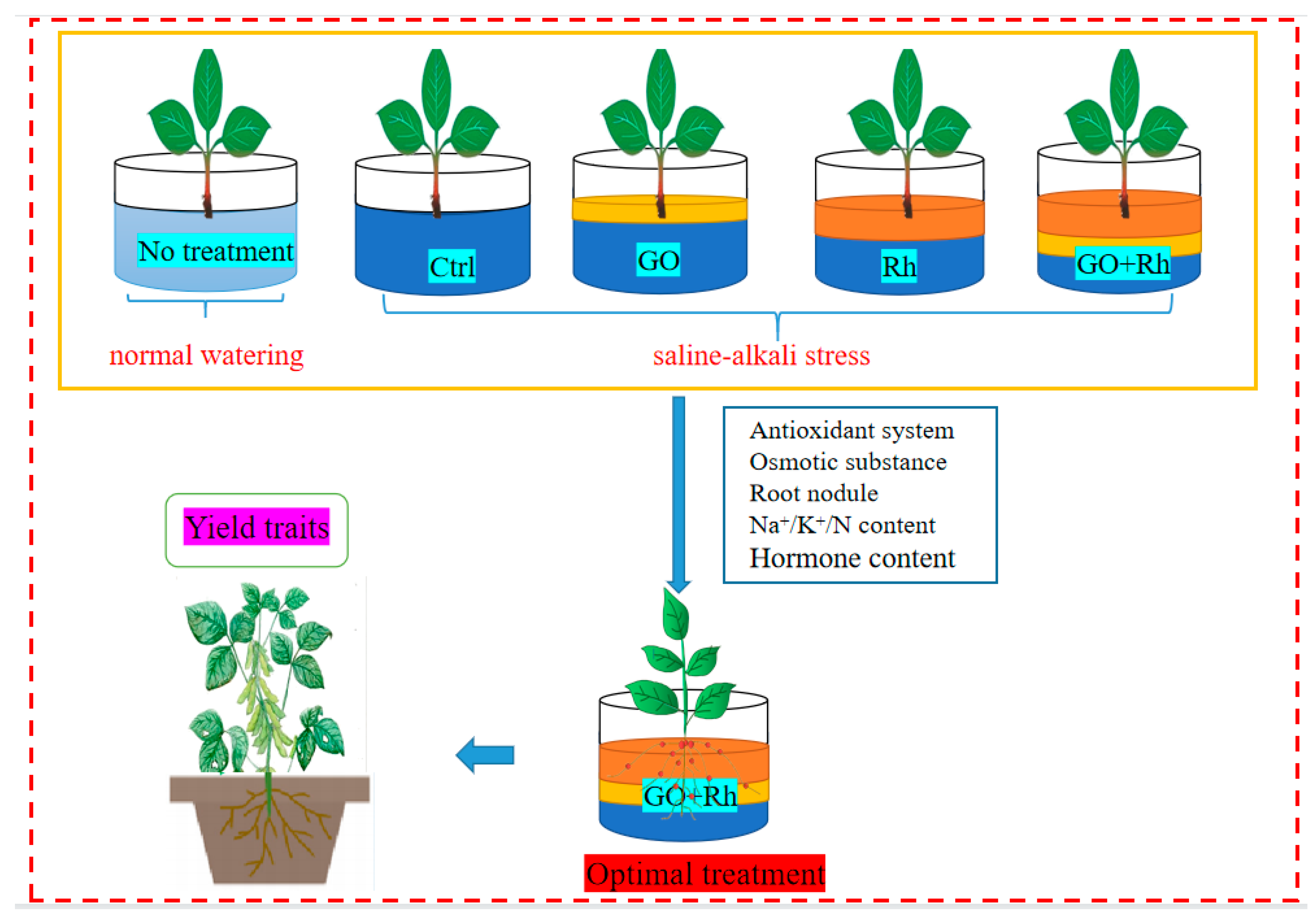


| Abbreviation | Mixture Components |
|---|---|
| No treatment | Hoagland solution |
| Ctrl | Hoagland solution+ saline-alkali solution |
| GO | Hoagland solution + saline-alkali solution + 100 mL GO |
| Rh | Hoagland solution+ saline-alkali solution + 3 mL Rh |
| GO + Rh | Hoagland solution+ saline-alkali solution + 100 mL GO + 3 mL Rh |
| Abbreviation | Mixture Components |
|---|---|
| No treatment | normal watering |
| Ctrl | saline-alkali solution |
| GO + Rh | saline-alkali solution + 100 mL GO + 3 mL Rh |
| Time | Treatment | Na+ Content (mg/g) |
|---|---|---|
| 0 d | No treatment | 6.40 ± 0.68 |
| Ctrl | 6.35 ± 0.82 | |
| GO | 6.44 ± 0.54 | |
| Rh | 6.51 ± 0.28 | |
| GO + Rh | 6.26 ± 0.46 | |
| 7 d | No treatment | 8.46 ± 0.92 |
| Ctrl | 219.08 ± 8.52 | |
| GO | 225.48 ± 5.73 | |
| Rh | 221.59 ± 4.79 | |
| GO + Rh | 237.87 ± 6.56 * |
| Time | Treatment | Root Length (m) | Root Surface (cm2) | Root Diameter (mm) | Root Volume (cm3) |
|---|---|---|---|---|---|
| 0 d | No treatment | 4.45± 0.96 | 55.23 ± 12.77 | 4.0 ± 0.18 | 0.57 ± 0.13 |
| Ctrl | 4.43 ± 0.82 | 52.97 ± 5.13 | 3.97 ± 0.38 | 0.50 ± 1.0 | |
| GO | 4.52 ± 0.43 | 53.53 ± 3.34 | 3.76 ± 0.36 | 0.51 ± 0.06 | |
| Rh | 4.41 ± 0.11 | 54.37 ± 10.82 | 4.04 ± 0.11 | 0.56 ± 0.11 | |
| GO + Rh | 4.51 ± 0.27 | 56.72 ± 4.33 | 4.02 ± 0.40 | 0.57 ± 0.09 | |
| 7 d | No treatment | 23.69 ± 0.44 | 278.46 ± 5.54 | 5.50 ± 0.48 | 2.61 ± 0.63 |
| Ctrl | 14.53 ± 0.56 | 178.23 ± 9.57 | 4.01 ± 0.12 | 1.74 ± 0.13 | |
| GO | 18.56 ± 3.21 | 235.50 ± 38.71 | 4.20 ± 0.20 | 2.38 ± 0.37 | |
| Rh | 21.25 ± 0.44 * | 261.49 ± 11.19 ** | 4.36 ± 0.40 | 2.56 ± 0.19 * | |
| GO + Rh | 22.84 ± 3.24 * | 276.24 ± 28.61 ** | 4.83 ± 0.13 ** | 2.66 ± 0.16 * |
| Treatment | Pods Number | Empty Pods Number | Seed Number | Seed Weight (g) | 100-Seed Weight (g) |
|---|---|---|---|---|---|
| No treatment | 16.25 ± 3.84 | 3.67 ± 1.83 | 27.92 ± 6.56 | 7.07 ± 1.89 | 30.44 ± 1.4 |
| Ctrl | 10.75 ± 3.62 | 2.42 ± 1.00 | 18.25 ± 6.22 | 3.89 ± 1.84 | 20.9 ± 1.76 |
| GO + Rh | 16.75 ± 6.59 ** | 4.17 ± 1.27 ** | 26.92 ± 12.63 * | 6.17 ± 3.37 * | 28.92 ± 3.04 * |
| Time | Treatment | Na+ Content (mg/g) |
|---|---|---|
| Seedings period | No treatment | 1.98 ± 0.42 |
| Ctrl | 1.66 ± 0.42 | |
| GO + Rh | 1.58 ± 0.28 | |
| Harvest period | No treatment | 2.58 ± 1.01 |
| Ctrl | 149.48 ± 1.97 | |
| GO + Rh | 165.7 ± 6.4 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.; Bian, D.; Gu, X.; Cao, J.; Liu, J. Combination of Graphene Oxide and Rhizobium Improved Soybean Tolerance in Saline-Alkali Stress. Agronomy 2023, 13, 1637. https://doi.org/10.3390/agronomy13061637
Fu X, Bian D, Gu X, Cao J, Liu J. Combination of Graphene Oxide and Rhizobium Improved Soybean Tolerance in Saline-Alkali Stress. Agronomy. 2023; 13(6):1637. https://doi.org/10.3390/agronomy13061637
Chicago/Turabian StyleFu, Xiaohong, Dahong Bian, Xuyang Gu, Jinfeng Cao, and Jianfeng Liu. 2023. "Combination of Graphene Oxide and Rhizobium Improved Soybean Tolerance in Saline-Alkali Stress" Agronomy 13, no. 6: 1637. https://doi.org/10.3390/agronomy13061637
APA StyleFu, X., Bian, D., Gu, X., Cao, J., & Liu, J. (2023). Combination of Graphene Oxide and Rhizobium Improved Soybean Tolerance in Saline-Alkali Stress. Agronomy, 13(6), 1637. https://doi.org/10.3390/agronomy13061637





