Agronomic Response to Irrigation and Biofertilizer of Peanut (Arachis hypogea L.) Grown under Mediterranean Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Experiments
2.2. Crop Physiological Measurements
2.3. Irrigation Management and Agricultural Water Productivity
2.4. Microbiological Analyses of Soil
2.5. Analysis of Peanut Storage Protein Composition
2.6. Experimental Design and Statistical Analysis
3. Results
3.1. Weather Variability and Crop Physiological Response Assessed by Spectral Phenotyping
3.2. Soil Microbiology
3.3. Peanut Yield, Yield Components and Water Productivity
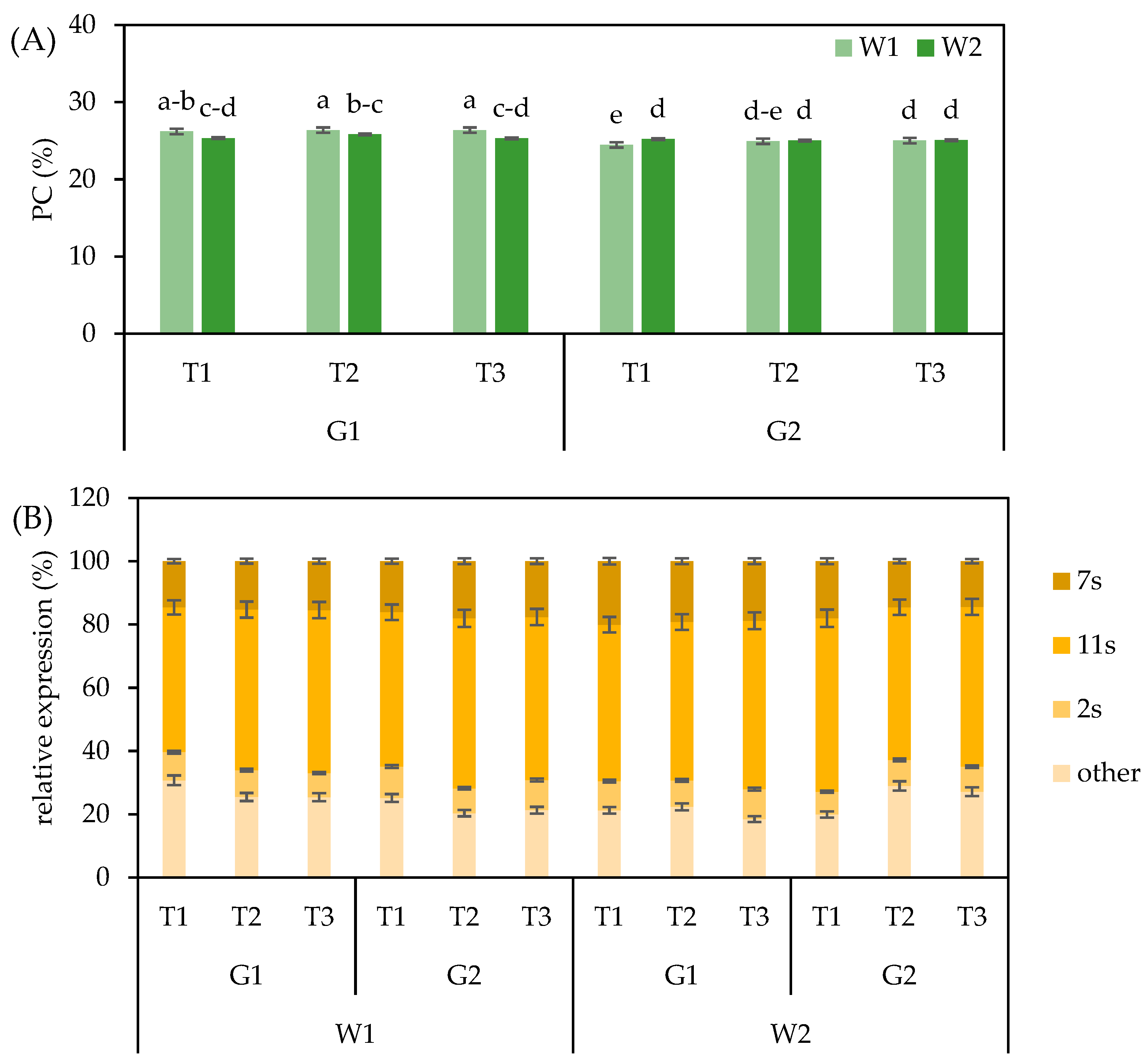
3.4. Seed Protein Content and Composition
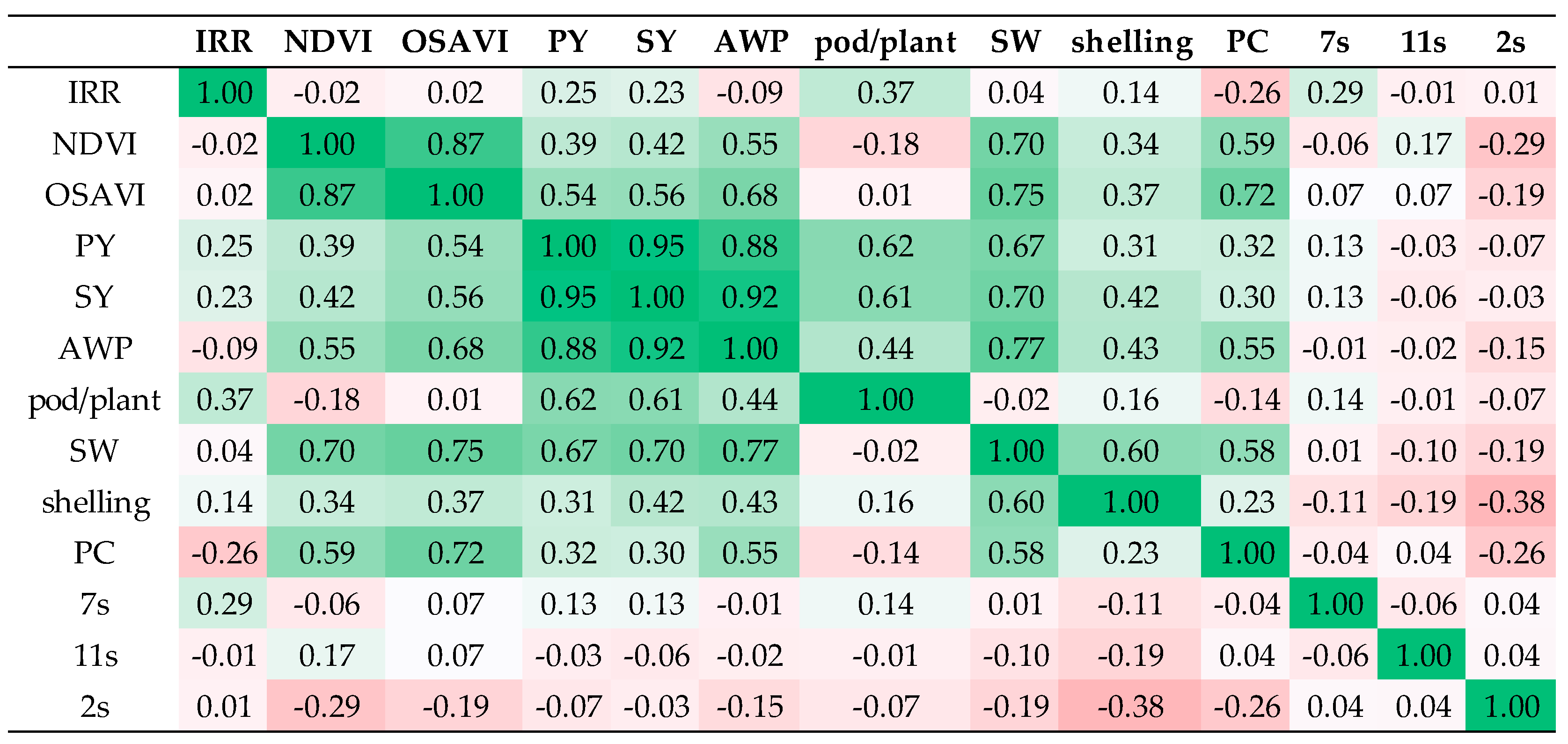
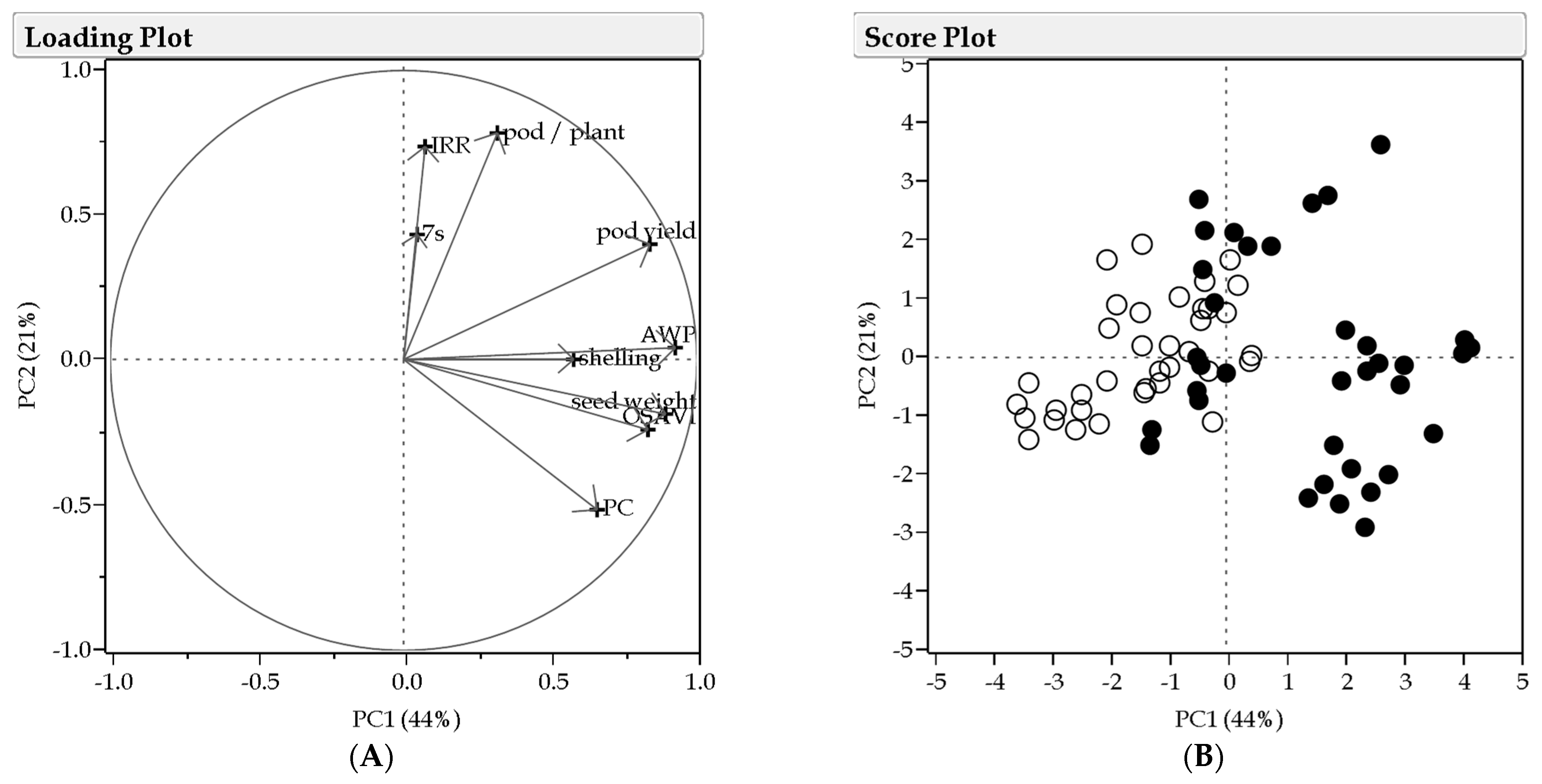
3.5. Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- dos Santos, A.F.; Corrêa, L.N.; Lacerda, L.N.; Tedesco-Oliveira, D.; Pilon, C.; Vellidis, G.; da Silva, R.P. High-resolution satellite image to predict peanut maturity variability in commercial fields. Precis. Agric. 2021, 22, 1464–1478. [Google Scholar] [CrossRef]
- Chen, X.; Lu, Q.; Liu, H.; Zhang, J.; Hong, Y.; Lan, H.; Li, H.; Wang, J.; Liu, H.; Li, S.; et al. Sequencing of cultivated peanut, Arachis hypogaea, yields insights into genome evolution and oil improvement. Mol. Plant 2019, 12, 920–934. [Google Scholar] [CrossRef] [PubMed]
- Nawade, B.; Mishra, G.P.; Radhakrishnan, T.; Dodia, S.M.; Ahmad, S.; Kumar, A.; Kumar, A.; Kundu, R. High oleic peanut breeding: Achievements, perspectives, and prospects. Trends Food Sci. Technol. 2018, 78, 107–119. [Google Scholar] [CrossRef]
- Çiftçi, S.; Suna, G. Functional components of peanuts (Arachis Hypogaea L.) and health benefits: A review. Future Foods 2022, 5, 100140. [Google Scholar] [CrossRef]
- Haro, R.J.; Baldessari, J.; Otegui, M.E. Genetic improvement of peanut in Argentina between 1948 and 2004: Seed yield and its components. Field Crops Res. 2013, 149, 76–83. [Google Scholar] [CrossRef]
- Hauser, A. Peanuts. J. Agric. Food Inf. 2018, 19, 195–202. [Google Scholar] [CrossRef]
- Muñoz-Arrieta, R.; Esquivel-Alvarado, D.; Alfaro-Viquez, E.; Alvarez-Valverde, V.; Krueger, C.G.; Reed, J.D. Nutritional and bioactive composition of Spanish, Valencia, and Virginia type peanut skins. J. Food Compos. Anal. 2021, 98, 103816. [Google Scholar] [CrossRef]
- Matsuo, H.; Yokooji, T.; Taogoshi, T. Common food allergens and their IgE-binding epitopes. Allergol. Int. 2015, 64, 332–343. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Wood, R.A. Advances in diagnosing peanut allergy. J. Allergy Clin. Immunol. Pract. 2013, 1, 1–13. [Google Scholar] [CrossRef]
- Sezen, S.M.; Yucel, S.; Tekin, S.; Yıldız, M. Determination of optimum irrigation and effect of deficit irrigation strategies on yield and disease rate of peanut irrigated with drip system in Eastern Mediterranean. Agric. Water Manag. 2019, 221, 211–219. [Google Scholar] [CrossRef]
- Putto, W.; Patanothai, A.; Jogloy, S. Hoogenboom, GDetermination of mega-environments for peanut breeding using the CSM-CROPGRO-Peanut model. Crop Sci. 2008, 48, 973–982. [Google Scholar] [CrossRef]
- Kokkanti, R.R.; Vemuri, H.; Gaddameedi, A.; Rayalacheruvu, U. Variability in drought stress-induced physiological, biochemical responses and expression of DREB2A, NAC4 and HSP70 genes in groundnut (Arachis Hypogaea L.). S. Afr. J. Bot. 2022, 144, 448–457. [Google Scholar] [CrossRef]
- Sullivan, D.G.; Holbrook, C.C. Using ground-based reflectance measurements as selection criteria for droughtߚ and aflatoxin-resistant peanut genotypes. Crop Sci. 2007, 47, 1040–1050. [Google Scholar] [CrossRef]
- Buchaillot, M.L.; Soba, D.; Shu, T.; Liu, J.; Aranjuelo, I.; Araus, J.L.; Runion, G.B.; Prior, S.A.; Kefauver, S.C.; Sanz-Saez, A. Estimating peanut and soybean photosynthetic traits using leaf spectral reflectance and advance regression models. Planta 2022, 255, 93. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.; Xia, G.; Wu, Q.; Chi, D. Continuous regulated deficit irrigation enhances peanut water use efficiency and drought resistance. Agric. Water Manag. 2021, 255, 106997. [Google Scholar] [CrossRef]
- Khodadadi Dehkordi, D. The effect of different irrigation treatments on yield and water productivity of Arachis Hypogaea L. under semi-arid conditions in Iran. Irrig. Drain. 2020, 69, 646–657. [Google Scholar] [CrossRef]
- Zhen, X.; Huo, W.; Tian, D.; Zhang, Q.; Sanz-Saez, A.; Chen, C.Y.; Batchelor, W.D. County level calibration strategy to evaluate peanut irrigation water use under different climate change scenarios. Eur. J. Agron. 2023, 143, 126693. [Google Scholar] [CrossRef]
- Awal, M.A.; Ikeda, T. Effects of changes in soil temperature on seedling emergence and phenological development in field-grown stands of peanut (Arachis Hypogaea). Environ. Exp. Bot. 2002, 47, 101–113. [Google Scholar] [CrossRef]
- De Santis, M.A.; Satriani, A.; De Santis, F.; Flagella, Z. Water use efficiency, spectral phenotyping and protein composition of two chickpea genotypes grown in Mediterranean environments under different water and nitrogen supply. Agriculture 2022, 12, 2026. [Google Scholar] [CrossRef]
- Allen, R.G. Crop Evapotranspiration: Guidelines for Computing Crop Water Requirements; FAO Irrigation and Drainage Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 1998; ISBN 978-92-5-104219-9. [Google Scholar]
- Satriani, A.; Loperte, A.; Soldovieri, F. Integrated geophysical techniques for sustainable management of water resource. A case study of local dry bean versus commercial common bean cultivars. Agric. Water Manag. 2015, 162, 57–66. [Google Scholar] [CrossRef]
- Brown, M.E.; Burlingham, S.K.; Jackson, R.M. Studies onAzotobacter species in soil. Plant Soil 1962, 17, 320–332. [Google Scholar] [CrossRef]
- Walczyk, N.E.; Smith, P.M.C.; Tovey, E.; Wright, G.C.; Fleischfresser, D.B.; Roberts, T.H. Analysis of crude protein and allergen abundance in peanuts (Arachis Hypogaea cv. Walter) from three growing regions in Australia. J. Agric. Food Chem. 2013, 61, 3714–3725. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhou, N.; Xiong, F.; Li, X.; Yang, A.; Tong, P.; Tang, R.; Chen, H. Allergen composition analysis and allergenicity assessment of Chinese peanut cultivars. Food Chem. 2016, 196, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Mamone, G.; Di Stasio, L.; De Caro, S.; Picariello, G.; Nicolai, M.A.; Ferranti, P. Comprehensive analysis of the peanut allergome combining 2-DE gel-based and gel-free proteomics. Food Res. Int. 2019, 116, 1059–1065. [Google Scholar] [CrossRef]
- Kumar, N.; Haak, D.C.; Dunne, J.C.; Balota, M. Multilocation evaluation of Virginia and Runner-type peanut cultivars for yield and grade in Virginia–Carolina region. Agronomy 2022, 12, 3206. [Google Scholar] [CrossRef]
- Nautiyal, P.C.; Ravindra, V.; Rathnakumar, A.L.; Ajay, B.C.; Zala, P.V. Genetic variations in photosynthetic rate, pod yield and yield components in Spanish groundnut cultivars during three cropping seasons. Field Crops Res. 2012, 125, 83–91. [Google Scholar] [CrossRef]
- Haro, R.J.; Baldessari, J.; Otegui, M.E. Genetic improvement of peanut in Argentina between 1948 and 2004: Links between phenology and grain yield determinants. Field Crops Res. 2015, 174, 12–19. [Google Scholar] [CrossRef]
- Zhang, Q.; Dang, P.; Chen, C.; Feng, Y.; Batchelor, W.; Lamb, M.; Sanz-Saez, A. Tolerance to mid-season drought in peanut can be achieved by high water use efficiency or high efficient use of water. Crop Sci. 2022, 62, 1948–1966. [Google Scholar] [CrossRef]
- Mozingo, R.W.; Coffelt, T.A.; Wynne, J.C. Genetic improvement in large-seeded Virginia-type peanut cultivars since 1941. Crop Sci. 1987, 27, 228–231. [Google Scholar] [CrossRef]
- Yol, E.; Furat, S.; Upadhyaya, H.D.; Uzun, B. Characterization of groundnut (Arachis Hypogaea L.) collection using quantitative and qualitative traits in the Mediterranean basin. J. Integr. Agric. 2018, 17, 63–75. [Google Scholar] [CrossRef]
- Reddy, T.Y.; Reddy, V.R.; Anbumozhi, V. Physiological responses of groundnut (Arachis hypogea L.) to drought stress and its amelioration: A critical review. Plant Growth Regul. 2003, 41, 75–88. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.K.; Mallick, S.; Rana, S.K. Water balance and crop coefficients of summer-grown peanut (Arachis Hypogaea L.) in a humid tropical region of India. Irrig. Sci. 2005, 23, 161–169. [Google Scholar] [CrossRef]
- Abou Kheira, A.A. Macromanagement of deficit-irrigated peanut with sprinkler irrigation. Agric. Water Manag. 2009, 96, 1409–1420. [Google Scholar] [CrossRef]
- Puangbut, D.; Jogloy, S.; Vorasoot, N.; Akkasaeng, C.; Kesmala, T.; Rachaputi, R.C.N.; Wright, G.C.; Patanothai, A. Association of root dry weight and transpiration efficiency of peanut genotypes under early season drought. Agric. Water Manag. 2009, 96, 1460–1466. [Google Scholar] [CrossRef]
- Craufurd, P.Q.; Wheeler, T.R.; Ellis, R.H.; Summerfield, R.J.; Williams, J.H. Effect of temperature and water deficit on water-use efficiency, carbon isotope discrimination, and specific leaf area in peanut. Crop Sci. 1999, 39, 136–142. [Google Scholar] [CrossRef]
- Scott Tubbs, R.; Harris, G.H.; Beasley, J.P.; Smith, A.R.; Smith, N.B. Effect of inoculant and nitrogen application at planting on peanut production in Georgia. Crop Manag. 2012, 11, 1–11. [Google Scholar] [CrossRef]
- Tekulu, K.; Taye, G.; Assefa, D. Effect of starter nitrogen and phosphorus fertilizer rates on yield and yield components, grain protein content of groundnut (Arachis Hypogaea L.) and residual soil nitrogen content in a semiarid north Ethiopia. Heliyon 2020, 6, e05101. [Google Scholar] [CrossRef]
- Xia, G.; Wu, Q.; Chi, D.; Chen, J.; Wang, S. Enhancing water productivity while improving peanut kernel quality by water regulation under different nitrogen levels. Irrig. Drain. 2020, 69, 86–94. [Google Scholar] [CrossRef]
- Carley, D.S.; Jordan, D.L.; Dharmasri, L.C.; Sutton, T.B.; Brandenburg, R.L.; Burton, M.G. Peanut response to planting date and potential of canopy reflectance as an indicator of pod maturation. Agron. J. 2008, 100, 376–380. [Google Scholar] [CrossRef]
- Song, Y.; Rowland, D.L.; Tillman, B.L.; Wilson, C.H.; Sarnoski, P.J.; Zurweller, B.A. Impact of seed maturity on season-long physiological performance and offspring seed quality in peanut (Arachis Hypogaea L.). Field Crops Res. 2022, 288, 108674. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Boote, K.J.; Thomas, J.M.G.; Allen Jr, L.H.; Gorbet, D.W. Influence of soil temperature on seedling emergence and early growth of peanut cultivars in field conditions. J. Agron. Crop Sci. 2006, 192, 168–177. [Google Scholar] [CrossRef]
- Yao, X.; Chen, S.; Ding, S.; Zhang, M.; Cui, Z.; Linghu, S.; Xu, J. Temperature, moisture, hyperspectral vegetation indexes, and leaf traits regulated soil respiration in different crop planting fields. J. Soil Sci. Plant Nutr. 2021, 21, 3203–3220. [Google Scholar] [CrossRef]
- Mondal, M.; Skalicky, M.; Garai, S.; Hossain, A.; Sarkar, S.; Banerjee, H.; Kundu, R.; Brestic, M.; Barutcular, C.; Erman, M.; et al. Supplementing nitrogen in combination with rhizobium inoculation and soil mulch in peanut (Arachis Hypogaea L.) production system: Part I. Effects on productivity, soil moisture, and nutrient dynamics. Agronomy 2020, 10, 1582. [Google Scholar] [CrossRef]
- Podile, A.R. Survival of Bacillus subtilis AF 1 in the bacterized peanut rhizosphere and its influence on native microflora and seedling growth. World J. Microbiol. Biotechnol. 1994, 10, 700–703. [Google Scholar] [CrossRef] [PubMed]
- Hinsu, A.T.; Panchal, K.J.; Pandit, R.J.; Koringa, P.G.; Kothari, R.K. Characterizing rhizosphere microbiota of peanut (Arachis Hypogaea L.) from pre-sowing to post-harvest of crop under field conditions. Sci. Rep. 2021, 11, 17457. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, X.; Yu, J.; Guo, Z.; Wu, J.; Li, X.; Chi, Y.; Wan, S. Biological control of peanut southern blight (Sclerotium rolfsii) by the strain Bacillus pumilus LX11. Biocontrol Sci. Technol. 2020, 30, 485–489. [Google Scholar] [CrossRef]
- Masood, S.; Zhao, X.Q.; Shen, R.F. Bacillus pumilus promotes the growth and nitrogen uptake of tomato plants under nitrogen fertilization. Sci. Hortic. 2020, 272, 109581. [Google Scholar] [CrossRef]
- Abdelghany, A.M.; El-Banna, A.A.A.; Salama, E.A.A.; Ali, M.M.; Al-Huqail, A.A.; Ali, H.M.; Paszt, L.S.; El-Sorady, G.A.; Lamlom, S.F. The individual and combined effect of nanoparticles and biofertilizers on growth, yield, and biochemical attributes of peanuts (Arachis hypogea L.). Agronomy 2022, 12, 398. [Google Scholar] [CrossRef]
- Dey, R.; Pal, K.K.; Bhatt, D.M.; Chauhan, S.M. Growth promotion and yield enhancement of peanut (Arachis Hypogaea L.) by application of plant growth-promoting rhizobacteria. Microbiol. Res. 2004, 159, 371–394. [Google Scholar] [CrossRef]
- Bi, Y.; Zhou, H. Changes in peanut canopy structure and photosynthetic characteristics induced by an arbuscular mycorrhizal fungus in a nutrient-poor environment. Sci. Rep. 2021, 11, 14832. [Google Scholar] [CrossRef]
- Gravel, A.; Doyen, A. Pulse globulins 11S and 7S: Origins, purification methods, and techno-functional properties. J. Agric. Food Chem. 2023, 71, 2704–2717. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Liang, X.; Chung, S.-Y.; Holbrook, C.C.; Maleki, J.S. Proteomic analysis of peanut seed storage proteins and genetic variation in a potential peanut allergen. Protein Pept. Lett. 2008, 15, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Asero, R.; Nucera, E.; Rizzi, A.; Aruanno, A.; Uasuf, C.G.; Manzotti, G.; Villalta, D.; Conte, M.; Pastorello, E.A.; Losappio, L.; et al. Peanut allergy in Italy: A unique Italian perspective. J. Allergy Clin. Immunol. Glob. 2022, 1, 61–66. [Google Scholar] [CrossRef]
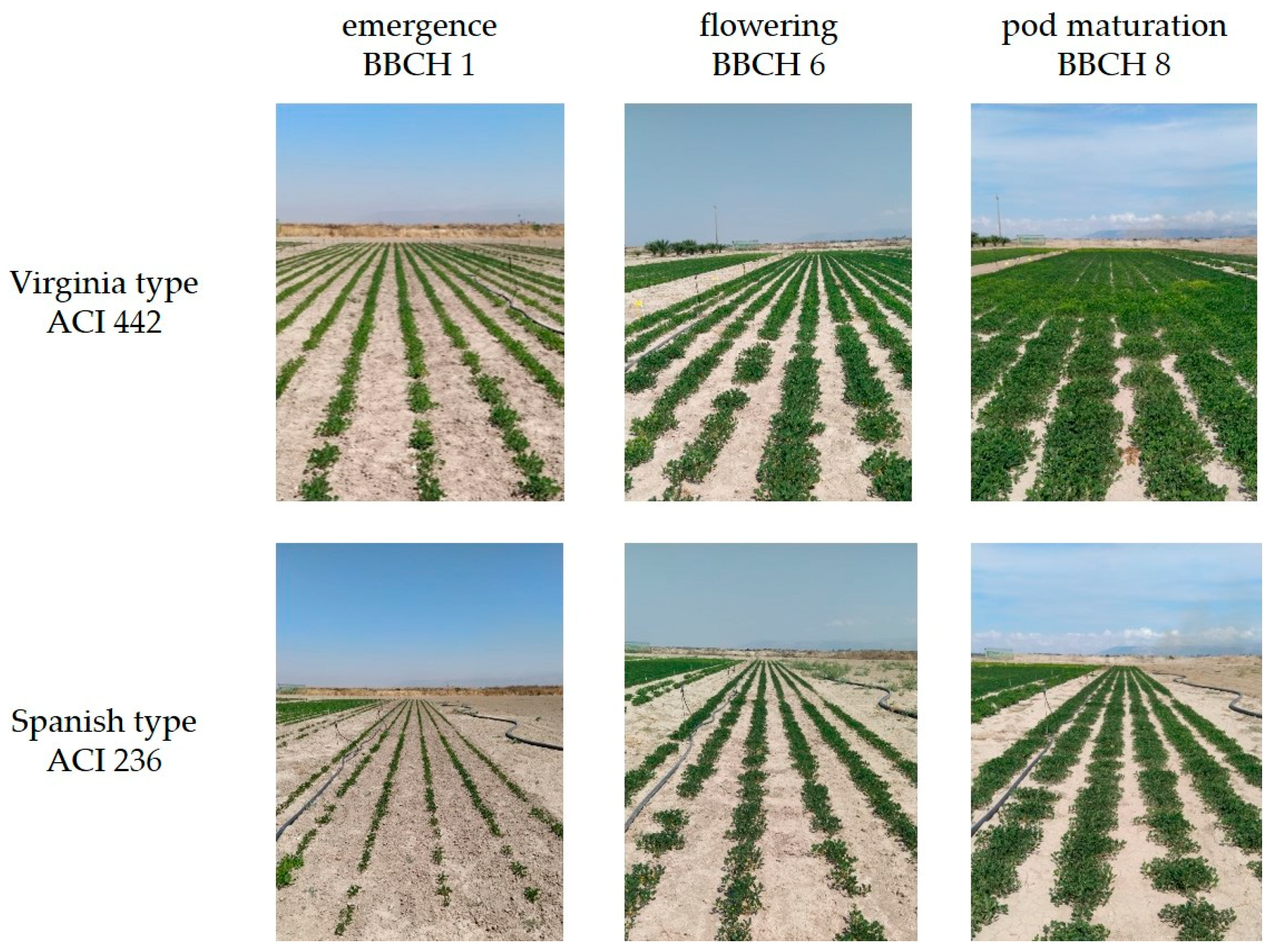
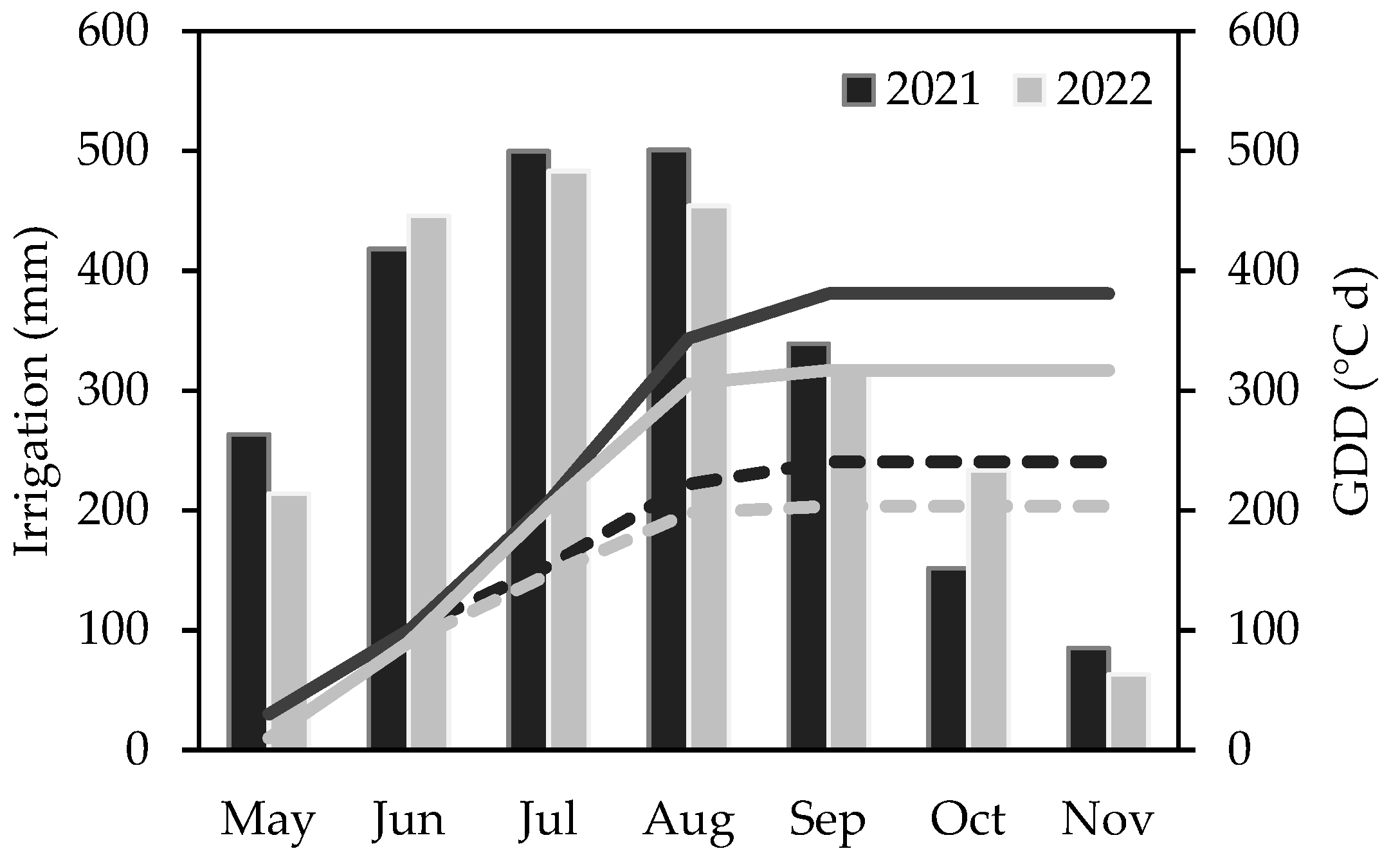
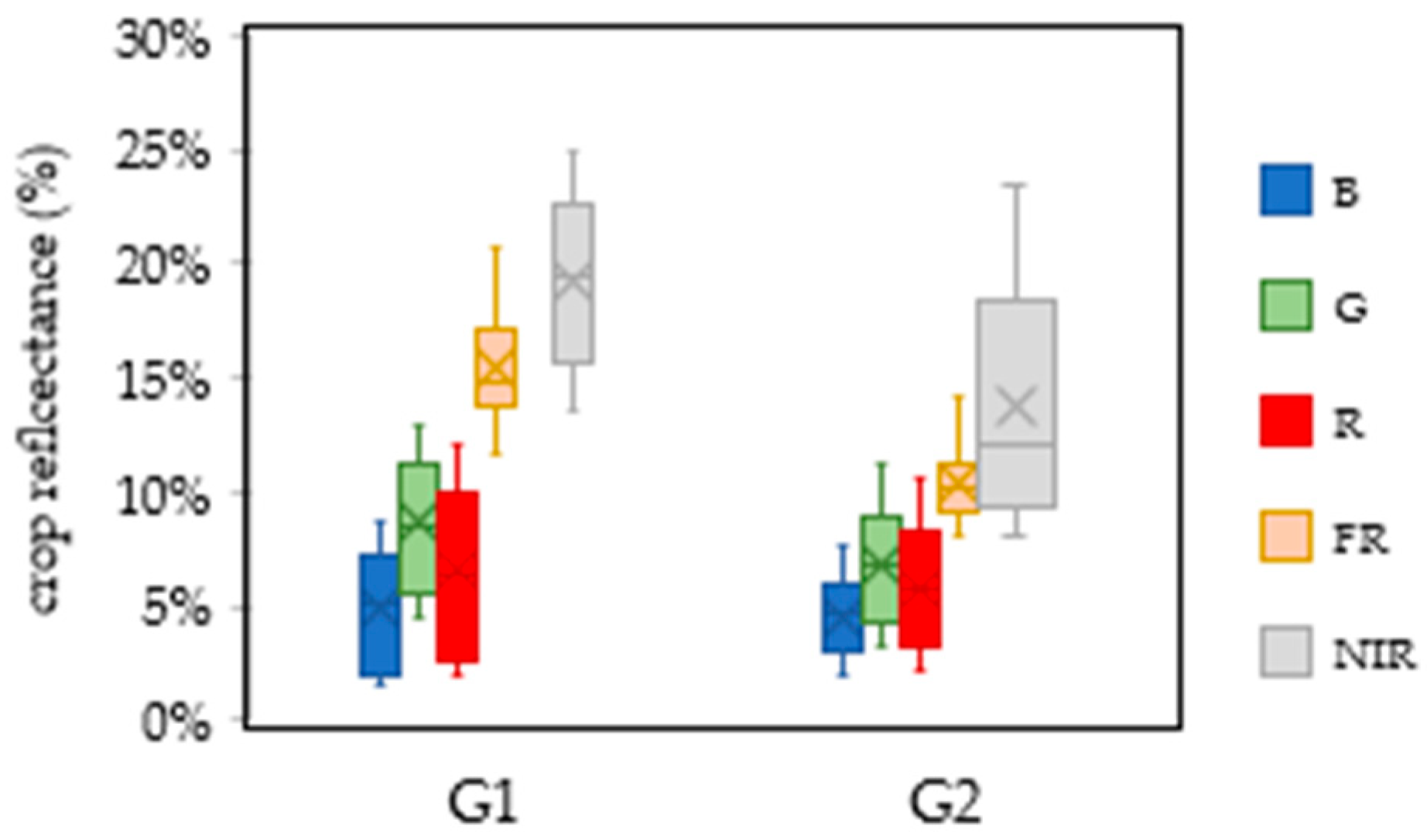

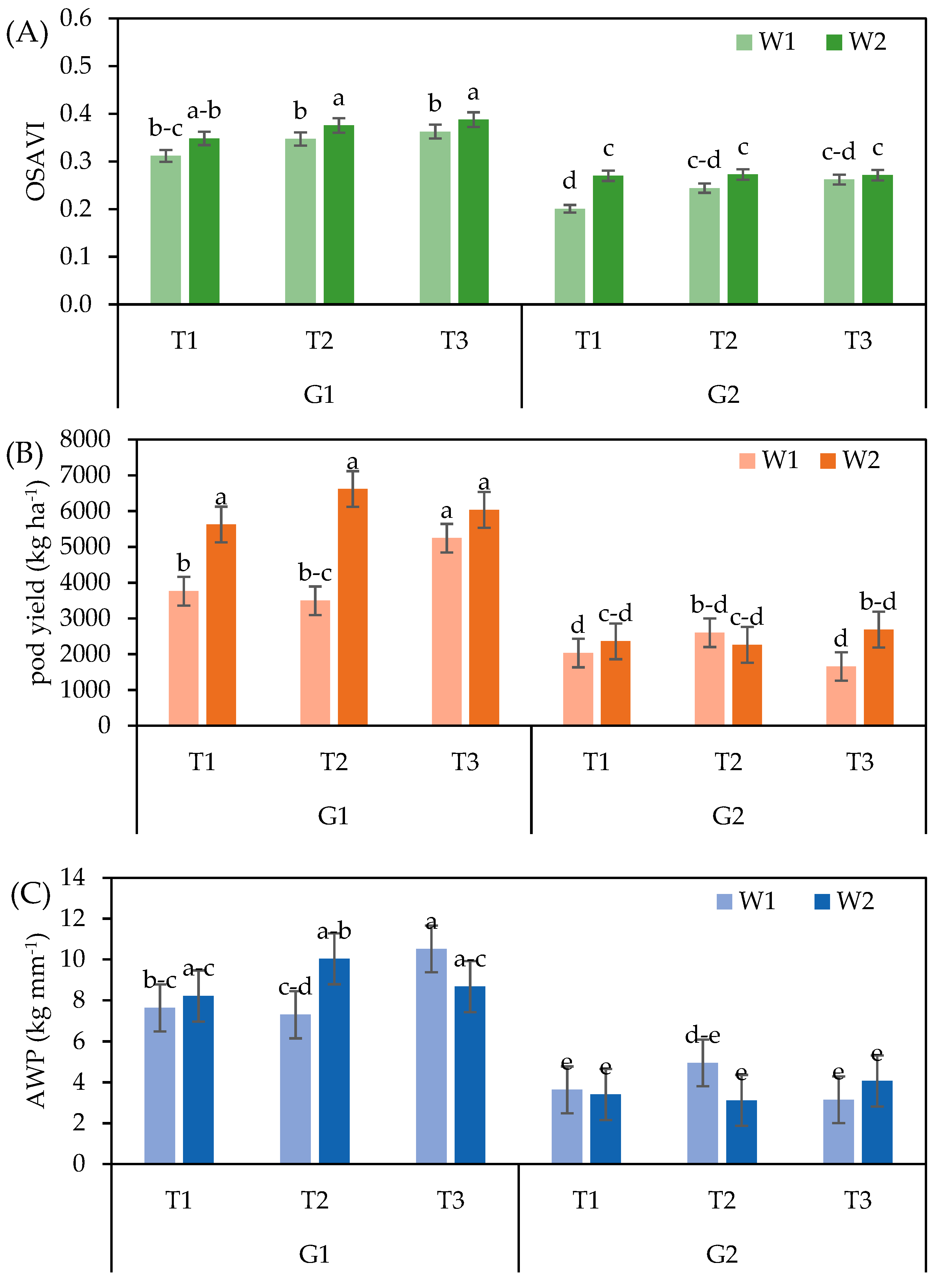

| Parameter | Year | Month | ||||||
|---|---|---|---|---|---|---|---|---|
| May | Jun | Jul | Aug | Sep | Oct | Nov | ||
| SRAD (W m−2) | 2021 | 254 | 256 | 252 | 227 | 207 | 131 | 70 |
| 2022 | 259 | 281 | 277 | 221 | 151 | 114 | 58 | |
| LT | 252 | 279 | 285 | 254 | 181 | 124 | 79 | |
| T min (°C) | 2021 | 11.0 | 16.1 | 19.0 | 19.3 | 15.5 | 10.9 | 11.6 |
| 2022 | 13.2 | 18.3 | 19.1 | 19.1 | 15.1 | 11.7 | 8.3 | |
| LT | 13.1 | 17.6 | 20.8 | 21.3 | 17.3 | 13.2 | 8.8 | |
| T max (°C) | 2021 | 26.8 | 33.8 | 35.3 | 35.0 | 29.2 | 20.9 | 18.1 |
| 2022 | 28.4 | 33.5 | 34.1 | 32.2 | 28.2 | 25.4 | 17.9 | |
| LT | 25.0 | 30.3 | 33.6 | 33.7 | 28.3 | 23.4 | 17.6 | |
| T ave (°C) | 2021 | 18.9 | 24.9 | 27.1 | 27.2 | 22.3 | 15.9 | 14.9 |
| 2022 | 20.8 | 25.9 | 26.6 | 25.6 | 21.7 | 18.5 | 13.1 | |
| LT | 19.1 | 23.9 | 27.2 | 27.5 | 22.8 | 18.3 | 13.2 | |
| P (mm) | 2021 | 11 | 9 | 8 | 28 | 12 | 53 | 75 |
| 2022 | 2 | 2 | 15 | 16 | 15 | 7 | 27 | |
| LT | 38 | 30 | 26 | 25 | 47 | 51 | 66 | |
| PET (mm) | 2021 | 93 | 112 | 115 | 100 | 74 | 36 | 11 |
| 2022 | 100 | 117 | 119 | 87 | 54 | 38 | 14 | |
| LT | 83 | 104 | 119 | 104 | 63 | 39 | 19 | |
| RH (%) | 2021 | 60 | 53 | 56 | 58 | 67 | 78 | 91 |
| 2022 | 65 | 58 | 60 | 67 | 67 | 78 | 85 | |
| LT | 57 | 48 | 44 | 48 | 60 | 70 | 75 | |
| WS (m s−1) | 2021 | 3.2 | 2.7 | 3.0 | 3.3 | 2.8 | 3.3 | 2.4 |
| 2022 | 2.8 | 3.0 | 2.8 | 3.2 | 2.7 | 2.3 | 2.3 | |
| LT | 3.7 | 3.6 | 3.7 | 3.5 | 3.6 | 3.6 | 3.9 |
| Factors | NDVI | OSAVI | Pod Yield | Seed Yield | AWP | Pod/Plant | Seed Weight | Shelling | |
|---|---|---|---|---|---|---|---|---|---|
| GS 8 | GS 8 | kg ha−1 | kg ha−1 | kg mm−1 | n. | mg | % | ||
| Y | 2021 | 0.36 b | 0.27 b | 3756 a | 2285 a | 5.4 b | 37.0 a | 512 b | 58.1 a |
| 2022 | 0.59 a | 0.34 a | 3639 a | 2264 a | 7.0 a | 22.9 b | 735 a | 59.6 a | |
| SE | 0.008 | 0.006 | 198 | 151 | 0.38 | 2.0 | 13 | 1.2 | |
| p | *** | *** | ns | ns | ** | *** | *** | ns | |
| W | W1 | 0.43 b | 0.29 b | 3131 b | 1915 b | 6.2 a | 27.0 b | 565 b | 57.0 b |
| W2 | 0.52 a | 0.32 a | 4264 a | 2634 a | 6.3 a | 32.9 a | 683 a | 60.6 a | |
| SE | 0.008 | 0.006 | 198 | 151 | 0.38 | 2.0 | 13 | 1.2 | |
| p | *** | ** | ** | ** | ns | * | *** | * | |
| G | G1 | 0.52 a | 0.36 a | 5129 a | 3166 a | 8.7 a | 32.0 a | 850 a | 60.6 a |
| G2 | 0.42 b | 0.25 b | 2266 b | 1383 b | 3.7 b | 27.9 a | 397 b | 57.0 b | |
| SE | 0.008 | 0.006 | 198 | 151 | 0.38 | 2.0 | 13 | 1.2 | |
| p | *** | *** | *** | *** | *** | ns | *** | * | |
| T | T1 | 0.45 b | 0.29 b | 3445 a | 2081 a | 5.7 a | 28.0 b | 616 a | 58.7 a |
| T2 | 0.47 ab | 0.30 ab | 3744 a | 2313 a | 6.4 a | 29.3 b | 650 a | 59.6 a | |
| T3 | 0.49 a | 0.32 a | 3905 a | 2428 a | 6.6 a | 32.3 a | 605 a | 58.1 a | |
| SE | 0.010 | 0.007 | 243 | 184 | 0.46 | 2.5 | 16 | 1.4 | |
| p | ns | ns | ns | ns | ns | ns | ns | ns | |
| Y × W | ns | * | * | * | * | ns | * | * | |
| Y × G | *** | *** | * | * | ** | ns | * | ns | |
| W × G | ns | ns | * | * | ns | ns | ns | * | |
| Y × W × G | ns | * | ns | ns | ns | ns | ns | ns | |
| Y × T | ** | ** | ns | ns | ns | ns | ns | ns | |
| W × T | ** | * | ns | ns | ns | ns | ns | ns | |
| Y × W × T | ns | ns | ns | ns | ns | ns | ns | ns | |
| G × T | ns | ns | ns | ns | ns | ns | ns | ns | |
| Y × G × T | ns | ns | ns | ns | ns | ns | ns | ns | |
| W × G × T | ns | ns | * | ns | * | ns | ns | ns | |
| Y × W × G × T | ns | ns | ns | ns | ns | ns | ns | * |
| Factors | PC | 7s vicilin | 11s legumin | 2s albumin | |
|---|---|---|---|---|---|
| % | % | % | % | ||
| Y | 2021 | 25.0 b | 17.6 a | 49.6 b | 9.0 a |
| 2022 | 25.8 a | 16.1 b | 51.8 a | 8.1 b | |
| SE | 0.05 | 0.07 | 0.18 | 0.06 | |
| p | * | *** | * | ** | |
| W | W1 | 25.5 a | 16.2 b | 50.3 a | 8.7 a |
| W2 | 25.2 a | 17.5 a | 51.1 a | 8.4 a | |
| SE | 0.05 | 0.07 | 0.18 | 0.06 | |
| p | ns | ** | ns | ns | |
| G | G1 | 25.9 a | 16.5 b | 51.3 a | 8.4 a |
| G2 | 25.0 b | 17.2 a | 50.1 a | 8.7 a | |
| SE | 0.05 | 0.07 | 0.18 | 0.06 | |
| p | * | * | ns | ns | |
| T | T1 | 25.0 a | 17.2 a | 49.7 a | 8.8 a |
| T2 | 25.5 a | 16.8 a | 50.7 a | 8.2 a | |
| T3 | 25.5 a | 16.6 a | 51.7 a | 8.6 a | |
| SE | 0.06 | 0.09 | 0.21 | 0.08 | |
| p | ns | ns | ns | ns | |
| Y × W | * | *** | ns | ns | |
| Y × G | * | ns | ns | ns | |
| W × G | * | *** | ns | ** | |
| Y × W × G | * | ** | * | ns | |
| Y × T | ns | ** | ** | ns | |
| W × T | * | ** | ** | ns | |
| Y × W × T | * | *** | ns | ns | |
| G × T | ns | ns | * | ns | |
| Y × G × T | * | *** | * | * | |
| W × G × T | * | ns | ns | * | |
| Y × W × G × T | * | * | ** | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Santis, M.A.; Campaniello, D.; Tozzi, D.; Giuzio, L.; Corbo, M.R.; Bevilacqua, A.; Sinigaglia, M.; Flagella, Z. Agronomic Response to Irrigation and Biofertilizer of Peanut (Arachis hypogea L.) Grown under Mediterranean Environment. Agronomy 2023, 13, 1566. https://doi.org/10.3390/agronomy13061566
De Santis MA, Campaniello D, Tozzi D, Giuzio L, Corbo MR, Bevilacqua A, Sinigaglia M, Flagella Z. Agronomic Response to Irrigation and Biofertilizer of Peanut (Arachis hypogea L.) Grown under Mediterranean Environment. Agronomy. 2023; 13(6):1566. https://doi.org/10.3390/agronomy13061566
Chicago/Turabian StyleDe Santis, Michele Andrea, Daniela Campaniello, Damiana Tozzi, Luigia Giuzio, Maria Rosaria Corbo, Antonio Bevilacqua, Milena Sinigaglia, and Zina Flagella. 2023. "Agronomic Response to Irrigation and Biofertilizer of Peanut (Arachis hypogea L.) Grown under Mediterranean Environment" Agronomy 13, no. 6: 1566. https://doi.org/10.3390/agronomy13061566
APA StyleDe Santis, M. A., Campaniello, D., Tozzi, D., Giuzio, L., Corbo, M. R., Bevilacqua, A., Sinigaglia, M., & Flagella, Z. (2023). Agronomic Response to Irrigation and Biofertilizer of Peanut (Arachis hypogea L.) Grown under Mediterranean Environment. Agronomy, 13(6), 1566. https://doi.org/10.3390/agronomy13061566









