Effects of Rice Husk Biochar and Compost Amendments on Soil Phosphorus Fractions, Enzyme Activities and Rice Yields in Salt-Affected Acid Soils in the Mekong Delta, Viet Nam
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Description
2.2. Experimental Designs
2.3. Sample Analysis
2.3.1. Soil Chemical Properties
2.3.2. Soil Enzyme Activities
2.4. Statistical Analysis
3. Results
3.1. Effects of Biochar and Compost Amendments on Chemical Properties of Fertilized Salt-Affected Acid Soils
3.1.1. pH, Total C, Labile C, Non-Labile C, Total N, and Available N
3.1.2. P Fractionation
3.1.3. Effect on Labile P Pools
3.1.4. Effect on Moderately Labile P Pools (NaOH-Pi, NaOH-Po, and H2SO4-Pi)
3.1.5. Effect on Non-Labile P Pools (Residual P)
3.2. Effects of Biochar and Compost Amendment on Enzyme Activities of Fertilized Salt-Affected Acid Soils
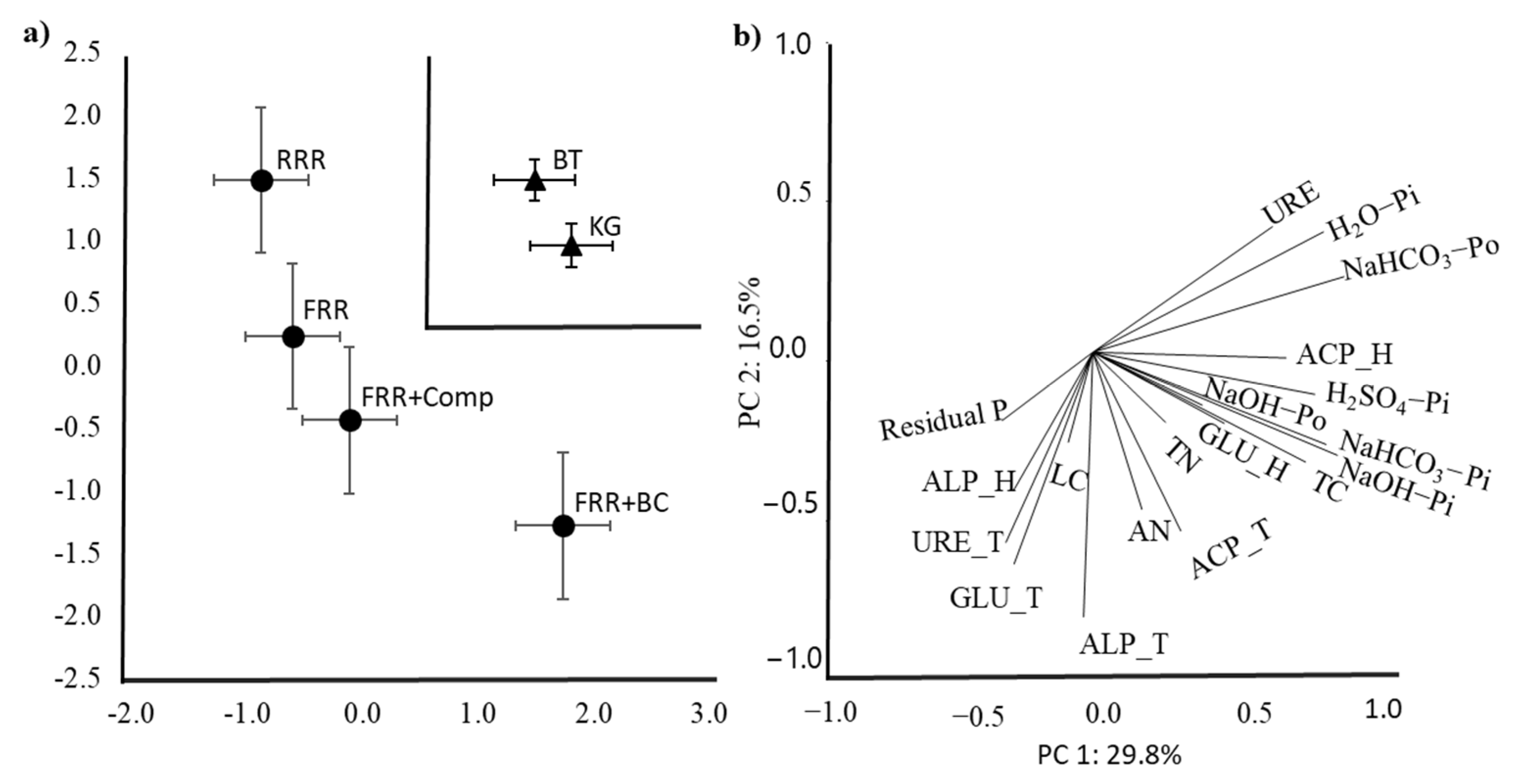
3.3. Principal Component Analysis
3.4. Relationships to Grain Yield
3.5. Triple versus Double Rice Cropping
4. Discussion
4.1. Response of P Fractions, Labile C, and Available N to Biochar or Compost Amendments to Fertilized Salt-Affected Acid Soils
4.2. Response of Enzyme Activities to Biochar or Compost Amendments to Fertilized Salt-Affected Acid Soils
4.3. Relationship between Rice Yield and Biochemical Properties
4.4. Triple versus Double Rice Cropping
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooperation, V.N.N. Towards a Mekong Delta Plan. Synthesis of Water Sector Assessment; Programme Partners for Water; Deltares: Delft, The Netherlands, 2011. [Google Scholar]
- Parfitt, R. Anion Adsorption by Soils and Soil Materials. Adv. Agron. 1979, 30, 1–50. [Google Scholar] [CrossRef]
- Phuong, N.T.K.; Khoi, C.M.; Ritz, K.; Van Sinh, N.; Tarao, M.; Toyota, K. Potential Use of Rice Husk Biochar and Compost to Improve P Availability and Reduce GHG Emissions in Acid Sulfate Soil. Agronomy 2020, 10, 685. [Google Scholar] [CrossRef]
- Pierzynski, G.M.; McDowell, R.W.; Sims, J.T. Chemistry, cycling, and potential movement of inorganic phosphorus in soils. In Phosphorus: Agriculture and the Environment; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2005; Volume 46, pp. 51–86. [Google Scholar]
- Oelkers, E.H.; Valsami-Jones, E. Phosphate Mineral Reactivity and Global Sustainability. Elements 2008, 4, 83–87. [Google Scholar] [CrossRef]
- Hedley, M.; Stewart, J.; Chauhan, B. Changes in Inorganic and Organic Soil Phosphorus Fractions Induced by Cultivation Practices and by Laboratory Incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Cross, A.F.; Schlesinger, W.H. A literature review and evaluation of the Hedley fractionation: Applications to the biogeo-chemical cycle of soil phosphorus in natural ecosystems. Geoderma 1995, 64, 197–214. [Google Scholar] [CrossRef]
- Song, C.; Han, X.; Wang, E. Phosphorus budget and organic phosphorus fractions in response to long-term applications of chemical fertilisers and pig manure in a Mollisol. Soil Res. 2011, 49, 253–260. [Google Scholar] [CrossRef]
- Saleque, M.A.; Naher, U.A.; Islam, A.; Pathan, A.B.M.B.U.; Hossain, A.T.M.S.; Meisner, C.A. Inorganic and Organic Phosphorus Fertilizer Effects on the Phosphorus Fractionation in Wetland Rice Soils. Soil Sci. Soc. Am. J. 2004, 68, 1635–1644. [Google Scholar] [CrossRef]
- Gao, S.; DeLuca, T.H.; Cleveland, C.C. Biochar additions alter phosphorus and nitrogen availability in agricultural ecosystems: A meta-analysis. Sci. Total Environ. 2019, 654, 463–472. [Google Scholar] [CrossRef]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y.; et al. How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. GCB Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Ghorbani, M.; Neugschwandtner, R.W.; Konvalina, P.; Asadi, H.; Kopecký, M.; Amirahmadi, E. Comparative effects of biochar and compost applications on water holding capacity and crop yield of rice under evaporation stress: A two-years field study. Paddy Water Environ. 2023, 21, 47–58. [Google Scholar] [CrossRef]
- Blair, G.; Lefroy, R.; Lisle, L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Lewis, D.B.; Kaye, J.P.; Jabbour, R.; Barbercheck, M.E. Labile carbon and other soil quality indicators in two tillage systems during transition to organic agriculture. Renew. Agric. Food Syst. 2011, 26, 342–353. [Google Scholar] [CrossRef]
- Jobbágy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Haynes, R. Labile Organic Matter Fractions as Central Components of the Quality of Agricultural Soils: An Overview. Adv. Agron. 2005, 5, 221–268. [Google Scholar] [CrossRef]
- Adetunji, A.T.; Lewu, F.; Mulidzi, R.; Ncube, B. The biological activities of β-glucosidase, phosphatase and urease as soil quality indicators: A review. J. Soil Sci. Plant Nutr. 2017, 17, 794–807. [Google Scholar] [CrossRef]
- Demisie, W.; Liu, Z.; Zhang, M. Effect of biochar on carbon fractions and enzyme activity of red soil. Catena 2014, 121, 214–221. [Google Scholar] [CrossRef]
- Khadem, A.; Raiesi, F. Response of soil alkaline phosphatase to biochar amendments: Changes in kinetic and thermodynamic characteristics. Geoderma 2018, 337, 44–54. [Google Scholar] [CrossRef]
- Liu, S.; Meng, J.; Jiang, L.; Yang, X.; Lan, Y.; Cheng, X.; Chen, W. Rice husk biochar impacts soil phosphorous availability, phosphatase activities and bacterial community characteristics in three different soil types. Appl. Soil Ecol. 2017, 116, 12–22. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, G.; Feng, H.; Sun, B.; Zhao, Y.; Chen, H.; Chen, J.; Dyck, M.; Wang, X.; Zhang, J.; et al. Effects of straw and biochar amendments on aggregate stability, soil organic carbon, and enzyme activities in the Loess Plateau, China. Environ. Sci. Pollut. Res. 2017, 24, 10108–10120. [Google Scholar] [CrossRef]
- Foster, E.J.; Hansen, N.; Wallenstein, M.; Cotrufo, M.F. Biochar and manure amendments impact soil nutrients and microbial enzymatic activities in a semi-arid irrigated maize cropping system. Agric. Ecosyst. Environ. 2016, 233, 404–414. [Google Scholar] [CrossRef]
- Wu, F.; Jia, Z.; Wang, S.; Chang, S.X.; Startsev, A. Contrasting Effects of Wheat Straw and Its Biochar on Greenhouse Gas Emissions and Enzyme Activities in a Chernozemic Soil. Biol. Fertil. Soils 2013, 49, 555–565. [Google Scholar] [CrossRef]
- Huang, M.; Zhou, X.; Chen, J.; Cao, F.; Jiang, L.; Zou, Y. Interaction of Changes in pH and Urease Activity Induced by Biochar Addition Affects Ammonia Volatilization on an Acid Paddy Soil Following Application of Urea. Commun. Soil Sci. Plant Anal. 2017, 48, 107–112. [Google Scholar] [CrossRef]
- Singh, B.; Singh, B.P.; Cowie, A.L. Characterisation and evaluation of biochars for their application as a soil amendment. Soil Res. 2010, 48, 516–525. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Phuong, N.T.K.; Khoi, C.M.; Ritz, K.; Linh, T.B.; Minh, D.D.; Duc, T.A.; Van Sinh, N.; Linh, T.T.; Toyota, K. Influence of Rice Husk Biochar and Compost Amendments on Salt Contents and Hydraulic Properties of Soil and Rice Yield in Salt-Affected Fields. Agronomy 2020, 10, 1101. [Google Scholar] [CrossRef]
- Wardle, D.A.; Walker, L.R.; Bardgett, R.D. Ecosystem Properties and Forest Decline in Contrasting Long-Term Chronosequences. Science 2004, 305, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Elser, J.J.; Bracken, M.E.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef]
- Farrell, M.; Macdonald, L.M.; Butler, G.; Chirino-Valle, I.; Condron, L.M. Biochar and fertiliser applications influence phosphorus fractionation and wheat yield. Biol. Fertil. Soils 2013, 50, 169–178. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Nayak, A.K.; Shahid, M.; Tripathi, R.; Mohanty, S.; Kumar, A.; Raja, R.; Panda, B.B.; Lal, B.; Gautam, P.; et al. Effects of 42-year long-term fertilizer management on soil phosphorus availability, fractionation, adsorption–desorption isotherm and plant uptake in flooded tropical rice. Crops J. 2015, 3, 387–395. [Google Scholar] [CrossRef]
- Alotaibi, K.D.; Arcand, M.; Ziadi, N. Effect of biochar addition on legacy phosphorus availability in long-term cultivated arid soil. Chem. Biol. Technol. Agric. 2021, 8, 47. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, C.; Gray, E.M.; Boyd, S.E.; Yang, H.; Zhang, D. Roles of biochar in improving phosphorus availability in soils: A phosphate adsorbent and a source of available phosphorus. Geoderma 2016, 276, 1–6. [Google Scholar] [CrossRef]
- Murphy, J.A.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Weil, R.R.; Islam, K.R.; Stine, M.A.; Gruver, J.B.; Samson-Liebig, S.E. Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use. Am. J. Altern. Agric. 2003, 18, 3–17. [Google Scholar] [CrossRef]
- Saito, M. Estimation of nitrogen availability indices based on UV absorption of soil extracts. Jpn. J. Soil Sci. Plant Nutr. 1988, 59, 493–495. [Google Scholar]
- Alef, K.; Nannipieri, P. Methods in Applied Soil Microbiology and Biochemistry; Academic Press: Cambridge, MA, USA, 1995; pp. 311–373. [Google Scholar]
- Gianfreda, L.; Bollag, J. Influence of natural and anthropogenic factors on enzyme activity in soil. In Soil Biochemistry; Marcel Dekker: New York, NY, USA, 1996; Volume 9, pp. 123–193. [Google Scholar]
- Bandick, A.K.; Dick, R.P. Field management effects on soil enzyme activities. Soil Biol. Biochem. 1999, 31, 1471–1479. [Google Scholar] [CrossRef]
- Tabatabai, M.; Dick, W. Enzymes in soil: Research and developments in measuring activities. In Enzymes in the Environment: Activity, Ecology, and Applications; Burns, R.G., Dick, R.P., Eds.; Marcel Dekker: New York, NY, USA, 2002; pp. 567–596. [Google Scholar]
- Zornoza, R.; Guerrero, C.; Mataix-Solera, J.; Arcenegui, V.; García-Orenes, F.; Mataix-Beneyto, J. Assessing air-drying and rewetting pre-treatment effect on some soil enzyme activities under Mediterranean conditions. Soil Biol. Biochem. 2006, 38, 2125–2134. [Google Scholar] [CrossRef]
- Tabatabai, M. Soil enzymes. In Methods of Soil Analysis: Part 2 Microbiological and Biochemical Properties; Soil Science Society of America: Madison, WI, USA, 1994; Volume 5, pp. 775–833. [Google Scholar]
- Nannipieri, P.; Johnson, R.; Paul, E. Criteria for measurement of microbial growth and activity in soil. Soil Biol. Biochem. 1978, 10, 223–229. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, J.; Rong, X.; Han, Y.; Yang, Z.; Hou, K.; Zhao, H.; Hu, W. Responses of maize yield, nitrogen and phosphorus runoff losses and soil properties to biochar and organic fertilizer application in a light-loamy fluvo-aquic soil. Agric. Ecosyst. Environ. 2021, 314, 107433. [Google Scholar] [CrossRef]
- Schneider, F.; Haderlein, S.B. Potential effects of biochar on the availability of phosphorus—Mechanistic insights. Geoderma 2016, 277, 83–90. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Z.-Q.; He, X.-S.; Yang, C.; Du, Y.-Q.; Huang, Y.-D.; Su, P.; Wang, S.; Zheng, X.-X.; Xue, Y.-J. Mechanisms of rice straw biochar effects on phosphorus sorption characteristics of acid upland red soils. Chemosphere 2018, 207, 267–277. [Google Scholar] [CrossRef]
- Rashmi, I.; Jha, P.; Biswas, A.K. Phosphorus Sorption and Desorption in Soils Amended with Subabul Biochar. Agric. Res. 2019, 9, 371–378. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, S.; Guo, D.; Zhang, S.; Song, X.; Yue, K.; Zhang, K.; Bao, D. Phosphorus adsorption and desorption char-acteristics of different textural fluvo-aquic soils under long-term fertilization. J. Soils Sed. 2019, 19, 1306–1318. [Google Scholar] [CrossRef]
- Xu, G.; Shao, H.; Zhang, Y.; Junna, S. Nonadditive effects of biochar amendments on soil phosphorus fractions in two con-trasting soils. Land Degrad. Dev. 2018, 29, 2720–2727. [Google Scholar] [CrossRef]
- Hong, C.; Lu, S.G. Does biochar affect the availability and chemical fractionation of phosphate in soils? Environ. Sci. Pollut. Res. 2018, 25, 8725–8734. [Google Scholar] [CrossRef]
- Gagnon, B.; Demers, I.; Ziadi, N.; Chantigny, M.H.; Parent, L.E.; Forge, T.A.; Larney, F.L.; Buckley, K.E. Forms of phos-phorus in composts and in compost-amended soils following incubation. Can. J. Soil Sci. 2012, 92, 711–721. [Google Scholar] [CrossRef]
- Rivaie, A.A.; Tillman, R.W. Phosphorus fractions of fertiliser-derived P in an allophanic soil under Pinus radiata seedlings grown with broom and ryegrass. J. For. Res. 2009, 20, 229–236. [Google Scholar] [CrossRef]
- Chintala, R.; Schumacher, T.E.; McDonald, L.M.; Clay, D.E.; Malo, D.D.; Papiernik, S.K.; Clay, S.A.; Julson, J.L. Phosphorus Sorption and Availability from Biochars and Soil/Biochar Mixtures. CLEAN–Soil Air Water 2014, 42, 626–634. [Google Scholar] [CrossRef]
- Jiang, J.; Yuan, M.; Xu, R.; Bish, D.L. Mobilization of phosphate in variable-charge soils amended with biochars derived from crop straws. Soil Tillage Res. 2015, 146, 139–147. [Google Scholar] [CrossRef]
- Sui, Y.; Gao, J.; Liu, C.; Zhang, W.; Lan, Y.; Li, S.; Meng, J.; Xu, Z.; Tang, L. Interactive effects of straw-derived biochar and N fertilization on soil C storage and rice productivity in rice paddies of Northeast China. Sci. Total. Environ. 2016, 544, 203–210. [Google Scholar] [CrossRef]
- Zhang, A.; Bian, R.; Pan, G.; Cui, L.; Hussain, Q.; Li, L.; Zheng, J.; Zheng, J.; Zhang, X.; Han, X.; et al. Effects of biochar amendment on soil quality, crop yield and greenhouse gas emission in a Chinese rice paddy: A field study of 2 consecutive rice growing cycles. Field Crops Res. 2012, 127, 153–160. [Google Scholar] [CrossRef]
- Singh, B.P.; Cowie, A.L. Long-term influence of biochar on native organic carbon mineralisation in a low-carbon clayey soil. Sci. Rep. 2014, 4, 3687. [Google Scholar] [CrossRef]
- Bolan, N.; Kunhikrishnan, A.; Choppala, G.; Thangarajan, R.; Chung, J. Stabilization of carbon in composts and biochars in relation to carbon sequestration and soil fertility. Sci. Total. Environ. 2012, 424, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Abhishek, K.; Sarswat, A.; Patel, M.; Singh, P.; Pittman, C.U. Biochar production and applications in soil fertility and carbon sequestration—A sustainable solution to crop-residue burning in India. RSC Adv. 2018, 8, 508–520. [Google Scholar] [CrossRef]
- Xie, Z.; Xu, Y.; Liu, G.; Liu, Q.; Zhu, J.; Tu, C.; Amonette, J.E.; Cadisch, G.; Yong, J.W.H.; Hu, S. Impact of biochar application on nitrogen nutrition of rice, greenhouse-gas emissions and soil organic carbon dynamics in two paddy soils of China. Plant Soil 2013, 370, 527–540. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Jing, Y.; Zhang, Y.; Han, I.; Wang, P.; Mei, Q.; Huang, Y. Effects of different straw biochars on soil organic carbon, nitrogen, available phosphorus, and enzyme activity in paddy soil. Sci. Rep. 2020, 10, 8837. [Google Scholar] [CrossRef]
- Ahmed, W.; Qaswar, M.; Jing, H.; Wenjun, D.; Geng, S.; Kailou, L.; Ying, M.; Ao, T.; Mei, S.; Chao, L.; et al. Tillage practices improve rice yield and soil phosphorus fractions in two typical paddy soils. J. Soils Sediments 2020, 20, 850–861. [Google Scholar] [CrossRef]
- Elzobair, K.A.; Stromberger, M.E.; Ippolito, J.A.; Lentz, R.D. Contrasting effects of biochar versus manure on soil microbial communities and enzyme activities in an Aridisol. Chemosphere 2016, 142, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Steinweg, J.M.; Dukes, J.S.; Wallenstein, M.D. Modeling the effects of temperature and moisture on soil enzyme activity: Linking laboratory assays to continuous field data. Soil Biol. Biochem. 2012, 55, 85–92. [Google Scholar] [CrossRef]
- Kotroczó, Z.; Veres, Z.; Fekete, I.; Krakomperger, Z.; Tóth, J.A.; Lajtha, K.; Tóthmérész, B. Soil enzyme activity in response to long-term organic matter manipulation. Soil Biol. Biochem. 2014, 70, 237–243. [Google Scholar] [CrossRef]
- Dick, R.; Rasmussen, P.; Kerle, E. Influence of long-term residue management on soil enzyme activities in relation to soil chemical properties of a wheat-fallow system. Biol. Fertil. Soils 1988, 6, 159–164. [Google Scholar] [CrossRef]
- Zhang, A.; Cui, L.; Pan, G.; Li, L.; Hussain, Q.; Zhang, X.; Zheng, J.; Crowley, D. Effect of biochar amendment on yield and methane and nitrous oxide emissions from a rice paddy from Tai Lake plain, China. Agric. Ecosyst. Environ. 2010, 139, 469–475. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, J.; Wang, S.; Xing, G. Successive straw biochar application as a strategy to sequester carbon and improve fertility: A pot experiment with two rice/wheat rotations in paddy soil. Plant Soil 2014, 378, 279–294. [Google Scholar] [CrossRef]
- Chen, D.; Guo, H.; Li, R.; Li, L.; Pan, G.; Chang, A.; Joseph, S. Low uptake affinity cultivars with biochar to tackle Cd-tainted rice—A field study over four rice seasons in Hunan, China. Sci. Total. Environ. 2016, 541, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Fan, L.; Jiang, L.-G.; Yang, S.-Y.; Zou, Y.-B.; Uphoff, N. Continuous applications of biochar to rice: Effects on grain yield and yield attributes. J. Integr. Agric. 2019, 18, 563–570. [Google Scholar] [CrossRef]
- Rafiq, M.K.; Bai, Y.; Aziz, R.; Mašek, O.; Bachmann, R.T.; Joseph, S.; Shahbaz, M.; Qayyum, A.; Shang, Z.; Danaee, M.; et al. Biochar amendment improves alpine meadows growth and soil health in Tibetan plateau over a three year period. Sci. Total. Environ. 2020, 717, 135296. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, S.; Chen, M.; Liu, J.; Ding, X. A sustainable option: Biochar addition can improve soil phosphorus retention and rice yield in a saline–alkaline soil. Environ. Technol. Innov. 2021, 24, 102070. [Google Scholar] [CrossRef]
- Sushanta, S.; Bholanath, S.; Sidhu, M.; Sajal, P.; Partha, D.R.; Saha, S.; Murm, S.; Pati, S.; Roy, P.D. Grain yield and phosphorus uptake by wheat as influenced by long-term phosphorus fertilization. Afr. J. Agric. Res. 2014, 9, 607–612. [Google Scholar] [CrossRef]



| Organic Materials | Soils | |||
|---|---|---|---|---|
| Compost | Biochar | Ben Tre | Kien Giang | |
| pH (H2O) * (1:2.5) | 4.64 | 4.65 | ||
| pH (H2O) * (1:5) | 8.7 | 7.7 | ||
| EC * (mS cm−1) (1:2.5) | 1.24 | 1.16 | ||
| EC * (mS cm−1) (1:5) | 17.1 | 4.1 | ||
| Total C * (g kg−1) | 154 | 471 | 15.7 | 15.0 |
| Labile C (g kg−1) | 3.13 | 1.18 | 0.59 | 0.39 |
| Total N * (g kg−1) | 26 | 4.72 | 1.55 | 1.32 |
| Available N (mg kg−1) | 528 | 8.13 | 24.7 | 22.5 |
| Available P * (mg kg−1) | 3600 | 800 | 6.91 | 20.9 |
| Soluble K * (mg kg−1) | 20 | 3.35 | 0.15 | 0.07 |
| Exchangeable K * (mg kg−1) | 15 | 12.9 | 0.63 | 0.52 |
| Soluble Ca * (mg kg−1) | 7.29 | 0.15 | 1.70 | 0.96 |
| Exchangeable Ca * (mg kg−1) | 61.6 | 0.16 | 6.01 | 3.44 |
| P Fractions | H2O- Pi | NaHCO3- Pi | NaHCO3- Po | NaOH- Pi | NaOH- Po | H2SO4- Pi | Residual P |
|---|---|---|---|---|---|---|---|
| Ben Tre | 0.48 | 26.3 | 65.5 | 78.1 | 62.9 | 35.6 | 219 |
| Kien Giang | 0.73 | 36.0 | 70.5 | 146 | 72.5 | 54.2 | 216 |
| Sites (S) | Treatments (T) | pH (H2O) | Total C | Labile C | Non-Labile C | Total N | Available N |
|---|---|---|---|---|---|---|---|
| g C kg−1 | g C kg−1 | g C kg−1 | g N kg−1 | mg N kg−1 | |||
| Ben Tre | RRR | 5.05 | 16.3 b | 0.55 | 15.8 b | 2.03 | 22.3 |
| FRR | 5.21 | 16.2 b | 0.54 | 15.7 b | 1.98 | 19.0 | |
| FRR + Comp | 4.97 | 17.9 b | 0.54 | 17.3 b | 2.09 | 23.8 | |
| FRR + BC | 5.18 | 23.3 a | 0.56 | 22.7 a | 2.23 | 20.8 | |
| Kien Giang | RRR | 4.85 | 19.7 b | 0.55 | 19.1 b | 2.15 | 19.5 |
| FRR | 4.91 | 19.7 b | 0.52 | 19.2 b | 2.13 | 20.9 | |
| FRR + Comp | 5.04 | 17.4 b | 0.49 | 16.9 b | 1.97 | 20.5 | |
| FRR + BC | 4.96 | 25.7 a | 0.53 | 25.2 a | 2.11 | 21.9 | |
| ANOVA | |||||||
| S | ns | * | ns | * | ns | ns | |
| T | ns | *** | ns | *** | ns | ns | |
| S × T | ns | ns | ns | ns | ns | ns |
| Sites | Treatments | Labile P | Moderately Labile P | Non-Labile P | ||||
|---|---|---|---|---|---|---|---|---|
| H2O- Pi | NaHCO3- Pi | NaHCO3- Po | NaOH- Pi | NaOH- Po | H2SO4- Pi | Residual- P | ||
| mg kg−1 | ||||||||
| Ben Tre | RRR | 0.38 | 14.1 b | 30.2 | 83.7 b | 32.6 b | 38.2 | 232 |
| FRR | 0.41 | 17.3 b | 34.4 | 88.8 b | 37.0 ab | 44.3 | 220 | |
| FRR + Comp | 0.33 | 31.0 ab | 31.5 | 126 ab | 54.9 a | 55.5 | 245 | |
| FRR + BC | 0.41 | 48.8 a | 24.3 | 133 a | 50.1 ab | 51.1 | 232 | |
| Kien Giang | RRR | 0.90 ab | 30.7 b | 82.5 b | 133 b | 47.8 b | 55.4 b | 209 ab |
| FRR | 0.86 b | 33.6 b | 82.7 b | 131 b | 74.4 a | 55.3 b | 193 b | |
| FRR + Comp | 1.18 ab | 42.3 b | 71.4 b | 143 b | 44.3 b | 60.5 b | 229 a | |
| FRR + BC | 1.39 a | 66.2 a | 123 a | 192 a | 39.5 b | 78.1 a | 209 ab | |
| ANOVA | ||||||||
| S | *** | * | *** | *** | ns | *** | * | |
| T | ns | *** | ns | *** | ns | ** | ns | |
| S × T | ns | ns | * | ns | * | ns | ns | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linh, D.T.T.; Khoi, C.M.; Ritz, K.; Sinh, N.V.; Phuong, N.T.K.; My, H.M.T.; Linh, T.B.; Minh, D.D.; Linh, T.T.; Toyota, K. Effects of Rice Husk Biochar and Compost Amendments on Soil Phosphorus Fractions, Enzyme Activities and Rice Yields in Salt-Affected Acid Soils in the Mekong Delta, Viet Nam. Agronomy 2023, 13, 1593. https://doi.org/10.3390/agronomy13061593
Linh DTT, Khoi CM, Ritz K, Sinh NV, Phuong NTK, My HMT, Linh TB, Minh DD, Linh TT, Toyota K. Effects of Rice Husk Biochar and Compost Amendments on Soil Phosphorus Fractions, Enzyme Activities and Rice Yields in Salt-Affected Acid Soils in the Mekong Delta, Viet Nam. Agronomy. 2023; 13(6):1593. https://doi.org/10.3390/agronomy13061593
Chicago/Turabian StyleLinh, Doan Thi Truc, Chau Minh Khoi, Karl Ritz, Nguyen Van Sinh, Nguyen Thi Kim Phuong, Huynh Mach Tra My, Tran Ba Linh, Dang Duy Minh, Thi Tu Linh, and Koki Toyota. 2023. "Effects of Rice Husk Biochar and Compost Amendments on Soil Phosphorus Fractions, Enzyme Activities and Rice Yields in Salt-Affected Acid Soils in the Mekong Delta, Viet Nam" Agronomy 13, no. 6: 1593. https://doi.org/10.3390/agronomy13061593
APA StyleLinh, D. T. T., Khoi, C. M., Ritz, K., Sinh, N. V., Phuong, N. T. K., My, H. M. T., Linh, T. B., Minh, D. D., Linh, T. T., & Toyota, K. (2023). Effects of Rice Husk Biochar and Compost Amendments on Soil Phosphorus Fractions, Enzyme Activities and Rice Yields in Salt-Affected Acid Soils in the Mekong Delta, Viet Nam. Agronomy, 13(6), 1593. https://doi.org/10.3390/agronomy13061593






