Forage Radish Cover Crops Improve Soil Quality and Fruit Yield of Lycium barbarum L. in an Arid Area of Northwest China
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Site Description
2.2. Experimental Design and Yield Estimation
2.3. Soil Sampling and Laboratory Analysis
2.4. Soil Quality Assessment
2.4.1. MDS Determination
2.4.2. MDS Verification
2.5. Statistical Analysis
3. Results
3.1. Soil Physicochemical Properties
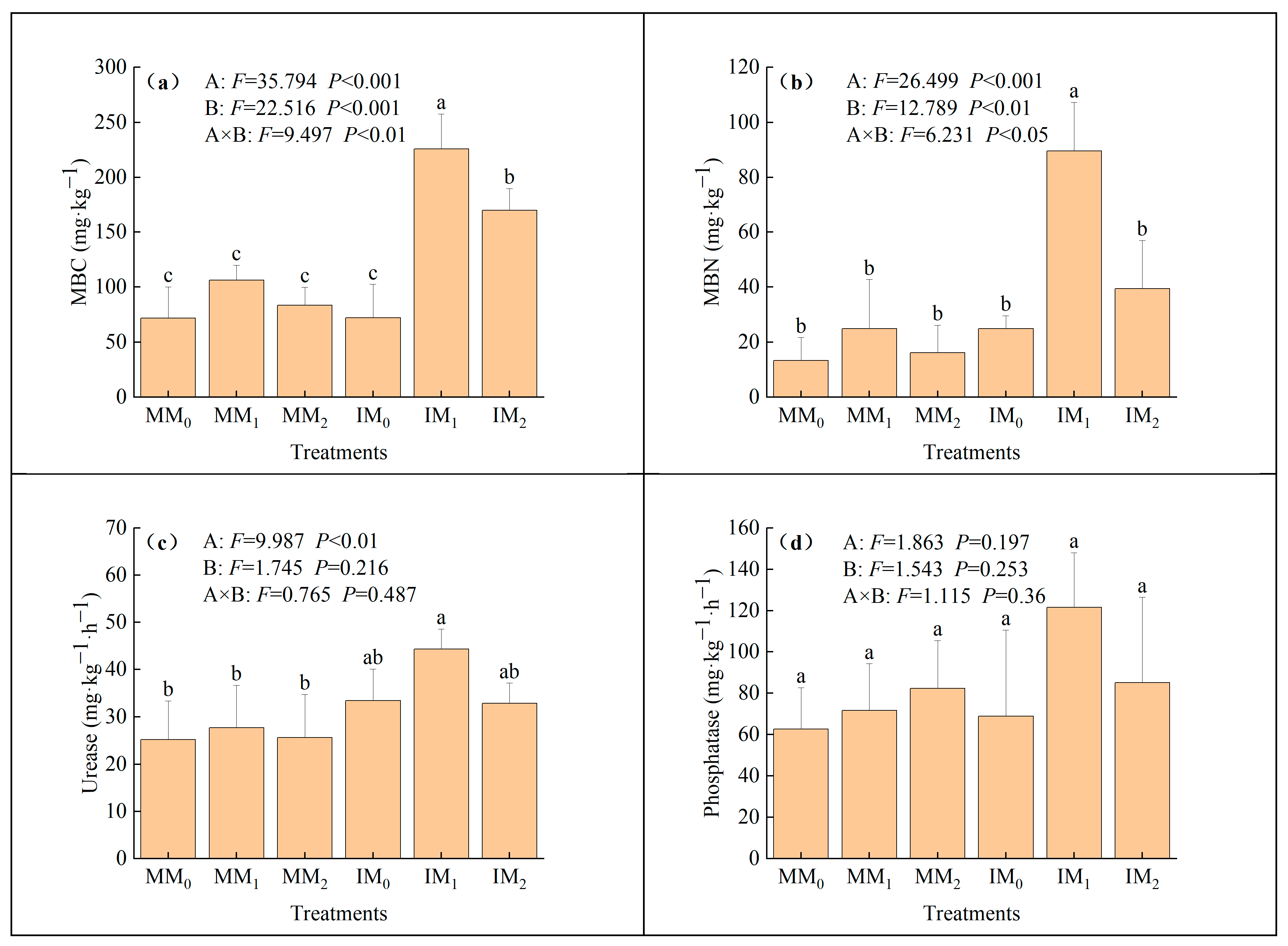
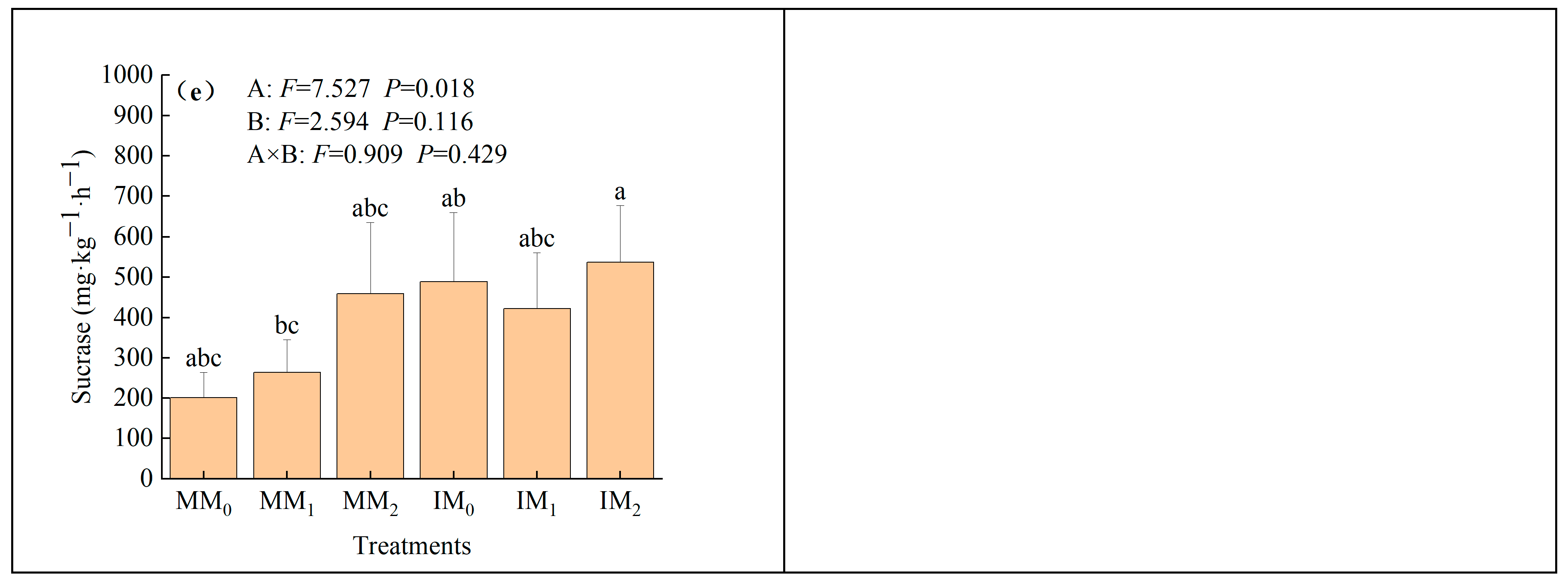
3.2. Soil Biological Properties
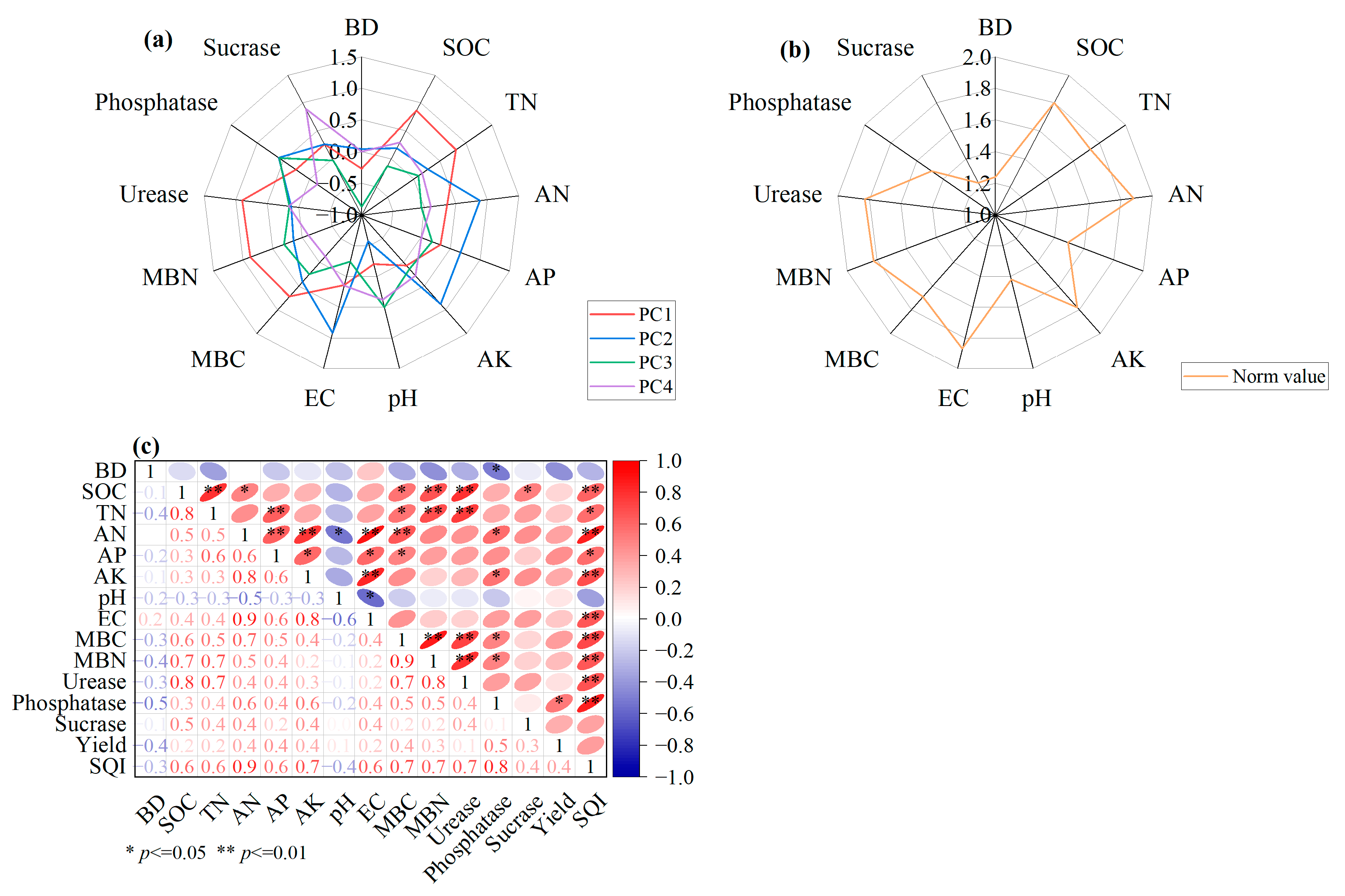
3.3. MDS Construction for Soil Quality Indicators
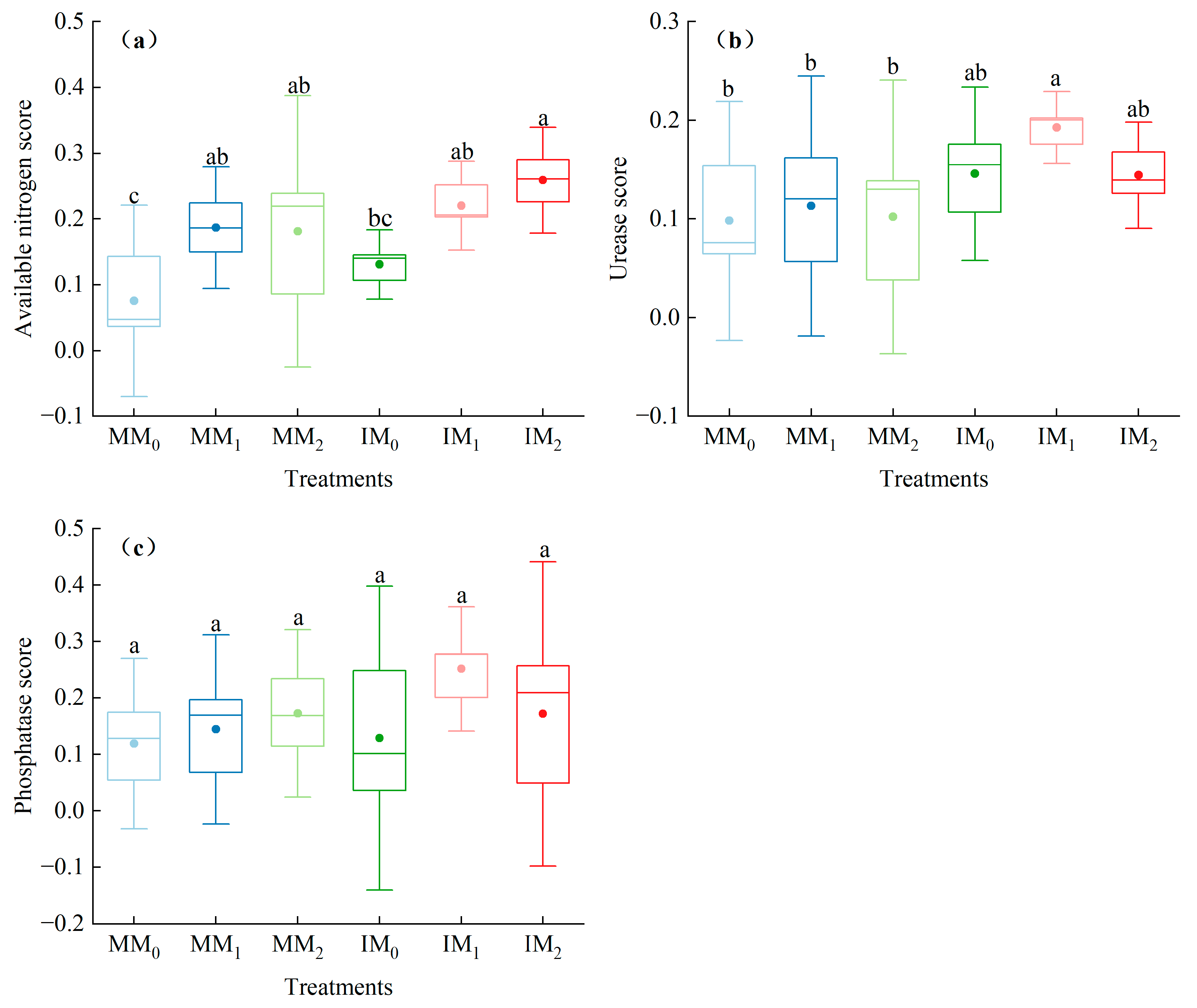
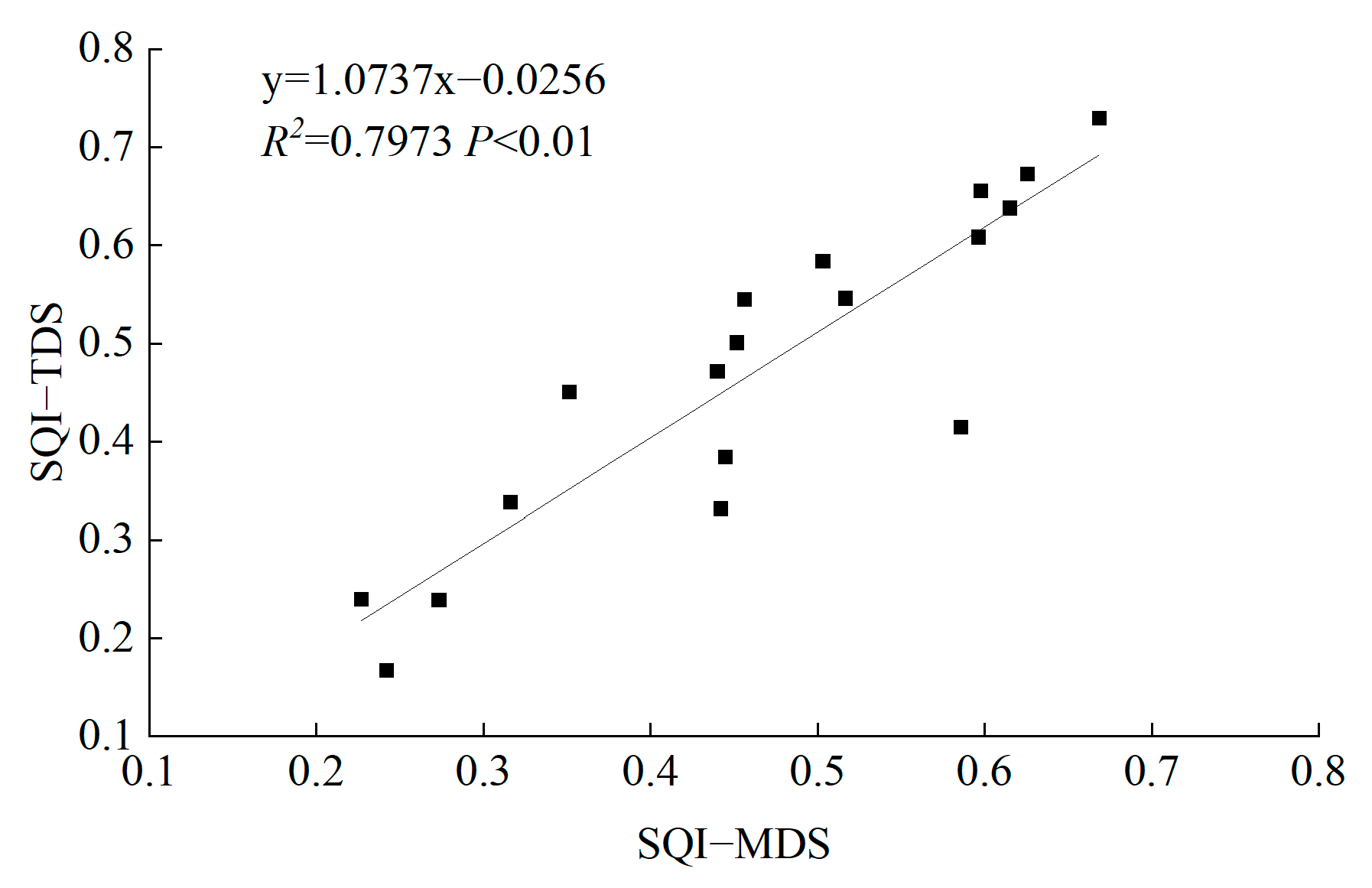
3.4. MDS-Based Soil Quality Assessment
3.5. Soil Quality and Fruit Yield
4. Discussion
4.1. Amelioration of Soil Physicochemical Properties by Cover Cropping with Organic Fertilizer
4.2. Enhancement of Soil Biochemical Properties by Cover Cropping with Organic Fertilizer
4.3. Improvement of Soil Quality by Cover Cropping with Organic Fertilizer
4.4. Yield Validation of Soil Quality Assessment
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, F.; Li, W.; Lin, Y.; Nan, X.; Yuan, J. Soil organic carbon pool and the production of Goji berry (Lycium barbarum L.) as affected by different fertilizer combinations under drip fertigation. Front. Environ. Sci. 2022, 10, 933124. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Ke, Y.; Luo, J.; Chen, X.; Zhang, X. Characteristics of soil nutrients and present situation of fertilization in the major wolfberry producing areas of Ningxia. Agric. Res. Arid. Areas 2016, 34, 113–118. [Google Scholar]
- Wang, Y.; Liu, L.; Tian, Y.; Wu, X.; Yang, J.; Luo, Y.; Li, H.; Awasthi, M.K.; Zhao, Z. Temporal and spatial variation of soil microorganisms and nutrient under white clover cover. Soil Tillage Res. 2020, 202, 104666. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A. Carbon sequestration in agricultural soils via cultivation of cover crops—A meta-analysis. Agric. Ecosyst. Environ. 2015, 200, 33–41. [Google Scholar] [CrossRef]
- Li, L. Intercropping enhances agroecosystem services and functioning: Current knowledge and perspectives. Chin. J. Ecol. 2016, 24, 403–415. [Google Scholar]
- Hu, Y.; Zhan, P.; Thomas, B.W.; Zhao, J.; Zhang, X.; Yan, H.; Zhang, Z.; Chen, S.; Shi, X.; Zhang, Y. Organic carbon and nitrogen accumulation in orchard soil with organic fertilization and cover crop management: A global meta-analysis. Sci. Total Environ. 2022, 852, 158402. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; De Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Jia, R.; Zhou, J.; Chu, J.; Shahbaz, M.; Yang, Y.; Jones, D.L.; Zang, H.; Razavi, B.S.; Zeng, Z. Insights into the associations between soil quality and ecosystem multifunctionality driven by fertilization management: A case study from the North China Plain. J. Clean. Prod. 2022, 362, 132265. [Google Scholar] [CrossRef]
- Serri, D.L.; Pérez-Brandan, C.; Meriles, J.M.; Salvagiotti, F.; Bacigaluppo, S.; Malmantile, A.; Vargas-Gil, S. Development of a soil quality index for sequences with different levels of land occupation using soil chemical, physical and microbiological properties. Agric. Ecosyst. Environ. Appl. Soil Ecol. 2022, 180, 104621. [Google Scholar] [CrossRef]
- Zhang, J.; Nie, J.; Cao, W.; Gao, Y.; Lu, Y.; Liao, Y. Long-term green manuring to substitute partial chemical fertilizer simultaneously improving crop productivity and soil quality in a double-rice cropping system. Eur. J. Agron. 2023, 142, 126641. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Z.; Zhou, J.; Xu, X.; Zhu, Y. Long-term straw mulching with nitrogen fertilization increases nutrient and microbial determinants of soil quality in a maize–wheat rotation on China’s Loess Plateau. Sci. Total Environ. 2021, 775, 145930. [Google Scholar] [CrossRef]
- Jin, H.; Shi, D.; Lou, Y.; Zhang, J.; Ye, Q.; Jiang, N. Evaluation of the quality of cultivated-layer soil based on different degrees of erosion in sloping farmland with purple soil in China. Catena 2021, 198, 105048. [Google Scholar] [CrossRef]
- Liu, T.; Wu, X.; Li, H.; Ning, C.; Li, Y.; Zhang, X.; He, J.; Filimonenko, E.; Chen, S.; Chen, X.; et al. Soil quality and r–K fungal communities in plantations after conversion from subtropical forest. Catena 2022, 219, 106584. [Google Scholar] [CrossRef]
- Bao, S. Soil Analysis Manual, 3rd ed.; China Agriculture Press: Beijing, China, 2005. [Google Scholar]
- Guan, S.; Meng, Z. Agrochemical characters and enzyme activities of black soils with different Years of reclamation. Chin. J. Soil Sci. 1986, 157–159. [Google Scholar]
- Zhou, Y.; Ma, H.; Xie, Y.; Jia, X.; Su, T.; Li, J.; Shen, Y. Assessment of soil quality indexes for different land use types in typical steppe in the loess hilly area, China. Ecol. Indic. 2020, 118, 106743. [Google Scholar] [CrossRef]
- Raiesi, F. A minimum data set and soil quality index to quantify the effect of land use conversion on soil quality and degradation in native rangelands of upland arid and semiarid regions. Ecol. Indic. 2017, 75, 307–320. [Google Scholar] [CrossRef]
- Xiong, P.; Zhang, Z.; Hallett, P.D.; Peng, X. Variable responses of maize root architecture in elite cultivars due to soil compaction and moisture. Plant Soil 2020, 455, 79–91. [Google Scholar] [CrossRef]
- Calonego, J.C.; Raphael, J.P.A.; Rigon, J.P.G.; de Oliveira, N.L.; Rosolem, C.A. Soil compaction management and soybean yields with cover crops under no-till and occasional chiseling. Eur. J. Agron. 2017, 85, 31–37. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, X. Bio-tillage: A new perspective for sustainable agriculture. Soil Tillage Res. 2021, 206, 104844. [Google Scholar] [CrossRef]
- Chen, G.; Weil, R.R. Root growth and yield of maize as affected by soil compaction and cover crops. Soil Tillage Res. 2011, 117, 17–27. [Google Scholar] [CrossRef]
- Kaye, J.P.; Quemada, M. Using cover crops to mitigate and adapt to climate change. A review. Agron. Sustain. Dev. 2017, 37, 2–17. [Google Scholar] [CrossRef]
- Abdalla, M.; Hastings, A.; Cheng, K.; Yue, Q.; Chadwick, D.; Espenberg, M.; Truu, J.; Rees, R.M.; Smith, P. A critical review of the impacts of cover crops on nitrogen leaching, net greenhouse gas balance and crop productivity. Glob. Chang. Biol. 2019, 25, 2530–2543. [Google Scholar] [CrossRef] [PubMed]
- Weil, R.; White, C.; Lawley, Y. Forage radish: New multi-purpose cover crop for the Mid-Atlantic. Fact Sheet 2009, 824, 1–8. [Google Scholar]
- Weil, R.; Kremen, A. Thinking across and beyond disciplines to make cover crops pay. J. Sci. Food Agric. 2007, 87, 551–557. [Google Scholar] [CrossRef]
- Yang, J.; Gao, W.; Ren, S. Long-term effects of combined application of chemical nitrogen with organic materials on crop yields, soil organic carbon and total nitrogen in fluvo-aquic soil. Soil Tillage Res. 2015, 151, 67–74. [Google Scholar] [CrossRef]
- Das, P.P.; Singh, K.R.B.; Nagpure, G.; Mansoori, A.; Singh, R.P.; Ghazi, I.A.; Kumar, A.; Singh, J. Plant-soil-microbes: A tripartite interaction for nutrient acquisition and better plant growth for sustainable agricultural practices. Environ. Res. 2022, 214, 113821. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, M.; Yan, J. Effect of green manure combined with organic fertilizer on the improvement of red soil in the newly reclaimed orchard. Chin. J. Soil Sci. 2020, 51, 164–170. [Google Scholar]
- Ma, Q.; Wen, Y.; Wang, D.; Sun, X.; Hill, P.W.; Macdonald, A.; Chadwick, D.R.; Wu, L.; Jones, D.L. Farmyard manure applications stimulate soil carbon and nitrogen cycling by boosting microbial biomass rather than changing its community composition. Soil Biol. Biochem. 2020, 144, 107760. [Google Scholar] [CrossRef]
- Muhammad, I.; Wang, J.; Sainju, U.M.; Zhang, S.; Zhao, F.; Khan, A. Cover cropping enhances soil microbial biomass and affects microbial community structure: A meta-analysis. Geoderma 2021, 381, 114696. [Google Scholar] [CrossRef]
- Zheng, W.; Gong, Q.; Lv, F.; Yin, Y.; Li, Z.; Zhai, B. Tree-scale spatial responses of extracellular enzyme activities and stoichiometry to different types of fertilization and cover crop in an apple orchard. Eur. J. Soil Biol. 2020, 99, 103207. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Ruis, S.J. Cover crop impacts on soil physical properties: A review. Soil Sci. Soc. Am. J. 2020, 84, 1527–1576. [Google Scholar] [CrossRef]
- Qian, X.; Gu, J.; Pan, H.; Zhang, K.; Sun, W.; Wang, X.; Gao, H. Effects of living mulches on the soil nutrient contents, enzyme activities, and bacterial community diversities of apple orchard soils. Eur. J. Soil Biol. 2015, 70, 23–30. [Google Scholar] [CrossRef]
- Ren, F.; Sun, N.; Xu, M.; Zhang, X.; Wu, L.; Xu, M. Changes in soil microbial biomass with manure application in cropping systems: A meta-analysis. Soil Tillage Res. 2019, 194, 104291. [Google Scholar] [CrossRef]
- Ma, D.; Yin, L.; Ju, W.; Li, H.; Liu, X.; Deng, X.; Wang, S. Meta-analysis of green manure effects on soil properties and crop yield in northern China. Field Crops Res. 2021, 266, 108146. [Google Scholar] [CrossRef]
- Ablimit, R.; Li, W.; Zhang, J.; Gao, H.; Zhao, Y.; Cheng, M.; Meng, X.; An, L.; Chen, Y. Altering microbial community for improving soil properties and agricultural sustainability during a 10-year maize-green manure intercropping in Northwest China. J. Environ. Manag. 2022, 321, 115859. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, S.; Chen, T.; Fang, W.; Ma, X.; Guo, W.; Huang, C. Effects of Different Mulching Patterns on Soil Active Organic Carbon Components and Related Enzyme Activities in Cherry Orchard. Southwest China 2021, 34, 2465–2472. [Google Scholar]
- Song, Y.; Yu, J.; Chen, S.; Xiao, C.; Li, Y.; Su, X.; Ding, F. Effects of reduced chemical fertilizer with application of bio-organic fertilizer on rape growth, microorganism and enzymes activities in soil. J. Soil Water Conserv. 2018, 32, 352–360. [Google Scholar]
- Kumar, M.; Mitra, S.; Mazumdar, S.; Majumdar, B.; Saha, A.R.; Singh, S.R.; Pramanick, B.; Gaber, A.; Walaa, F.; Alsanie, W.F.; et al. Improvement of soil health and system productivity through crop diversification and residue incorporation under jute-based different cropping systems. Agronomy 2021, 11, 1622. [Google Scholar] [CrossRef]
- Alvarez, R.; Steinbach, H.S.; De Paepe, J.L. Cover crop effects on soils and subsequent crops in the pampas: A meta-analysis. Soil Tillage Res. 2017, 170, 53–65. [Google Scholar] [CrossRef]
- Malone, L.C.; Mourtzinis, S.; Gaska, J.M.; Lauer, J.G.; Ruark, M.D.; Conley, S.P. Cover crops in a Wisconsin annual cropping system: Feasibility and yield effects. Agron. J. 2022, 114, 1052–1067. [Google Scholar] [CrossRef]
- Lawley, Y.E.; Weil, R.R.; Teasdale, J.R. Forage radish cover crop suppresses winter annual weeds in fall and before corn planting. Agron. J. 2011, 103, 137–144. [Google Scholar] [CrossRef]




| Treatment | BD (g·cm3) | SOC (g·kg−1) | TN (g·kg−1) | AN (mg·kg−1) | AP (mg·kg−1) | AK (mg·kg−1) | pH | EC (μS·cm−1) |
|---|---|---|---|---|---|---|---|---|
| MM0 | 1.45 ± 0.01 a | 9.04 ± 2.52 a | 0.76 ± 0.24 b | 19.71 ± 7.87 b | 15.41 ± 9.74 b | 52.65 ± 25.71 b | 8.97 ± 0.28 a | 109.37 ± 12.81 c |
| MM1 | 1.45 ± 0.01 a | 9.11 ± 2.79 a | 0.71 ± 0.25 b | 35.20 ± 5.74 ab | 17.05 ± 4.92 b | 38.89 ± 11.42 b | 8.85 ± 0.15 a | 133.33 ± 16.98 bc |
| MM2 | 1.45 ± 0.02 a | 11.25 ± 3.38 a | 0.88 ± 0.44 ab | 35.2 ± 12.03 ab | 15.84 ± 7.12 b | 78.04 ± 35.74 ab | 8.93 ± 0.34 a | 155.6 ± 30.32 b |
| IM0 | 1.42 ± 0.02 ab | 11.04 ± 0.80 a | 1.34 ± 0.12 ab | 27.21 ± 2.71 bc | 35.96 ± 10.51 ab | 51.00 ± 0.72 b | 9.01 ± 0.02 a | 116.80 ± 4.79 c |
| IM1 | 1.40 ± 0.03 b | 13.21 ± 1.85 a | 1.51 ± 0.55 a | 40.69 ± 5.28 ab | 31.39 ± 15.55 ab | 74.59 ± 18.55 ab | 8.97 ± 0.17 a | 136.23 ± 22.61 bc |
| IM2 | 1.45 ± 0.01 a | 11.39 ± 0.64 a | 1.33 ± 0.28 ab | 49.50 ± 8.37 a | 47.63 ± 15.77 a | 114.47 ± 31.43 a | 8.85 ± 0.13 a | 191.07 ± 10.57 a |
| A | * | ns | ** | * | ** | ns | ns | ns |
| B | ns | ns | ns | ** | ns | * | ns | *** |
| Indicator | TDS | MDS | ||
|---|---|---|---|---|
| Communality | Weight | Communality | Weight | |
| BD | 0.824 | 0.076 | ||
| SOC | 0.878 | 0.081 | ||
| TN | 0.770 | 0.071 | ||
| AN | 0.911 | 0.084 | 0.716 | 0.370 |
| AP | 0.590 | 0.055 | ||
| AK | 0.876 | 0.081 | ||
| pH | 0.764 | 0.071 | ||
| EC | 0.946 | 0.088 | ||
| MBC | 0.770 | 0.071 | ||
| MBN | 0.895 | 0.083 | ||
| Urease | 0.864 | 0.080 | 0.535 | 0.276 |
| Phosphatase | 0.773 | 0.072 | 0.686 | 0.354 |
| Sucrase | 0.926 | 0.086 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Li, W.; Chen, H.; Weil, R.R.; Zhu, L.; Nan, X. Forage Radish Cover Crops Improve Soil Quality and Fruit Yield of Lycium barbarum L. in an Arid Area of Northwest China. Agronomy 2023, 13, 1634. https://doi.org/10.3390/agronomy13061634
Wang F, Li W, Chen H, Weil RR, Zhu L, Nan X. Forage Radish Cover Crops Improve Soil Quality and Fruit Yield of Lycium barbarum L. in an Arid Area of Northwest China. Agronomy. 2023; 13(6):1634. https://doi.org/10.3390/agronomy13061634
Chicago/Turabian StyleWang, Fang, Wenhui Li, Haonan Chen, Ray R. Weil, Lizhen Zhu, and Xiongxiong Nan. 2023. "Forage Radish Cover Crops Improve Soil Quality and Fruit Yield of Lycium barbarum L. in an Arid Area of Northwest China" Agronomy 13, no. 6: 1634. https://doi.org/10.3390/agronomy13061634
APA StyleWang, F., Li, W., Chen, H., Weil, R. R., Zhu, L., & Nan, X. (2023). Forage Radish Cover Crops Improve Soil Quality and Fruit Yield of Lycium barbarum L. in an Arid Area of Northwest China. Agronomy, 13(6), 1634. https://doi.org/10.3390/agronomy13061634







