Discovery of Oscheius myriophilus (Nematoda: Rhabditidae) in Gastropods and Its Similar Virulence to Phasmarhabditis papillosa against Arion vulgaris, Deroceras reticulatum, and Cernuella virgata
Abstract
1. Introduction
2. Materials and Methods
2.1. Nematode Isolation from Slugs and Culture Maintenance
2.2. Molecular Identification
2.3. Laboratory Bioassay
2.4. Statistical Analyses
3. Results
3.1. Molecular Identification
3.2. Laboratory Bioassay
3.2.1. The Spanish Slug (A. vulgaris)
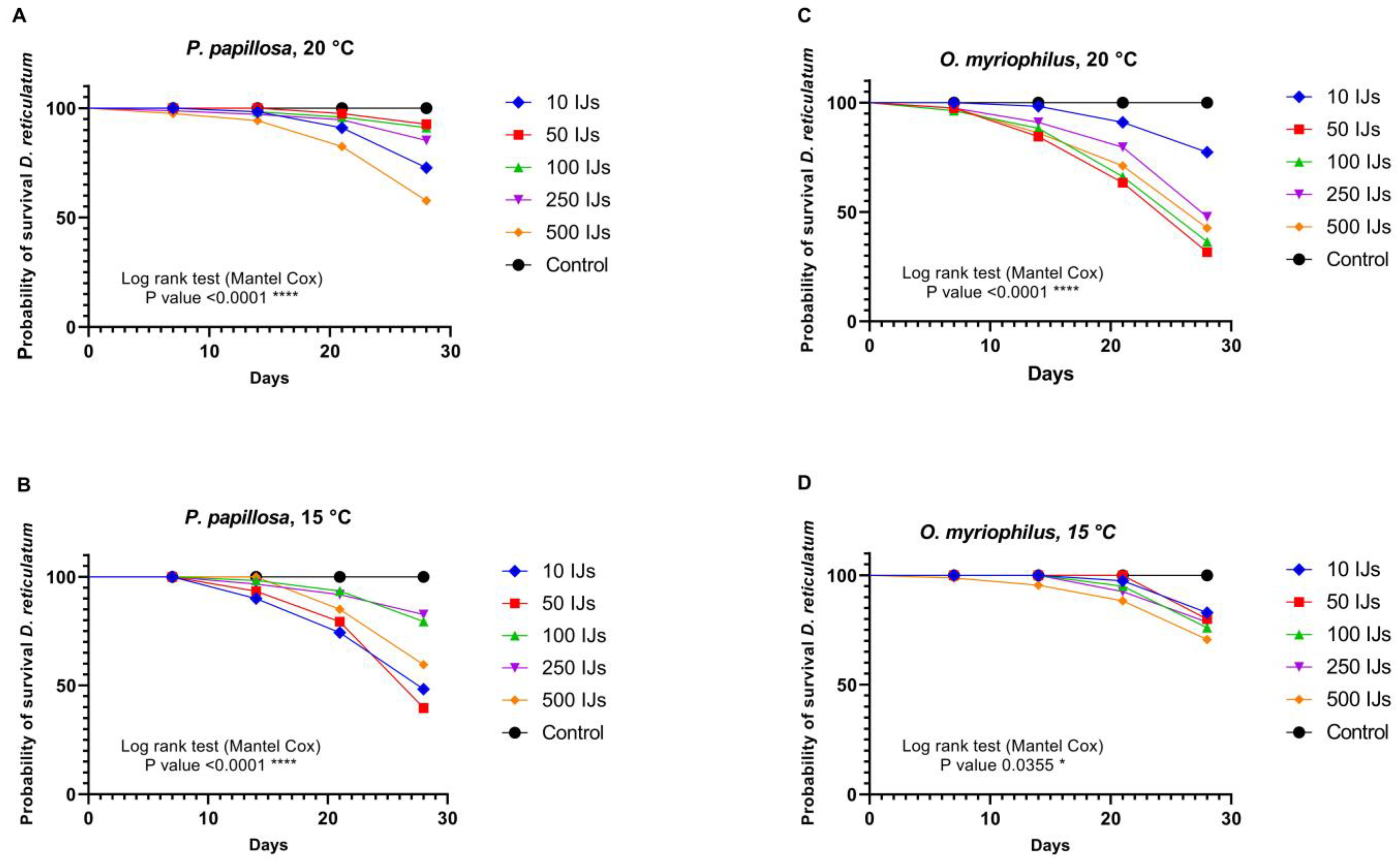
3.2.2. The Grey Field Slug (Deroceras reticulatum)
3.2.3. The Vineyard Snail (Cernuella virgata)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barker, G.M. Molluscs as Crop Pests; CABI Publishing: Wallingford, UK, 2002. [Google Scholar]
- Rowson, B.; Turner, J.; Anderson, R.; Symondson, B. Slugs of Britain and Ireland; Rowson, B., Turner, J., Anderson, R., Symondson, B., Eds.; FSC Publications: Telford, UK, 2014; p. 136. [Google Scholar]
- Glen, D.M.; Moens, R. Agriolimacidae, Arionidae and Milacidae as pests in west European cereals. In Molluscs as Crop Pests; Barker, G.M., Ed.; CABI Publishing: Wallingford, UK, 2002; pp. 271–300. [Google Scholar]
- Ross, J.L. Riding the Slime Wave: Gathering Global Data on Slug Control; Nuffield Farming Scholarships Trust Report; The Nuffield Farming Scholarships Trust Southill Farm, Staple Fitzpaine: Taunton, UK, 2019. [Google Scholar]
- Purvis, G.; Bannon, J.W. Non-target effects of repeated methiocarb slug pellets application on carabid beetle (Coleoptera, Carabidae) activity in winter-sown cereals. Ann. Appl. Biol. 1992, 121, 215–223. [Google Scholar] [CrossRef]
- Buhl, K.; Bond, C.; Stone, D. Iron Phosphate General Fact Sheet; National Pesticide Information Center, Oregon State University Extension Services: Corvallis, OR, USA, 2013; Available online: http://npic.orst.edu/factsheets/ironphosphategen.html (accessed on 8 January 2019).
- Langan, A.M.; Shaw, E.M. Responses of the earthworm Lumbricus terrestris (L.) to iron phosphate and metaldehyde slug pellet formulations. Appl. Soil Ecol. 2006, 34, 184–189. [Google Scholar] [CrossRef]
- Wilson, M.J.; Rae, R. Phasmarhabditis hermaphrodita as a control agent for Slugs. In Nematode Pathogenesis of Insects and Other Pests; Campos-Herrera, R., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 509–521. [Google Scholar]
- Laznik, Ž.; Trdan, S. Is a combination of different natural substances suitable for slug (Arion spp.) control? Span. J. Agric. Res. 2016, 14, e1004. [Google Scholar] [CrossRef]
- Dörler, D.; Scheucher, A.; Zaller, J.G. Efficacy of chemical and biological slug control measures in response to watering and earthworms. Sci. Rep. 2019, 9, 2954. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, A.; Tiedt, L.R.; Malan, A.P.; Ross, J.L. First record of Phasmarhabditis papillosa (Nematoda: Rhabditidae) in South Africa and its virulence against the invasive slug, Deroceras panormitanum. Nematology 2017, 19, 1035–1050. [Google Scholar] [CrossRef]
- Laznik, Ž.; Majić, I.; Trdan, S.; Malan, A.; Pieterse, A.; Ross, J.L. Is Phasmarhabditis papillosa (Nematoda: Rhabditidae) a possible biological control agent against the Spanish slug, Arion vulgaris (Gastropoda: Arionidae)? Nematology 2020, 23, 577–585. [Google Scholar] [CrossRef]
- Tandingan De Ley, I.; Holovachov, O.; Mc Donnell, R.J.; Bert, W.; Paine, T.D.; De Ley, P. Description of Phasmarhabditis californica n. sp. and first report of P. papillosa (Nematoda: Rhabditidae) from invasive slugs in the USA. Nematology 2016, 18, 175–193. [Google Scholar] [CrossRef]
- Wilson, M.J.; Glen, D.M.; George, S.K. The rhabditid nematode Phasmarhabditis hermaphrodita as a potential biological control agent for slugs. Biocontrol Sci. Technol. 1993, 3, 503–511. [Google Scholar] [CrossRef]
- Rae, R.; Verdun, C.; Grewal, P.S.; Robertson, J.F.; Wilson, M.J. Biological control of terrestrial molluscs using Phasmarhabditis hermaphrodita—Progress and prospects. Pest Manag. Sci. 2007, 63, 1153–1164. [Google Scholar] [CrossRef]
- Nermut’, J.; Půža, V.; Mekete, T.; Mráček, Z. Phasmarhabditis bohemica n. sp. (Nematoda: Rhabditidae), a slug-parasitic nematode from the Czech Republic. Nematology 2016, 19, 93–107. [Google Scholar] [CrossRef]
- McDonnell, R.J.; Colton, A.J.; Howe, D.K.; Denver, D.R. Lethality of four species of Phasmarhabditis (Nematoda: Rhabditidae) to the invasive slug, Deroceras reticulatum (Gastropoda: Agriolimacidae) in laboratory infectivity trials. Biol. Control 2020, 150, e104349. [Google Scholar] [CrossRef]
- Tandingan De Ley, I.; Schurkman, J.; Wilen, C.; Dillman, A.R. Mortality of the invasive white garden snail Theba pisana exposed to three US isolates of Phasmarhabditis spp (P. hermaphrodita, P. californica, and P. papillosa). PLoS ONE 2020, 15, e0225244. [Google Scholar] [CrossRef] [PubMed]
- Mengert, H. Nematoden und Schnecken. Z. Morphol. Ökol. Tiere 1953, 4, 311–349. [Google Scholar] [CrossRef]
- Andrássy, I. A Taxonomic Review of the Suborder Rhabditina (Nematoda: Secernentia); ORSTOM: Paris, France, 1983; p. 241. [Google Scholar]
- Sudhaus, W.; Hooper, D.J. Rhabditis (Oscheius) guentheri sp. n., an unusual species with reduced posterior ovary, with observations on the Dolichura and Insectivora groups (Nematoda: Rhabditidae). Nematologica 1994, 40, 508–533. [Google Scholar] [CrossRef]
- Kumar, P.; Jamal, W.; Somvanshi, V.S.; Chauhan, K.; Mumtaz, S. Description of Oscheius indicus n. sp. (Rhabditidae: Nematoda) from India. J. Nematol. 2019, 51, e2019-04. [Google Scholar] [CrossRef]
- Campos-Herrera, R.; Půža, V.; Jaffuel, G.; Blanco-Pérez, R.; Čepulyte Rakauskiene, R.; Turlings, T. Unraveling the intraguild competition between Oscheius spp. nematodes and entomopathogenic nematodes: Implications for their natural distribution in Swiss agricultural soils. J. Invertebr. Pathol. 2015, 132, 216–227. [Google Scholar] [CrossRef]
- Castro-Ortega, I.R.; Caspeta-Mandujano, J.M.; Suárez-Rodríguez, R.; Peña-Chora, G.; Ramírez Trujillo, J.A.; Cruz-Pérez, K.; Sosa, I.A.; Hernández-Velázquez, V.M. Oscheius myriophilus (Nematoda: Rhabditida) isolated in sugar cane soils in Mexico with potential to be used as entomopathogenic nematode. J. Nematol. 2020, 52, 1–8. [Google Scholar] [CrossRef]
- Poinar, G.O. Rhabditis myriophila sp. n., (Rhabditidae: Rhabditida), associated with the millipede, Oxidis gracilis (Polydesmida: Diplepoda). Proc. Helminthol. Soc. Wash. 1986, 53, 232–236. [Google Scholar]
- Sudhaus, W.; Schulte, F. Rhabditis (Rhabditis) necronema sp. n., (Nematoda: Rhabditidae), from southern Australian diplopoda with notes on its riblings R. myriophila, Poinar, 1986 and R. caulloryi, Maupas, 1919. Nematologica 1989, 35, 15–24. [Google Scholar]
- Erbaş, Z.; Demir, İ.; Demirbağ, Z. Isolation and characterization of a parasitic nematode, Oscheius myriophila (Nematoda: Rhabditida), associated with European Mole Cricket, Gryllotalpa gryllotalpa (Orthoptera: Gryllotalpidae). Hacet. J. Biol. Chem. 2017, 45, 197–203. [Google Scholar] [CrossRef]
- Zemanova, M.A.; Knop, E.; Heckel, G. Phylogeographic past and invasive presence of Arion pest slugs in Europe. Mol. Ecol. 2016, 25, 5747–5764. [Google Scholar] [CrossRef] [PubMed]
- Hominick, W.M.; Briscoe, B.R.; del Pino, F.G.; Heng, J.; Hunt, D.J.; Kozodoy, E.; Mracek, Z.; Nguyen, K.B.; Reid, A.P.; Spiridonov, S.; et al. Biosystematics of entomopathogenic nematodes: Current status, protocols and definitions. J. Helminthol. 1997, 71, 271–298. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Berry, R.E.; Moldenke, A.F. Phylogenetic relationships of entomopathogenic nematodes (Heterorhabditidae and Steinernematidae) inferred from partial 18S rRNA gene sequences. J. Invertebr. Pathol. 1997, 69, 246–252. [Google Scholar] [CrossRef]
- Rae, R.; Sommer, R.J. Bugs don’t make worms kill. J. Exp. Biol. 2011, 214, 1053. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Foye, S.H.; MacGuidwin, A.E.; Steffan, S.H. Incidence of Oscheius onirici (Nematoda: Rhabditidae), a potentially entomopathogenic nematode from the marshlands of Wisconsin, USA. J. Nematol. 2018, 50, 9–26. [Google Scholar] [CrossRef]
- Speiser, B.; Zaller, J.G.; Neudecker, A. Size-specific susceptibility of the pest slugs Deroceras reticulatum and Arion lusitanicus to the nematode biocontrol agent Phasmarhabditis hermaphrodita. Biol. Control 2001, 46, 311–320. [Google Scholar]
- Grimm, B.; Schaumberger, K. Daily activity of the pest slug Arion lusitanicus under laboratory conditions. Ann. Appl. Biol. 2002, 141, 35–44. [Google Scholar] [CrossRef]
- Cutler, J.; Williamson, S.M.; Rae, R. The effect of sertraline, haloperidol and apomorphine on the behavioural manipulation of slugs (Deroceras invadens) by the nematode Phasmarhabditis hermaphrodita. Behav. Process. 2019, 165, 1–3. [Google Scholar] [CrossRef]
- Nermut’, J.; Půža, V.; Mráček, Z. The effect of intraspecific competition on the development and quality of Phasmarhabditis hermaphrodita (Rhabditida: Rhabditidae). Biocontrol Sci. Technol. 2012, 22, 1389–1397. [Google Scholar] [CrossRef]
- Koppenhöfer, A.M.; Kaya, H.K. Density-dependent effects on Steinernema glaseri (Nematoda: Steinernematidae) within an insect host. J. Parasitol. 1995, 81, 797–799. [Google Scholar] [CrossRef]
- Půža, V.; Mráček, Z. Seasonal dynamics of entomopathogenic nematodes of the genera Steinernema and Heterorhabditis as a response to abiotic factors and abundance of insect hosts. J. Invertebr. Pathol. 2005, 89, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Kruitbos, L.M.; Heritage, S.; Hapca, S.; Wilson, M.J. Influence of substrate on the body–waving behaviour of nematodes. Nematology 2009, 11, 917–925. [Google Scholar] [CrossRef]
- Majić, I.; Sarajlić, A.; Lakatos, T.; Tóth, T.; Raspudić, E.; Puškadija, Z.; Kanižai Šarić, G.; Laznik, Ž. Virulence of new strain of Heterorhabditis bacteriophora from Croatia against Lasioptera rubi. Plant Prot. Sci. 2019, 55, 134–141. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- Glare, T.; Caradus, J.; Gelernter, W.; Jackson, T.; Keyhani, N.; Köhl, J.; Marrone, P.; Morin, L.; Stewart, A. Have biopesticides come of age? Trends Biotechnol. 2012, 30, 250–258. [Google Scholar] [CrossRef]


| 15 °C Spanish Slug (A. vulgaris) | ||||
|---|---|---|---|---|
| Nematode Dose (IJs Gastropod−1) | Phasmarhabditis papillosa | |||
| 7 days | 14 days | 21 days | 28 days | |
| 0 | 100 ± 0 Ac | 100 ± 0 Ad | 100 ± 0 Ac | 100 ± 0 Ac |
| 10 | 95 ± 5 Bbc | 45 ± 11 Bc | 0 ± 0 Aa | 0 ± 0 Aa |
| 50 | 75 ± 10 Ca | 15 ± 8 Bab | 0 ± 0 Aa | 0 ± 0 Aa |
| 100 | 95 ± 5 Bbc | 5 ± 5 Aa | 0 ± 0 Aa | 0 ± 0 Aa |
| 250 | 100 ± 0 C | 40 ± 11 Bc | 20 ± 9 Bb | 0 ± 0 Aa |
| 500 | 90 ± 7 Cab | 30 ± 11 Bbc | 20 ± 9 ABb | 10 ± 7 Ab |
| Nematode dose (IJs gastropod−1) | Oscheius myriophilus | |||
| 7 days | 14 days | 21 days | 28 days | |
| 0 | 100 ± 0 Ab | 100 ± 0 Ad | 100 ± 0 Ac | 100 ± 0 Ab |
| 10 | 80 ± 9 Ca | 35 ± 11 Bab | 5 ± 5 Aa | 0 ± 0 Aa |
| 50 | 80 ± 9 Da | 30 ± 11 Ca | 10 ± 7 Bab | 0 ± 0 Aa |
| 100 | 100 ± 0 Db | 70 ± 11 Cc | 15 ± 8 Bab | 0 ± 0 Aa |
| 250 | 90 ± 7 Da | 55 ± 11 Cbc | 15 ± 8 Bab | 0 ± 0 Aa |
| 500 | 90 ± 7 Da | 60 ± 11 Cc | 20 ± 9 Bb | 0 ± 0 Aa |
| 20 °C | ||||
|---|---|---|---|---|
| Spanish Slug (A. vulgaris) | ||||
| Nematode dose (IJs gastropod−1) | Phasmarhabditis papillosa | |||
| 7 day | 14 days | 21 days | 28 days | |
| 0 | 100 ± 0 Aa | 100 ± 0 Aa | 100 ± 0 Ab | 100 ± 0 Ab |
| 10 | 100 ± 0 Aa | 100 ± 0 Aa | 100 ± 0 Ab | 100 ± 0 Ab |
| 50 | 100 ± 0 Aa | 100 ± 0 Aa | 100 ± 0 Ab | 100 ± 0 Ab |
| 100 | 100 ± 0 Ca | 95 ± 5 BCa | 95 ± 5 BCab | 85 ± 8 Aa |
| 250 | 100 ± 0 Ba | 95 ± 5 ABa | 90 ± 7 Aa | 90 ± 7 Aa |
| 500 | 100 ± 0 Ba | 100 ± 0 Ba | 95 ± 5 ABab | 85 ± 8 Aa |
| Nematode dose (IJs gastropod−1) | Oscheius myriophilus | |||
| 7 days | 14 days | 21 days | 28 days | |
| 0 | 100 ± 0 Aa | 100 ± 0 Ac | 100 ± 0 Ac | 100 ± 0 Ab |
| 10 | 100 ± 0 Ba | 85 ± 8 Aab | 85 ± 8 Aa | 75 ± 10 Aa |
| 50 | 100 ± 0 Ba | 100 ± 0 Bc | 90 ± 7 Aab | 80 ± 9 Aa |
| 100 | 100 ± 0 Ba | 80 ± 9 Aa | 90 ± 7 Aab | 80 ± 9 Aa |
| 250 | 100 ± 0 Ba | 95 ± 5 ABbc | 100 ± 0 Bc | 90 ± 7 Aa |
| 500 | 100 ± 0 Ba | 95 ± 5 ABbc | 95 ± 5 ABbc | 90 ± 7 Aa |
| 15 °C | ||||
|---|---|---|---|---|
| Grey Field Slug (D. reticulatum) | ||||
| Nematode dose (IJs gastropod−1) | Phasmarhabditis papillosa | |||
| 7 days | 14 days | 21 days | 28 days | |
| 0 | 100 ± 0 Ac | 100 ± 0 Ac | 100 ± 0 Ac | 100 ± 0 Ac |
| 10 | 45 ± 11 Aab | 30 ± 11 Aa | 30 ± 11 Aa | 35 ± 11 Aab |
| 50 | 60 ± 11 Ab | 50 ± 12 Aab | 45 ± 11 Aab | 40 ± 11 Aab |
| 100 | 30 ± 11 Aa | 35 ± 11 Aab | 40 ± 11 Aa | 55 ± 11 Aab |
| 250 | 65 ± 11 Bb | 55 ± 11 Bb | 65 ± 11 Bb | 20 ± 9 Aa |
| 500 | 40 ± 11 Aab | 40 ± 11 Aab | 40 ± 11 Aa | 35 ± 11 Aab |
| Nematode dose (IJs gastropod−1) | Oscheius myriophilus | |||
| 7 days | 14 days | 21 days | 28 days | |
| 0 | 100 ± 0 Ac | 100 ± 0 Ac | 100 ± 0 Ad | 100 ± 0 Ab |
| 10 | 55 ± 11 Aa | 45 ± 11 Ab | 55 ± 11 Aab | 40 ± 11 Aa |
| 50 | 45 ± 11 Aa | 40 ± 11 Aab | 75 ± 10 Bbc | 30 ± 11 Aa |
| 100 | 60 ± 11 Bab | 35 ± 11 Aab | 40 ± 11 ABa | 45 ± 11 ABa |
| 250 | 80 ± 9 Cb | 20 ± 9 Aa | 80 ± 9 Cc | 45 ± 11 Ba |
| 500 | 45 ± 11 Aa | 35 ± 11 Aab | 40 ± 11 Aa | 35 ± 11 Aa |
| 20 °C | ||||
|---|---|---|---|---|
| Grey Field Slug (D. reticulatum) | ||||
| Nematode dose (IJs gastropod−1) | Phasmarhabditis papillosa | |||
| 7 days | 14 days | 21 days | 28 days | |
| 0 | 100 ± 0 Ab | 100 ± 0 Ad | 100 ± 0 Ac | 100 ± 0 Ab |
| 10 | 55 ± 11 Ba | 65 ± 11 Bc | 65 ± 11 Bb | 25 ± 10 Aa |
| 50 | 60 ± 11 Ba | 60 ± 11 Bbc | 35 ± 11 Aa | 25 ± 10 Aa |
| 100 | 65 ± 11 Ba | 55 ± 11 Babc | 55 ± 11 Bab | 30 ± 11 Aa |
| 250 | 70 ± 11 Ba | 35 ± 11 Aa | 50 ± 12 Bab | 30 ± 11 Aa |
| 500 | 65 ± 11 Ba | 40 ± 11 Aab | 45 ± 11 ABab | 35 ± 11 Aa |
| Nematode dose (IJs gastropod−1) | Oscheius myriophilus | |||
| 7 days | 14 days | 21 days | 28 days | |
| 0 | 100 ± 0 Ac | 100 ± 0 Ad | 100 ± 0 Ab | 100 ± 0 Ab |
| 10 | 75 ± 10 Bb | 70 ± 11 Bc | 60 ± 11 ABa | 40 ± 11 Aa |
| 50 | 70 ± 11 Bb | 40 ± 11 Aa | 45 ± 11 Aa | 50 ± 12 ABa |
| 100 | 55 ± 11 Bb | 55 ± 11 Babc | 40 ± 11 ABa | 30 ± 11 Aa |
| 250 | 55 ± 11 Ab | 65 ± 11 Abc | 55 ± 11 Aa | 45 ± 11 Aa |
| 500 | 25 ± 10 Aa | 45 ± 11 Aab | 45 ± 11 Aa | 35 ± 11 Aa |
| 15 °C | ||||
|---|---|---|---|---|
| Vineyard Snail (C. virgata) | ||||
| Nematode dose (IJs gastropod−1) | Phasmarhabditis papillosa | |||
| 7 days | 14 days | 21 days | 28 days | |
| 0 | 100 ± 0 Ad | 100 ± 0 Ac | 100 ± 0 Ac | 100 ± 0 Ac |
| 10 | 75 ± 10 Cc | 40 ± 11 Bab | 30 ± 11 Ab | 30 ± 11 Aab |
| 50 | 50 ± 12 Bb | 30 ± 11 ABab | 20 ± 9 Aab | 25 ± 10 Aab |
| 100 | 20 ± 9 Aa | 45 ± 11 Bab | 10 ± 7 Aa | 15 ± 8 Aa |
| 250 | 20 ± 9 Aa | 55 ± 11 Bb | 10 ± 7 Aa | 20 ± 9 Aab |
| 500 | 25 ± 10 Aa | 25 ± 10 Aa | 30 ± 11 Ab | 40 ± 11 Ab |
| Nematode dose (IJs gastropod−1) | Oscheius myriophilus | |||
| 7 days | 14 days | 21 days | 28 days | |
| 0 | 100 ± 0 Ad | 100 ± 0 Ac | 100 ± 0 Ab | 100 ± 0 Ab |
| 10 | 75 ± 9 Bc | 30 ± 11 Aa | 40 ± 11 Aa | 45 ± 11 Aa |
| 50 | 70 ± 11 Bc | 45 ± 11 Aab | 45 ± 11 Aa | 60 ± 11 ABa |
| 100 | 35 ± 11 Aa | 60 ± 11 Bb | 35 ± 11 Aa | 65 ± 11 Ba |
| 250 | 40 ± 11 ABab | 45 ± 11 BCab | 20 ± 9 Aa | 65 ± 11 Ca |
| 500 | 60 ± 11 Bbc | 45 ± 11 ABab | 30 ± 11 Aa | 65 ± 11 Ba |
| 20 °C | ||||
|---|---|---|---|---|
| Vineyard Snail (C. virgata) | ||||
| Nematode dose (IJs gastropod−1) | Phasmarhabditis papillosa | |||
| 7 days | 14 days | 21 days | 28 days | |
| 0 | 100 ± 0 Ab | 100 ± 0 Ad | 100 ± 0 Ac | 100 ± 0 Ac |
| 10 | 75 ± 10 Aa | 25 ± 10 Aab | 30 ± 11 Aab | 30 ± 11 Ab |
| 50 | 55 ± 11 Ba | 60 ± 11 Bc | 30 ± 11 Aab | 45 ± 11 ABb |
| 100 | 60 ± 11 Ba | 45 ± 11 Bbc | 45 ± 11 Bb | 10 ± 7 Aa |
| 250 | 95 ± 5 Bb | 20 ± 9 Aa | 15 ± 8 Aa | 30 ± 11 Ab |
| 500 | 100 ± 0 Bb | 35 ± 11 Aab | 20 ± 9 Aa | 30 ± 11 Ab |
| Nematode dose (IJs gastropod−1) | Oscheius myriophilus | |||
| 7 days | 14 days | 21 days | 28 days | |
| 0 | 100 ± 0 Ac | 100 ± 0 Ab | 100 ± 0 Ac | 100 ± 0 Ab |
| 10 | 65 ± 11 Ca | 40 ± 11 ABa | 45 ± 11 ABCb | 25 ± 10 Aa |
| 50 | 70 ± 11 Ca | 50 ± 12 BCa | 25 ± 10 Aab | 30 ± 11 ABa |
| 100 | 65 ± 11 Ba | 45 ± 11 Ba | 20 ± 9 Aa | 15 ± 8 Aa |
| 250 | 90 ± 7 Bb | 35 ± 11 Aa | 25 ± 10 Aab | 30 ± 11 Aa |
| 500 | 55 ± 11 Ca | 45 ± 11 Ba | 10 ± 7 Aa | 20 ± 9 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laznik, Ž.; Trdan, S.; Tóth, T.; Ádám, S.; Lakatos, T.; Majić, I. Discovery of Oscheius myriophilus (Nematoda: Rhabditidae) in Gastropods and Its Similar Virulence to Phasmarhabditis papillosa against Arion vulgaris, Deroceras reticulatum, and Cernuella virgata. Agronomy 2023, 13, 1386. https://doi.org/10.3390/agronomy13051386
Laznik Ž, Trdan S, Tóth T, Ádám S, Lakatos T, Majić I. Discovery of Oscheius myriophilus (Nematoda: Rhabditidae) in Gastropods and Its Similar Virulence to Phasmarhabditis papillosa against Arion vulgaris, Deroceras reticulatum, and Cernuella virgata. Agronomy. 2023; 13(5):1386. https://doi.org/10.3390/agronomy13051386
Chicago/Turabian StyleLaznik, Žiga, Stanislav Trdan, Tímea Tóth, Szabolcs Ádám, Tamás Lakatos, and Ivana Majić. 2023. "Discovery of Oscheius myriophilus (Nematoda: Rhabditidae) in Gastropods and Its Similar Virulence to Phasmarhabditis papillosa against Arion vulgaris, Deroceras reticulatum, and Cernuella virgata" Agronomy 13, no. 5: 1386. https://doi.org/10.3390/agronomy13051386
APA StyleLaznik, Ž., Trdan, S., Tóth, T., Ádám, S., Lakatos, T., & Majić, I. (2023). Discovery of Oscheius myriophilus (Nematoda: Rhabditidae) in Gastropods and Its Similar Virulence to Phasmarhabditis papillosa against Arion vulgaris, Deroceras reticulatum, and Cernuella virgata. Agronomy, 13(5), 1386. https://doi.org/10.3390/agronomy13051386








