Overexpression of Chalcone Isomerase-like Genes, GmCHI4A and GmCHI4B, Enhances Salt Tolerance of Cotyledon Hairy Roots and Composite Plant in Soybean (Glycine max (L.) Merr.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Isolation and Sequence Analysis of GmCHI4 Genes
2.3. RNA Extraction and qRT-PCR Performance
2.4. Transformation and Identification of Transformed Hairy Roots and Composite Plants of Soybean
2.5. Salt Treatment and Tolerance Evaluation of Transformed Hairy Roots and Composite Plants
2.6. Extraction and Determination of Isoflavone of Transformed Hairy Roots
2.7. Statistical Analysis and Phylogenetic Analysis
3. Results
3.1. Characterization and Spatial Expression of the GmCHI4s in Soybean
3.2. Expression Level of Hairy Roots That Overexpress GmCHI4s
3.3. GmCHI4s Alleviated Salt Tolerance in Transformed Soybean Hairy Roots and Composite Plants
3.4. Transcription Patterns of Stress-Related Genes in Soybean Hair Root after the Overexpression of GmCHI4s
3.5. Overexpression of GmCHI4s Increased Isoflavone Content in Transformed Soybean Hairy Root
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shuang, M.; Zhou, W.L.; Yang, F. Research progress on the mechanism of plant root response to saline-alkali stress. Zhejiang J. Agric. Sci. 2021, 33, 10. [Google Scholar]
- Leung, H.S.; Chan, L.Y.; Law, C.H.; Li, M.W.; Lam, H.M. Twenty years of mining salt tolerance genes in soybean. Mol. Breed. 2023, 43, 6. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2017, 217, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Duan, L.; Tian, X.; Wang, B.; Egrinya Eneji, A.; Li, Z. Coronatine alleviates salinity stress in cotton by improving the antioxidative defense system and radical-scavenging activity. J. Plant Physiol. 2008, 165, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Dietz, K.J. Tuning of redox regulatory mechanisms, reactive oxygen species and redox homeostasis under salinity stress. Front. Plant Sci. 2016, 7, 548. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.H.; Li, W.; Niu, C.F.; Wei, W.; Hu, Y.; Han, J.Q.; Lu, X.; Tao, J.J.; Jin, M.; Qin, H.; et al. A class B heat shock factor selected for during soybean domestication contributes to salt tolerance by promoting flavonoid biosynthesis. New Phytol. 2019, 225, 268–283. [Google Scholar] [CrossRef]
- Goormachtig, S.; Lievens, S.; Herman, S.; Van Montagu, M.; Holsters, M. Chalcone reductase-homologous transcripts accumulate during development of stem-borne nodules on the tropical legume Sesbania rostrata. Planta 1999, 209, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Dinkins, R.D.; Hancock, J.; Coe, B.L.; May, J.B.; Goodman, J.P.; Bass, W.T.; Liu, J.; Fan, Y.; Zheng, Q.; Zhu, H. Isoflavone levels, nodulation and gene expression profiles of a CRISPR/Cas9 deletion mutant in the isoflavone synthase gene of red clover. Plant Cell Rep. 2021, 40, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.Y.; Jiang, T.; Zhou, C.; Yang, X.F.; Fang, Y.; Zhang, Z.S.; Sun, S.S.; Miao, M.X.; Shi, S.Z. The OsmiR396-OsGRF8-OsF3H flavonoid pathway mediates resistance to the brown planthopper in rice (Oryza sativa). Plant Biotechnol. J. 2019, 17, 1657–1669. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, X.; Gao, Z.; Hu, T.; Liu, Y. The Research Progress of Chalcone Isomerase (CHI) in Plants. Mol. Biotechnol. 2019, 61, 32–52. [Google Scholar] [CrossRef]
- Jia, T.; An, J.; Liu, Z.; Yu, B.; Chen, J. Salt stress induced soybean GmIFS1 expression and isoflavone accumulation and salt tolerance in transgenic soybean cotyledon hairy roots and tobacco. Plant Cell Tissue Organ Cult. 2016, 128, 469–477. [Google Scholar] [CrossRef]
- Pi, E.; Qu, L.; Hu, J.; Huang, Y.; Qiu, L.; Lu, H.; Jiang, B.; Liu, C.; Peng, T.; Zhao, Y.; et al. Mechanisms of soybean roots’ tolerances to salinity revealed by proteomic and phosphoproteomic comparisons between two cultivars. Mol. Cell. Proteom. 2016, 15, 266–288. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Pinto, D.C.G.A.; Freitas, H.; Santos, C.; Silva, A.M.S. The antioxidant system in Olea europaea to enhanced UV-B radiation also depends on flavonoids and secoiridoids. Phytochemistry 2020, 170, 11299. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Yuan, H.; Dong, X.; Peng, M.; Jing, X.; Xu, Q.; Tang, T.; Wang, Y.; Zha, S.; Gao, M.; et al. Genome-wide dissection of co-selected UV-B responsive pathways in the UV-B adaptation of Qingke. Mol. Plant 2020, 13, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lv, J.; Liu, Z.; Wang, J.; Yang, B.; Chen, W.; Ou, L.; Dai, X.; Zhang, Z.; Zou, X. Integrative analysis of metabolome and transcriptome reveals the mechanism of color formation in pepper fruit (Capsicum annuum L.). Food Chem. 2020, 141, 290492. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Huang, Y.; Wu, W.; Zhu, C.; Zhang, R.; Chen, J.; Zeng, J. Metabolomics analysis of the peels of different colored citrus fruits (Citrus reticulata cv. ‘Shatangju’) during the maturation period based on UHPLC-QQQ-MS. Molecules 2020, 25, 396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, X.; Wilson, I.W.; Shao, F.; Qiu, D. Identification of the Genes Involved in anthocyanin biosynthesis and accumulation in Taxus chinensis. Genes 2019, 10, 982. [Google Scholar] [CrossRef] [PubMed]
- Alcalde-Eon, C.; Ferreras-Charro, R.; Ferrer-Gallego, R.; Rivero, F.J.; Heredia, F.J.; Escribano-Bailón, M.T. Monitoring the effects and side-effects on wine colour and flavonoid composition of the combined post-fermentative additions of seeds and mannoproteins. Food Res. Int. 2019, 126, 108650. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, M.; Qasim, M.; Ali, S.; Yousaf, H.K.; Waqas, M.; Ali, E.; Ahmad, M.A.; Jan, S.; Bashir, M.A.; Noman, A.; et al. Expression and functional analysis of P450 gene induced tolerance/resistance to lambda-cyhalothrin in quercetin fed larvae of beet armyworm Spodoptera exigua (Hübner). Saudi J. Biol. Sci. 2020, 27, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Prasifka, J.R.; Wallis, C.M. Concentrations of sunflower phenolics appear insufficient to explain resistance to floret- and seed-feeding caterpillars. Arthropod-Plant Interact. 2019, 13, 915–921. [Google Scholar] [CrossRef]
- Shan, T.; Wang, Y.; Wang, S.; Xie, Y.; Cui, Z.; Wu, C.; Sun, J.; Wang, J.; Mao, Z. A new p-terphenyl derivative from the insect-derived fungus Aspergillus candidus Bdf-2 and the synergistic effects of terphenyllin. PeerJ 2020, 8, e8211. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.Y.; Li, F.; Nan, Z.B.; Coulter, J.A.; Wei, W.J. Do Epichloë Endophytes and their grass symbiosis only produce toxic alkaloids to insects and livestock? J. Agric. Food Chem. 2020, 68, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B. An analyses of flavonoids present in the inflorescence of sunflower. Braz. J. Bot. 2019, 42, 421–429. [Google Scholar] [CrossRef]
- Zhou, X.; Shi, F.; Zhou, L.; Zhou, Y.; Liu, Z.; Ji, R.; Feng, H. iTRAQ-based proteomic analysis of fertile and sterile flower buds from a genetic male sterile line ‘AB01′ in Chinese cabbage (Brassica campestris L. ssp. pekinensis). J. Proteom. 2019, 204, 103395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; He, R.R.; Lian, J.P.; Zhou, Y.F.; Zhang, F.; Li, Q.F.; Yu, Y.; Feng, Y.Z.; Yang, Y.W.; Lei, M.Q.; et al. OsmiR528 regulates rice-pollen intine formation by targeting an uclacyanin to influence flavonoid metabolism. Proc. Natl. Acad. Sci. USA 2019, 117, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; An, Y.; Wang, L. 24-Epibrassinolide enhances 5-ALA-induced anthocyanin and flavonol accumulation in calli of ‘Fuji’ apple flesh. Plant Cell Tissue Organ Cult. 2018, 134, 319–330. [Google Scholar] [CrossRef]
- Boonkerd, S.; Yompakdee, C.; Miyakawa, T.; Chavasiri, W. A flavonoid, 5-hydroxy-3,7-dimethoxyflavone, from Kaempferia parviflora Wall. Ex. Baker as an inhibitor of Ca2+ signal-mediated cell-cycle regulation in yeast. Ann. Microbiol. 2013, 64, 1049–1054. [Google Scholar] [CrossRef]
- Md-Mustafa, N.; Khalid, N.; Gao, H.; Peng, Z.; Alimin, M.F.; Bujang, N.; Ming, W.S.; Mohd-Yusuf, Y.; Harikrishna, J.A.; Othman, R.Y. Transcriptome profiling shows gene regulation patterns in a flavonoid pathway in response to exogenous phenylalanine in Boesenbergia rotunda cell culture. BMC Genom. 2014, 15, 984. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.H.; Jeong, B.R.; Hawes, M.C. Flavonoids: From cell cycle regulation to biotechnology. Biotechnol. Lett. 2005, 27, 365–374. [Google Scholar] [CrossRef]
- Jayaraman, K.; Venkat, R.K.; Sevanthi, A.M.; Gayatri; Viswanathan, C.; Mohapatra, T.; Mandal, P.K. Stress-inducible expression of chalcone isomerase2 gene improves accumulation of flavonoids and imparts enhanced abiotic stress tolerance to rice. Environ. Exp. Bot. 2021, 190, 104582. [Google Scholar] [CrossRef]
- Gomaa, N.H.; Hassan, M.O.; Fahmy, G.M.; González, L.; Hammouda, O.; Atteya, A.M. Flavonoid profiling and nodulation of some legumes in response to the allelopathic stress of Sonchus oleraceus L. Acta Bot. Bras. 2015, 29, 553–560. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Qin, X.M.; Xiao, J.X.; Tang, L.; Wei, C.Z.; Wei, J.J.; Zheng, Y. Intercropping influences component and content change of flavonoids in root exudates and nodulation of Faba bean. J. Plant Interact. 2017, 12, 187–192. [Google Scholar] [CrossRef]
- Zhang, J.; Subramanian, S.; Stacey, G.; Yu, O. Flavones and flavonols play distinct critical roles during nodulation of Medicago truncatula by Sinorhizobium meliloti. Plant J. 2008, 57, 171–183. [Google Scholar] [CrossRef]
- Abdel-Aleem, E.R.; Attia, E.Z.; Farag, F.F.; Samy, M.N.; Desoukey, S.Y. Total phenolic and flavonoid contents and antioxidant, anti-inflammatory, analgesic, antipyretic and antidiabetic activities of Cordia myxa L. leaves. Clin. Phytosci. 2019, 5, 29. [Google Scholar] [CrossRef]
- Jung, Y.J.; Park, J.H.; Cho, J.G.; Seo, K.H.; Lee, D.S.; Kim, Y.C.; Kang, H.C.; Song, M.C.; Baek, N.-I. Lignan and flavonoids from the stems of Zea mays and their anti-inflammatory and neuroprotective activities. Arch. Pharmacal Res. 2014, 38, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Quilantang, N.G.; Kim, H.Y.; Lee, S.; Cho, E.J. Attenuation of hydrogen peroxide-induced oxidative stress in SH-SY5Y cells by three flavonoids from Acer okamotoanum. Chem. Pap. 2018, 73, 1135–1144. [Google Scholar] [CrossRef]
- Magiera, S.; Zaręba, M. Chromatographic determination of phenolic acids and flavonoids in Lycium barbarum L. and evaluation of antioxidant activity. Food Anal. Methods 2015, 8, 2665–2674. [Google Scholar] [CrossRef]
- Chakrabarti, M.; Ray, S.K. Anti-tumor activities of luteolin and silibinin in glioblastoma cells: Overexpression of miR-7-1-3p augmented luteolin and silibinin to inhibit autophagy and induce apoptosis in glioblastoma in vivo. Apoptosis 2015, 21, 312–328. [Google Scholar] [CrossRef]
- Huang, M.; Wang, Y.; Xu, L.; You, M. Anti-tumor properties of Prunella vulgaris. Curr. Pharmacol. Rep. 2015, 1, 401–419. [Google Scholar] [CrossRef]
- Pavlova, S.I.; Albegova, D.Z.; Vorob’eva, Y.S.; Laptev, O.S.; Kozlov, I.G. Flavonoids as potential immunosuppressants affecting intracellular signaling pathways. Pharm. Chem. J. 2016, 49, 645–652. [Google Scholar] [CrossRef]
- Kang, C.H.; Rhie, S.J.; Kim, Y.C. Antioxidant and skin Anti-aging effects of marigold methanol extract. Toxicol. Res. 2018, 34, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Cho, L.Y.; Yang, J.J.; Ko, K.-P.; Ma, S.H.; Shin, A.; Choi, B.Y.; Kim, H.J.; Han, D.S.; Song, K.S.; Kim, Y.S.; et al. Gene polymorphisms in the ornithine decarboxylase–polyamine pathway modify gastric cancer risk by interaction with isoflavone concentrations. Gastric Cancer 2014, 18, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Ralston, L.; Subramanian, S.; Matsuno, M.; Yu, O. Partial reconstruction of flavonoid and isoflavonoid biosynthesis in yeast using soybean type I and type II chalcone isomerases. Plant Physiol. 2005, 137, 1375–1388. [Google Scholar] [CrossRef] [PubMed]
- Shimada, N.; Aoki, T.; Sato, S.; Nakamura, Y.; Tabata, S.; Ayabe, S.-I. A cluster of genes encodes the two types of chalcone isomerase involved in the biosynthesis of general flavonoids and legume-specific 5-Deoxy(iso)flavonoids in Lotus japonicus. Plant Physiol. 2003, 131, 941–951. [Google Scholar] [CrossRef]
- Ngaki, M.N.; Louie, G.V.; Philippe, R.N.; Manning, G.; Pojer, F.; Bowman, M.E.; Li, L.; Larsen, E.; Wurtele, E.S.; Noel, J.P. Evolution of the chalcone-isomerase fold from fatty-acid binding to stereospecific catalysis. Nature 2012, 485, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Yin, Q.; Wu, R.; Zheng, G.; Liu, J.; Dixon, R.A.; Pang, Y. Role of a chalcone isomerase-like protein in flavonoid biosynthesis in Arabidopsis thaliana. J. Exp. Bot. 2015, 66, 7165–7179. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Takagi, K.; Fukuchi-Mizutani, M.; Ishiguro, K.; Tanaka, Y.; Nitasaka, E.; Nakayama, M.; Saito, N.; Kagami, T.; Hoshino, A.; et al. A chalcone isomerase-like protein enhances flavonoid production and flower pigmentation. Plant J. 2014, 78, 294–304. [Google Scholar] [CrossRef]
- Dastmalchi, M.; Dhaubhadel, S. Soybean chalcone isomerase: Evolution of the fold, and the differential expression and localization of the gene family. Planta 2014, 241, 507–523. [Google Scholar] [CrossRef]
- Wei, P.; Wang, L.; Liu, A.; Yu, B.; Lam, H.-M. GmCLC1 confers enhanced salt tolerance through regulating chloride accumulation in soybean. Front. Plant Sci. 2016, 7, 1082. [Google Scholar] [CrossRef]
- Zhao, Y.; Shao, S.; Li, X.; Zhai, Y.; Zhang, Q.; Qian, D.; Wang, Q. Isolation and activity analysis of a Seed-Abundant soyAP1 Gene promoter from soybean. Plant Mol. Biol. Rep. 2012, 30, 1400–1407. [Google Scholar] [CrossRef]
- Qu, Y.N.; Zhou, Q.; Yu, B.J. Effects of Zn2+ and niflumic acid on photosynthesis in Glycine soja and Glycine max seedlings under NaCl stress. Environ. Exp. Bot. 2009, 65, 304–309. [Google Scholar] [CrossRef]
- Puckette, M.C.; Weng, H.; Mahalingam, R. Physiological and biochemical responses to acute ozone-induced oxidative stress in Medicago truncatula. Plant Physiol. Biochem. 2007, 45, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Jia, B.; Cui, N.; Wen, Y.; Duanmu, H.; Yu, Q.; Xiao, J.; Sun, X.; Zhu, Y. Functional characterization of a Glycine soja Ca2+ATPase in salt–alkaline stress responses. Plant Mol. Biol. 2016, 90, 419–434. [Google Scholar] [CrossRef]
- Li, X.W.; Li, J.W.; Zhai, Y.; Zhao, Y.; Zhao, X.; Zhang, H.J.; Su, L.-T.; Wang, Y.; Wang, Q.-Y. A R2R3-MYB transcription factor, GmMYB12B2, affects the expression levels of flavonoid biosynthesis genes encoding key enzymes in transgenic Arabidopsis plants. Gene 2013, 532, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, G.K.; Tuan, P.A.; Renault, S.; Daayf, F.; Ayele, B.T. Polyamine-mediated transcriptional regulation of enzymatic antioxidative response to excesssoil moisture during early seedling growth in soybean. Biology 2020, 9, 185. [Google Scholar] [CrossRef]
- An, J.; Hu, Z.; Che, B.; Chen, H.; Yu, B.; Cai, W. Heterologous expression of panax ginseng PgTIP1 confers enhanced salt tolerance of soybean cotyledon hairy roots, composite, and whole plants. Front. Plant Sci. 2017, 8, 1232. [Google Scholar] [CrossRef]
- Li, S.F.; Huang, Y.Z.; Xuan, H.D.; Huang, L.; Zhao, J.M.; Wang, H.T.; Guo, N.; Xing, H. Cloning and function analysis of GmTIP1-1 gene in soybean. J. Nanjing Agric. Univ. 2019, 42, 793–801. [Google Scholar]
- Yan, J.; Wang, B.; Jiang, Y.; Cheng, L.; Wu, T. GmFNSII-Controlled Soybean flavone metabolism responds to abiotic stresses and regulates plant salt tolerance. Plant Cell Physiol. 2014, 55, 74–86. [Google Scholar] [CrossRef]
- Dolatabadian, A.; Modarres Sanavy, S.A.M.; Ghanati, F.; Gresshoff, P.M. Agrobacterium rhizogenes transformed soybean roots differ in their nodulation and nitrogen fixation response to genistein and salt stress. World J. Microbiol. Biotechnol. 2013, 29, 1327–1339. [Google Scholar] [CrossRef]
- Wu, Y.M.; Zhou, Q.; Yu, B.J. Physiological effect of soybean isoflavone soaking on soybean seedlings under salt stress. Acta Ecol. Sin. 2011, 31, 6669–6676. [Google Scholar]
- Golkar, P.; Taghizadeh, M. In vitro evaluation of phenolic and osmolite compounds, ionic content, and antioxidant activity in safflower (Carthamus tinctorius L.) under salinity stress. Plant Cell Tissue Organ Cult. 2018, 134, 357–368. [Google Scholar] [CrossRef]
- Golkar, P.; Taghizadeh, M.; Yousefian, Z. The effects of chitosan and salicylic acid on elicitation of secondary metabolites and antioxidant activity of safflower under in vitro salinity stress. Plant Cell Tissue Organ Cult. 2019, 137, 575–585. [Google Scholar] [CrossRef]
- López-Gómez, M.; Hidalgo-Castellanos, J.; Iribarne, C.; Lluch, C. Proline accumulation has prevalence over polyamines in nodules of medicago sativa in symbiosis with Sinorhizobium meliloti during the initial response to salinity. Plant Soil 2013, 374, 149–159. [Google Scholar] [CrossRef]
- Qu, L.; Huang, Y.; Zhu, C.; Zeng, H.; Shen, C.; Liu, C.; Zhao, Y.; Pi, E. Rhizobia-inoculation enhances the soybean’s tolerance to salt stress. Plant Soil 2015, 400, 209–222. [Google Scholar] [CrossRef]
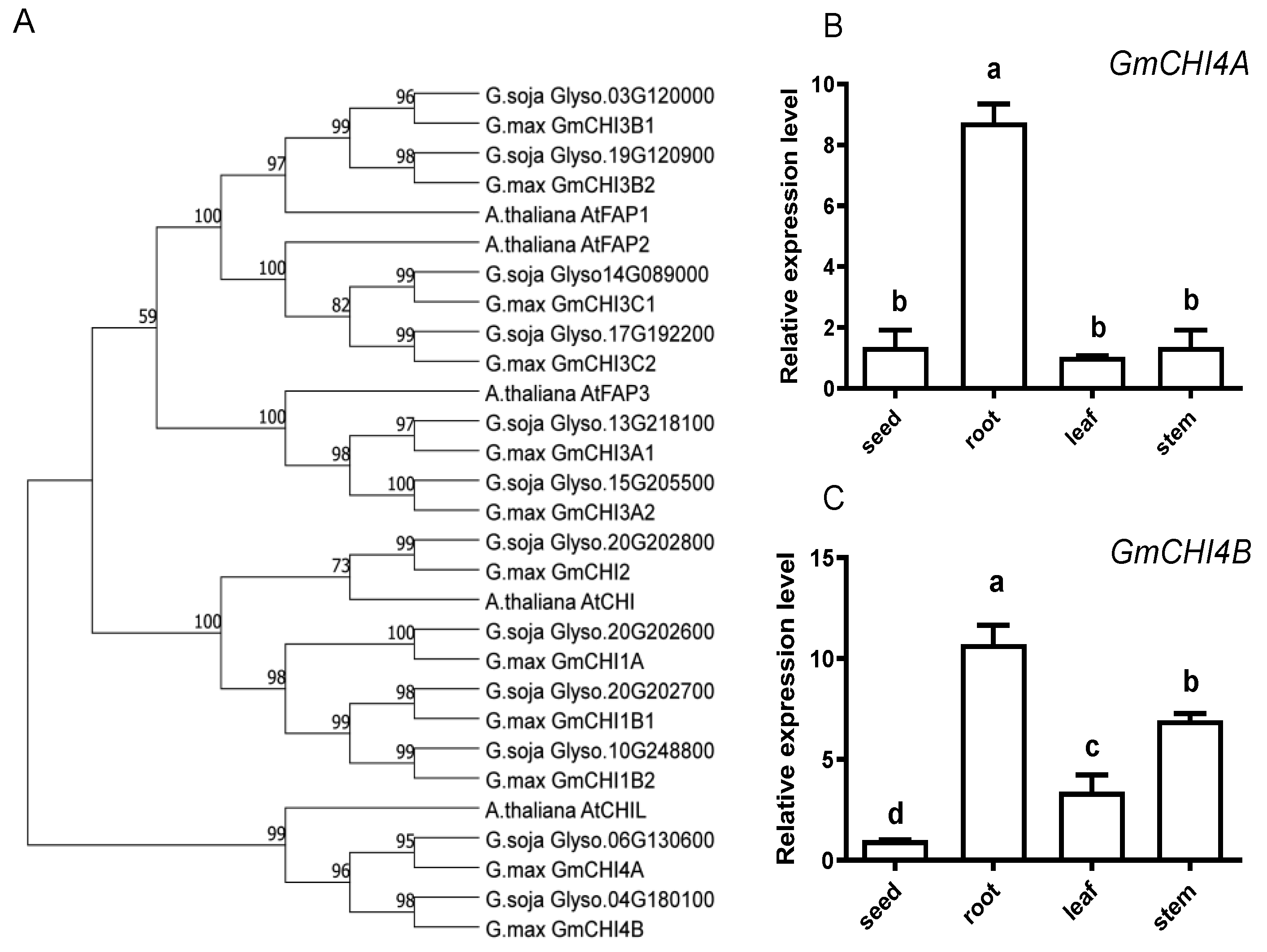
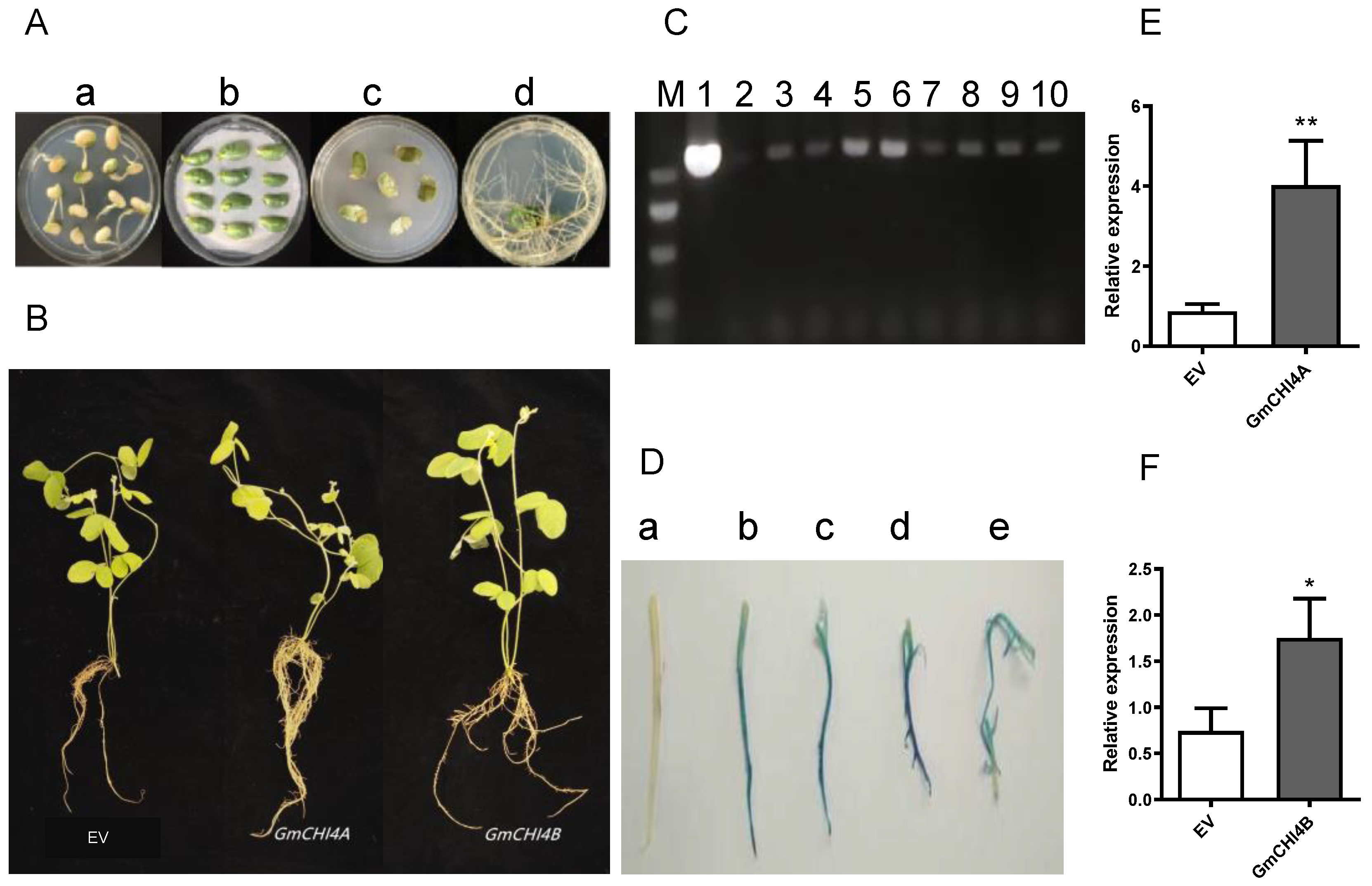

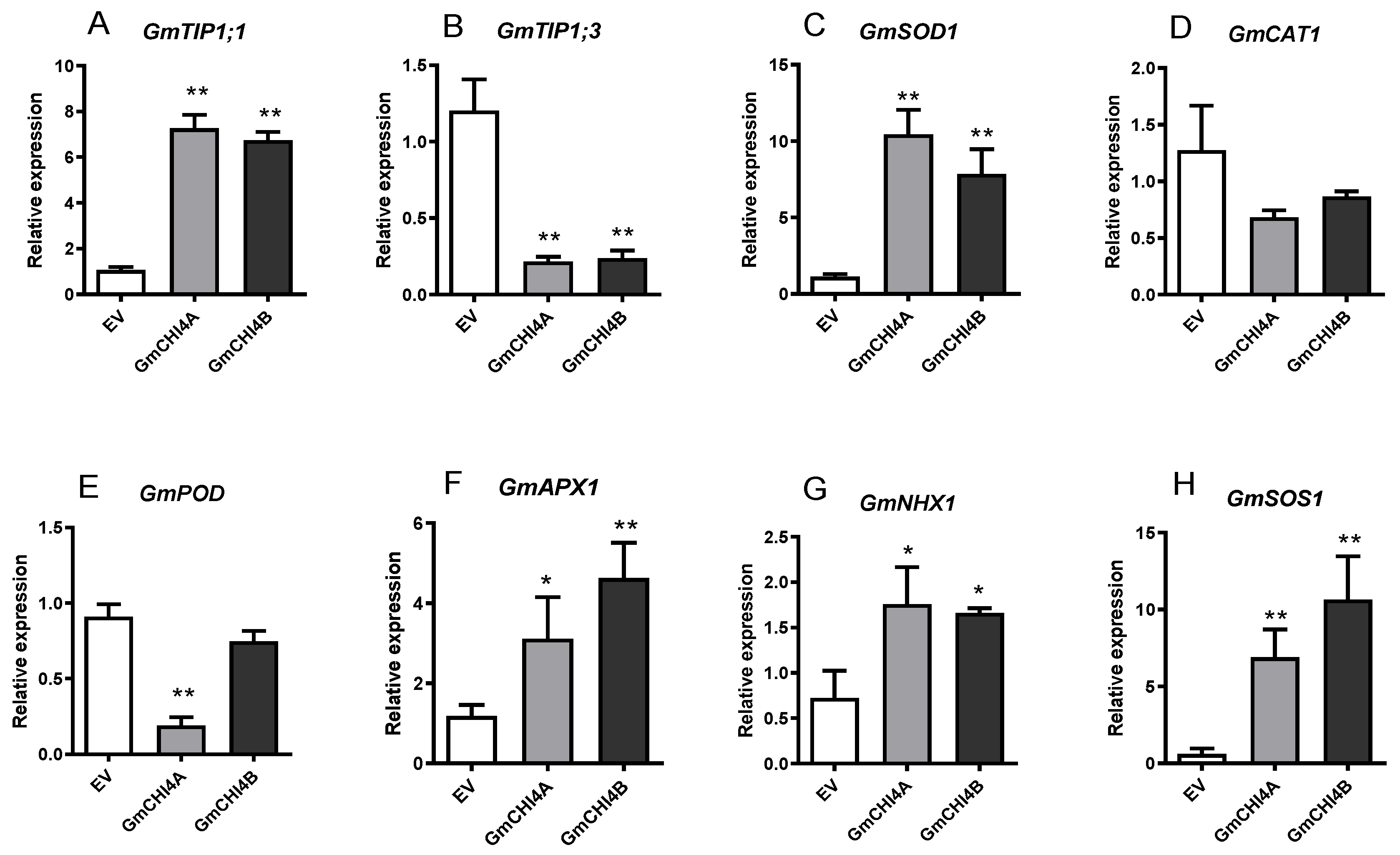
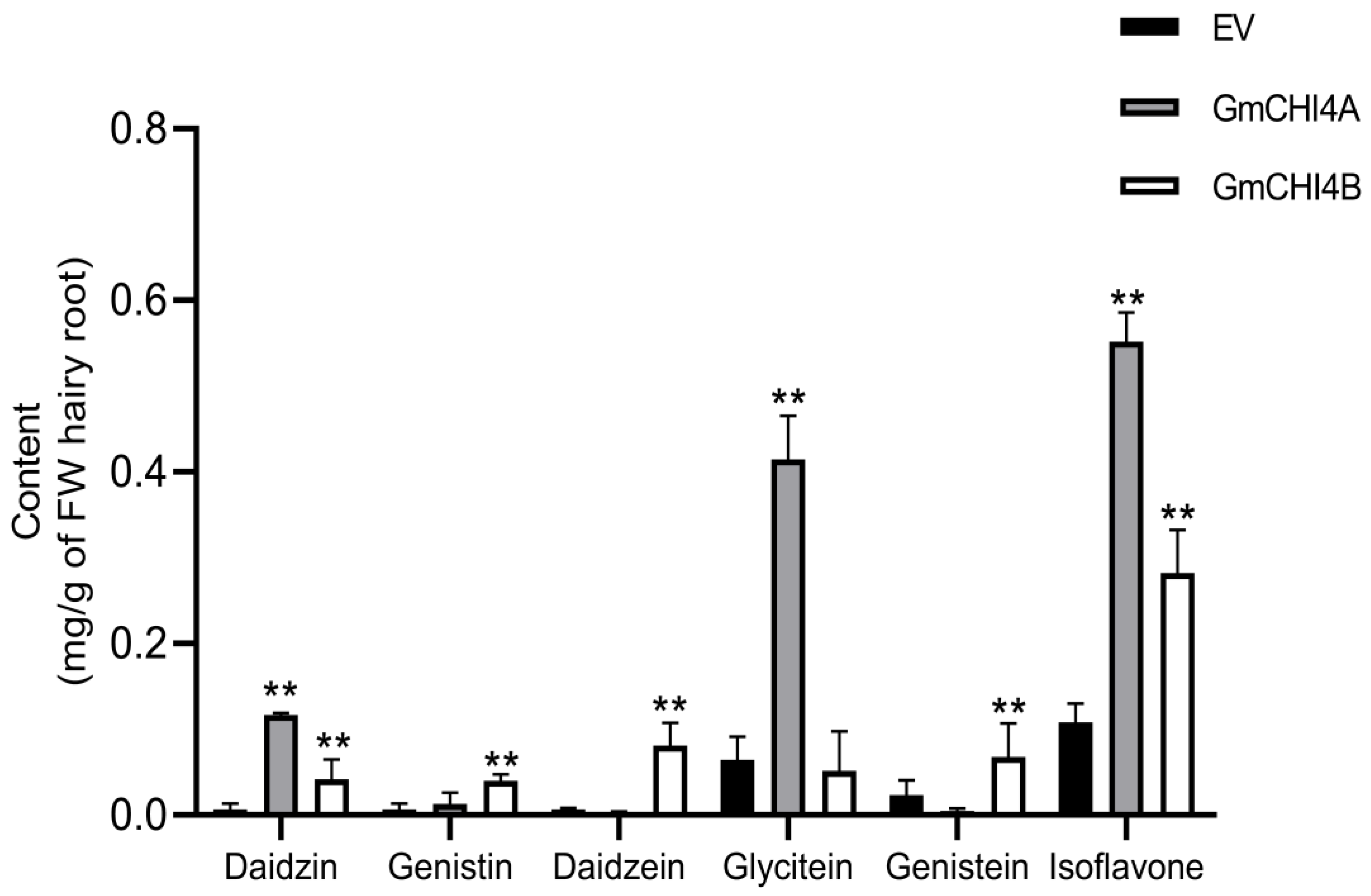
| Gene Name | Forward Primer Sequences (5′-3′) | Reverse Primer Sequences (5′-3′) | Accession Number | Product Size |
|---|---|---|---|---|
| GmCHI4A | TTTCAGTGATCACATACCATTTCCCAG | CACCAAGGTACCATTTCTTGATG | NM_001249853.2 | 138 bp |
| GmCHI4B | ATTTATTTGGAGCCTGAAGTAGTTG | GAGAGCATTGAAGAACTCATCGTTC | NM_001255112.2 | 96 bp |
| GmTIP1;1 | AACCCTGCAGTCACATTTGGT | TCCACCGGTTGCAGACTTG | XM_003518610.5 | 129 bp |
| GmTIP1;3 | TCTTGGTTGGTGGGGCTTTT | ATGGCAGCAGTAGCTGAACC | XM_003536723.4 | 136 bp |
| GmSOD1 | GCTTCAGTATTACCGACAGTCA | CACAAGCTACTCTGCCACCA | NM_001248369 | 153 bp |
| GmCAT1 | CCAAGTCCCACATCCAGGAG | GGTGTTGACACCGAAGCCAT | NM_001250627 | 143 bp |
| GmPOD | CTTCAGCGGCACAGGAAGTC | AAGTGTCAGGGGTTGAAGGATC | XM_006575142.4 | 128 bp |
| GmAPX1 | AACCTTTGACAAGGGCACGA | AACAGCGATGTCAAGACCGT | NM_001250856 | 100 bp |
| GmNHX1 | AAGCAGCATCCGTGCTTTAC | CCTGCCACCAAAAACAGGAC | AY972078 | 100 bp |
| GmSOS1 | GGTACTCATCATCGGCTGGG | ACCAGGGCCAGCTAGTAAGA | NM_001258010 | 177 bp |
| GmActin | AGGTCAACAGAGAAAGTGCC | CAAACGAAGGATGGCATGGG | V00450.1 | 194 bp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wang, Y.; Li, J.; Zhu, Y.; Wang, L.; Li, Z.; Liu, Y.; Yan, F.; Wang, Q. Overexpression of Chalcone Isomerase-like Genes, GmCHI4A and GmCHI4B, Enhances Salt Tolerance of Cotyledon Hairy Roots and Composite Plant in Soybean (Glycine max (L.) Merr.). Agronomy 2024, 14, 731. https://doi.org/10.3390/agronomy14040731
Zhang J, Wang Y, Li J, Zhu Y, Wang L, Li Z, Liu Y, Yan F, Wang Q. Overexpression of Chalcone Isomerase-like Genes, GmCHI4A and GmCHI4B, Enhances Salt Tolerance of Cotyledon Hairy Roots and Composite Plant in Soybean (Glycine max (L.) Merr.). Agronomy. 2024; 14(4):731. https://doi.org/10.3390/agronomy14040731
Chicago/Turabian StyleZhang, Jinhao, Ying Wang, Jingwen Li, Youcheng Zhu, Le Wang, Zhiqi Li, Yajing Liu, Fan Yan, and Qingyu Wang. 2024. "Overexpression of Chalcone Isomerase-like Genes, GmCHI4A and GmCHI4B, Enhances Salt Tolerance of Cotyledon Hairy Roots and Composite Plant in Soybean (Glycine max (L.) Merr.)" Agronomy 14, no. 4: 731. https://doi.org/10.3390/agronomy14040731
APA StyleZhang, J., Wang, Y., Li, J., Zhu, Y., Wang, L., Li, Z., Liu, Y., Yan, F., & Wang, Q. (2024). Overexpression of Chalcone Isomerase-like Genes, GmCHI4A and GmCHI4B, Enhances Salt Tolerance of Cotyledon Hairy Roots and Composite Plant in Soybean (Glycine max (L.) Merr.). Agronomy, 14(4), 731. https://doi.org/10.3390/agronomy14040731








