Abstract
Crambe abyssinica Hochst defatted seed meals were used to produce protein hydrolysates through a mild enzymatic two-step hydrolysis process. The resulting hydrolysates were rich in free amino acids, low-molecular-weight peptides, and potential bioactive compounds such as phenols, glucosinolates, or their derivatives. These hydrolysates were tested in bioassays, performed under controlled conditions, on mung bean (Vigna radiata) cuttings, to investigate a possible auxin effect, and on maize (Zea mays L.) in an aeroponic/hydroponic system during the first two weeks of growth. In both assays, crambe hydrolysates revealed a stimulating effect on root development at a dose corresponding to nitrogen concentration of 4.8 mM, promoting lateral root formation and altering root architecture. Furthermore, they exhibited a positive impact on nitrogen content in both maize roots and shoots, along with an increase in the chlorophyll SPAD index. Notably, the observed effects were similar to those induced by a commercial biostimulant based on an animal-derived hydrolysate, tested under the same conditions on maize. The present work underscores the potential of crambe seed by-products for new sustainable and environmentally safe agro-inputs aimed at enhancing crop performance within the framework of a circular economy.
Keywords:
crambe by-product; enzymatic hydrolysis; amino acids; peptides; auxin; plant rooting; mung bean; maize 1. Introduction
In recent years, there has been a surge in interest in the use of plant biostimulants in agriculture. This heightened interest is driven by increasing attention to eco-friendly and sustainable solutions aimed at enhancing crop productivity in the face of environmental and climate change challenges [1]. Biostimulants comprise a diverse group of natural substances and microorganisms that, when applied to plants or the surrounding soil, stimulate physiological processes in plants, resulting in improved nutrient uptake, stress tolerance, growth, and overall health [2,3,4]. Through reducing the reliance on conventional inputs, biostimulants work via enhancing plant’s inherent capabilities, making it more resilient and enabling it to better withstand environmental stressors, such as drought, extreme temperatures, or salinity. Furthermore, through improving soil health and nutrient cycling, biostimulants contribute to carbon sequestration, aligning with the global climate agenda [5].
The global biostimulant market has experienced significant growth due to the increasing investments by the private sector and the emergence of regulatory support in various countries worldwide. The recent EU Fertilising Products Regulation (Regulation UE 2019/1009), in effect since July 2022, clearly includes and regulates biostimulant products. Since manufacturers must be able to prove the claims on their labels, great efforts have been put into the standardization of tests, with the final goal of having easier access to the EU Single Market. This regulation opens many opportunities for the R&D of biostimulants, starting from the search for new raw materials, which have to be effective, safe, and sustainable.
Biostimulants include several classes of compounds based on their primary modes of action and chemical composition, and among them, protein hydrolysates have recently been the object of numerous research studies. Protein hydrolysates derive from different vegetal or animal sources, such as agricultural waste and by-products, through the enzymatic or chemical breakdown of proteins into smaller peptides and amino acids. They elicit various beneficial effects on plant nutrient uptake and utilization, root development, crop yield and quality, stress mitigation and soil microbial activity, and are generally compatible with other agricultural inputs such as fertilizers and pesticides, making them versatile tools in integrated crop management [6,7]. The use of hydrolysates produced from vegetal biomasses is particularly attractive due to their higher sustainability, environmental safety, and tolerability in plants with respect to animal-derived hydrolysates [8]. Amino acids, whose uptake by the plant roots has been demonstrated [9], are the most studied hydrolysate components, considered partly responsible for the biostimulant effects. Recently, amino acids have been shown to improve the growth, elemental and bioactive content, and essential oil profile of coriander [10], while the foliar application of glycine and glutamine showed beneficial effects in lettuce [11]. Legume-derived protein hydrolysates were studied for their biostimulant properties on greenhouse tomato, wall and baby rocket, and greenhouse spinach, enhancing the yield and nutritional and functional quality of crops [12,13,14]. Furthermore, the key role of hydrolysate small peptides, such as signaling peptides with auxin-like activities in root development, is emerging in many studies [15,16,17].
Crambe abyssinica Hochst is a plant belonging to the family of Brassicaceae, cultivated mainly for its rusticity, requiring low-input cultivation, and the distinct characteristics of its oil, rich in erucic acid, used for industrial purposes and, recently, in cosmetics, too [18,19]. Defatted seed meals (DSMs), the main by-products of oil extraction, have been proven to be a valuable source of bioactive molecules [20], among which proteins are very abundant and versatile for the production of protein hydrolysates. Moreover, DSMs also contain phenolic compounds with potential antioxidant properties and glucosinolates (GSLs), whose hydrolysis products are known for their biological activity against plant pathogens and beneficial effects on human health, but that are less studied for their effect when applied as an exogenous treatment on plants [21,22]. Epi-progoitrin ((R)-2-hydroxybut-3-enyl GSL-EPI) in particular is the main GSL in crambe seeds [23,24], and it is hydrolyzed, upon tissue disruption, by the endogenous enzyme myrosinase in different products, among which is (5R)-5-vinyl-1,3-oxazolidine-2-thione (VOT), which have been tested for their effect on living tissues of Lactuca sativa [25]. Katz et al. observed that allyl-GSL, one of the most common GSLs of Brassicaceae, and its metabolites had a synergistic action with auxin on root growth, with different biological effects and mechanisms in function of the plant species tested and the environment [26]. At the same time, among the myrosinase hydrolysis products, allyl-isothiocyanate had no effect on root length, while allyl-nitrile had a similar effect to allyl GSL. Further, the GSL breakdown product indole-3-carbinol was shown to act as an auxin antagonist in roots of Arabidopsis thaliana [27].
The aim of the present study was to explore the possibility to hydrolyze the proteins in crambe DSMs and to test the effect of the produced hydrolysates throughout reproducible bioassays in controlled conditions on mung bean cuttings and on maize grown in soilless cultivation.
2. Materials and Methods
2.1. Crambe Defatted Seed Meals
Crambe abyssinica Hochst VAR. Mario, sourced from the CREA-CI Brassicales collection [28], was cultivated in a CREA-CI experimental plot (Budrio, Bologna 44°32′00′′ N; 11°29′33′′ E, 28 m above sea level). The low-input cultivation techniques described by Fanigliulo et al. [18] were employed. After harvesting, cleaned and partially dried seeds were mechanically defatted using two processes, differing in the application or not of a heat pre-treatment of the seeds. A MIG srl (Fornovo San Giovanni, Bergamo, Italy) screw press, equipped with a pre-heater (80 °C), and a pilot mechanical extraction unit at CREA-CI, without a pre-heater, were used. The resulting defatted seed meals (DSMs), named hot and cold crambe DSMs, were ground to a size of 0.5 mm and characterized for their main components as previously described [18].
2.2. Protein Hydrolysis
Protein hydrolysates from cold and hot crambe DSMs (CCH and HCH, respectively) were obtained as described by Ugolini et al. [29], with some modifications. Enzymatic hydrolysis was applied on DSMs using a two-step procedure involving first the enzyme, Alcalase (from Bacillus licheniformis, 2.4 AU g−1, Sigma Aldrich, St. Louis, MO, USA), followed by Flavourzyme (from Aspergillus oryzae, 500 LAPU g−1, Sigma Aldrich). A suspension of DSM in water (10% w/v) was equilibrated for 30 min at 50 °C and pH 8 under stirring in a pH-stat titration unit (Mettler-Toledo DL50), before the addition of 0.3 AU g−1 of Alcalase/DSM protein and incubation at the same conditions. After 60 min of hydrolysis by Alcalase, pH was adjusted to 7 with citric acid and Flavourzyme was added at 50 LAPU g−1 enzyme/DSM protein. Hydrolysis continued at pH 7 and 50 °C for a total of 180 min, keeping the pH constant with the addition of 2.0 M KOH. At the end of the hydrolysis, the pH was lowered to 6 with citric acid and the hydrolysate was centrifuged (17.700✕ g, 30 min), paper-filtered, and frozen at −20 °C. Hydrolysate in solid form was obtained via freeze-drying for 72 h and stored at room temperature, protected from light and moisture.
The degree of hydrolysis (DH), defined as the proportion of cleaved peptide bonds in a protein hydrolysate, was determined using the pH-stat and the TNBS method for Alcalase and Flavourzyme hydrolysis, respectively. In the first case, the consumed volume of KOH, automatically registered by the Mettler-Toledo titration station, was used for DH calculation according to Adler-Nissen [30]. In the second one, DH was determined via the quantification of the reaction products of 2,4,6-Trinitrobenzene Sulfonic Acid (TNBS) with N-terminal amino groups formed at different times of hydrolysis in the hydrolysates and subtracted from the T0 starting point. A UV-VIS spectrophotometer set at 340 nm of absorbance (Varian Cary-300 BIO) and the standard L-leucine at different concentrations (0.5, 1.0, 1.5, 2.0 mM) were used for the calibration curve construction, quantification, and DH calculation [31].
Peptide molecular weight (MW) estimation and distribution of hydrolysates sampled at T0 and different hydrolysis times was determined via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using the PhastSystem separation and control unit, PhastGel homogeneous 12.5 gels (12.5% polyacrylamide), PhastGel SDS buffer strips (0.2 M Tricine, 0.2 M Tris, 0.55% SDS, pH 8.1), and following the GE HealthCare user manual instructions (GE healthcare Bio-Sciences AB, Upsala, Sweden). Low-molecular-weight protein standards (14.4–97 kDa) and the Coomassie staining technique were used for peptide size calibration and detection.
2.3. Hydrolysate Characterization
2.3.1. Chemical Characterization
The hydrolysate moisture content was determined using the difference in weight of the sample before and after drying in an oven at 105 °C for 12 h. Nitrogen and carbon content were determined using the Elemental analyzer LECO CHN TruSpec (St. Joseph, MI, USA). Nitrogen content was used to obtain the protein content percentage multiplying it by the standard conversion factor 6.25.
The hydrolysate determination of mineral content, the macro-elements (Na, K, P, Ca, and Mg) and micro-elements (Mn, Fe, Cu, Zn, Ni, Se, and Mo) was performed according to Beleggia et al. [32] with few modifications. Briefly, thirty milligrams of five replicates of each sample were digested with concentrated HNO3 and H2O2 using a microwave oven (Ethos Touch Control; Milestone, Arlington, MA, USA) and then diluted to 50 mL with high-purity deionized water. Then, the mineral content was analyzed using inductively coupled plasma mass spectrometry (Agilent 7700x; Agilent Technologies, Santa Clara, CA, USA), equipped with an auto-sampler (ASX-500), with the following operating conditions: the ICP-MS was tuned to standard mode and with collision gas (He) to remove many of the simple solvents and the argon-based polyatomic spectral interference; the plasma power was operated at 1550 ± 50 W, and the carrier and make-up gases were typically set at 0.83 L min−1 and 0.17 L min−1, respectively. Sample uptake was maintained at approximately 0.1 mL min−1 using a self-aspirating nebulizer. Data were processed using the MassHunter WorkStation software, version A.01.02 of 2012 (Agilent Technologies, Santa Clara, CA, USA).
2.3.2. Amino Acid Composition
The amino acid composition of the hydrolysates was assessed via HPLC-diod array detector (DAD) analysis and automatic precolumn derivatization with o-phtalaldehyde-3-mercaptopropionic acid (OPA) and 9-fluorenylmethylchloroformate (FMOC) according to Ugolini et al. [29]. A Hewlett-Packard Model series 1100 system (Hewlett-Packard, Palo Alto, CA, USA) was used, coupled with a DAD and a Gemini C18 column (4.6 × 250 mm, 5 μm; Phenomenex, Torrance, CA, USA). Hydrolysates were solubilized in 0.1 M HCl and filtered (first with paper filter Whatman 1 and then with Acrodisk syringe filter 0.2 μm) prior to injection. Norvaline and sarcosine were used as internal standards for quantitative determination of primary and secondary amino acids, respectively [33]. The limit of quantification ranged from 0.11 to 0.63 mg g−1 for single amino acids.
2.3.3. Phytochemical Content and Antioxidant Capacity
The glucosinolate content of crambe DSMs and hydrolysates was determined via HPLC-DAD desulfo GSL analysis according to the ISO 9167-1 method [34], with some minor modifications [35]. Epi-progoitrin ((R)-2-hydroxybut-3-enyl GSL-EPI) and 4-hydroxyglucobrassicin (4-hydroxy-3-indolylmethylglucosinolate-4-OH GBS) were identified according to UV spectra and standard retention times. Sinigrin (2-propenyl GSL) internal standard and relative response factors were used for quantification [36]. Sinigrin standard was isolated from Brassica juncea with a purity of 98.7% (HPLC-UV) and >96% on a weight basis [37].
(5R)-5-vinyl-1,3-oxazolidine-2-thione (VOT) hydrolysate content was determined via HPLC-DAD analysis as described by Leoni et al. [38]. VOT quantification was determined via an external calibration curve using purified VOT [25].
HPLC analyses were performed on a Hewlett-Packard Model series 1100 system (Hewlett-Packard, Palo Alto, CA, USA) equipped with a DAD and a Pursuit RXs5 C18 (250 × 3.0 mm, 5 μm) (Agilent Technologies, Santa Clara, CA, USA) or an Inertsil 5ODS-3 (250 × 3.0 mm, 5 μm) (GL Sciences, Torrance, CA, USA) column for GSL and VOT analysis, respectively.
The total phenolic content of DSM extracts and hydrolysates was determined by means of acidified methanolic solvent extraction and the Folin–Ciocalteu method using an Infinite M200 PRO microplate reader (Tecan, Männedorf, Switzerland) [22]. Results were expressed as mg g−1 gallic acid equivalent (GAE).
Antiradical capacity measured via testing DSM and hydrolysate extract quenching activity against 2,2′ -azinobis-(3-ethylbenzot hiazoline-6-sulfonic acid (ABTS•+) synthetic radical was determined according to Ugolini et al. [22] and using an Infinite M200 PRO microplate reader (Tecan, Männedorf, Switzerland). EC50 was determined as the amount of extract (mg mL−1) causing 50% inhibition of the radical initial color production in the assay conditions [39]. Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) was used as the reference standard.
2.4. In Vivo Assays
2.4.1. Mung Bean Rooting Assay
Auxin-like activity was evaluated with the mung bean (Vigna radiata) assay according to Sharma et al. [40] with some modifications.
Seeds of mung bean were surface sterilized with 5% sodium hypochlorite for 10 min, rinsed with plenty of water and germinated in moist vermiculite in the dark at 24 °C and 50% relative humidity (RU) for 3 days. Seedlings were transferred in a growth chamber (Percival Scientific, Perry, IA, USA) at 24 °C and 50% RU, with a 16/8 h photoperiod. After 7 days, uniform seedlings were selected and cut 3 cm below the cotyledonary node. Uniform cuttings were placed in vials containing hydrolysates solubilized in 11 mL of distilled water at the desired concentration (1–10–100–1000 ppm) and kept in the growth chamber for 48 h. After that time solutions were substituted with water or Hoagland nutritive solution (HS) modified according to Ertani et al. [41] and kept in the growth chamber for another 7 days. Vials kept for two days in water or an aqueous solution of KNO3 at the concentration corresponding to the nitrogen content of the higher hydrolysate concentration tested (4.8 and 5.0 mM for CCH and HCH, respectively) were used for control. Water was added daily to maintain the initial content in vials. For each treatment, eighteen plants were used. Adventitious roots length and number (>1 mm) were registered, and root dry weight (DW) was determined after drying in an oven at 105 °C overnight in triplicate.
2.4.2. Maize Assay
The maize assay was performed according to Ertani et al. [41] with some modifications. Maize seeds (Zea mays L., Kampius; SIS S.p.A., Bologna, Italy) were soaked overnight in deionized water, surface-sterilized with sodium hypochlorite 5% for 10 min, rinsed with water, and germinated for 72 h at 25 °C on filter paper. Homogeneous seedlings (about 1 cm) were selected and grown in a mixed aeroponic/hydroponic system in pots filled with expanded clay. Three plants per pot and 20 pots per 10 L tank were used for every treatment. A modified HS was used and changed every 48 h. Spray nozzles, controlled by a cycling timer (10 min on every hour) pumped the nutritive solution below the growing surface and aerated the solution. A 14/10 h light/dark cycle, air temperature of 28/19 °C, 50% RU, and photosynthetically active radiation of 230 μmol m−2 s−1 were applied. Treatments with different hydrolysate concentrations were applied for 48 h, 3 and 12 days after transplanting. In the first experiment, HCH was applied at 474–948–1896 ppm, corresponding to a nitrogen concentration of 2.4–4.8–9.6 mM, respectively, in HS. In the second one, CCH was tested at 537 and 1074 ppm, corresponding to nitrogen concentrations of 2.4 and 4.8 mM, and the commercial biostimulant COMH was also applied at the highest nitrogen content of CCH, 4.8 mM, i.e., 440 ppm of dry matter (0.6 mL L−1 applied as the liquid formulation present on the market; density, 1.23 g mL−1). At the end of the second treatment, plants were harvested, rinsed with water to remove debris from the substrate and analyzed for physiological parameters. Total shoot and root fresh weight (FW) and DW were determined in three pools of 16 plants for each treatment. Nitrogen and carbon content was determined in dried shoots and roots as described above (Section 2.3.1). Primary root and shoot length were manually determined in 30 plants per treatment. For root evaluation, 20 roots per treatment were also scanned with the flatbed scanner of an HP LaserJet 100 color MFP M175a. The open-source software RhizoVision Explorer v. 2.0.3 [42] was then used to retrieve the total root length, the length of roots with diameter below and above 0.5 mm, the average root diameter, the number of branch points, and the branching frequency, using the setting for broken roots. For shoot evaluation, the soil plant analysis development (SPAD) index was measured using the chlorophyll meter SPAD-502 Plus (Konica Minolta, Tokyo, Japan) on the middle portion of fully expanded leaves of 60 plants per treatment.
2.5. Statistical Analysis
The analyses were replicated a minimum of three times and the statistical analysis of data (mean ± SD) was performed with R (Foundation for Statistical Computing, Vienna, Austria). Data were subjected to a one-way analysis of variance employing the least significant difference (LSD) test to assess significant differences between samples (p < 0.05).
3. Results
3.1. Hydrolysate Production
Crambe seeds were mechanically defatted through a cold- and a hot-press method, and two DSMs were obtained with similar residual oil, protein, and carbon content (20.7, 23.8, and 51.2% for cold-extracted DSMs and 20.4, 24.4, and 53.1% for hot-extracted DSMs, respectively).
A direct hydrolysis process, combining the extraction and hydrolysis step and using two enzymes in sequence, an endopeptidase (Alcalase), and a mixture of endo- and exo-peptidases (Flavourzyme), was followed to obtain an extensive hydrolysis and preventing bioactive compound losses by preliminary protein isolations steps as previously reported [29,43,44,45]. Indeed, the hydrolysis protocol applied on sunflower DSM proposed by Ugolini et al. [29] was slightly modified, only increasing the Alcalase concentration from 0.2 to 0.3 AU g−1 to obtain a higher final DH, while the other hydrolysis conditions and Flavourzyme concentration were found optimal also for crambe DSMs. A good DH of proteins was obtained (41.2%) starting from both DSMs, and hydrolysis progress was highly reproducible (CV 3.0%) (Figure 1).
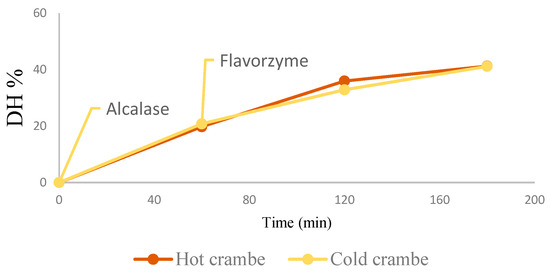
Figure 1.
Hydrolysis progress, DH (%) determined at different times from Alcalase addition to 60 min (Flavourzyme addition), 120 and 180 min, for hot and cold crambe DSMs in water suspensions.
DH was not influenced by the DSM residual lipid content, around 20% in both cold and hot crambe DSMs, and was comparable to the DH determined in seed meals defatted via exhaustive hexane extraction.
Peptide MW distribution of CCH was determined via SDS-PAGE and the result is shown in Figure S1. The bigger peptides extracted from DSM before hydrolysis (T0) disappeared in the final hydrolysate (CCH) in which peptides MWs were lower than 30 kDa. A similar distribution was obtained with HCH (results not shown).
From 100 g of DSM, about 31 g of fine powder of freeze-dried hydrolysates was obtained.
3.2. Hydrolysate Characterization
3.2.1. Chemical Characterization
CCH and HCH were chemically characterized for their main components and compared with a liquid commercial biostimulant based on protein hydrolysate of animal origin (COMH) (Table 1).

Table 1.
Chemical characterization of cold and hot hydrolysates (CCH and HCH, respectively) and a commercial hydrolysate of animal origin (COMH). Moisture, nitrogen, and free amino acid are expressed as % (w/w); elements are reported as ppm (w/w). Data are the means of a minimum of three measures ± standard deviation and are expressed on dry matter basis. Mean values (n > 3) followed by different letters are significantly different according to the LSD test (p < 0.05).
Nitrogen content was slightly higher in HCH than in CCH, despite the similar nitrogen content of the initial DSMs, proving a higher extraction efficiency of proteins from the heat-treated meal. Protein concentration estimated using Jones’ conversion factor of 6.25, in fact, was 43.8 and 50.0% for CCH and HCH, respectively. Instead, COMH nitrogen concentration, calculated on dry matter, was almost twice the nitrogen concentrations of crambe hydrolysates, corresponding to 95.6% of protein content. Carbon content was similar among the three hydrolysates, slightly lower for CCH and COMH.
Regarding the elemental analysis, the high content of K in the crambe hydrolysates was expected, as KOH was used as a titrant to maintain constant pH during hydrolysis. A higher K content for CCH than HCH was also expected as, in the equilibration step, a higher amount of titrant was consumed to compensate for the decrease in pH due to GSL hydrolysis, a reaction that produces protons [46]. Instead, COMH had a lower K content but a higher Na content, 30 and 44 times the CCH and HCH content, respectively. Overall, the crambe hydrolysates had a higher content of different micro and macro nutrients respect to COMH and CCH had a higher or equal content respect to HCH for all elements, apart for Mn, which was higher in HCH.
3.2.2. Free Amino Acid Composition and Bioactive Content
Free amino acid content was higher in HCH than CCH, reflecting the hydrolysates nitrogen content and even higher in COMH (Table 1). On the contrary, free amino acid content expressed on total proteins was about 23.0 for the two hydrolysates and 15.6% for COMH.
Free amino acid composition was also similar between the two crambe hydrolysates as both the initial seeds and the applied hydrolysis protocol were the same (Table 2).

Table 2.
Free amino acid content (mg g−1) expressed on dry matter basis and composition (% of total free amino acids) of cold and hot crambe hydrolysates (CCH and HCH) and of a commercial biostimulant (COMH).
A large diversity of amino acids was present as 19 different amino acids were detected in the two crambe hydrolysates. The most abundant was glutamine, as for the source crambe DSM [20], arginine, leucine, and lysine. Tryptophan was also present because of the mild hydrolysis performed on DSMs. Instead, COMH’s main amino acids were glutamic acid, glycine, alanine, and a higher percentage of proline, while many others were not detected, such as glutamine, arginine, and tryptophan, for instance.
Other potentially bioactive compounds were also quantified in DSMs and hydrolysates (Table 3).

Table 3.
Bioactive content and antiradical capacity of crambe defatted seed meals (DSMs) and cold and hot crambe hydrolysates (CCH and HCH): total glucosinolates (GSLs) expressed as μmol g−1; (5R)-5-vinyl-1,3-oxazolidine-2-thione (VOT) expressed as μmol g−1; total phenolic content, TPC, expressed as mg gallic acid equivalent (GAE) g−1; antiradical activity, AR, expressed as EC50 (mg mL−1 of DSM or hydrolysate methanolic extract required to obtain 50% of radicals ABTS•+ scavenging in the assay conditions). Trolox EC50 for ABTS assay was 0.04 ± 0.01 mg mL−1. Mean values (n > 3) followed by different letters are significantly different according to the LSD test (p < 0.05).
GSLs, compounds characteristic of the plants of the Brassicaceae family, were identified in crambe DSMs, where EPI accounted for more than 90% of the total, while 4-OH GBS was found at 5.6% (2.01 ± 0.02 μmol g−1) and 1.1% (0.31 ± 0.02 μmol g−1), in cold and hot crambe DSM, respectively. Total GSL content was higher in cold crambe than hot crambe DSM (−23.6%).
Between the hydrolysates, GSLs were found only in HCH, highly concentrated respect to the starting DSM, and the total GSL extraction yield from DSM was determined as 72%. A 0.6% (0.39 ± 0.05 μmol g−1) of the total GSLs was still represented by 4-OH GBS.
GSLs were not detected in CCH, while VOT, the myrosinase-dependent degradation product of EPI, was found in high amount in CCH, and only in traces in HCH (<1.4 μmol g−1). Considering a conversion factor GSL:VOT of 1:1, the VOT accounted for 76% of the starting EPI in cold crambe DSM. Most (>90%) of EPI conversion to VOT took place during the first 30 min of DSM suspension equilibration time. Indeed, part of VOT (15%) was lost during the final hydrolysate freeze-drying process, so the theoretical yield of EPI to VOT conversion was even higher (>90%).
Total phenol content and antiradical capacity were also quantified in DSM and hydrolysates, to evaluate the potential antioxidant activity of the products. Phenols were slightly higher in cold DSM compared to hot DSM, while this trend was reversed for hydrolysates. This means that phenol extraction yield from DSM was 77.8 and 93.8% for CCH and HCH, respectively. At the same time, antioxidant activity, measured according to the ABTS radical quenching, reflected the TPC and was higher for HCH than for CCH.
3.3. Mung Bean Assay
Hydrolysates were tested for their effects on plant roots through treating mung bean cuttings in controlled conditions. Results of the adventitious root length and number and the DW recorded after two days of cutting treatments and growth in HS for seven days are reported in Figure 2 for the two hydrolysates.

Figure 2.
Adventitious root number, length, and dry weight of mung bean (Vigna radiata) recordings after treatment of cuttings with crambe hydrolysate for two days at different concentrations (ppm), grown in modified Hoagland solution (HS). Untreated cuttings were kept in water and HS as control. KNO3 samples were treated for two days with KNO3 and grown in HS; (A1–A3) cold crambe hydrolysate (CCH); (B1–B3) hot crambe hydrolysate (HCH). Mean values (n > 3) followed by different letters are significantly different according to the LSD test (p < 0.05).
CCH at the highest concentration determined a significant shortening (65%) of adventitious roots respect to HS control, similarly to water control and KNO3 treatment (at nitrogen concentration equivalent to 1000 ppm), which caused a 70–80% reduction (Figure 2(A1)). A clear effect of CCH was observed on the number of lateral roots at the highest concentration, which determined an increase of 138% compared to HS control (Figure 2(A2)). Lateral roots were increased by number but also had a different distribution along the main root as shown in Figure 3.
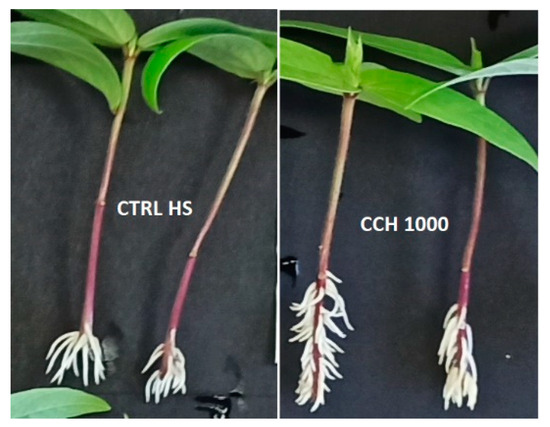
Figure 3.
The effect of CCH treatment at 1000 ppm on lateral roots, which increased in number but had also a different distribution along the main root.
Other treatments, including KNO3, did not affect the root number or caused a little reduction. Looking at the root DW no significant effect was observed for all treatments, which means that the root shortening effect of the hydrolysate at 1000 ppm was compensated by the development of new lateral roots (Figure 2(A3)).
HCH treatments determined, similarly to CCH, a shortening of lateral roots from 50 to 70%, with no significant differences among the applied concentrations and not different from water control and KNO3-treated roots (Figure 2(B1)). As for CCH, HCH treatment significantly increased the lateral root number at the highest concentration (1000 ppm), which determined an even higher increase of 194% compared to HS control (Figure 2(B2)). Other treatments had no significant effect on the number of roots. The root DW did not significantly change among treatments apart for the water control, in which it was lower (Figure 2(B3)).
3.4. Maize Assay
The effect of crambe hydrolysates was also tested on the roots and shoots of maize plants grown in a soilless aeroponic/hydroponic system and evaluated in the early stage of growth. Plants were treated two times for 48 h, 3 and 12 days after transplanting the seedlings into pots, at three different nitrogen concentrations in HS, 2.4–4.8–9.6 mM. The middle concentration was chosen according to the most effective concentration found with the mung bean assay reported above (1000 ppm).
3.4.1. HCH Effect on Root and Shoot System
Results obtained with HCH treatments on maize roots are reported in Table 4.

Table 4.
Effect on roots of maize after aeroponic/hydroponic treatment with hot crambe hydrolysate (HCH) at three nitrogen concentrations (N doses, mM), in modified Hoagland solution, for two days at 3 and 12 days after transplanting. FW and DW, root fresh and dry weight; N, nitrogen root content; L, root length; Ø, root diameter; L up to 0.5 mm Ø (m), length of roots with diameter up to 0.5 mm (m); L > 0.5 mm Ø (m); length of roots with diameter above 0.5 mm (m). Mean values followed by different letters are significantly different according to the LSD test (p < 0.05).
Root FW and DW were not significantly affected by treatments, while nitrogen content in roots notably increased respect to control, by 45% with the lower dose and 64–74% with the two higher doses, without significant differences between them. All treatments determined a significant reduction in the primary root length, from 18 to 27%, more pronounced at higher doses, and a reduction in root total length and length of roots with different diameters, of about 12–17%, similarly for 4.8 and 9.6 mM. Root average diameter increased with treatments (6–11%), as did the number of branch points (16% for 4.8 mM) and branching frequency (32% with 4.8 and 9.6 mM). As root DW weight remained almost constant for the three tested doses, it can be suggested that the root shortening effect was balanced by hair root development, as observed in the mung bean assay.
Regarding the effect of HCH on the maize shoot system, results are reported in Table 5.

Table 5.
Effect on shoots of maize after aeroponic/hydroponic treatment with hot crambe hydrolysate (HCH) at three nitrogen concentrations (N doses, mM), in modified Hoagland solution, for two days at 3 and 12 days after transplanting. FW and DW, fresh and dry weight; N, nitrogen; C, carbon; Soil Plant Analysis Development, SPAD index. Mean values followed by different letters are significantly different according to the LSD test (p < 0.05).
Treatments did not affect shoot FW, while the two higher doses determined an increase in DW by 10%. Shoot total length was slightly reduced, by 3–4%, in treated plants at 4.8–9.6 mM. The more evident effect was on SPAD index which increased with treatment concentration by 8–22–27% and on the nitrogen shoot content incremented by 22–25–30% for 2.4–4.8–9.6 mM, respectively. The elemental carbon content, which could represent structural (e.g., cellulose) and non-structural (e.g., soluble sugars, starch) carbohydrates, increased slightly but significantly at the higher concentrations, reflecting the DW trend.
Considering a cost–benefit balance, the HCH concentration that gave the best results on maize roots and shoots was the middle nitrogen concentration at 4.8 mM as a double concentration determined a little improvement of the maize performance.
3.4.2. CCH Effect on Root and Shoot System
The maize assay was also used to test the efficacy of CCH. CCH doses were chosen maintaining the two lower nitrogen concentrations of the previous test performed with HCH, 2.4 and 4.8 mM. The highest concentration was not included as its effect on maize plants did not justify the high dose application. Furthermore, the commercial biostimulant COMH was also applied at the concentration of nitrogen equal to the higher CCH concentration of 4.8 mM. Results on roots are reported in Table 6.

Table 6.
Effect on roots of maize after aeroponic/hydroponic treatment with cold crambe hydrolysate (CCH) at two nitrogen concentrations (N doses, mM), and a commercial hydrolysate (COMH), in modified Hoagland solution, for two days at 3 and 12 days after transplanting. FW and DW, root fresh and dry weight; N, nitrogen root content; L, root length; Ø, root diameter; L up to 0.5 mm Ø (m), length of roots with diameter up to 0.5 mm (m); L > 0.5 mm Ø (m); length of roots with diameter above 0.5 mm (m). Mean values followed by different letters are significantly different according to the LSD test (p < 0.05).
The CCH application determined a similar effect on maize roots as HCH, causing the development of shorter roots (−22%) but more branched (+32 and +48% of branch points and branching frequency at 4.8 mM), and did not significantly change the root DW, respect to control. Anyway, no effect was recorded on root diameter. Nitrogen root content was 21 and 55% higher compared to control with 2.4 and 4.8 mM CCH, respectively. COMH determined, in a similar way, a reduction in primary root length, even if less marked than CCH (−18%), and an increase in root branching. It caused in fact a 36% increase in branch points respect to control, higher than CCH 2.4 mM, but comparable to CCH 4.8 mM, and 26% increase in branching frequency, comparable to CCH 2.4 mM but lower than CCH 4.8 mM. As in HCH, but not CCH, it caused an increase in root diameter (9%). Nitrogen root content was increased by 73% with respect to control, more than for the two crambe hydrolysates.
Regarding the effect of CCH and COMH hydrolysates on shoots, results are shown on Table 7.

Table 7.
Effect on shoots of maize after aeroponic/hydroponic treatment with cold crambe hydrolysate (CCH) at two nitrogen concentrations (N doses, mM), and a commercial hydrolysate (COMH), in modified Hoagland solution, for two days at 3 and 12 days after transplanting. FW and DW, fresh and dry weight; N, nitrogen; C, carbon; Soil Plant Analysis Development, SPAD index. Mean values followed by different letters are significantly different according to the LSD test (p < 0.05).
CCH influenced SPAD index, stronger than HCH but also COMH, determining an increment with respect to the control of 23 and 36% at 2.4 and 4.8 mM, respectively, versus 21% of COMH 4.8 mM. On the contrary, the nitrogen content was more influenced by COMH than CCH (+20 vs. +16% compared to control). Carbon content slightly increased with the highest concentration of CCH, while COMH did not caused any changes.
Overall, CCH had a more marked effect on root branching and leave SPAD index, while HCH had a major effect on root and shoot nitrogen content, root average diameter, and shoot DW. COMH had a similar effect, more marked for root nitrogen content, average diameter, and number of branch points. Furthermore, the root shortening action seemed to be slightly milder than the two crambe hydrolysates.
4. Discussion
Two crambe DSMs were the by-products of oil extraction performed by means of a cold- and a hot-press method. A seed pre-heating process was applied to increase the oil extraction efficiency and rate [18] but partially destroyed some of bioactive content, such as GSLs, in hot crambe DSM. Indeed, indole GSLs, such as 4-OH GBS, have shown to be more susceptible to thermal degradation than the aliphatic ones [47]. Phenol content and antiradical activity were also lower in hot crambe DSM compared to cold DSM. Nevertheless, the two DSMs’ protein content was similar and was exploited for hydrolysate production by applying a mild two-step enzyme hydrolysis process. Two hydrolysates rich in peptides with MW lower than 30 KDa and free amino acids at 10–11% (w/w) were obtained according to other works where combinations of hydrolytic enzymes were used [48,49]. Hydrolysates were then freeze-dried to obtain a fine powder and to increase product shelf-life, even though more economical and sustainable processes for full-scale production could be alternatively applied to reduce the overall energy consumption rate of the production process, such as liquid concentration or freeze concentration [50,51].
The amino acid composition of the hydrolysates reflected the substrate origin and the hydrolytic process applied, as Alcalase hydrolyses peptide bonds from non-terminal amino acids, with preference for large uncharged residues, while Flavourzyme breaks N-terminal amino acids [52,53]. Crambe hydrolysate’s most represented amino acids, such as glutamine, arginine, and tryptophane, deserve particular attention for their possible role in plant biostimulation [54,55]. On the other hand, the amino acid composition of the COMH of animal origin was quite different, with abundant thermostable amino acids like glycine, alanine, and proline, typical of animal tissues [56]. Among them, proline is considered a key amino acid in plant physiology as a water balance regulator and protector from saline stress [57]. Instead, the absence of the more thermolabile amino acid tryptophane, the precursor of plant auxin phytohormones known to influence root morphology, could be an indication of high-energy chemical/thermal processes often used for hydrolysates production from animal wastes [8]. In any case, the amino acid composition of hydrolysates could not alone explain the biostimulant effect. Santi et al. [58], for instance, observed that a protein hydrolysate from animal tissue induced root growth in a stronger manner with respect to inorganic nitrogen supply and a mixture of free amino acids equalized for nitrogen content.
Protein and nitrogen content was higher in COMH compared to the crambe hydrolysates, but COMH was poorer in macro- (Mg, P, K, and Ca) and microelements (Mn, Fe, Cu, Zn, Se, and Mo) known to improve the nutrient use efficiency and yield/quality of crop plants [59].
Furthermore, bioactive components, such as GSLs, or derived compounds, and phenols, were extracted from DSMs during hydrolysis and were present in crambe hydrolysates. Overall, the two hydrolysates, HCH and CCH, had similar composition, with HCH slightly richer in nitrogen, free amino acids, and phenols, and with a higher antiradical activity, too, measured against ABTS. Nevertheless, the main difference was related to the VOT presence in CCH and the GSL presence, mainly EPI, in HCH. This finding suggests that in cold crambe DSM the presence of active myrosinase determined EPI conversion to VOT [60]. On the contrary, in hot crambe DSM, the enzyme myrosinase was deactivated by the temperature and pressure applied during oil extraction, and, therefore, GSLs were maintained in the final hydrolysate, HCH. The low HCH VOT content was probably due to some residual myrosinase activity in the DSM or other degradation mechanisms.
Crambe hydrolysates tested on cuttings of the model plant mung bean showed possible auxin-like activity on root development. The highest tested concentration revealed a root shortening effect but also a stimulant action on lateral root development, an effect resembling that of indol-3-acetic acid (IAA), previously tested with the same assay [61]. The effect was more evident for HCH than CCH, and it seems that it could not be ascribed to the nitrogen content alone, as KNO3 treatment had a similar root-shortening effect but did not determine an increase in adventitious root number. Commercial formulations containing algal/plant extracts, humic and amino acids, lipids, and inorganic components were previously tested using the mung bean rooting assay, giving positive results linked to auxin-like compound content [62,63]. IAA was detected at a low concentration in protein hydrolysate with auxin-like activity produced from sunflower DSM, and IAA conjugates were found in many plant seeds [29,64]. At the same time, L-tryptophane was detected in the same sunflower hydrolysate but also in CCH and HCH at a comparable concentration and could be considered a possible rooting co-factor [65,66]. Nevertheless, hydrolysates could also contain bioactive peptides eliciting hormone-like activities, like those exerted by plant endogenous signaling peptides promoting root and shoot growth, as previously demonstrated [15,67]. In a different experiment, Ceccarelli et al. showed the effect of hydrolysates produced from Brassicaceae biomasses on tomato cuttings following the quick dipping of leaves in a nitrogen concentration of about 20 mM which increased adventitious root length by 68% with respect to water control, while root number was not significantly affected by treatment [68]. In the same work, an untargeted metabolomics approach revealed that auxin, gibberellin, and brassinosteroid production was downregulated in treated plants.
The effect of crambe hydrolysates on roots was confirmed on maize in the first weeks of growth in aeroponic/hydroponic solution. The best cost/benefit balance was obtained with a concentration of nitrogen of 4.8 mM, similarly to the results obtained with the mung bean test and was compared with the COMH at the same nitrogen concentration. The three hydrolysates determined, even if with different percentages, root shortening and increased branching, thus modifying the root architecture system and consequently stimulating nitrogen uptake in the roots and shoots, too. Furthermore, treatments increased the SPAD index in leaves, which is correlated with the nitrogen and chlorophyll content and thus plant photosynthesis [69]. Previously, Ertani et al. [41,70,71] showed the effect of protein hydrolysates of vegetal or animal origin and phenol-containing organic substances on nitrogen and carbon metabolisms and nutrient uptake in maize hydroponic culture, suggesting a possible role of endogenous IAA, phenols, amino acids, and small peptides. Other authors studied the effect of vegetal protein hydrolysates produced from different sources, including Brassicaceae plant biomass, applied as seed priming or foliar distribution on Arabidopsis seeds or in lettuce and tomato plants, respectively [72,73,74]. Using high-throughput phenotyping and metabolomics approaches, they showed the growth promotor and stress alleviator effects of those hydrolysates through the coordinated action of plant growth regulators and antioxidant compounds, such as phenolics and carotenoids. Ambrosini et al. showed that the removal of free amino acids and short peptides through filtration and dialyses of a commercial collagen-derived protein hydrolysate lowered its biostimulating effect on the roots of maize plants, grown in hydroponic solution, showing that smaller compounds were particularly active in lateral root development [75].
Due to the complex composition of protein-based hydrolysates, it is difficult to attribute the observed effects to a single compound or class of compounds. For crambe hydrolysates, further studies should be carried out to investigate the mechanism of action and component fractionation could be a valuable strategy. In any case, considering the similar behaviors observed among the two crambe hydrolysates and even the commercial one, it is possible to speculate that the EPI GSL or VOT content of crambe hydrolysates has a little role in the final biostimulant effect. In a study by Galletti et al., VOT was shown to have root growth inhibition activity at concentrations higher than 4 mM and induce root branching at low doses in lettuce (0.4–0.8 mM) [25]. However, the VOT concentrations tested in our maize assays using CCH were 0.047 and 0.094 mM for 2.4 and 4.8 mM doses, thus, far lower than the VOT concentration tested by those authors.
On the contrary, amino acids such as L-tryptophane and L-glutamate, but also nitrate, peptides, mineral elements, and antioxidants, could have contributed to the positive regulation of lateral root development, acting as signaling molecules in a complex dialogue between them [76,77].
5. Conclusions
The use of biostimulants fosters closed-loop systems, where agricultural waste and by-products can be converted into valuable inputs, further reducing the environmental footprint of farming practices. As DSMs are by-products of oil extraction, their valorization through new uses aligns perfectly with the goals of circularity and sustainability in production. Here, we show that protein hydrolysates obtained from Crambe abysinnica DSMs, tested on two representative families in crop science, such as legumes and cereals, and using different bioassays and methodologies, proved to exert a biostimulant effect on plant root development. Furthermore, the effect was similar to the one of a commercial biostimulant based on a hydrolysate of proteins of animal origin, making them valuable components for the development of new formulations. Further studies are necessary to elucidate the hydrolysates’ mechanism of action and verify the effect on plants at different growth stages. However, these preliminary data suggested the potential application, alone or in combination with other bioactive products, as biostimulants in the case, for instance, of plant transplantation, thanks to their positive action on root development.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy13112755/s1, Figure S1: SDS-PAGE of hydrolysed protein from cold crambe defatted seed meal.
Author Contributions
Conceptualization, L.U., L.M. and L.R.; methodology, L.U., L.M., R.M., E.P., R.B. and L.R.; formal analysis, L.U., L.M., R.B. and L.R.; investigation, L.U., L.M., R.M., E.P. and L.R.; data curation, L.U. and L.R.; writing—original draft preparation, L.U. and L.R.; writing—review and editing, L.U., R.M., E.P., R.B. and L.R.; supervision, L.U. and L.R.; project administration, L.U. and L.R.; funding acquisition, L.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been carried out in the ambit of the AGROENER Research Project, WP4—Integrated biorefineries in agro-food production chains, granted by MiPAAF, the Italian Ministry of Agriculture, Food and Forestry Policies (D.D. n. 26329) and the National Research Center for “Agriculture Technologies—Agritech”, funded by the European Union—Next-GenerationEU and MUR (Italian Ministry of University and Research) and the Agritech National Research Center receiving funding from the European Union Next-GenerationEU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR)—MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4—D.D. 1032 17/06/2022, CN00000022). This manuscript reflects only the authors’ views and opinions; neither the European Union nor the European Commission can be considered responsible for them.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Nerio Casadei (CREA-CI) for supporting crambe reproduction in Bologna and crambe oil extraction from seeds.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bhupenchandra, I.; Chongtham, S.K.; Devi, E.L.; Ramesh, R.; Choudhary, A.K.; Salam, M.D.; Sahoo, M.R.; Bhutia, T.L.; Devi, S.H.; Thounaojam, A.S.; et al. Role of biostimulants in mitigating the effects of climate change on crop performance. Front. Plant Sci. 2022, 13, 967665. [Google Scholar] [CrossRef] [PubMed]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant Biostimulants: Importance of the Quality and Yield of Horticultural Crops and the Improvement of Plant Tolerance to Abiotic Stress—A Review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef]
- Rajabi Hamedani, S.; Rouphael, Y.; Colla, G.; Colantoni, A.; Cardarelli, M. Biostimulants as a Tool for Improving Environmental Sustainability of Greenhouse Vegetable Crops. Sustainability 2020, 12, 5101. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef]
- Malécange, M.; Sergheraert, R.; Teulat, B.; Mounier, E.; Lothier, J.; Sakr, S. Biostimulant Properties of Protein Hydrolysates: Recent Advances and Future Challenges. Int. J. Mol. Sci. 2023, 24, 9714. [Google Scholar] [CrossRef]
- Rouphael, Y.; Carillo, P.; Cristofano, F.; Cardarelli, M.; Colla, G. Effects of vegetal- versus animal-derived protein hydrolysate on sweet basil morpho-physiological and metabolic traits. Sci. Hortic. 2021, 284, 110123. [Google Scholar] [CrossRef]
- Yao, X.; Nie, J.; Bai, R.; Sui, X. Amino acid transporters in plants: Identification and function. Plants 2020, 9, 972. [Google Scholar] [CrossRef]
- Sowmya, R.S.; Warke, V.G.; Mahajan, G.B.; Annapure, U.S. Effect of amino acids on growth, elemental content, functional groups, and essential oils composition on hydroponically cultivated coriander under different conditions. Ind. Crops Prod. 2023, 197, 116577. [Google Scholar] [CrossRef]
- Noroozlo, Y.A.; Souri, M.K.; Delshad, M. Stimulation Effects of Foliar Applied Glycine and Glutamine Amino Acids on Lettuce Growth. Open Agric. 2019, 4, 164–172. [Google Scholar] [CrossRef]
- Rouphael, Y.; Giordano, M.; Cardarelli, M.; Cozzolino, E.; Mori, M.; Kyriacou, M.C.; Bonini, P.; Colla, G. Plant-and seaweed-based extracts increase yield but differentially modulate nutritional quality of greenhouse spinach through biostimulant action. Agronomy 2018, 8, 126. [Google Scholar] [CrossRef]
- Caruso, G.; De Pascale, S.; Cozzolino, E.; Giordano, M.; El-Nakhel, C.; Cuciniello, A.; Cenvinzo, V.; Colla, G.; Rouphael, Y. Protein hydrolysate or plant extract-based biostimulants enhanced yield and quality performances of greenhouse perennial wall rocket grown in different seasons. Plants 2019, 8, 208. [Google Scholar] [CrossRef] [PubMed]
- Di Mola, I.; Ottaiano, L.; Cozzolino, E.; Senatore, M.; Giordano, M.; El-nakhel, C.; Sacco, A.; Rouphael, Y.; Colla, G.; Mri, M. Plant-Based Biostimulants Influence the Agronomical, Physiological, and Qualitative Responses of Baby Rocket Leaves under Diverse Nitrogen Conditions. Plants 2019, 8, 522. [Google Scholar] [CrossRef]
- Matsumiya, Y.; Kubo, M. Soybean Peptide: Novel Plant Growth Promoting Peptide from Soybean; El-Shemy, H., Ed.; IntechOpen: Rijeka, Croatia, 2011; pp. 215–231. [Google Scholar]
- Lucini, L.; Miras-Moreno, B.; Rouphael, Y.; Cardarelli, M.; Colla, G. Combining Molecular Weight Fractionation and Metabolomics to Elucidate the Bioactivity of Vegetal Protein Hydrolysates in Tomato Plants. Front. Plant Sci. 2020, 11, 976. [Google Scholar] [CrossRef]
- Oh, E.; Seo, P.J.; Kim, J. Signaling Peptides and Receptors Coordinating Plant Root Development. Trends Plant Sci. 2018, 23, 337–351. [Google Scholar] [CrossRef]
- Fanigliulo, R.; Pochi, D.; Bondioli, P.; Grilli, R.; Fornaciari, L.; Folegatti, L.; Malaguti, L.; Matteo, R.; Ugolini, L.; Lazzeri, L. Semi-refined Crambe abyssinica (Hochst. EX R.E.Fr.) oil as a biobased hydraulic fluid for agricultural applications. Biomass Convers. Biorefinery 2023, 13, 1859–1871. [Google Scholar] [CrossRef]
- de Aguiar, C.M.; Santos, K.A.; Sampaio, S.C.; Martin, C.A. Crambe Abyssinica Hochst. In Oil BT—Fruit Oils: Chemistry and Functionality; Ramadan, M.F., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 433–450. ISBN 978-3-030-12473-1. [Google Scholar]
- Lazzeri, L.; Leoni, O.; Conte, L.S.; Palmieri, S. Some technological characteristics and potential uses of Crambe abyssinica products. Ind. Crop. Prod. 1994, 3, 103–112. [Google Scholar] [CrossRef]
- Kissen, R.; Øverby, A.; Winge, P.; Bones, A.M. Allyl-isothiocyanate treatment induces a complex transcriptional reprogramming including heat stress, oxidative stress and plant defence responses in Arabidopsis thaliana. BMC Genom. 2016, 17, 740. [Google Scholar] [CrossRef]
- Ugolini, L.; Pagnotta, E.; Matteo, R.; Malaguti, L.; Di Francesco, A.; Lazzeri, L. Brassica meal-derived allyl-isothiocyanate postharvest application: Influence on strawberry nutraceutical and biochemical parameters. J. Sci. Food Agric. 2019, 99, 4235–4241. [Google Scholar] [CrossRef]
- Leoni, O.; Bernardi, R.; Gueyrard, D.; Rollin, P.; Palmieri, S. Chemo-enzymatic preparation from renewable resources of enantiopure 1,3-oxazolidine-2-thiones. Tetrahedron Asymmetry 1999, 10, 4775–4780. [Google Scholar] [CrossRef]
- Daubos, P.; Grumel, V.; Iori, R.; Leoni, O.; Palmieri, S.; Rollin, P. Crambe abyssinica meal as starting material for the production of enantiomerically pure fine chemicals. Ind. Crop. Prod. 1998, 7, 187–193. [Google Scholar] [CrossRef]
- Galletti, S.; Bernardi, R.; Leoni, O.; Rollin, P.; Palmieri, S. Preparation and Biological Activity of Four Epiprogoitrin Myrosinase-Derived Products. J. Agric. Food Chem. 2001, 49, 471–476. [Google Scholar] [CrossRef]
- Katz, E.; Bagchi, R.; Jeschke, V.; Rasmussen, A.R.M.; Hopper, A.; Burow, M.; Estelle, M.; Kliebenstein, D.J. Diverse Allyl Glucosinolate Catabolites Independently Influence Root Growth and Development. Plant Physiol. 2020, 183, 1376–1390. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.; Nisani, S.; Yadav, B.S.; Woldemariam, M.G.; Shai, B.; Obolski, U.; Ehrlich, M.; Shani, E.; Jander, G.; Chamovitz, D.A. The glucosinolate breakdown product indole-3-carbinol acts as an auxin antagonist in roots of A rabidopsis thaliana. Plant J. 2015, 82, 547–555. [Google Scholar] [CrossRef]
- Lazzeri, L.; Malaguti, L.; Bagatta, M.; D’Avino, L.; Ugolini, L.; De Nicola, G.R.; Casadei, N.; Cinti, S.; Matteo, R.; Iori, R. Characterization of the main glucosinolate content and fatty acid composition in non-food Brassicaceae seeds. Acta Hortic. 2013, 1005, 331–338. [Google Scholar] [CrossRef]
- Ugolini, L.; Cinti, S.; Righetti, L.; Stefan, A.; Matteo, R.; D’Avino, L.; Lazzeri, L. Production of an enzymatic protein hydrolyzate from defatted sunflower seed meal for potential application as a plant biostimulant. Ind. Crop. Prod. 2015, 75, 15–23. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Enzymic Hydrolysis of Food Proteins; Elsevier Applied Science Publishers: London, UK, 1986; ISBN 0-85334-386-1. [Google Scholar]
- Adler-Nissen, J. Determination of the Degree of Hydrolysis of Food Protein Hydrolysates by Trinitrobenzenesulfonic Acid. J. Agric. Food Chem. 1979, 27, 1256–1262. [Google Scholar] [CrossRef]
- Beleggia, R.; Fragasso, M.; Miglietta, F.; Cattivelli, L.; Menga, V.; Nigro, F.; Pecchioni, N.; Fares, C. Mineral composition of durum wheat grain and pasta under increasing atmospheric CO2 concentrations. Food Chem. 2018, 242, 53–61. [Google Scholar] [CrossRef]
- Henderson, J.W.; Brooks, A. Improved Amino Acid Methods Using Agilent ZORBAX Eclipse Plus C18 Columns for a Variety of Agilent LC Instrumentation and Separation Goals; Application Note 5990-4547EN; Agilent Technologies, Inc.: Wilmington, DE, USA, 2010. [Google Scholar]
- ISO 9167-1:1992/Amd 1:2013; Graines de Colza—Dosage des Glucosinolates—Partie 1: Methode Par Chromatographie Liquide à Haute Performance. ISO: Geneva, Switzerland, 1992.
- Pagnotta, E.; Agerbirk, N.; Olsen, C.E.; Ugolini, L.; Cinti, S.; Lazzeri, L. Hydroxyl and Methoxyl Derivatives of Benzylglucosinolate in Lepidium densiflorum with Hydrolysis to Isothiocyanates and non-Isothiocyanate Products: Substitution Governs Product Type and Mass Spectral Fragmentation. J. Agric. Food Chem. 2017, 65, 3167–3178. [Google Scholar] [CrossRef]
- Whatelet, J.-P.; Iori, R.; Leoni, O.; Quinsac, A.; Palmieri, S. Guidelines for glucosinolate analysis in green tissues used for biofumigation. Agroindustria 2004, 3, 257–266. [Google Scholar]
- Citi, V.; Piragine, E.; Pagnotta, E.; Ugolini, L.; Di Cesare Mannelli, L.; Testai, L.; Ghelardini, C.; Lazzeri, L.; Calderone, V.; Martelli, A. Anticancer properties of erucin, an H2 S-releasing isothiocyanate, on human pancreatic adenocarcinoma cells (AsPC-1). Phyther. Res. 2019, 33, 845–855. [Google Scholar] [CrossRef]
- Leoni, O.; Cinti, S.; Aliano, N.; Tittonel, E.D. A rapid chromatographic method for determining the glucosinolate content in crambe seed. Plant Breed. 2003, 122, 517–520. [Google Scholar] [CrossRef]
- Bobo-García, G.; Davidov-Pardo, G.; Arroqui, C.; Vírseda, P.; Marín-Arroyo, M.R.; Navarro, M. Intra-laboratory validation of microplate methods for total phenolic content and antioxidant activity on polyphenolic extracts, and comparison with conventional spectrophotometric methods. J. Sci. Food Agric. 2015, 95, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.H.S.; Lyons, G.; McRoberts, C.; McCall, D.; Carmichael, E.; Andrews, F.; Swan, R.; McCormack, R.; Mellon, R. Biostimulant activity of brown seaweed species from Strangford Lough: Compositional analyses of polysaccharides and bioassay of extracts using mung bean (Vigno mungo L.) and pak choi (Brassica rapa chinensis L.). J. Appl. Phycol. 2012, 24, 1081–1091. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghello, D.; Altissimo, A.; Nardi, S. Use of meat hydrolyzate derived from tanning residues as plant biostimulant for hydroponically grown maize. J. Plant Nutr. Soil Sci. 2013, 176, 287–295. [Google Scholar] [CrossRef]
- Seethepalli, A.; Dhakal, K.; Griffiths, M.; Guo, H.; Freschet, G.T.; York, L.M. RhizoVision Explorer: Open-source software for root image analysis and measurement standardization. AoB Plants 2021, 13, plab056. [Google Scholar] [CrossRef]
- Parrado, J.; Bautista, J.; Romero, E.J.; García-Martínez, A.M.; Friaza, V.; Tejada, M. Production of a carob enzymatic extract: Potential use as a biofertilizer. Bioresour. Technol. 2008, 99, 2312–2318. [Google Scholar] [CrossRef]
- Villanueva, A.; Vioque, J.; Sánchez-Vioque, R.; Clemente, A.; Bautista, J.; Millán, F. Production of an extensive sunflower protein hydrolysate by sequential hydrolysis with endo- and exo-proteases. Grasas Y Aceites 1999, 50, 472–476. [Google Scholar] [CrossRef]
- Parrado, J.; Bautista, J.; Machado, A. Production of soluble enzymic protein hydrolyzate from industrially defatted nondehulled sunflower meal. J. Agric. Food Chem. 1991, 39, 447–450. [Google Scholar] [CrossRef]
- Parchem, K.; Piekarska, A.; Bartoszek, A. Chapter 3—Enzymatic Activities behind Degradation of Glucosinolates. In Galanakis Recovery, and Applications; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 79–106. ISBN 978-0-12-816493-8. [Google Scholar] [CrossRef]
- Oerlemans, K.; Barrett, D.M.; Suades, C.B.; Verkerk, R.; Dekker, M. Thermal degradation of glucosinolates in red cabbage. Food Chem. 2006, 95, 19–29. [Google Scholar] [CrossRef]
- Moreno-Hernández, J.M.; Benítez-García, I.; Mazorra-Manzano, M.A.; Ramírez-Suárez, J.C.; Sánchez, E. Strategies for production, characterization and application of protein-based biostimulants in agriculture: A review. Chil. J. Agric. Res. 2020, 80, 274–289. [Google Scholar] [CrossRef]
- Kamnerdpetch, C.; Weiss, M.; Kasper, C.; Scheper, T. An improvement of potato pulp protein hydrolyzation process by the combination of protease enzyme systems. Enzyme Microb. Technol. 2007, 40, 508–514. [Google Scholar] [CrossRef]
- Khan, M.U.; Tolstorebrov, I.; Widell, K.N.; Hafner, A.; Nordtvedt, T.S. Modelling of Crystallization During Freeze-Concentration of Hydrolysates. In Proceedings of the IIR International Conference on Sustainability and the Cold Chain Online, Online, 11–13 April 2022. [Google Scholar] [CrossRef]
- Petrova, I.; Tolstorebrov, I.; Eikevik, T.M. Production of fish protein hydrolysates step by step: Technological aspects, equipment used, major energy costs and methods of their minimizing. Int. Aquat. Res. 2018, 10, 223–241. [Google Scholar] [CrossRef]
- Vioque, J.; Sánchez-Vioque, R.; Clemente, A.; Pedroche, J.; Bautista, J.; Millan, F. Production and characterization of an extensive rapeseed protein hydrolysate. J. Am. Oil Chem. Soc. 1999, 76, 819–823. [Google Scholar] [CrossRef]
- Mokni Ghribi, A.; Maklouf Gafsi, I.; Sila, A.; Blecker, C.; Danthine, S.; Attia, H.; Bougatef, A.; Besbes, S. Effects of enzymatic hydrolysis on conformational and functional properties of chickpea protein isolate. Food Chem. 2015, 187, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Ravelo-Ortega, G.; López-Bucio, J.S.; Ruiz-Herrera, L.F.; Pelagio-Flores, R.; Ayala-Rodríguez, J.Á.; de la Cruz, H.R.; Guevara-García, A.; López-Bucio, J. The growth of Arabidopsis primary root is repressed by several and diverse amino acids through auxin-dependent and independent mechanisms and MPK6 kinase activity. Plant Sci. 2021, 302, 110717. [Google Scholar] [CrossRef]
- Shukry, W.M.; Haroun, S.A. Asparagine and Glutamine affect the Growth and Cause Metabolic Changes in Phaseolus vulgaris in Vivo. Middle East. Russ. J. Plant Sci. Biotechnol. 2008, 2, 9–28. [Google Scholar]
- Wilson, H.T.; Amirkhani, M.; Taylor, A.G. Evaluation of gelatin as a biostimulant seed treatment to improve plant performance. Front. Plant Sci. 2018, 9, 1006. [Google Scholar] [CrossRef]
- El Moukhtari, A.; Cabassa-Hourton, C.; Farissi, M.; Savouré, A. How Does Proline Treatment Promote Salt Stress Tolerance During Crop Plant Development? Front. Plant Sci. 2020, 11, 1127. [Google Scholar] [CrossRef]
- Santi, C.; Zamboni, A.; Varanini, Z.; Pandolfini, T. Growth stimulatory effects and genome-wide transcriptional changes produced by protein hydrolysates in maize seedlings. Front. Plant Sci. 2017, 8, 433. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, S.; Mohapatra, T. Interaction Between Macro- and Micro-Nutrients in Plants. Front. Plant Sci. 2021, 12, 665583. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, R.; Finiguerra, M.G.; Rossi, A.A.; Palmieri, S. Isolation and biochemical characterization of a basic myrosinase from ripe Crambe abyssinica seeds, highly specific for epi-progoitrin. J. Agric. Food Chem. 2003, 51, 2737–2744. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Gemma, H.; Sobajima, Y.; Masago, H. Rooting Cofactor Activity of Plant Phytoalexins. Plant Physiol. 1986, 82, 864–866. [Google Scholar] [CrossRef]
- Sharma, H.S.S.; Selby, C.; Carmichael, E.; McRoberts, C.; Rao, J.R.; Ambrosino, P.; Chiurazzi, M.; Pucci, M.; Martin, T. Physicochemical analyses of plant biostimulant formulations and characterisation of commercial products by instrumental techniques. Chem. Biol. Technol. Agric. 2016, 3, 13. [Google Scholar] [CrossRef]
- Briceño-Domínguez, D.; Hernández-Carmona, G.; Moyo, M.; Stirk, W.; van Staden, J. Plant growth promoting activity of seaweed liquid extracts produced from Macrocystis pyrifera under different pH and temperature conditions. J. Appl. Phycol. 2014, 26, 2203–2210. [Google Scholar] [CrossRef]
- Bandurski, R.S.; Schulze, A. Concentration of Indole-3-acetic Acid and Its Derivatives in Plants. Plant Physiol. 1977, 60, 211–213. [Google Scholar] [CrossRef]
- Sanada, A.; Agehara, S. Characterizing Root Morphological Responses to Exogenous Tryptophan in Soybean (Glycine max) Seedlings Using a Scanner-Based Rhizotron System. Plants 2023, 12, 186. [Google Scholar] [CrossRef]
- Maki, Y.; Soejima, H.; Sugiyama, T.; Sato, T.; Yamaguchi, J.; Watahiki, M.K. Conjugates of 3-phenyllactic acid and tryptophan enhance root-promoting activity without adverse effects in Vigna angularis. Plant Biotechnol. 2022, 39, 173–177. [Google Scholar] [CrossRef]
- Pituello, C.; Ambrosini, S.; Varanini, Z.; Pandolfini, T.; Zamboni, A.; Povolo, C.; Agnolon, F.; Franco, E.; Candido, M.C.; Neresini, M. Animal-Derived Hydrolyzed Protein and Its Biostimulant Effects BT—Biostimulants: Exploring Sources and Applications; Ramawat, N., Bhardwaj, V., Eds.; Springer Nature Singapore: Singapore, 2022; pp. 107–140. ISBN 978-981-16-7080-0. [Google Scholar]
- Ceccarelli, A.V.; Miras-Moreno, B.; Buffagni, V.; Senizza, B.; Pii, Y.; Cardarelli, M.; Rouphael, Y.; Colla, G.; Lucini, L. Foliar Application of Different Vegetal-Derived Protein Hydrolysates Distinctively Modulates Tomato Root Development and Metabolism. Plants 2021, 10, 326. [Google Scholar] [CrossRef]
- Pinzón-Sandoval, E.H.; Balaguera-López, H.E.; Almanza-Merchán, P.J. Evaluation of SPAD Index for Estimating Nitrogen and Magnesium Contents in Three Blueberry Varieties (Vaccinium corymbosum L.) on the Andean Tropics. Horticulturae 2023, 9, 269. [Google Scholar] [CrossRef]
- Ertani, A.; Cavani, L.; Pizzeghello, D.; Brandellero, E.; Altissimo, A.; Ciavatta, C.; Nardi, S. Biostimulant activity of two protein hydrolyzates in the growth and nitrogen metabolism of maize seedlings. J. Plant Nutr. Soil Sci. 2009, 172, 237–244. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Altissimo, A.; Franceschi, C.; Nardi, S. Phenol-containing organic substances stimulate phenylpropanoid metabolism in Zea mays. J. Plant Nutr. Soil Sci. 2011, 174, 496–503. [Google Scholar] [CrossRef]
- Paul, K.; Sorrentino, M.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Reynaud, H.; Canaguier, R.; Trtílek, M.; Panzarová, K.; et al. Understanding the biostimulant action of vegetal-derived protein hydrolysates by high-throughput plant phenotyping and metabolomics: A case study on tomato. Front. Plant Sci. 2019, 10, 47. [Google Scholar] [CrossRef]
- Sorrentino, M.; Panzarová, K.; Spyroglou, I.; Spíchal, L.; Buffagni, V.; Ganugi, P.; Rouphael, Y.; Colla, G.; Lucini, L.; De Diego, N. Integration of Phenomics and Metabolomics Datasets Reveals Different Mode of Action of Biostimulants Based on Protein Hydrolysates in Lactuca sativa L. and Solanum lycopersicum L. Under Salinity. Front. Plant Sci. 2022, 12, 808711. [Google Scholar] [CrossRef]
- Sorrentino, M.; De Diego, N.; Ugena, L.; Spíchal, L.; Lucini, L.; Miras-Moreno, B.; Zhang, L.; Rouphael, Y.; Colla, G.; Panzarová, K. Seed Priming With Protein Hydrolysates Improves Arabidopsis Growth and Stress Tolerance to Abiotic Stresses. Front. Plant Sci. 2021, 12, 626301. [Google Scholar] [CrossRef]
- Ambrosini, S.; Prinsi, B.; Zamboni, A.; Espen, L.; Zanzoni, S.; Santi, C.; Varanini, Z.; Pandolfini, T. Chemical Characterization of a Collagen-Derived Protein Hydrolysate and Biostimulant Activity Assessment of Its Peptidic Components. J. Agric. Food Chem. 2022, 70, 11201–11211. [Google Scholar] [CrossRef]
- Walch-Liu, P.; Ivanov, I.I.; Filleur, S.; Gan, Y.; Remans, T.; Forde, B.G. Nitrogen regulation of root branching. Ann. Bot. 2006, 97, 875–881. [Google Scholar] [CrossRef]
- Sutherland, W.J.; Stillman, R.A. The Foraging Tactics of Plants. Oikos 1988, 52, 239–244. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).