Increasing Legume Input through Interseeding Cover Crops: Soil and Crop Response as Affected by Tillage System
Abstract
1. Introduction
2. Materials and Methods
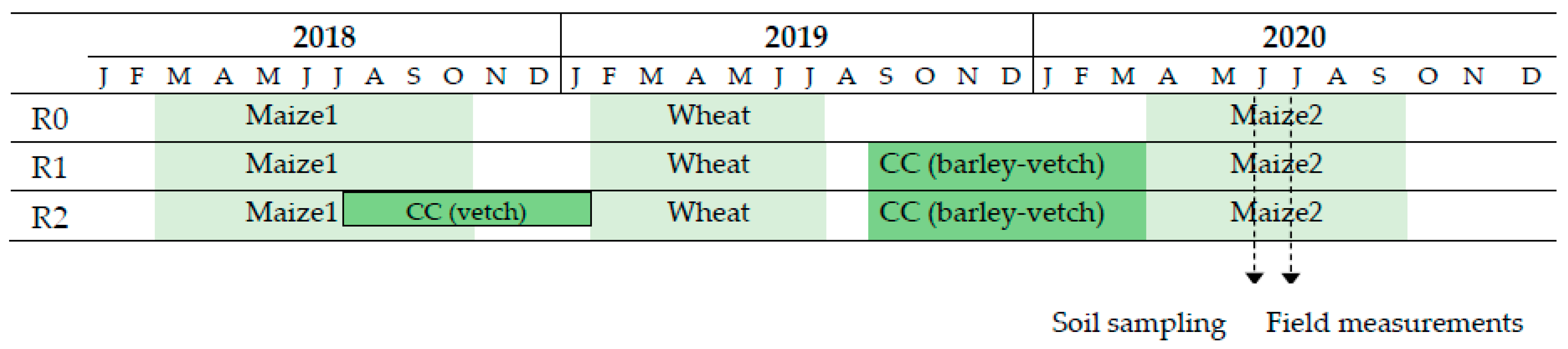
2.1. Study Area and Experimental Design
2.2. Experiment Management
2.3. Soil and Plant Sampling and Analyses
2.4. Data Analysis
3. Results
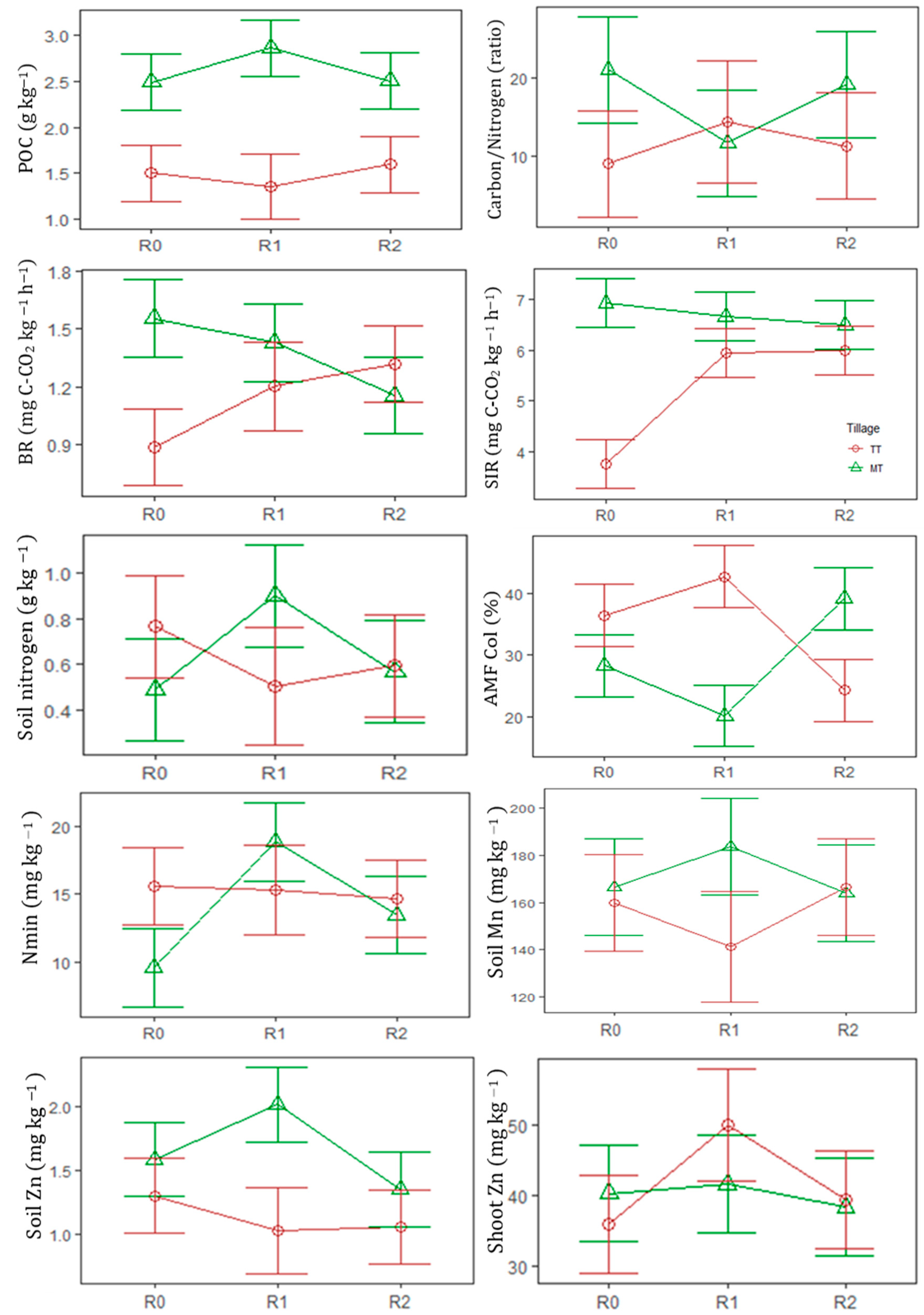
3.1. Soil and Crop Variables as Affected by Legume Input Level, Tillage System and Its Interaction
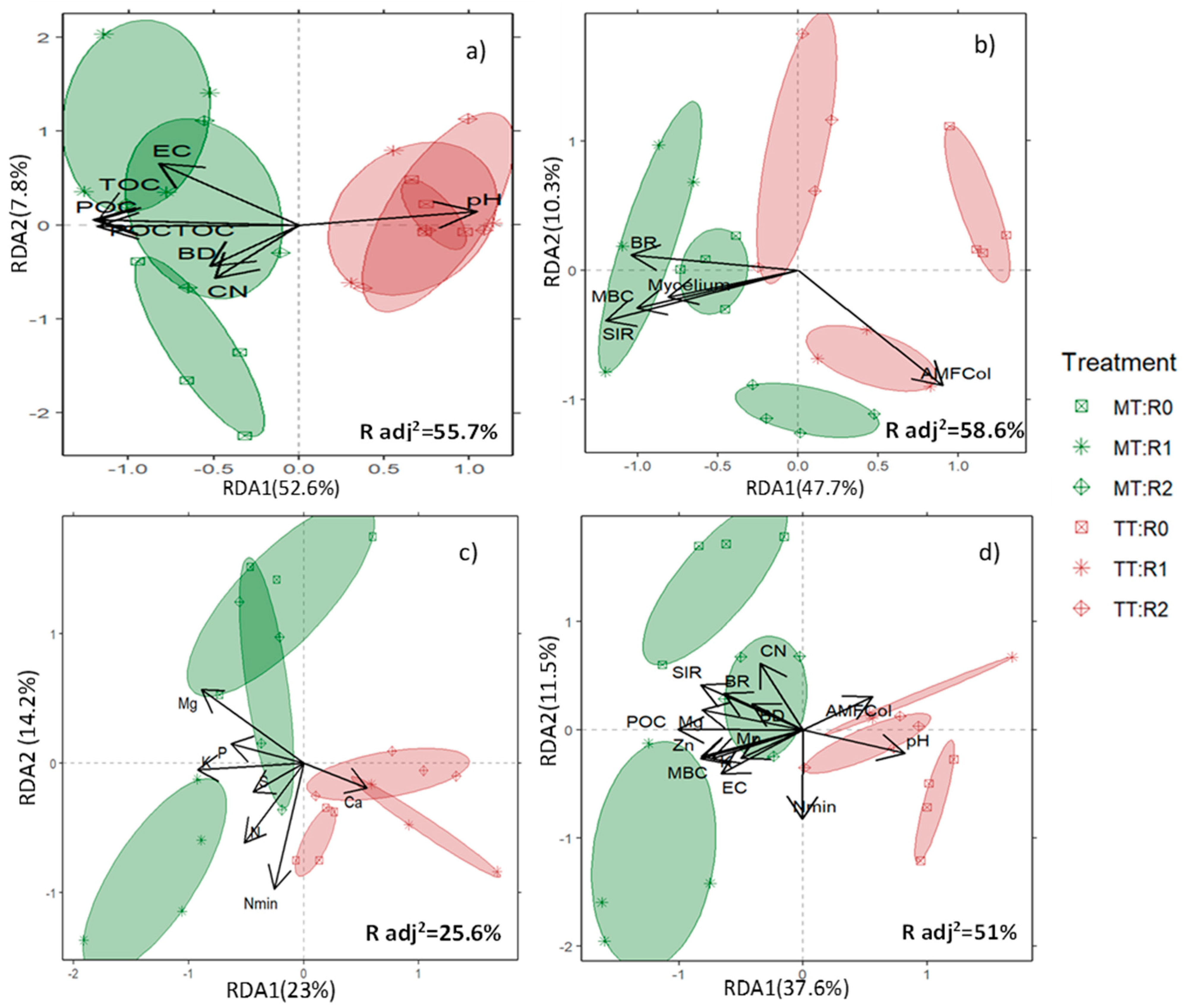
3.2. Soil Response to Treatments by Type of Variables—A Comprehensive Soil Response
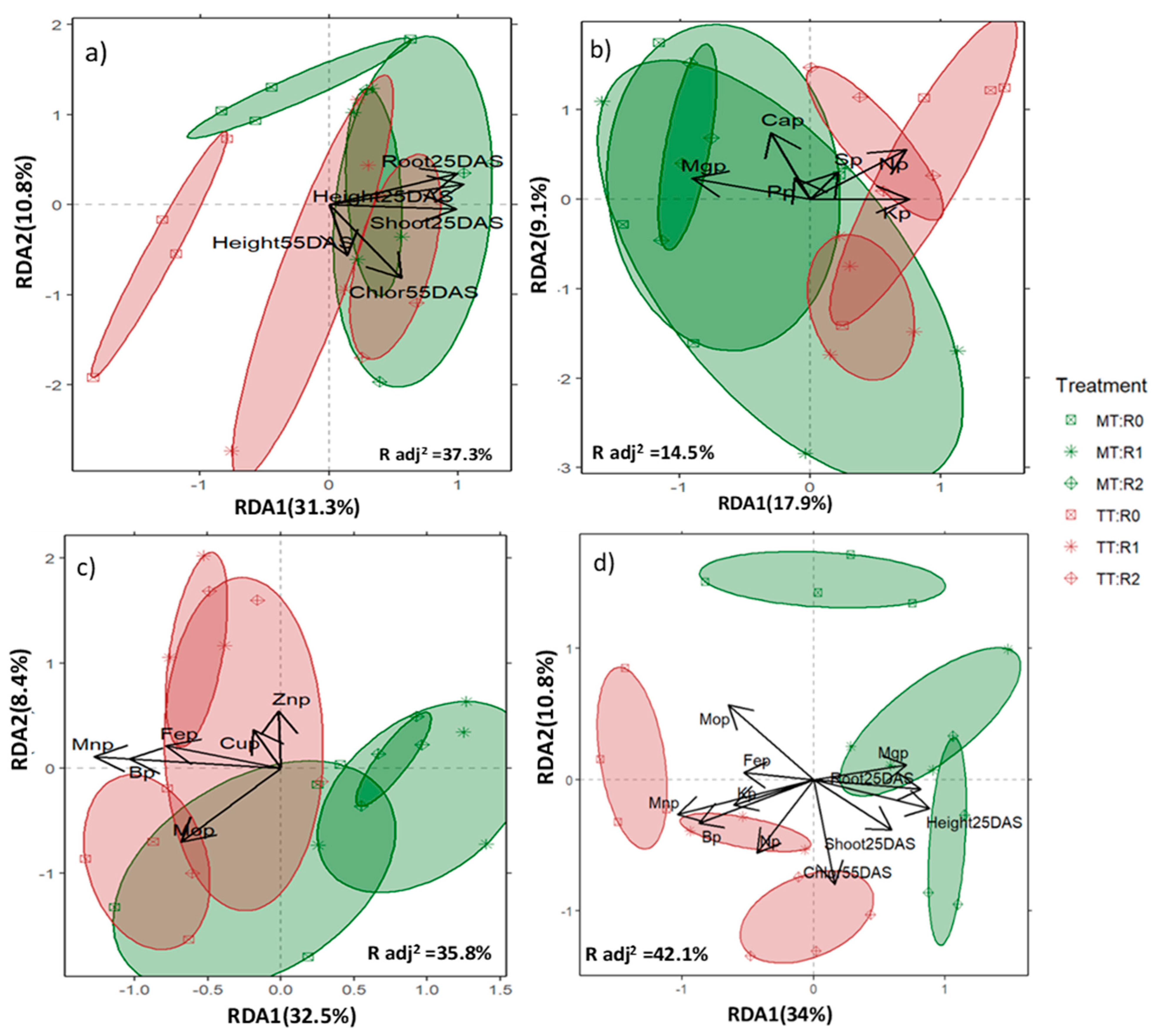
3.3. Crop Response to Treatments by Type of Variables—A Comprehensive Crop Response
4. Discussion
4.1. Soil Response to Treatments
4.2. Crop Response to Treatments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kopittke, P.M.; Menzies, N.W.; Wang, P.; McKenna, B.A.; Lombi, E. Soil and the intensification of agriculture for global food security. Environ. Int. 2019, 132, 105078. [Google Scholar] [CrossRef] [PubMed]
- Isbell, F.; Adler, P.R.; Eisenhauer, N.; Fornara, D.; Kimmel, K.; Kremen, C.; Letourneau, D.K.; Liebman, M.; Polley, H.W.; Quijas, S. Benefits of increasing plant diversity in sustainable agroecosystems. J. Ecol. 2017, 105, 871–879. [Google Scholar] [CrossRef]
- Schipanski, M.E.; Barbercheck, M.; Douglas, M.R.; Finney, D.M.; Haider, K.; Kaye, J.P.; Kemanian, A.R.; Mortensen, D.A.; Ryan, M.R.; Tooker, J.; et al. A framework for evaluating ecosystem services provided by cover crops in agroecosystems. Agric. Syst. 2014, 125, 12–22. [Google Scholar] [CrossRef]
- Kaye, J.P.; Quemada, M. Using cover crops to mitigate and adapt to climate change. A review. Agron. Sustain. Dev. 2017, 37, 4. [Google Scholar] [CrossRef]
- Stagnari, F.; Maggio, A.; Galieni, A.; Pisante, M. Multiple benefits of legumes for agriculture sustainability: An overview. Chem. Biol. Technol. Agric. 2017, 4, 2. [Google Scholar] [CrossRef]
- Coombs, C.; Lauzon, J.D.; Deen, B.; Van Eerd, L.L. Legume cover crop management on nitrogen dynamics and yield in grain corn systems. Field Crops Res. 2017, 201, 75–85. [Google Scholar] [CrossRef]
- Li, F.; Sørensen, P.; Li, X.; Olesen, J.E. Carbon and nitrogen mineralization differ between incorporated shoots and roots of legume versus non-legume based cover crops. Plant Soil 2020, 446, 243–257. [Google Scholar] [CrossRef]
- Bowles, T.M.; Jackson, L.E.; Loeher, M.; Cavagnaro, T.R. Ecological intensification and arbuscular mycorrhizas: A meta-analysis of tillage and cover crop effects. J. Appl. Ecol. 2017, 54, 1785–1793. [Google Scholar] [CrossRef]
- Uchino, H.; Iwama, K.; Jitsuyama, Y.; Ichiyama, K.; Sugiura, E.; Yudate, T.; Nakamura, S.; Gopal, J. Effect of interseeding cover crops and fertilization on weed suppression under an organic and rotational cropping system: 1. Stability of weed suppression over years and main crops of potato, maize and soybean. Field Crops Res. 2012, 127, 9–16. [Google Scholar] [CrossRef]
- Noland, R.L.; Wells, M.S.; Sheaffer, C.C.; Baker, J.M.; Martinson, K.L.; Coulter, J.A. Establishment and function of cover crops interseeded into corn. Crop Sci. 2018, 58, 863–873. [Google Scholar] [CrossRef]
- Curran, W.S.; Hoover, R.J.; Mirsky, S.B.; Roth, G.W.; Ryan, M.R.; Ackroyd, V.J.; Wallace, J.M.; Dempsey, M.A.; Pelzer, C.J. Evaluation of cover crops drill interseeded into corn across the Mid-Atlantic region. Agron. J. 2018, 110, 435–443. [Google Scholar] [CrossRef]
- Bybee-Finley, K.A.; Ryan, M.R. Advancing intercropping research and practices in industrialized agricultural landscapes. Agriculture 2018, 8, 80. [Google Scholar] [CrossRef]
- Alonso-Ayuso, M.; Gabriel, J.L.; Pancorbo, J.L.; Quemada, M. Interseeding cover crops into maize: Characterization of species performance under Mediterranean conditions. Field Crops Res. 2020, 249, 107762. [Google Scholar] [CrossRef]
- García-González, I.; Hontoria, C.; Gabriel, J.L.; Alonso-Ayuso, M.; Quemada, M. Cover crops to mitigate soil degradation and enhance soil functionality in irrigated land. Geoderma 2018, 322, 81–88. [Google Scholar] [CrossRef]
- Jian, J.; Lester, B.J.; Du, X.; Reiter, M.S.; Stewart, R.D. A calculator to quantify cover crop effects on soil health and productivity. Soil Tillage Res. 2020, 199, 104575. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Jasa, P.J. Do grass and legume cover crops improve soil properties in the long term? Soil Sci. Soc. Am. J. 2019, 83, 1181–1187. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Shaver, T.M.; Lindquist, J.L.; Shapiro, C.A.; Elmore, R.W.; Francis, C.A.; Hergert, G.W. Cover crops and ecosystem services: Insights from studies in temperate soils. Agron. J. 2015, 107, 2449–2474. [Google Scholar] [CrossRef]
- White, C.M.; DuPont, S.T.; Hautau, M.; Hartman, D.; Finney, D.M.; Bradley, B.; LaChance, J.C.; Kaye, J.P. Managing the trade off between nitrogen supply and retention with cover crop mixtures. Agric. Ecosyst. Environ. 2017, 237, 121–133. [Google Scholar] [CrossRef]
- Abdalla, M.; Hastings, A.; Cheng, K.; Yue, Q.; Chadwick, D.; Espenberg, M.; Truu, J.; Rees, R.M.; Smith, P. A critical review of the impacts of cover crops on nitrogen leaching, net greenhouse gas balance and crop productivity. Glob. Change Biol. 2019, 25, 2530–2543. [Google Scholar] [CrossRef]
- Kramberger, B.; Gselman, A.; Kristl, J.; Lešnik, M.; Šuštar, V.; Muršec, M.; Podvršnik, M. Winter cover crop: The effects of grass–clover mixture proportion and biomass management on maize and the apparent residual N in the soil. Eur. J. Agron. 2014, 55, 63–71. [Google Scholar] [CrossRef]
- Daryanto, S.; Fu, B.; Wang, L.; Jacinthe, P.; Zhao, W. Quantitative synthesis on the ecosystem services of cover crops. Earth-Sci. Rev. 2018, 185, 357–373. [Google Scholar] [CrossRef]
- Kim, N.; Zabaloy, M.C.; Guan, K.; Villamil, M.B. Do cover crops benefit soil microbiome? A meta-analysis of current research. Soil Biol. Biochem. 2020, 142, 107701. [Google Scholar] [CrossRef]
- Farmaha, B.S.; Sekaran, U.; Franzluebbers, A.J. Cover cropping and conservation tillage improve soil health in the southeastern United States. Agron. J. 2022, 114, 296–316. [Google Scholar] [CrossRef]
- Mbuthia, L.W.; Acosta-Martínez, V.; DeBruyn, J.; Schaeffer, S.; Tyler, D.; Odoi, E.; Mpheshea, M.; Walker, F.; Eash, N. Long term tillage, cover crop, and fertilization effects on microbial community structure, activity: Implications for soil quality. Soil Biol. Biochem. 2015, 89, 24–34. [Google Scholar] [CrossRef]
- Büchi, L.; Wendling, M.; Amossé, C.; Necpalova, M.; Charles, R. Importance of cover crops in alleviating negative effects of reduced soil tillage and promoting soil fertility in a winter wheat cropping system. Agric. Ecosyst. Environ. 2018, 256, 92–104. [Google Scholar] [CrossRef]
- Nunes, M.R.; van Es, H.M.; Schindelbeck, R.; Ristow, A.J.; Ryan, M. No-till and cropping system diversification improve soil health and crop yield. Geoderma 2018, 328, 30–43. [Google Scholar] [CrossRef]
- Adetunji, A.T.; Ncube, B.; Mulidzi, R.; Lewu, F.B. Management impact and benefit of cover crops on soil quality: A review. Soil Tillage Res. 2020, 204, 104717. [Google Scholar] [CrossRef]
- Mirsky, S.B.; Curran, W.S.; Mortensen, D.A.; Ryan, M.R.; Shumway, D.L. Control of cereal rye with a roller/crimper as influenced by cover crop phenology. Agron. J. 2009, 101, 1589–1596. [Google Scholar] [CrossRef]
- Alonso-Ayuso, M.; Gabriel, J.L.; Hontoria, C.; Ibáñez, M.Á.; Quemada, M. The cover crop termination choice to designing sustainable cropping systems. Eur. J. Agron. 2020, 114, 126000. [Google Scholar] [CrossRef]
- Centurión, N.; Ulcuango, K.; Navas, M.; Mariscal-Sancho, I.; Ibáñez, M.A.; Moliner, A.; Hontoria, C. Soil Microbial Response to Cover Crop Termination Methods under Two Water Levels. Agronomy 2022, 12, 3002. [Google Scholar] [CrossRef]
- García-González, I.; Quemada, M.; Gabriel, J.L.; Alonso-Ayuso, M.; Hontoria, C. Legacy of eight-year cover cropping on mycorrhizae, soil, and plants. J. Plant Nutr. Soil Sci. 2018, 181, 818–826. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Part 3 Chemical Methods; SSSA Book Series No. 5, SSSA and ASA; John Wiley: Hoboken, NJ, USA; Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar] [CrossRef]
- Bremner, J.M. Nitrogen-total. In Methods of Soil Analysis: Part 3 Chemical Methods; SSSA Book Series No. 5, SSSA and ASA; John Wiley: Madison, WI, USA, 1996; pp. 1085–1121. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Elliott, E.T. Particulate soil organic-matter changes across a grassland cultivation sequence. Soil Sci. Soc. Am. J. 1992, 56, 777–783. [Google Scholar] [CrossRef]
- Keeney, D.R.; Nelson, D.W. Nitrogen—Inorganic forms. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; Agronomy Monograph 9; Wiley: Madison, WI, USA, 1983; pp. 643–698. [Google Scholar] [CrossRef]
- Solorzano, L. Determination of ammonia in natural waters by the phenolhypochlorite method 1 1. This research was fully supported by US Atomic Energy Commission. Contract No. ATS (11-1) GEN 10, PA20. Limnol. Oceanogr. 1969, 14, 799–801. [Google Scholar] [CrossRef]
- Kemper, W.D.; Rosenau, R.C. Aggregate stability and size distribution. In Methods of Soil Analysis: Part 1 Physical and Mineralogical Methods; Agronomy Monograph 9; Wiley: Madison, WI, USA, 1986; pp. 425–442. [Google Scholar] [CrossRef]
- Alef, K.; Nannipieri, P. Methods in Applied Soil Microbiology and Biochemistry; Academic Press: London, UK, 1995. [Google Scholar]
- Anderson, J.; Domsch, K.H. Mineralization of bacteria and fungi in chloroform-fumigated soils. Soil Biol. Biochem. 1978, 10, 207–213. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Yakovchenko, V.P.; Sikora, L.J. Modified dichromate method for determining low concentrations of extractable organic carbon in soil. Commun. Soil Sci. Plant Anal. 1998, 29, 421–433. [Google Scholar] [CrossRef]
- García-González, I.; Quemada, M.; Gabriel, J.L.; Hontoria, C. Arbuscular mycorrhizal fungal activity responses to winter cover crops in a sunflower and maize cropping system. Appl. Soil Ecol. 2016, 102, 10–18. [Google Scholar] [CrossRef]
- Vierheilig, H.; Coughlan, A.P.; Wyss, U.; Piché, Y. Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl. Environ. Microbiol. 1998, 64, 5004–5007. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular—Arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Grossman, R.B.; Reinsch, T.G. Bulk density and linear extensibility. In Methods of Soil Analysis: Part 4 Physical Methods; SSSA Book Series No. 5; SSSA, Inc.: Madison, WI, USA, 2002; pp. 201–228. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2022; Available online: http://www.rstudio.com/ (accessed on 16 January 2023).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P. Vegan: Community Ecology Package, R package version 2.5; Viena, Austria, 2020. Available online: https://www.researchgate.net/publication/346579465_vegan_community_ecology_package_version_25-7_November_2020 (accessed on 16 January 2023).
- Wickham, H. Data Analysis, in Anonymous Ggplot2; Springer: Berlin/Heidelberg, Germany, 2016; pp. 189–201. [Google Scholar]
- Álvaro-Fuentes, J.; López, M.V.; Cantero-Martínez, C.; Arrúe, J.L. Tillage effects on soil organic carbon fractions in Mediterranean dryland agroecosystems. Soil Sci. Soc. Am. J. 2008, 72, 541–547. [Google Scholar] [CrossRef]
- Bienes, R.; Marques, M.J.; Sastre, B.; García-Díaz, A.; Esparza, I.; Antón, O.; Navarrete, L.; Hernánz, J.L.; Sánchez-Girón, V.; Sánchez del Arco María, J. Tracking changes on soil structure and organic carbon sequestration after 30 years of different tillage and management practices. Agronomy 2021, 11, 291. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A. Carbon sequestration in agricultural soils via cultivation of cover crops–A meta-analysis. Agric. Ecosyst. Environ. 2015, 200, 33–41. [Google Scholar] [CrossRef]
- Wooliver, R.; Jagadamma, S. Response of soil organic carbon fractions to cover cropping: A meta-analysis of agroecosystems. Agric. Ecosyst. Environ. 2023, 351, 108497. [Google Scholar] [CrossRef]
- Jilling, A.; Keiluweit, M.; Gutknecht, J.L.; Grandy, A.S. Priming mechanisms providing plants and microbes access to mineral-associated organic matter. Soil Biol. Biochem. 2021, 158, 108265. [Google Scholar] [CrossRef]
- Zhang, C.; Xue, W.; Xue, J.; Zhang, J.; Qiu, L.; Chen, X.; Hu, F.; Kardol, P.; Liu, M. Leveraging functional traits of cover crops to coordinate crop productivity and soil health. J. Appl. Ecol. 2022, 59, 2627–2641. [Google Scholar] [CrossRef]
- Conant, R.T.; Ogle, S.M.; Paul, E.A.; Paustian, K. Measuring and monitoring soil organic carbon stocks in agricultural lands for climate mitigation. Front. Ecol. Environ. 2011, 9, 169–173. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Change Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Clarholm, M.; Skyllberg, U.; Rosling, A. Organic acid induced release of nutrients from metal-stabilized soil organic matter–the unbutton model. Soil Biol. Biochem. 2015, 84, 168–176. [Google Scholar] [CrossRef]
- Navas, M.; Martín-Lammerding, D.; Hontoria, C.; Ulcuango, K.; Mariscal-Sancho, I. The distinct responses of bacteria and fungi in different-sized soil aggregates under different management practices. Eur. J. Soil Sci. 2021, 72, 1177–1189. [Google Scholar] [CrossRef]
- Peralta, N.R.; Costa, J.L.; Balzarini, M.; Franco, M.C.; Córdoba, M.; Bullock, D. Delineation of management zones to improve nitrogen management of wheat. Comput. Electron. Agric. 2015, 110, 103–113. [Google Scholar] [CrossRef]
- Veenstra, J.; Horwath, W.; Mitchell, J.; Munk, D. Conservation tillage and cover cropping influence soil properties in San Joaquin Valley cotton-tomato crop. Calif. Agric. 2006, 60, 146–153. [Google Scholar] [CrossRef]
- Soane, B.D.; Ball, B.C.; Arvidsson, J.; Basch, G.; Moreno, F.; Roger-Estrade, J. No-till in northern, western and south-western Europe: A review of problems and opportunities for crop production and the environment. Soil Tillage Res. 2012, 118, 66–87. [Google Scholar] [CrossRef]
- Gabriel, J.L.; García-González, I.; Quemada, M.; Martin-Lammerding, D.; Alonso-Ayuso, M.; Hontoria, C. Cover crops reduce soil resistance to penetration by preserving soil surface water content. Geoderma 2021, 386, 114911. [Google Scholar] [CrossRef]
- Muhammad, I.; Wang, J.; Sainju, U.M.; Zhang, S.; Zhao, F.; Khan, A. Cover cropping enhances soil microbial biomass and affects microbial community structure: A meta-analysis. Geoderma 2021, 381, 114696. [Google Scholar] [CrossRef]
- Marinari, S.; Mancinelli, R.; Brunetti, P.; Campiglia, E. Soil quality, microbial functions and tomato yield under cover crop mulching in the Mediterranean environment. Soil Tillage Res. 2015, 145, 20–28. [Google Scholar] [CrossRef]
- Chen, B.; Liu, E.; Tian, Q.; Yan, C.; Zhang, Y. Soil nitrogen dynamics and crop residues. A review. Agron. Sustain. Dev. 2014, 34, 429–442. [Google Scholar] [CrossRef]
- Liu, A.; Ku, Y.; Contador, C.A.; Lam, H. The impacts of domestication and agricultural practices on legume nutrient acquisition through symbiosis with rhizobia and arbuscular mycorrhizal fungi. Front. Genet. 2020, 11, 583954. [Google Scholar] [CrossRef]
- Murrell, E.G.; Ray, S.; Lemmon, M.E.; Luthe, D.S.; Kaye, J.P. Cover crop species affect mycorrhizae-mediated nutrient uptake and pest resistance in maize. Renew. Agric. Food Syst. 2020, 35, 467–474. [Google Scholar] [CrossRef]
- Turmel, M.; Speratti, A.; Baudron, F.; Verhulst, N.; Govaerts, B. Crop residue management and soil health: A systems analysis. Agric. Syst. 2015, 134, 6–16. [Google Scholar] [CrossRef]
- Hoeksema, J.D.; Chaudhary, V.B.; Gehring, C.A.; Johnson, N.C.; Karst, J.; Koide, R.T.; Pringle, A.; Zabinski, C.; Bever, J.D.; Moore, J.C. A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol. Lett. 2010, 13, 394–407. [Google Scholar] [CrossRef]
- Brito, I.; Goss, M.J.; De Carvalho, M. Effect of tillage and crop on arbuscular mycorrhiza colonization of winter wheat and triticale under Mediterranean conditions. Soil Use Manag. 2012, 28, 202–208. [Google Scholar] [CrossRef]
- Shackelford, G.E.; Kelsey, R.; Dicks, L.V. Effects of cover crops on multiple ecosystem services: Ten meta-analyses of data from arable farmland in California and the Mediterranean. Land Use Policy 2019, 88, 104204. [Google Scholar] [CrossRef]
- Grzyb, A.; Wolna-Maruwka, A.; Niewiadomska, A. Environmental factors affecting the mineralization of crop resi-dues. Agronomy 2020, 10, 1951. [Google Scholar] [CrossRef]
- Marcillo, G.S.; Miguez, F.E. Corn yield response to winter cover crops: An updated meta-analysis. J. Soil Water Conserv. 2017, 72, 226–239. [Google Scholar] [CrossRef]
- Abdollahi, L.; Munkholm, L.J. Tillage system and cover crop effects on soil quality: I. Chemical, mechanical, and biological properties. Soil Sci. Soc. Am. J. 2014, 78, 262–270. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. The Anthroposphere. In Trace Elements in Soils and Plants, 2nd ed.; CRC Press Inc.: Boca Raton, FL, USA, 1992. [Google Scholar]
- Grant, C.A.; Monreal, M.A.; Irvine, R.B.; Mohr, R.M.; McLaren, D.L.; Khakbazan, M. Preceding crop and phosphorus fertilization affect cadmium and zinc concentration of flaxseed under conventional and reduced tillage. Plant Soil 2010, 333, 337–350. [Google Scholar] [CrossRef]
| Soil Property | Unit | Rotation (F1) | Tillage (F2) | Factors | |||||
|---|---|---|---|---|---|---|---|---|---|
| R0 | R1 | R2 | TT | MT | F1 | F2 | F1 × F2 | ||
| Physicochemicals | |||||||||
| pH 1:2.5 | 8.24(0.03) a | 8.21(0.02) a | 8.26(0.03) a | 8.30(0.01) b | 8.17(0.01) a | ns | *** | ns | |
| EC 1:2.5 | µS cm−1 | 297(5.91) a | 331(11.9) b | 310(8.89) ab | 298(5.04) a | 327(8.55) b | ** | ** | ns |
| TOC | g kg−1 | 7.83(0.41) a | 8.14(0.68) a | 7.54(0.50) a | 6.61(0.17) a | 9.06(0.25) b | ns | *** | ns |
| POC | g kg−1 | 2.00(0.21) a | 2.11(0.32) a | 2.05(0.19) a | 1.48(0.04) a | 2.62(0.11) b | ns | *** | * |
| MAOC | g kg−1 | 5.31(0.15) a | 5.28(0.38) a | 5.14(0.18) a | 4.71(0.11) a | 5.74(0.14) b | ns | *** | ns |
| POC/TOC | % | 26.9(1.57) a | 27.6(1.93) a | 28.1(1.23) a | 23.8(0.44) a | 31.2(0.67) b | ns | *** | ns |
| C/N | 15.0(3.09) a | 13.0(1.86) a | 15.2(2.85) a | 11.5(1.20) a | 17.3(2.46) b | ns | * | + | |
| BD | g cm−3 | 1.44(0.03) b | 1.36(0.04) ab | 1.34(0.03) a | 1.33(0.02) a | 1.42(0.03) b | + | * | ns |
| WSA | % | 41.8(1.84) a | 44.6(3.65) a | 40.8(2.11) a | 40.8(1.77) a | 44.0(2.25) a | ns | ns | ns |
| Microbial | |||||||||
| Mycelium L | cm g−1 | 20.8(1.54) a | 23.7(3.15) a | 18.8(1.95) a | 17.6(1.21) a | 24.6(1.80) b | ns | ** | ns |
| AMF Col | % | 32.3(2.17) a | 31.7(4.61) a | 30.8(3.25) a | 34.0(2.59) b | 29.2(2.67) a | ns | + | *** |
| BR | mg C-CO2 kg−1h−1 | 1.22(0.14) a | 1.32(0.08) a | 1.24(0.04) a | 1.13(0.07) a | 1.38(0.07) b | ns | ** | *** |
| SIR | mg C-CO2 kg−1h−1 | 5.33(0.60) a | 6.24(0.21) b | 6.24(0.21) b | 5.19(0.34) a | 6.69(0.14) b | *** | *** | *** |
| MBC | mg kg−1 | 149(7.64) a | 178(17.5) b | 189(12.23) b | 148(7.71) a | 197(9.68) b | * | *** | ns |
| MBN | mg kg−1 | 26.0(1.78) a | 28.6(3.59) a | 27.3(2.03) a | 22.2(1.08) a | 32.4(1.52) b | ns | *** | + |
| Macronutrients | |||||||||
| N | g kg−1 | 0.62(0.07) a | 0.70(0.11) a | 0.58(0.07) a | 0.62(0.04) a | 0.65(0.08) a | ns | ns | * |
| Nmin | mg kg−1 | 12.6(1.19) a | 17.1(1.61) b | 14.1(0.74) b | 15.2(0.66) b | 14(1.37) a | ** | ** | *** |
| P | mg kg−1 | 32.1(1.82) a | 28.3(2.55) a | 30.1(2.30) a | 27.7(1.60) a | 32.6(1.73) b | ns | + | ns |
| K | mg kg−1 | 227(12.16) a | 246(23.1) a | 219(8.27) a | 208(6.66) a | 253(13.1) b | ns | ** | ns |
| Ca | g kg−1 | 2.34(0.10) a | 2.39(0.22) a | 2.29(0.09) a | 2.51(0.14) b | 2.17(0.05) a | ns | + | ns |
| Mg | mg kg−1 | 682(29.0) a | 648(30.7) a | 666(16.1) a | 613(13.5) a | 717(13.6) b | ns | *** | ns |
| S | mg kg−1 | 44.4(1.92) a | 45.7(1.35) a | 41.5(1.64) a | 42.9(1.56) a | 44.8(1.27) a | ns | ns | ns |
| Micronutrients | |||||||||
| Cu | mg kg−1 | 1.92(0.04) a | 1.99(0.14) a | 1.89(0.05) a | 1.90(0.06) a | 1.96(0.07) a | ns | ns | * |
| Fe | mg kg−1 | 42.2(1.13) a | 42.5(2.74) a | 42.4(1.30) a | 40.2(1.66) a | 44.5(0.77) b | ns | + | ns |
| Mn | mg kg−1 | 163(4.96) a | 162(13.0) a | 165(4.24) a | 156(7.51) a | 171(4.09) a | ns | ns | + |
| Zn | mg kg−1 | 1.45(0.10) ab | 1.53(0.23) b | 1.21(0.07) a | 1.13(0.05) a | 1.65(0.12) b | + | *** | * |
| Crop Variables | Unit | Rotation (F1) | Tillage (F2) | F1 | F2 | F1 × F2 | |||
|---|---|---|---|---|---|---|---|---|---|
| R0 | R1 | R2 | TT | MT | |||||
| Development variables | |||||||||
| Height 25DAS | cm | 37.4(1.52) a | 40.9(1.01) b | 44.1(1.12) a | 38.9(1.16) a | 42.7(1.18) b | ** | ** | ns |
| Shoot biomass 25DAS | g plant−1 | 3.32(0.32) a | 4.30(0.19) b | 4.51(0.20) b | 4.00(0.31) a | 4.09(0.19) a | ** | ns | * |
| Root biomass 25DAS | g plant−1 | 5.45(0.38) a | 6.78(0.31) b | 6.62(0.27) b | 5.88a(0.20) | 6.68(0.37) b | ** | * | ns |
| Height 55DAS | cm | 83.9(2.56) a | 87.0(5.24) a | 90.2(3.95) a | 89.5(2.69) a | 84.6(3.47) a | ns | ns | ns |
| Chlorophyl 55DAS | % | 42.4(0.90) a | 44.5(0.86) ab | 46.9(0.86) b | 45.2(0.68) a | 44.0(1.02) a | ** | ns | ns |
| Macronutrients | |||||||||
| N | g 100 g−1 | 4.20(0.17) a | 4.06(0.13) a | 4.35(0.10) a | 4.39(0.11) b | 4.01(0.09) a | ns | ** | ns |
| P | 0.37(0.02) a | 0.35(0.01) a | 0.37(0.01) a | 0.36(0.01) a | 0.37(0.37) a | ns | ns | ns | |
| K | 4.15(0.19) a | 4.10(0.14) a | 3.96(0.12) a | 4.27(0.12) b | 3.87(0.09) a | ns | * | ns | |
| Ca | 0.50(0.004) b | 0.46(0.010) a | 0.49(0.007) b | 0.48(0.005) a | 0.49(0.008) a | * | ns | ns | |
| Mg | 0.56(0.02) a | 0.55(0.02) a | 0.61(0.02) a | 0.53(0.01) a | 0.61(0.02) b | ns | ** | ns | |
| S | 0.28(0.011) a | 0.28(0.005) a | 0.29(0.006) a | 0.28(0.005) a | 0.28(0.007) a | ns | ns | ns | |
| Micronutrients | |||||||||
| B | mg kg-−1 | 7.34(0.25) a | 7.02(0.27) a | 7.05(0.16) a | 7.60(0.12) b | 6.67(0.13) a | ns | *** | ns |
| Cu | 8.82(0.23) a | 9.01(0.16) a | 9.18(0.26) a | 9.19(0.23) a | 8.81(0.10) a | ns | ns | ns | |
| Fe | 757(144) a | 622(121) a | 526(50.8) a | 706(67.4) b | 564(109) a | ns | + | + | |
| Mn | 91.3(3.80) b | 81.7(5.01) a | 82.5(3.23) a | 94.6(1.71) b | 76.0(2.22) a | ** | *** | ns | |
| Mo | 0.42(0.02) b | 0.29(0.03) a | 0.27(0.01) a | 0.34(0.01) a | 0.32(0.03) a | ** | ns | ns | |
| Zn | 38.1(2.24) a | 45.8(2.66) a | 38.9(2.37) a | 41.8(2.59) a | 40.1(1.67) a | ns | ns | ns | |
| Potentially toxic elements | |||||||||
| Pb | mg kg−1 | 2.97(0.28) a | 2.55(0.24) a | 2.41(0.12) a | 2.79(0.12) a | 2.49(0.23) a | ns | ns | ns |
| Cd | 0.07(0.003) a | 0.06(0.004) a | 0.07(0.000) a | 0.06(0.001) a | 0.07(0.002) b | ns | ** | * | |
| Cr | 2.04(0.35) a | 1.79(0.25) a | 1.56(0.09) a | 1.78(0.16) a | 1.82(0.24) a | ns | ns | * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Centurión, N.; Mariscal-Sancho, I.; Navas, M.; Gabriel, J.L.; Ulcuango, K.; Ibáñez, M.Á.; Moliner, A.; Hontoria, C. Increasing Legume Input through Interseeding Cover Crops: Soil and Crop Response as Affected by Tillage System. Agronomy 2023, 13, 1388. https://doi.org/10.3390/agronomy13051388
Centurión N, Mariscal-Sancho I, Navas M, Gabriel JL, Ulcuango K, Ibáñez MÁ, Moliner A, Hontoria C. Increasing Legume Input through Interseeding Cover Crops: Soil and Crop Response as Affected by Tillage System. Agronomy. 2023; 13(5):1388. https://doi.org/10.3390/agronomy13051388
Chicago/Turabian StyleCenturión, Nelly, Ignacio Mariscal-Sancho, Mariela Navas, José Luis Gabriel, Kelly Ulcuango, Miguel Ángel Ibáñez, Ana Moliner, and Chiquinquirá Hontoria. 2023. "Increasing Legume Input through Interseeding Cover Crops: Soil and Crop Response as Affected by Tillage System" Agronomy 13, no. 5: 1388. https://doi.org/10.3390/agronomy13051388
APA StyleCenturión, N., Mariscal-Sancho, I., Navas, M., Gabriel, J. L., Ulcuango, K., Ibáñez, M. Á., Moliner, A., & Hontoria, C. (2023). Increasing Legume Input through Interseeding Cover Crops: Soil and Crop Response as Affected by Tillage System. Agronomy, 13(5), 1388. https://doi.org/10.3390/agronomy13051388











