Investigation of Flower Yield and Quality in Different Color Safflower Genotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Methods
2.2.1. Soil and Climate Characteristics of the Research Field
2.2.2. Trial Fields
2.2.3. Chemical Analysis of Flowers
2.3. Statistical Analysis
3. Results and Discussion
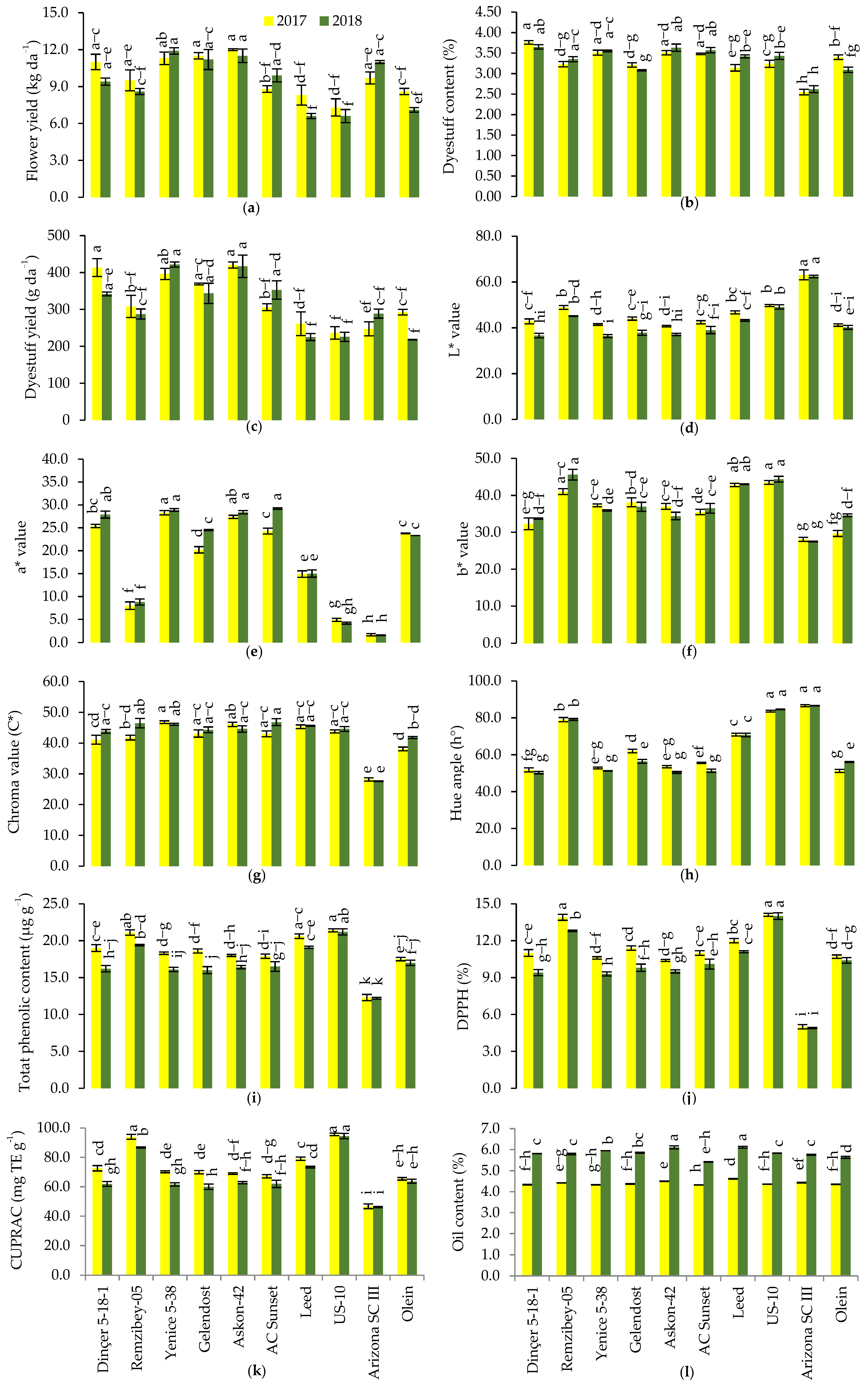
3.1. Flower Yield
3.2. Dyestuff Content
3.3. Dyestuff Yield
3.4. Color Values
3.5. The TPC and Antioxidant Capacity
3.6. Oil Content
3.7. Principal Component Analysis (PCA) of Characters
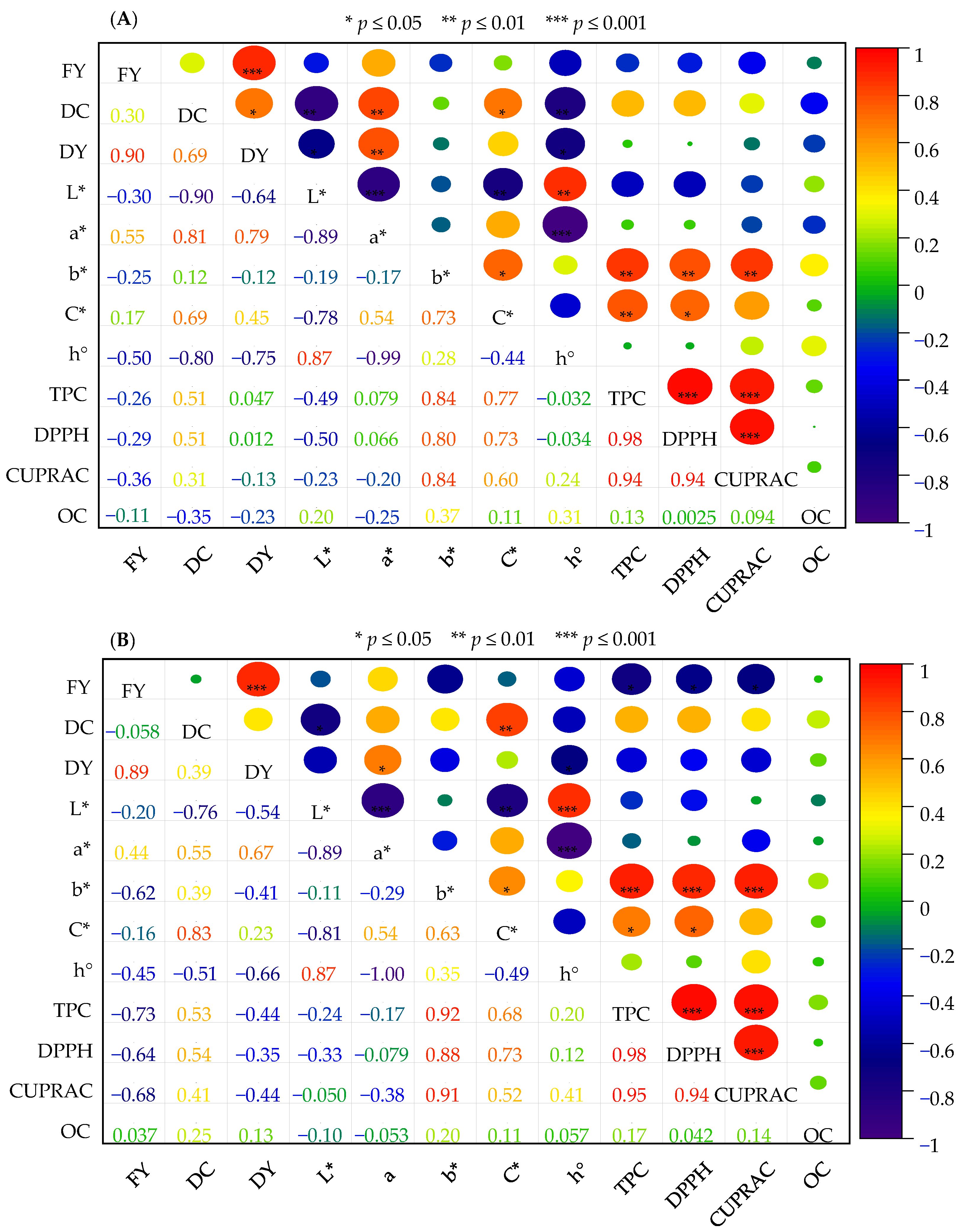
3.8. Correlation Analysis of Characters
3.9. Phenolic Compounds
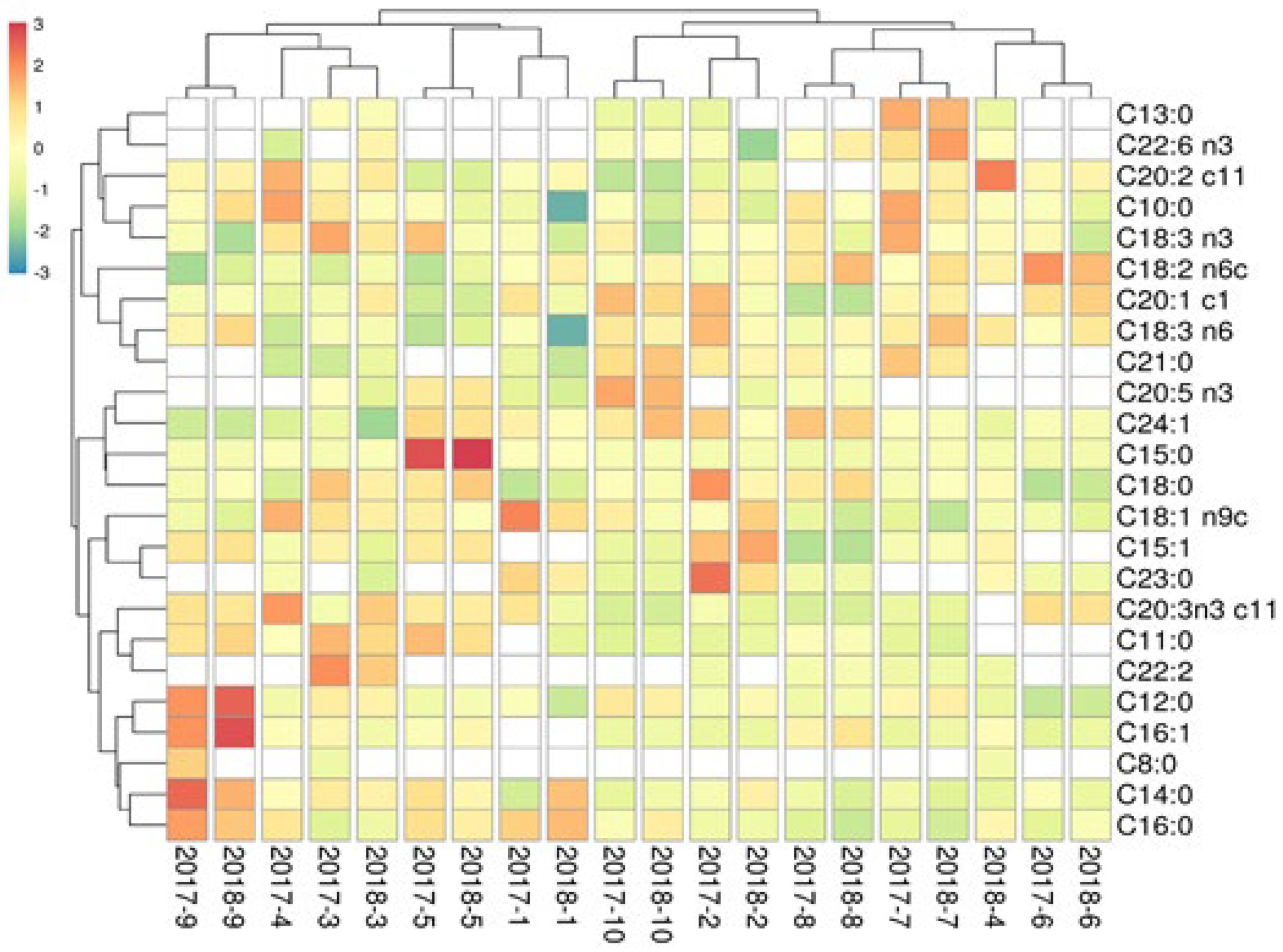
3.10. Fatty Acid Composition
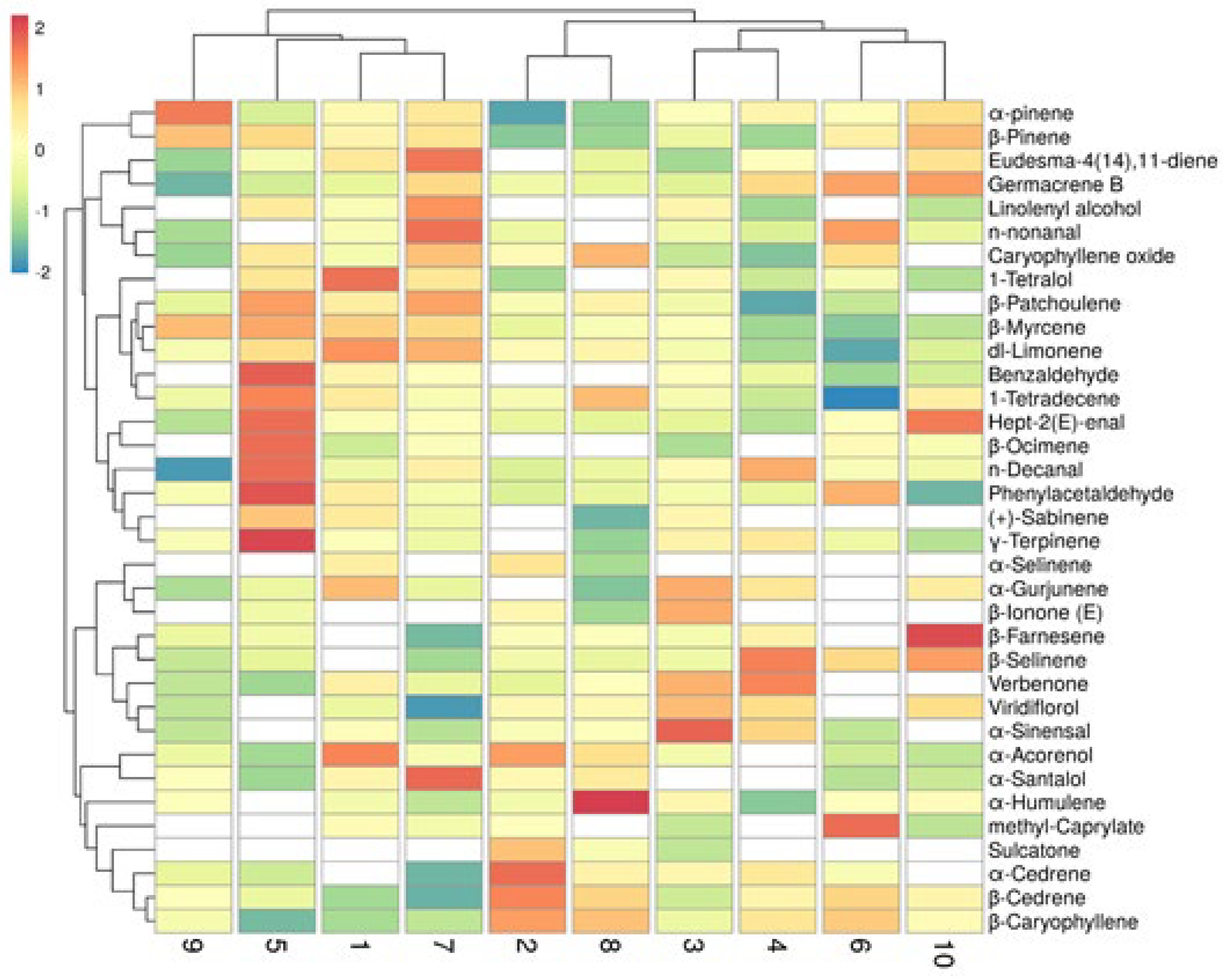
3.11. Floral Scent Compositions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT. 2023. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 23 January 2023).
- Aşkın, E.; Erbaş, S. Superior lines for agro-technological traits in safflower (Carthamus tinctorius L.). Turk. J. Field Crops 2020, 25, 50–56. [Google Scholar] [CrossRef]
- Weiss, E.A. Oilseed Crops, 2nd ed.; Blackwell Science Ltd.: Shoalhaven, NSW, Australia, 2000. [Google Scholar]
- Jingzhong, S.A. A probe into the safflower extract in cosmetics. In Proceedings of the 3rd International Safflower Conference, Beijing, China, 14–18 June 1993. [Google Scholar]
- Salem, N.; Msaada, K.; Elkahoui, S.; Mangano, G.; Azaeiz, S.; Ben Slimen, I.; Kefi, S.; Pintore, G.; Limam, F.; Marzouk, B. Evaluation of antibacterial, antifungal, and antioxidant activities of safflower natural dyes during flowering. BioMed Res. Int. 2014, 2014, 762397. [Google Scholar] [CrossRef]
- Lu, J.X.; Zhang, C.X.; Hu, Y.; Zhang, M.H.; Wang, Y.N.; Qian, Y.X.; Yang, J.; Yang, W.Z.; Jiang, M.M.; Guo, D.A. Application of multiple chemical and biological approaches for quality assessment of Carthamus tinctorius L. (safflower) by determining both the primary and secondary metabolites. Phytomedicine 2019, 58, 152826. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, G.; Devi, G.N.; Srinivas, C.V.S. Safflower petals and their chemical composition. In Proceedings of the 5th International Safflower Conference, Williston, ND, USA, 23–27 July 2001. [Google Scholar]
- Kim, M.N.; Le Scao- Bogaert, F.; Paris, M. Flavonoids from Carthamus tinctorius Flowers. Planta Med. 1992, 58, 285–286. [Google Scholar] [CrossRef]
- Kırıcı, S.; İnan, M. The Effects of different flower harvest dates on flower and seed yields and total dyestuff and oil ratios in safflower (Carthamus tinctorius L.). In Proceedings of the Turkey 4th Field Crops Congress, Tekirdag, Turkey, 17–21 September 2001. [Google Scholar]
- Dajue, L.; Mündel, H.H. Safflower, Promoting the Conservation and Use of Underutilized and Neglected Crops; Institute of Plant Genetics and Crop Plant Research, Gatersleben/International Plant Genetic Resources Institute: Rome, Italy, 1996; Volume 7, p. 83. [Google Scholar]
- Jiang, T.F.; Lv, Z.H.; Wang, Y.H. Separation and determination of chalcones from Carthamus tinctorius L. and its medicinal preparation by capillary zone electrophoresis. J. Sep. Sci. 2005, 28, 1244–1247. [Google Scholar] [CrossRef]
- Kim, E.O.; Oh, J.H.; Lee, S.K.; Lee, J.Y.; Choi, S.W. Antioxidant Properties and Quantification of Phenolic Compounds from Safflower (Carthamus tinctorius L.) Seeds. Food Sci. Biotechnol. 2007, 16, 71–77. [Google Scholar]
- Salem, N.; Msaada, K.; Hamdaoui, G.; Limam, F.; Marzouk, B. Variation in Phenolic Composition and Antioxidant Activity during Flower Development of Safflower (Carthamus tinctorius L.). J. Agric. Food Chem. 2011, 59, 4455–4463. [Google Scholar] [CrossRef]
- Si, W.; Yang, W.; Guo, D.; Wu, J.; Zhang, J.; Qiu, S.; Yao, C.; Cui, Y.; Wu, W. Selective ion monitoring of quinochalcone C-glycoside markers for the simultaneous identification of Carthamus tinctorius L. In eleven Chinese patent medicines by UHPLC/QTOF MS. J. Pharm. Biomed. Anal. 2016, 117, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.J.; Tang, Y.P.; Li, S.J.; Duan, J.A. Chemical and biological properties of quinochalcone C-glycosides from the florets of Carthamus tinctorius. Molecules 2013, 18, 15220–15254. [Google Scholar] [CrossRef] [PubMed]
- Mert, H.; Doğan, Y.; Başlar, S. Some plants used to obtain natural dye. J. Ecol. 1992, 5, 14–17. [Google Scholar]
- Kumar, J.K.; Sinha, A.K. Resurgence of natural colourants: A holistic view. Nat. Prod. Res. 2004, 18, 59–84. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Shabbir, M.; Mohammad, F. Natural colorants: Historical, processing and sustainable prospects. Nat. Prod. Bioprospect. 2017, 7, 123–145. [Google Scholar] [CrossRef] [PubMed]
- Algelini, L.G.; Pistelli, L.; Belloni, P.; Bertoli, A.; Panconesi, S. Rubia tinctorum a source of natural dyes: Agronomic evalotion, quantitative analysis of alizerin and industrial assays. Ind. Crops Prod. 1997, 6, 311–333. [Google Scholar]
- Bateman, B.; Warner, J.O.; Hutchinson, E.; Dean, T.; Rowlandson, P.; Gant, C.; Grundy, J.; Fitzgerald, C.; Stevenson, J. The effects of a double blind, placebo controlled artificial food colorings and benzoate preservative challenge on hyperactivity in a general population sample of preschool children. Arch. Dis. Child. 2004, 89, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Rudometova, N.V.; Pasovskij, A.P.; Blohina, E.A. Method of isolation and identification of carthamin from safflower: Application’s perspectives in Russian food products. In Proceedings of the 5th International Safflower Conference, Williston, ND, USA, 23–27 July 2001. [Google Scholar]
- Yoon, J.M.; Cho, M.H.; Park, J.E.; Kim, Y.H.; Hahn, T.R.; Paik, Y.S. Thermal stability of the pigments hydroxysafflor yellow A, safflor yellow B, and precarthamin from safflower (Carthamus tinctorius). J. Food Sci. 2003, 68, 839–843. [Google Scholar] [CrossRef]
- Kızıl, S.; Söğüt, T. A Study on the dyeing of wool carpet yarns with safflower (Carthamus tinctorius L.) flowers. In Proceedings of the Turkey 3rd Field Crops Congress, Adana, Turkey, 15–18 November 1999. [Google Scholar]
- Zheng, J.B.; Liu, X.X.; Zhou, Y.Z.; Suo, Z.R. Simultaneous determination of five phenolic compounds in dried flowers by LC using DAD combined electrochemical detection. Chromatographia 2007, 65, 707–712. [Google Scholar] [CrossRef]
- Ergönül, P.G.; Aksoylu Zeybek, O. Identification of bioactive compounds and total phenol contents of cold pressed oils from safflower and camelina seeds. J. Food Meas. Charact. 2018, 12, 2313–2323. [Google Scholar] [CrossRef]
- Yeloojeh, K.A.; Saeidi, G.; Sabzalian, M.R. Drought stress improves the composition of secondary metabolites in safflower flower at the expense of reduction in seed yield and oil content. Ind. Crops Prod. 2020, 154, 112496. [Google Scholar] [CrossRef]
- Qu, C.; Zhu, W.; Dong, K.; Pan, Z.; Chen, Y.; Chen, X.; Liu, X.; Xu, W.; Lin, H.; Zheng, Q.; et al. Inhibitory effect of hydroxysafflor yellow B on the proliferation of human breast cancer MCF-7 cells. Recent Pat. Anti-Cancer Drug Discov. 2019, 14, 187–197. [Google Scholar] [CrossRef]
- Yue, S.; Tang, Y.; Xu, C.; Li, S.; Zhu, Y.; Duan, J.A. Two new quinochalcone C-glycosides from the florets of Carthamus tinctorius. Int. J. Mol. Sci. 2014, 15, 16760–16771. [Google Scholar] [CrossRef]
- Bacchetti, T.; Morresi, C.; Bellachioma, L.; Ferretti, G. Antioxidant and pro-oxidant properties of Carthamus tinctorius, hydroxy safflor yellow A, and safflor yellow A. Antioxidants 2020, 9, 119. [Google Scholar] [CrossRef]
- Wincewicz, E.; Zawadzki, W.A.; Ostaszewska, M. Comparative study on the effect of evening primrose and safflower on selected morphological and biochemical blood parameters in experimental rats on a high-fat diet. Med. Weter. 2015, 71, 377–381. [Google Scholar]
- Zhu, H.; Wang, Z.; Ma, C.; Tian, J.; Fu, F.; Li, C.; Guo, D.; Roeder, E.; Liu, K. Neuroprotective effects of hydroxysaffor yellow A: In vivo and in vitro studies. Planta Med. 2003, 69, 429–433. [Google Scholar]
- Wang, J.; Zhang, Q.; Xie, H.; Gu, L.G.; Niu, X.Y.; Liu, L.T. Effect of hydroxy saffor Yellow A on the proliferation of human umbilical vein endothelial cells with the stimulus of tumor cell conditioned medium. CJTCMP 2009, 24, 572–575. [Google Scholar]
- Wang, C.C.; Choy, C.S.; Liu, Y.H.; Cheah, K.P.; Li, J.S.; Wang, J.T.; Yu, W.Y.; Lin, C.W.; Cheng, H.W.; Hu, C.M. Protective effect of dried saffower petal aqueous extract and its main constituent, carthamus yellow, against lipopolysaccharide-induced inflammation in RAW264.7 macrophages. J. Sci. Food Agric. 2011, 91, 218–225. [Google Scholar] [CrossRef]
- Chen, L.; Xiang, Y.; Kong, L.; Zhang, X.; Sun, B.; Wei, X.; Liu, H. Hydroxysaffor yellow A protects against cerebral ischemia-reperfusion injury by anti-apoptotic effect through PI3K/Akt/GSK3beta pathway in rat. Neurochem. Res. 2013, 38, 2268–2275. [Google Scholar] [CrossRef]
- Liu, L.; Si, N.; Ma, Y.; Ge, D.; Yu, X.; Fan, A.; Wang, X.; Hu, J.; Wei, P.; Ma, L.; et al. Hydroxysafflor-Yellow A Induces Human Gastric Carcinoma BGC-823 Cell Apoptosis by Activating Peroxisome Proliferator-Activated Receptor Gamma (PPARgamma). Med. Sci. Monit. 2018, 24, 803–811. [Google Scholar] [CrossRef]
- Kırıcı, S. Effects of gibberellic acid (GA3) on agronomic properties, flower yield and dyestuff ratio in two safflower cultivars. J. Field Crops Cent. Res. Inst. 1998, 7, 10–30. [Google Scholar]
- Azimi, S.; Chegini, G.; Kianmehr, M.H. Design and manufacture of safflower petal harvester machine. Mech. Ind. 2012, 13, 301–305. [Google Scholar] [CrossRef]
- Yun, G.; Lixin, Z.; Ying, Q.; Xiaopan, J.; Yuanbo, C. Dynamic model for sucking process of pneumatic cutting-type safflower harvest device. Int. J. Agric. Biol. Eng. 2016, 9, 43–50. [Google Scholar]
- Shin, Y.S.; Yoo, D.I. Storage stability and color reproducibility of yellow and red dyes extracted from Carthamus tinctorius L. Text. Color. Finish. 2012, 24, 165–172. [Google Scholar] [CrossRef]
- Rowell, D.L. Soil Science: Methods and Applications; Longman: London, UK, 1996. [Google Scholar]
- Koyuncu, M.A.; Dilmaçünal, T.; Bayindir, D.; Erbaş, S. The role of ethylene in determining oil rose (Rosa damascena Miller) storage life. Acta Hortic. 2013, 1012, 987–993. [Google Scholar] [CrossRef]
- Koyuncu, M.A.; Güneyli, A.; Erbaş, D.; Onursal, C.E.; Seçmen, T. Combined effects of MAP and postharvest salicylic acid treatment on quality attributes of dill (Anethum graveolens L.) bunches during storage. J. Agric. Sci. 2018, 24, 340–348. [Google Scholar] [CrossRef]
- Harborne, J.B. Phytochemical Methods a Guide to Modern Techniques of Plant Analysis; Chapman & Hall: London, UK, 1993. [Google Scholar]
- Singleton, V.L.; Rossi, J.R. Colorimetry of total phenolics with Phosphomolibdic-phosphothungstic acid. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Gülçin, İ. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute. INC SAS/STAT User’s Guide Release 9.0; SAS Institute: Cary, NC, USA, 1999. [Google Scholar]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, 566–570. [Google Scholar] [CrossRef]
- Patanè, C.; Cosentino, S.L.; Calcagno, S.; Pulvirentic, L.; Siracusac, L. How do sowing time and plant density affect the pigments safflomins and carthamin in florets of safflower. Ind. Crops Prod. 2020, 148, 112313. [Google Scholar] [CrossRef]
- El-Hamidi, A.; Ahmet, S.S.; El-Gawad, A.A.; Ezz El-Dın, A.A. The effect of nitrogen fertilizer and plant density on the production of carthamin. Planta Med. 1993, 59, 702. [Google Scholar] [CrossRef]
- Fatahi, N.; Carapetian, J.; Heidari, R. Comparing stability of carthamin and safflower yellow pigments at pH, temperature and light, from safflower (Carthamus tinctorius L.) florets. Res. J. Biol. Sci. 2009, 4, 250–253. [Google Scholar]
- Saito, K.; Takahashi, M. A new technique for the chemical processing of reddened florets from dyer’s saffron capitula. Food Chem. 1993, 48, 387–389. [Google Scholar] [CrossRef]
- Kulkarni, D.N.; Kulkarni, K.D.; Tathe, S. Studies on the extraction of safflower yellow B and carthamin red pigment from safflower florets as food colorant. In Proceedings of the 5th International Conference on Safflower, Williston, ND, USA, 23–27 July 2001. [Google Scholar]
- Vankar, P.; Tiwari, V.; Shanker, R.; Singh, S. Carthamus tintorius (Safflower), a commercially viable dye for textile. Asian Dye. 2004, 1, 25–29. [Google Scholar]
- Pu, Z.J.; Yue, S.J.; Zhou, G.S.; Yan, H.; Shi, X.Q.; Zhu, Z.H.; Huang, S.L.; Peng, G.P.; Chen, Y.Y.; Bai, J.Q.; et al. The comprehensive evaluation of safflowers in different producing areas by combined analysis of color, chemical compounds, and biological activity. Molecules 2019, 24, 3381. [Google Scholar] [CrossRef] [PubMed]
- Ekin, Z. Resurgence of safflower (Carthamus tinctorius L.) utilization: A global view. J. Agron. 2005, 4, 83–87. [Google Scholar] [CrossRef]
- Krížová, H. Natural dyes: Their past, present, future and sustainability. Recent Dev. Fibrous Mater. Sci. 2015, 12, 59–71. [Google Scholar]
- Erbaş, S.; Baydar, H.; Ünlüer, M.; Dalgıç, D. The effect of ontogenetic variability on essential oil content and composition, total phenolic content and antioxidant activity in Rosemary Rosmarinus officinalis L. In Proceedings of the Turkey 10th Field Crops Congress, Konya, Turkey, 10–13 September 2013; pp. 1090–1096. [Google Scholar]
- Baydar, H.; Özkan, G. Antioxidant activities of safflower (Carthamus tinctorius L.) petal extracts. In Proceedings of the VI International Safflower Conference, SAFFLOWER: A Unique Crop for Oil Spices and Health Consequently, a Better Life for You, Istanbul, Turkey, 6–10 June 2005. [Google Scholar]
- Hiramatsu, M.; Takahashi, T.; Komatsu, M.; Kido, T.; Kasahara, Y. Antioxidant and neuroprotective activities of Mogamibenibana (safflower, Carthamus tinctorius Linne). Neurochem. Res. 2009, 34, 795–805. [Google Scholar] [CrossRef]
- Karimkhani, M.M.; Shaddel, R.; Khodaparast, M.H.H.; Vazirian, M.; Piri-Gheshlaghi, S. Antioxidant and antibacterial activity of safflower (Carthamus tinctorius L.) extract from four different cultivars. Qual. Assur. Saf. Crop. Foods 2016, 8, 565–574. [Google Scholar] [CrossRef]
- Özkan, K.; Bekiroglu, H.; Bayram, Y.; Sagdic, O.; Erbaş, S. In vitro bioaccessibility, antioxidant and antibacterial activities of three different safflower (Carthamus tinctorius L.) genotypes. Food Sci. Technol. 2022, 42, 08921. [Google Scholar] [CrossRef]
- Bibi Sadeer, N.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The versatility of antioxidant assays in food science and safety—Chemistry, applications, strengths, and limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, P.; Liu, D.; Song, W.; Zhu, L.; Liu, X. Chemical Composition and In Vitro Antioxidant Activity of Sida rhombifolia L. Volatile Organic Compounds. Molecules 2022, 27, 7067. [Google Scholar] [CrossRef]
- Capanoglu, E.; Kamiloglu, S.; Ozkan, G.; Apak, R. Evaluation of antioxidant activity/capacity measurement methods for food products. In Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications; Apak, R., Capanoglu, E., Shahidi, F., Eds.; John Wiley & Sons: Chicester, UK, 2018; pp. 273–286. [Google Scholar] [CrossRef]
- Srinivas, C.V.S.; Praveena, B.; Nagaraj, G. Safflower petals: A source of gamma linolenic acid. Plant Foods Hum. Nutr. 1999, 54, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Machewad, G.M.; Ghatge, P.; Chappalwar, V.; Jadhav, B.; Chappalwar, A. Studies on extraction of safflower pigments and its utilization in ice cream. Int. J. Food Process. Technol. 2012, 3, 172. [Google Scholar]
- Öten, M.; Albayrak, S. Determination of variation between some alfalfa (Medicago sativa L.) genotypes by principal component and clustering analysis. Turk J. Agric. Res. 2018, 5, 222–228. [Google Scholar]
- Yilmaz, Y.; Toledo, R.T. Major flavonoids in grape seeds and skin: Antioxidant capacity of catechin, epicatechin, and gallic acid. J. Agric. Food Chem. 2004, 52, 255–260. [Google Scholar] [CrossRef]
- Kim, D.O.; Lee, C. Comprehensive study on vitamin C equivalent antioxidant capacity (VCEAC) of various polyphenolics in scavenging a free radical and its structural relationship. Crit. Rev. Food Sci. Nutr. 2004, 44, 253–273. [Google Scholar] [CrossRef]
- Manach, C.; Mazur, A.; Scalbert, A. Polyphenols and prevention of cardiovascular diseases. Curr. Opin. Lipidol. 2005, 16, 77–84. [Google Scholar] [CrossRef]
- Denny, A.; Buttriss, J. Plant foods and health: Focus on plant bioactives. Synth. Rep. 2007, 4, 1–64. [Google Scholar]
- Knekt, P.; Kumpulainen, J.; Järvinen, R.; Rissanen, H.; Heliövaara, M.; Reunanen, A.; Hakulinen, T.; Aromaa, A. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 2002, 76, 560–568. [Google Scholar] [CrossRef]
- Salvamani, S.; Gunasekaran, N.; Shaharuddin, N.A.; Ahmad, S.A.; Shukor, M.S. Antiartherosclerotic Effects of Plant Flavonoids. BioMed Res. Int. 2014, 2014, 480258. [Google Scholar] [CrossRef]
- Onder, S.; Tonguc, M.; Erbas, S.; Onder, D.; Mutlucan, M. Investigation of phenological, primary and secondary metabolites changes during flower developmental of Rosa damascena. Plant Physiol. Biochem. 2022, 192, 20–34. [Google Scholar] [CrossRef]
- Futehally, S.; Knowles, P.F. Inheritance of very high levels of linoleic acid in an introduction of safflower (Carthamus tinctorius L.) from Portugal. In First International Safflower Conference; Knowles, P.F., Ed.; University of California: Davis, CA, USA, 1981; pp. 56–61. [Google Scholar]
- Hamdan, Y.A.S.; Vich, B.P.; Fernandez-Martinez, J.; Velasco, L. Inheritance of very high linoleic acid content and relationship with nuclear male sterility in safflower. Plant Breed. 2008, 127, 507–509. [Google Scholar] [CrossRef]
- Erbas, S. Development of Safflower (Carthamus tinctorius L.) Lines with High Oil, Oleic Acid Content and Seed Yield through Hybridization Breeding. Ph.D. Thesis, Suleyman Demirel University, Isparta, Turkey, 2012. [Google Scholar]
- Murrin, L.C. Gamolenic Acid. In xPharm: The Comprehensive Pharmacology Reference; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–5. [Google Scholar]
- Qureshi, A.A.; Schnoes, H.K.; Din, Z.Z.; Peterson, D.M. Determination of the structure of cholesterol inhibitor II isolated from high-protein barley flour (HPBF). Fed. Proc. 1984, 43, 2626. [Google Scholar]
- Sunil, T.; Pai, M.D. Peripheral Neuropathy. In Integrative Medicine; Kakel, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Lapinskas, P. Oil Crops for the Pharmaceutical Industry in Seed Storage Compound; Shewny, P.R., Stobart, K., Eds.; Clarendon Press: Oxford, UK, 1993. [Google Scholar]
- Zheng, G.; Kenney, P.M.; Lam, L.K. Sesquiterpenes from clove (Eugenia caryophyllata) as potential anticarcinogenic agents. J. Nat. Prod. 1992, 55, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Sensch, O.; Vierling, W.; Brandt, W.; Reiter, M. Effects of inhibition of calcium and potassium currents in guinea-pig cardiac contraction: Comparison of β-caryophyllene oxide, eugenol, and nifedipine. Br. J. Pharmacol. 2000, 131, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Turgumbayeva, A.A.; Ustenova, G.O.; Yeskalieva, B.K.; Ramazanova, B.A.; Rahimov, K.D.; Aisa, H.; Juszkiewicz, K.T. Volatile oil composition of Carthamus tinctorius L. Flowers grown in Kazakhstan. AAEM 2018, 25, 87–89. [Google Scholar] [CrossRef]
- Ziarati, P.; Asgarpanah, J.; Kianifard, M. The essential oil composition of Carthamus tinctorius L. flowers growing in Iran. African J. Biotechnol. 2012, 11, 12921–12924. [Google Scholar]
- Shao, J.; Wang, Y.; Chen, Q.; Liu, Z.; Liu, Q.; Cai, W.; Wu, W. A Daily variations in essential oil components of flowers of different accessions from Carthamus tinctorius L. in Sichuan province of China. J. Med. Plants Res. 2011, 5, 3042–3051. [Google Scholar]



| Month | Total Precipitation, L m2 | Mean Temperature, °C | Mean Humidity, % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1950–2018 | 2017 | 2018 | 1950–2018 | 2017 | 2018 | 1950–2018 | 2017 | 2018 | |||||
| Min. | Max. | Mean | Min. | Max. | Mean | ||||||||
| March | 57.3 | 74.4 | 69.3 | 6.1 | −4.4 | 21.8 | 7.3 | −1.6 | 20.4 | 9.2 | 65.6 | 64.1 | 65.9 |
| April | 51.6 | 25.6 | 6.3 | 10.7 | −0.9 | 25.5 | 10.6 | 0.0 | 28.3 | 14.2 | 60.8 | 59.6 | 51.0 |
| May | 55.7 | 149.5 | 62.9 | 15.2 | 4.0 | 31.9 | 14.9 | 5.8 | 29.6 | 16.8 | 58.7 | 63.7 | 62.3 |
| June | 32.6 | 30.9 | 69.4 | 19.8 | 6.6 | 35.8 | 20.1 | 10.3 | 32.6 | 20.0 | 52.1 | 58.9 | 62.4 |
| July | 16.5 | 13.1 | 4.1 | 23.3 | 12.6 | 34.3 | 24.3 | 13.8 | 38.6 | 25.2 | 45.4 | 41.9 | 46.9 |
| August | 13.4 | 20.4 | 14.2 | 23.1 | 12.1 | 36.9 | 23.8 | 12.1 | 34.4 | 24.3 | 46.3 | 52.1 | 47.6 |
| TOTAL | 227.1 | 313.9 | 226.2 | ||||||||||
| MEAN | 16.4 | 5.2 | 31.8 | 17.0 | 6.5 | 29.9 | 18.1 | 54.8 | 56.7 | 56.0 | |||
| Sources of Variance | DF | Flower Yield (kg da−1) | Dyestuff Content (%) | Dyestuff Yield (g da−1) | L* Value | a* Value | b* Value | Chroma Value (C*) | Hue Angle (h°) | Total Phenolic Content (µg g−1) | DPPH | CUPRAC | Oil Content (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Block | 2 | 0.43 a | 0.01 | 1332.77 | 0.02 | 1.11 | 2.44 | 1.27 | 0.24 | 0.04 | 0.02 | 0.41 | 0.001 |
| Year (Y) | 1 | 2.89 * | 0.02 | 2483.26 | 174.14 ** | 26.40 ** | 7.76 | 30.37 ** | 16.08 ** | 32.25 ** | 11.58 ** | 486.89 ** | 30.64 ** |
| Genotype (G) | 9 | 18.26 ** | 0.58 ** | 29,000.80 ** | 327.78 ** | 639.54 ** | 166.75 ** | 176.96 ** | 1257.65 ** | 36.86 ** | 35.25 ** | 1173.35 ** | 0.11 ** |
| Y × G | 9 | 1.83 * | 0.05 ** | 2670.05 ** | 6.68 * | 5.77 ** | 9.15 ** | 6.94 ** | 13.05 ** | 1.34 ** | 0.47 ** | 19.53 ** | 0.03 ** |
| Error | 38 | 0.68 | 0.01 | 885.98 | 2.41 | 0.70 | 2.44 | 2.29 | 1.58 | 0.34 | 0.12 | 5.09 | 0.001 |
| CV (%) | 8.62 | 3.16 | 9.34 | 3.50 | 4.54 | 4.23 | 3.56 | 1.96 | 3.27 | 3.24 | 3.21 | 0.68 |
| RTa | Phenolic Compound | 2017 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| 5.2 | Gallic | 142.1 ± 3.3 | 71.3 ± 1.2 | 159.3 ± 4.6 | 83.0 ± 1.9 | 145.2 ± 2.5 | 89.0 ± 2.6 | 147.9 ± 3.4 | 66.2 ± 1.9 | 63.5 ± 1.5 | 110.0 ± 1.9 |
| 12.0 | Catechin | 21.4 ± 0.5 | 14.4 ± 0.2 | 16.9 ± 0.5 | 10.1 ± 0.2 | 13.5 ± 0.2 | 10.1 ± 0.3 | 17.4 ± 0.4 | 18.3 ± 0.5 | 3.6 ± 0.1 | 8.9 ± 0.2 |
| 14.0 | Chlorogenic | 19.7 ± 0.5 | 8.3 ± 0.1 | 24.7 ± 0.7 | 19.5 ± 0.4 | 36.1 ± 0.6 | 17.3 ± 0.5 | 29.6 ± 0.7 | 9.5 ± 0.3 | 4.3 ± 0.1 | 19.4 ± 0.3 |
| 20.0 | Syringic | 2.0 ± 0.0 | 4.2 ± 0.1 | 2.0 ± 0.1 | 2.7 ± 0.1 | 4.7 ± 0.1 | 3.5 ± 0.1 | 3.4 ± 0.1 | 3.6 ± 0.1 | - | 6.8 ± 0.1 |
| 45.0 | Rutine | 1.4 ± 0.0 | 3.1 ± 0.1 | 1.4 ± 0.0 | 1.1 ± 0.0 | 2.0 ± 0.0 | 2.0 ± 0.1 | 1.3 ± 0.0 | 4.1 ± 0.1 | 3.4 ± 0.1 | 3.9 ± 0.1 |
| 57.0 | Rosmarinic | 78.3 ± 1.8 | 69.3 ± 0.9 | 73.7 ± 2.5 | 54.8 ± 1.7 | 82.1 ± 1.4 | 78.0 ± 2.0 | 85.5 ± 2.1 | 75.7 ± 1.9 | 26.9 ± 0.6 | 45.7 ± 1.4 |
| 72.0 | Quercetin | 19.9 ± 0.5 | 9.8 ± 0.2 | 19.9 ± 0.6 | 16.4 ± 0.4 | 26.4 ± 0.5 | 14.2 ± 0.4 | 18.8 ± 0.4 | 12.0 ± 0.3 | 6.7 ± 0.2 | 14.2 ± 0.2 |
| 74.0 | Luteolin | 1.3 ± 0.0 | 5.3 ± 0.1 | 1.3 ± 0.0 | 2.5 ± 0.1 | 1.3 ± 0.0 | 3.4 ± 0.1 | 1.8 ± 0.0 | 4.4 ± 0.1 | - | 3.7 ± 0.1 |
| 77.0 | Kaempferol | 34.5 ± 0.8 | 33.2 ± 0.6 | 42.6 ± 1.2 | 51.6 ± 1.2 | 41.5 ± 0.7 | 52.2 ± 1.5 | 47.3 ± 1.1 | 29.5 ± 0.9 | 16.2 ± 0.4 | 55.8 ± 1.0 |
| RT* | Phenolic Compound | 2018 | |||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| 5.2 | Gallic | 147.8 ± 3.4 | 74.1 ± 1.3 | 165.7 ± 4.8 | 86.3 ± 2.0 | 151.0 ± 2.6 | 92.6 ± 2.7 | 153.9 ± 3.6 | 68.9 ± 2.0 | 66.0 ± 1.5 | 114.4 ± 2.0 |
| 12.0 | Catechin | 19.9 ± 0.5 | 13.4 ± 0.2 | 15.7 ± 0.5 | 9.4 ± 0.2 | 12.5 ± 0.2 | 9.4 ± 0.3 | 16.2 ± 0.4 | 17.0 ± 0.5 | 3.4 ± 0.1 | 8.3 ± 0.1 |
| 14.0 | Chlorogenic | 19.1 ± 0.4 | 8.0 ± 0.1 | 23.9 ± 0.7 | 18.9 ± 0.4 | 35.1 ± 0.6 | 16.7 ± 0.5 | 28.7 ± 0.7 | 9.2 ± 0.3 | 4.1 ± 0.1 | 18.8 ± 0.3 |
| 20.0 | Syringic | 2.2 ± 0.0 | 4.5 ± 0.1 | 2.2 ± 0.1 | 2.9 ± 0.1 | 5.1 ± 0.1 | 3.7 ± 0.1 | 3.7 ± 0.1 | 3.8 ± 0.1 | - | 7.3 ± 0.2 |
| 45.0 | Rutine | 1.3 ± 0.0 | 2.9 ± 0.1 | 1.3 ± 0.0 | 1.0 ± 0.0 | 1.9 ± 0.0 | 1.9 ± 0.1 | 1.2 ± 0.0 | 3.9 ± 0.1 | 3.2 ± 0.1 | 3.6 ± 0.1 |
| 57.0 | Rosmarinic | 75.9 ± 1.8 | 67.2 ± 0.9 | 71.5 ± 2.4 | 53.2 ± 1.7 | 79.6 ± 1.4 | 75.7 ± 1.9 | 82.9 ± 2.0 | 88.3 ± 1.8 | 26.1 ± 0.6 | 43.8 ± 1.3 |
| 72.0 | Quercetin | 21.8 ± 0.5 | 10.8 ± 0.2 | 21.8 ± 0.6 | 18.1 ± 0.4 | 29.0 ± 0.5 | 15.6 ± 0.5 | 20.7 ± 0.5 | 13.2 ± 0.4 | 7.3 ± 0.2 | 15.6 ± 0.3 |
| 74.0 | Luteolin | 1.4 ± 0.0 | 5.9 ± 0.1 | 1.5 ± 0.0 | 2.7 ± 0.1 | 1.5 ± 0.0 | 3.8 ± 0.1 | 2.0 ± 0.0 | 5.0 ± 0.1 | - | 4.1 ± 0.1 |
| 77.0 | Kaempferol | 36.9 ± 0.9 | 35.5 ± 0.6 | 45.5 ± 1.3 | 55.2 ± 1.3 | 44.4 ± 0.8 | 55.9 ± 1.6 | 50.6 ± 1.2 | 31.5 ± 0.9 | 17.3 ± 0.4 | 59.7 ± 1.0 |
| RTa | Fatty Acids | 2017 | 2018 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| 18.6 | C8:0 | - | - | 0.16 | - | - | - | - | - | 1.26 | - | - | - | - | 0.21 | - | - | - | - | - | - |
| 24.3 | C10:0 | 3.21 | 3.58 | 3.70 | 4.11 | 3.47 | 3.40 | 4.07 | 3.75 | 3.46 | 3.39 | 2.47 | 2.98 | 3.46 | 3.47 | 3.13 | 3.07 | 3.67 | 3.38 | 3.81 | 2.93 |
| 26.4 | C11:0 | - | 0.26 | 0.72 | 0.43 | 0.71 | - | 0.26 | 0.45 | 0.59 | 0.23 | 0.25 | 0.29 | 0.64 | - | 0.61 | - | 0.22 | 0.39 | 0.65 | 0.24 |
| 30.2 | C12:0 | 1.72 | 1.59 | 2.08 | 1.55 | 1.54 | 1.10 | 1.93 | 1.51 | 2.71 | 2.13 | 1.13 | 1.88 | 1.98 | 1.42 | 1.63 | 1.17 | 2.05 | 1.60 | 2.99 | 2.05 |
| 33.4 | C13:0 | - | 0.19 | 0.55 | - | - | - | 1.18 | - | - | 0.24 | - | - | 0.44 | 0.21 | - | - | 1.13 | - | - | 0.21 |
| 36.1 | C14:0 | 2.93 | 3.62 | 4.39 | 3.95 | 4.63 | 3.76 | 3.48 | 3.43 | 5.77 | 3.21 | 4.93 | 4.23 | 4.21 | 3.22 | 4.09 | 3.32 | 3.07 | 3.03 | 5.10 | 3.47 |
| 38.9 | C15:0 | 0.66 | 0.43 | 0.46 | 0.65 | 2.99 | 0.35 | 0.47 | 0.29 | 0.46 | 0.51 | 0.79 | 0.47 | 0.65 | 0.41 | 3.23 | 0.38 | 0.51 | 0.31 | 0.50 | 0.53 |
| 41.3 | C15:1 | - | 0.94 | 0.69 | 0.51 | 0.75 | - | 0.52 | 0.20 | 0.78 | 0.42 | - | 1.01 | 0.35 | 0.68 | 0.78 | - | 0.54 | 0.21 | 0.81 | 0.40 |
| 41.7 | C16:0 | 18.96 | 14.62 | 13.37 | 17.90 | 18.28 | 13.56 | 13.80 | 13.34 | 20.37 | 15.49 | 19.45 | 14.55 | 14.37 | 16.66 | 17.20 | 15.49 | 12.98 | 12.55 | 19.17 | 17.45 |
| 43.1 | C16:1 | - | 0.53 | 1.09 | 0.98 | 0.86 | 0.43 | 0.47 | 1.11 | 1.97 | 0.55 | - | 0.53 | 0.64 | 1.00 | 1.08 | 0.54 | 0.59 | 1.39 | 2.47 | 0.61 |
| 46.9 | C18:0 | 1.95 | 4.94 | 4.41 | 2.19 | 3.92 | 1.82 | 2.96 | 3.80 | 2.87 | 3.30 | 2.24 | 3.49 | 3.66 | 3.38 | 4.32 | 2.01 | 3.26 | 4.19 | 3.16 | 2.98 |
| 48.2 | C18:1 n9c | 7.52 | 5.63 | 6.33 | 6.97 | 6.03 | 5.08 | 4.56 | 4.72 | 5.01 | 6.05 | 6.45 | 6.64 | 5.98 | 5.22 | 5.44 | 4.58 | 4.12 | 4.26 | 4.52 | 5.23 |
| 50.1 | C18:2 n6c | 40.30 | 38.47 | 35.24 | 38.05 | 33.63 | 48.27 | 40.49 | 43.47 | 32.77 | 39.27 | 42.35 | 41.25 | 38.56 | 41.79 | 37.08 | 46.36 | 44.12 | 46.26 | 35.49 | 41.66 |
| 51.3 | C18:3 n6 | 2.85 | 3.54 | 2.84 | 2.32 | 2.24 | 2.94 | 3.18 | 2.74 | 3.07 | 3.24 | 1.85 | 2.98 | 2.77 | 3.24 | 2.47 | 3.24 | 3.51 | 3.02 | 3.38 | 3.11 |
| 52.5 | C20:1 c1 | 0.82 | 0.99 | 0.45 | 0.34 | 0.20 | 0.83 | 0.65 | 0.12 | 0.47 | 0.98 | 0.42 | 0.49 | 0.75 | - | 0.22 | 0.92 | 0.72 | 0.13 | 0.52 | 0.88 |
| 53.6 | C18:3 n3 | 16.43 | 16.54 | 18.50 | 17.56 | 18.16 | 16.87 | 18.46 | 17.39 | 16.36 | 17.18 | 15.29 | 16.65 | 17.42 | 16.84 | 16.39 | 15.23 | 16.66 | 15.69 | 14.76 | 14.86 |
| 55.1 | C21:0 | 0.49 | 0.76 | 0.38 | 0.38 | - | - | 0.89 | 0.72 | - | 0.84 | 0.35 | 0.69 | 0.48 | - | - | - | 0.77 | 0.62 | - | 0.89 |
| 55.9 | C20:2 c11 | 0.44 | 0.28 | 0.53 | 0.80 | 0.22 | 0.51 | 0.55 | - | 0.53 | 0.16 | 0.52 | 0.33 | 0.61 | 0.93 | 0.23 | 0.54 | 0.58 | - | 0.56 | 0.15 |
| 57.2 | C20:3 n3 c11 | 0.40 | 0.23 | 0.22 | 0.55 | 0.37 | 0.43 | 0.16 | 0.10 | 0.41 | 0.10 | 0.19 | 0.14 | 0.46 | - | 0.36 | 0.41 | 0.15 | 0.10 | 0.39 | 0.09 |
| 59.1 | C23:0 | 0.55 | 0.83 | - | 0.22 | - | 0.18 | - | 0.18 | - | 0.09 | 0.42 | 0.52 | 0.04 | 0.34 | - | 0.16 | - | 0.16 | - | 0.10 |
| 59.4 | C22:2 | - | 0.32 | 2.18 | - | - | - | 0.28 | 0.44 | - | - | - | - | 1.64 | 0.26 | - | - | 0.30 | 0.48 | - | - |
| 60.8 | C20:5 n3 | 0.16 | - | 0.42 | - | 0.62 | - | - | 0.35 | - | 0.91 | 0.09 | 0.23 | 0.14 | - | 0.65 | - | - | 0.36 | - | 0.86 |
| 61.8 | C24:1 | 0.61 | 0.78 | 0.37 | 0.26 | 0.75 | 0.47 | 0.50 | 0.81 | 0.22 | 0.66 | 0.54 | 0.49 | 0.09 | 0.33 | 0.71 | 0.44 | 0.47 | 0.76 | 0.21 | 0.83 |
| 61.9 | C22:6 n3 | - | 0.25 | - | 0.12 | - | - | 0.41 | 0.27 | - | 0.25 | - | 0.02 | 0.32 | 0.27 | - | - | 0.51 | 0.34 | - | 0.28 |
| ΣSFA | 30.5 | 30.8 | 30.2 | 31.4 | 35.5 | 24.2 | 29.0 | 27.5 | 37.5 | 29.4 | 32.0 | 29.1 | 29.9 | 29.3 | 34.2 | 25.6 | 27.7 | 26.2 | 35.4 | 30.9 | |
| ΣMUFA | 9.0 | 8.9 | 8.9 | 9.1 | 8.6 | 6.8 | 6.7 | 7.0 | 8.5 | 8.7 | 7.4 | 9.2 | 7.8 | 7.2 | 8.2 | 6.5 | 6.4 | 6.8 | 8.5 | 8.0 | |
| ΣPUFA | 60.6 | 59.6 | 59.9 | 59.4 | 55.2 | 69.0 | 63.5 | 64.8 | 53.1 | 61.1 | 60.3 | 61.6 | 61.9 | 63.3 | 57.2 | 65.8 | 25.8 | 66.3 | 54.6 | 61.0 | |
| ΣTUFA/ΣSFA | 2.0 | 1.9 | 2.0 | 1.9 | 1.6 | 2.9 | 2.2 | 2.4 | 1.4 | 2.1 | 1.9 | 2.1 | 2.1 | 2.2 | 1.7 | 2.6 | 0.9 | 2.5 | 1.5 | 2.0 | |
| LRI a | Compound | Formula | Class | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 902 | Hept-2(E)-enal | C7H12O | AA | 0.45 | 0.30 | 0.26 | 0.12 | 1.02 | 0.49 | 0.49 | 0.26 | 0.12 | 0.98 |
| 936.1 | α-pinene | C10H16 | MH | 14.95 | 4.65 | 13.74 | 15.23 | 9.95 | 14.36 | 16.59 | 6.84 | 22.05 | 17.65 |
| 964 | Benzaldehyde | C7H6O | AA | 0.84 | - | 0.74 | 0.49 | 1.56 | 0.14 | 0.74 | - | - | 0.35 |
| 973 | (+)-Sabinene | C10H16 | MH | 0.66 | - | 0.59 | - | 0.78 | - | 0.46 | 0.12 | - | - |
| 977.1 | β-Pinene | C10H16 | MH | 2.50 | 1.00 | 1.86 | 1.14 | 2.96 | 2.59 | 2.79 | 1.12 | 3.12 | 3.17 |
| 978.2 | Sulcatone | C8H14O | AK | - | 0.28 | 0.04 | - | - | - | - | 0.14 | - | - |
| 989.2 | β-Myrcene | C10H16 | MH | 6.09 | 3.51 | 4.52 | 2.25 | 6.64 | 1.98 | 5.91 | 4.35 | 6.37 | 2.71 |
| 1030 | dl-Limonene | C10H16 | MH | 2.15 | 1.36 | 1.12 | 0.49 | 1.74 | 0.14 | 1.98 | 1.45 | 1.15 | 0.79 |
| 1041 | Phenylacetaldehyde | C8H8O | AA | 1.38 | 0.67 | 0.96 | 0.77 | 2.25 | 1.78 | 0.97 | 0.78 | 1.03 | 0.14 |
| 1046 | β-Ocimene | C10H16 | MH | 0.58 | - | 0.41 | - | 2.05 | 1.14 | 1.02 | - | - | 0.99 |
| 1059.7 | γ-Terpinene | C10H16 | MH | 0.98 | - | 1.18 | 1.32 | 2.24 | 0.74 | 0.74 | 0.14 | 0.92 | 0.33 |
| 1103.3 | n-nonanal | C9H18O | AA | 0.46 | 0.36 | 0.39 | 0.26 | - | 0.88 | 1.00 | - | 0.12 | 0.33 |
| 1148 | methyl-Caprylate | C9H18O2 | OC | 0.45 | 0.43 | 0.14 | - | - | 0.88 | 0.33 | - | - | 0.12 |
| 1179 | Verbenone | C10H14O | OC | 0.75 | 0.37 | 1.04 | 1.19 | 0.12 | - | 0.42 | 0.62 | 0.24 | - |
| 1205.4 | n-Decanal | C10H20O | AA | 0.42 | 0.35 | 0.54 | 0.77 | 0.89 | 0.49 | 0.59 | 0.40 | 0.09 | 0.44 |
| 1408.6 | α-Gurjunene | C15H24 | SH | 2.72 | - | 2.86 | 2.28 | 1.16 | - | 1.14 | 0.15 | 0.49 | 2.14 |
| 1412.2 | α-Cedrene | C15H24 | SH | - | 6.91 | 4.21 | 4.75 | 2.21 | 3.49 | 0.78 | 4.36 | 2.76 | - |
| 1412.2 | β-Cedrene | C15H24 | SH | 8.12 | 19.29 | 9.58 | 14.35 | 11.12 | 16.49 | 6.36 | 16.47 | 13.13 | 14.26 |
| 1419 | β-Caryophyllene | C15H24 | SH | 23.40 | 34.75 | 26.79 | 31.42 | 21.59 | 33.00 | 24.21 | 33.49 | 27.85 | 29.47 |
| 1425.6 | β-Ionone (E) | C13H20O | AK | - | 0.34 | 0.47 | - | 0.26 | - | - | 0.14 | - | - |
| 1445.9 | β-Farnesene | C15H24 | SH | - | 1.04 | 0.98 | 1.19 | 0.96 | - | 0.49 | 1.06 | 0.89 | 1.79 |
| 1453.1 | α-Humulene | C15H24 | SH | 1.02 | 1.01 | 1.25 | 0.47 | - | 1.16 | 0.68 | 2.12 | 1.12 | 1.19 |
| 1457.2 | β-Patchoulene | C15H24 | SH | 3.34 | 2.58 | 2.34 | 0.49 | 4.41 | 1.55 | 4.33 | 3.14 | 1.96 | - |
| 1486.1 | β-Selinene | C15H24 | SH | - | 1.31 | 1.26 | 3.01 | 1.05 | 2.31 | 0.49 | 1.12 | 0.75 | 2.78 |
| 1493.4 | α-Selinene | C15H24 | SH | 0.43 | 0.44 | - | - | - | - | - | 0.35 | - | - |
| 1572 | 1-Tetradecene | C14H28 | ALH | 12.43 | 11.24 | 11.22 | 9.99 | 14.25 | 7.89 | 11.29 | 13.45 | 10.84 | 12.28 |
| 1590.8 | Viridiflorol | C15H26O | SA | 0.79 | 0.99 | 1.26 | 1.17 | - | - | 0.36 | 1.00 | 0.63 | 1.16 |
| 1630 | α-Acorenol | C15H26O | SA | 1.86 | 1.78 | 1.21 | - | 0.85 | 0.99 | 1.24 | 1.56 | 1.14 | 0.94 |
| 1688 | Eudesma-4(14),11-diene | C15H24 | SH | 1.42 | - | 0.47 | 1.14 | 1.03 | - | 2.00 | 0.83 | 0.41 | 1.49 |
| 1692 | α-Santalol | C15H24O | SAL | 1.12 | 1.07 | - | - | 0.33 | 0.44 | 1.86 | 1.23 | 1.01 | 0.51 |
| 1708.2 | Germacrene B | C15H24 | SH | 1.12 | 1.26 | 1.04 | 1.99 | 0.96 | 2.29 | 2.01 | 1.12 | 0.42 | 2.33 |
| 1753.5 | α-Sinensal | C15H22O | SALD | 0.45 | 0.39 | 0.97 | 0.67 | - | 0.12 | 0.09 | 0.41 | 0.12 | - |
| 1760 | Linolenyl alcohol | C18H32O | SAL | 0.93 | - | 1.14 | 0.22 | 1.26 | - | 1.79 | - | - | 0.37 |
| 1800 | 1-Tetralol | C20H22O | AH | 6.90 | 0.89 | 3.78 | 1.48 | 4.57 | 3.02 | 4.59 | - | - | 1.03 |
| 1986.2 | Caryophyllene oxide | C15H24O | OS | 1.26 | 1.41 | 0.99 | 0.77 | 1.56 | 1.65 | 1.74 | 1.78 | 0.84 | - |
| TOTAL (%) | 99.9 | 99.9 | 99.3 | 99.4 | 99.8 | 100.0 | 99.5 | 100.0 | 99.6 | 99.7 | |||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||||
| AA: Aromatic aldehyde: | 3.55 | 1.68 | 2.89 | 2.41 | 5.72 | 3.78 | 3.79 | 1.44 | 1.36 | 2.24 | |||
| MH: Monoterpene hydrocarbone: | 27.91 | 10.52 | 23.42 | 20.43 | 26.36 | 20.95 | 29.49 | 14.02 | 33.61 | 25.64 | |||
| AK: Aromatic ketone: | - | 0.62 | 0.51 | - | 0.26 | - | - | 0.28 | - | - | |||
| OC: Organic compound: | 1.20 | 0.80 | 1.18 | 1.19 | 0.12 | 0.88 | 0.75 | 0.62 | 0.24 | 0.12 | |||
| SH: Sesquiterpene hydrocarbone: | 40.15 | 68.59 | 50.31 | 59.95 | 43.46 | 60.29 | 40.49 | 63.38 | 49.37 | 53.96 | |||
| ALH: Aliphatic hydrocarbone: | 12.43 | 11.24 | 11.22 | 9.99 | 14.25 | 7.89 | 11.29 | 13.45 | 10.84 | 12.28 | |||
| SA: Sesquiterpene alkene: | 2.65 | 2.77 | 2.47 | 1.17 | 0.85 | 0.99 | 1.60 | 2.56 | 1.77 | 2.10 | |||
| SAL: Sesquiterpene alcohol: | 2.05 | 1.07 | 1.14 | 0.22 | 1.59 | 0.44 | 3.65 | 1.23 | 1.01 | 0.88 | |||
| SALD: Sesquiterpene aldehyde: | 0.45 | 0.39 | 0.97 | 0.67 | - | 0.12 | 0.09 | 0.41 | 0.12 | - | |||
| AH: Aromatic hydrocarbone: | 6.90 | 0.89 | 3.78 | 1.48 | 4.57 | 3.02 | 4.59 | - | - | 1.03 | |||
| OS: Oxygenated sesquiterpene: | 1.26 | 1.41 | 0.99 | 0.77 | 1.56 | 1.75 | 1.74 | 1.78 | 0.84 | - | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erbaş, S.; Mutlucan, M. Investigation of Flower Yield and Quality in Different Color Safflower Genotypes. Agronomy 2023, 13, 956. https://doi.org/10.3390/agronomy13040956
Erbaş S, Mutlucan M. Investigation of Flower Yield and Quality in Different Color Safflower Genotypes. Agronomy. 2023; 13(4):956. https://doi.org/10.3390/agronomy13040956
Chicago/Turabian StyleErbaş, Sabri, and Murat Mutlucan. 2023. "Investigation of Flower Yield and Quality in Different Color Safflower Genotypes" Agronomy 13, no. 4: 956. https://doi.org/10.3390/agronomy13040956
APA StyleErbaş, S., & Mutlucan, M. (2023). Investigation of Flower Yield and Quality in Different Color Safflower Genotypes. Agronomy, 13(4), 956. https://doi.org/10.3390/agronomy13040956





