Placing Management of Sunflower Downy Mildew (Plasmopara halstedii (Farl.) Berl. et de Toni) under an Integrated Pest Management (IPM) System Approach: Challenges and New Perspectives
Abstract
1. Current Situation and Plant Health Aspects of Sunflower Cultivation
2. Downy Mildew as a Major Threat to Sunflowers
2.1. Significance of Sunflower Downy Mildew
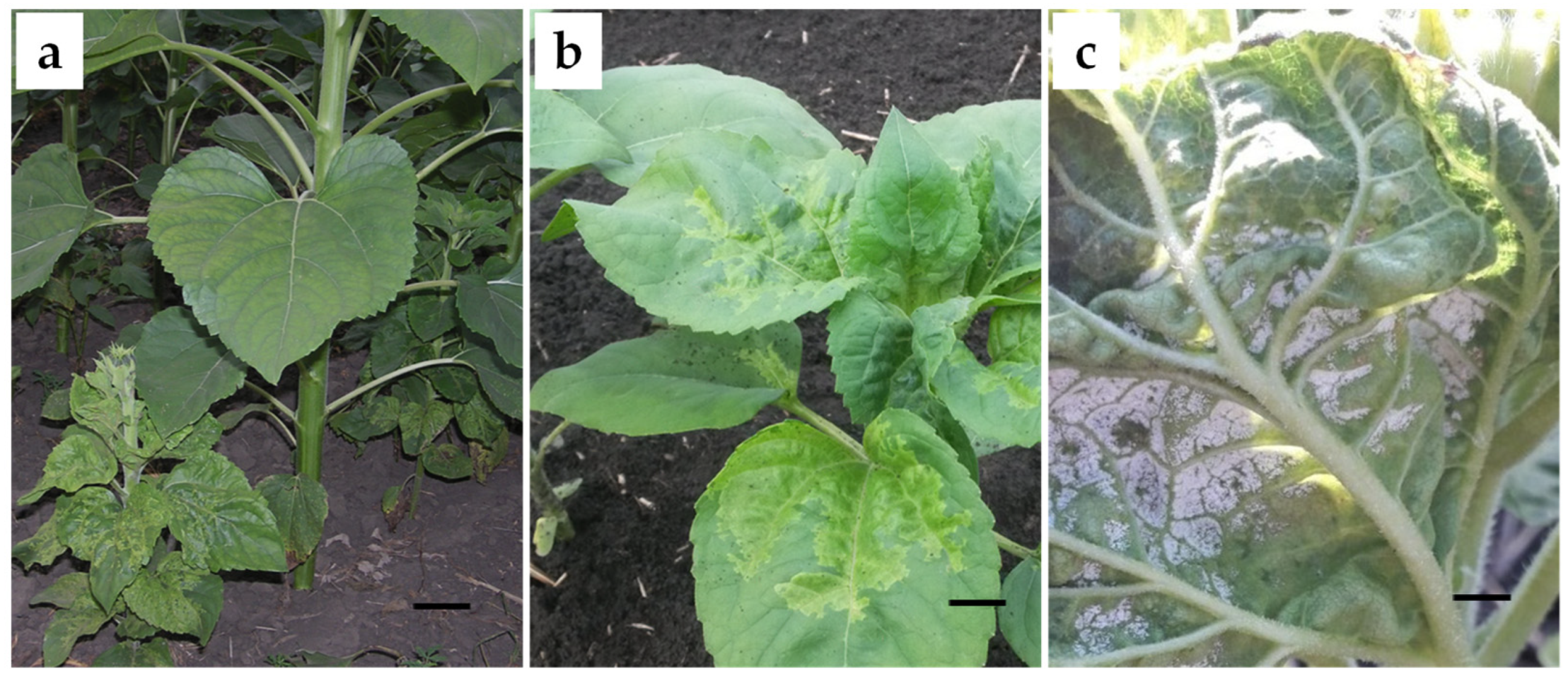
2.2. Biological Aspects of IPM against Plasmopara halstedii: Symptoms, Signs, and Life Cycle
2.3. Predisposing Environmental Conditions for Disease Development
3. Integrated Pest Management against Sunflower Downy Mildew
3.1. IPM as a Holistic Approach
- 1.
- Prevention and suppression;
- 2.
- Monitoring;
- 3.
- Decision-making process;
- 4–7.
- Control options;
- 8.
- Evaluation.
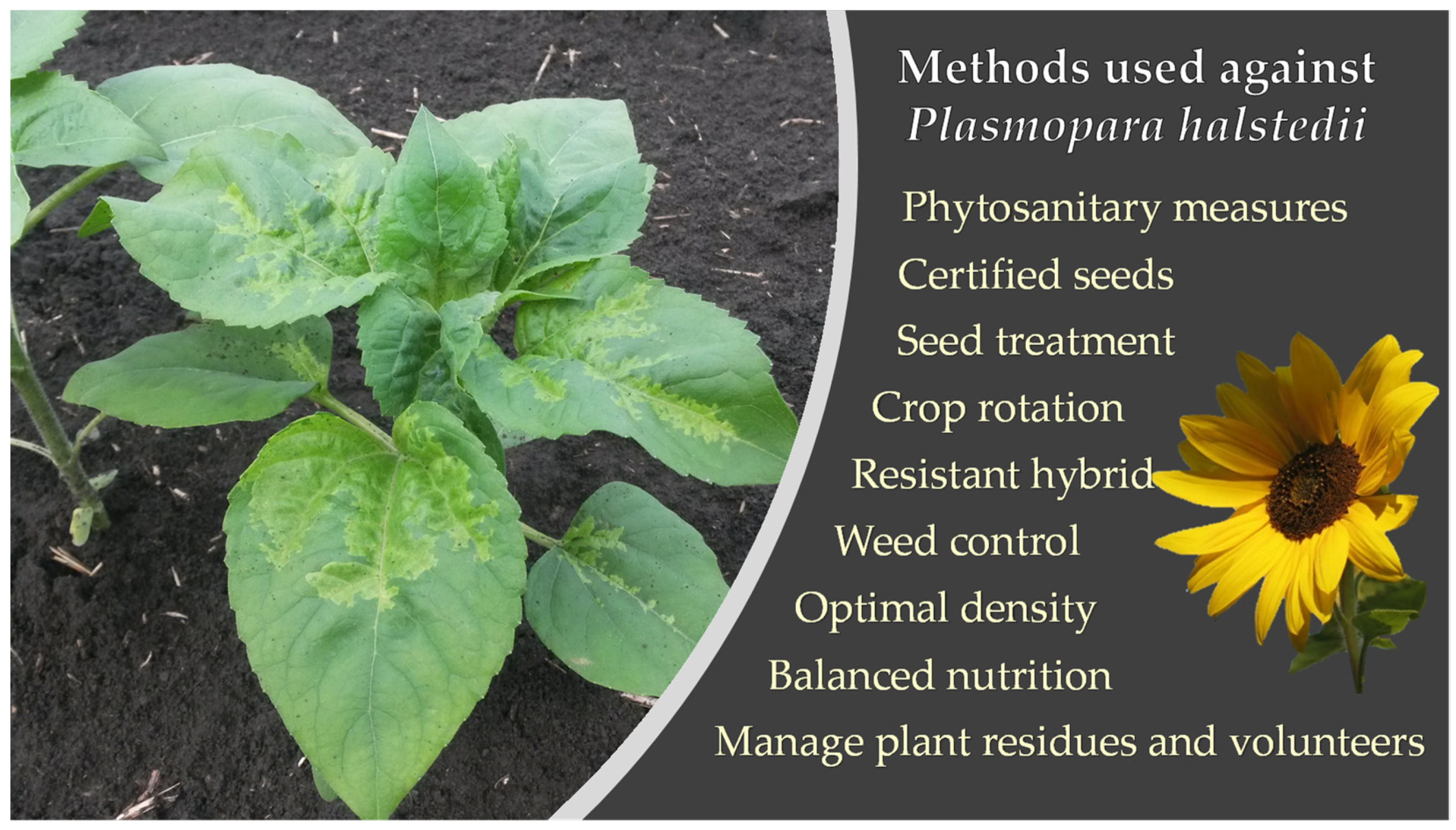
3.2. Phytosanitary Measures on Sunflower Downy Mildew and Seed Control
3.3. Combination of Cultural Measures: The Driving Force in IPM against Plasmopara halstedii
3.4. Other Measures to Control Sunflower Downy Mildew
4. Evaluating Current State, Future Challenges, and Perspectives: A SWOT Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adeleke, B.S.; Babalola, O.O. Oilseed crop sunflower (Helianthus annuus) as a source of food: Nutritional and health benefits. Food Sci. Nutr. 2020, 8, 4666–4684. [Google Scholar] [CrossRef]
- Edible Oils-Worldwide. Available online: https://www.statista.com/outlook/cmo/food/oils-fats/edible-oils/worldwide (accessed on 3 January 2023).
- Production Volume of Sunflower Seed in Major Producer Countries in 2021/2022. Available online: https://www.statista.com/statistics/263928/production-of-sunflower-seed-since-2000-by-major-countries/ (accessed on 15 December 2022).
- Miladinović, D.; Hladni, N.; Radanović, A.; Jocić, S.; Cvejić, S. Chapter 4 Sunflower and Climate Change: Possibilities of Adaptation Through Breeding and Genomic Selection. In Genomic Designing of Climate-Smart Oilseed Crops; Kole, C., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 173–238. ISBN 978-3-319-93535-5. [Google Scholar]
- Pilorgé, E. Sunflower in the global vegetable oil system: Situation, specificities and perspectives. OCL 2020, 27, 34. [Google Scholar] [CrossRef]
- Agüera, E.; de la Haba, P. Climate Change Impacts on Sunflower (Helianthus annuus L.) Plants. Plants 2021, 10, 2646. [Google Scholar] [CrossRef]
- Debaeke, P.; Mestries, E.; Desanlis, M.; Seassau, C. Effects of crop management on the incidence and severity of fungal diseases in sunflower. In Sunflowers: Growth and Development, Environmental Influences and Pests/Diseases; Arribas, J.I., Ed.; Botanical Research and Practices; Nova Science Publishers: New York, NY, USA, 2014; pp. 201–226. ISBN 978-1-63117-347-9. [Google Scholar]
- Leite, R.M.V.B.C. Disease Management in Sunflower. In Sunflowers: Growth and Development, Environmental Influences and Pests/Diseases; Arribas, J.I., Ed.; Botanical Research and Practices; Nova Publishers: New York, NY, USA, 2014; pp. 165–185. ISBN 978-1-63117-347-9. [Google Scholar]
- Bán, R.; Kovács, A.; Nisha, N.; Pálinkás, Z.; Zalai, M.; Yousif, A.I.A.; Körösi, K. New and High Virulent Pathotypes of Sunflower Downy Mildew (Plasmopara halstedii) in Seven Countries in Europe. JoF 2021, 7, 549. [Google Scholar] [CrossRef] [PubMed]
- Debaeke, P.; Casadebaig, P.; Flenet, F.; Langlade, N. Sunflower crop and climate change: Vulnerability, adaptation, and mitigation potential from case-studies in Europe. OCL 2017, 24, D102. [Google Scholar] [CrossRef]
- Molinero-Ruiz, L. Recent advances on the characterization and control of sunflower soilborne pathogens under climate change conditions. OCL 2019, 26, 2. [Google Scholar] [CrossRef]
- Gulya, T.J. Distribution of Plasmopara halstedii races from sunflower around the world. In Advances in Downy Mildew Research; Lebeda, A., Spencer-Phillips, P.T.N., Eds.; Palacký University in Olomouc and JOLA: Kostelec na Hané, Czech Republic, 2007; Volume 3, pp. 121–134. [Google Scholar]
- Gulya, T.; Harveson, R.; Mathew, F.; Block, C.; Thompson, S.; Kandel, H.; Berglund, D.; Sandbakken, J.; Kleingartner, L.; Markell, S. Comprehensive Disease Survey of U.S. Sunflower: Disease Trends, Research Priorities and Unanticipated Impacts. Plant Dis. 2019, 103, 601–618. [Google Scholar] [CrossRef]
- Picard, C.; Afonso, T.; Benko-Beloglavec, A.; Karadjova, O.; Matthews-Berry, S.; Paunovic, S.A.; Pietsch, M.; Reed, P.; Gaag, D.J.; Ward, M. Recommended regulated non-quarantine pests (RNQPs), associated thresholds and risk management measures in the European and Mediterranean region. EPPO Bull. 2018, 48, 552–568. [Google Scholar] [CrossRef]
- The European Commission. Commission Implementing Regulation (EU) 2019/2072 of 28 November 2019 Establishing Uniform Conditions for the Implementation of Regulation (EU) 2016/2031 of the European Parliament and the Council, as Regards Protective Measures against Pests of Plants, and Repealing Commission Regulation (EC) No 690/2008 and Amending Commission Implementing Regulation (EU) 2018/2019; The European Commission: Brussels, Belgium, 2019. [Google Scholar]
- EPPO Global Database: Plasmopara halstedii. Available online: https://gd.eppo.int/taxon/PLASHA/categorization (accessed on 15 December 2022).
- Albourie, J.-M.; Tourvieille, J.; Tourvieille de Labrouhe, D. Resistance to metalaxyl in isolates of the sunflower pathogen Plasmopara halstedii. Eur. J. Plant Pathol. 1998, 104, 235–242. [Google Scholar] [CrossRef]
- Vear, F. Changes in sunflower breeding over the last fifty years. OCL 2016, 23, D202. [Google Scholar] [CrossRef]
- Körösi, K.; Kovács, A.; Nisha, N.; Bóta, I.; Perczel, M.; Yousif, A.I.A.; Kiss, J.; Bán, R. New data on pathotype distribution and mefenoxam tolerance of Plasmopara halstedii in Hungary. Plant Protect. Sci. 2021, 57, 31–37. [Google Scholar] [CrossRef]
- Spring, O. Spreading and global pathogenic diversity of sunflower downy mildew—Review. Plant Protect. Sci. 2019, 55, 149–158. [Google Scholar] [CrossRef]
- Martín-Sanz, A.; Rueda, S.; García-Carneros, A.B.; Molinero-Ruiz, L. First Report of a New Highly Virulent Pathotype of Sunflower Downy Mildew (Plasmopara halstedii) Overcoming the Pl8 Resistance Gene in Europe. Plant Dis. 2020, 104, 597. [Google Scholar] [CrossRef]
- Viranyi, F.; Spring, O. Advances in sunflower downy mildew research. Eur. J. Plant. Pathol. 2011, 129, 207–220. [Google Scholar] [CrossRef]
- Ahmed, S.; Tourvieille de Labrouhe, D.; Delmotte, F. Emerging virulence arising from hybridisation facilitated by multiple introductions of the sunflower downy mildew pathogen Plasmopara halstedii. Fungal Genet. Biol. 2012, 49, 847–855. [Google Scholar] [CrossRef]
- Kitner, M.; Thines, M.; Sedlářová, M.; Vaculná, L.; Bán, R.; Körösi, K.; Iwebor, M.; Antonova, T.; Ali, T.; Nádvorník, P.; et al. Genetic structure of Plasmopara halstedii populations across Europe and South Russia. Plant Pathol. 2022, 72, 361–375. [Google Scholar] [CrossRef]
- Islam, M.T.; Tahara, S. Chemotaxis of Fungal Zoospores, with Special Reference to Aphanomyces cochlioides. Biosci. Biotechnol. Biochem. 2001, 65, 1933–1948. [Google Scholar] [CrossRef]
- Spring, O.; Gomez-Zeledon, J.; Hadziabdic, D.; Trigiano, R.N.; Thines, M.; Lebeda, A. Biological Characteristics and Assessment of Virulence Diversity in Pathosystems of Economically Important Biotrophic Oomycetes. Crit. Rev. Plant Sci. 2018, 37, 439–495. [Google Scholar] [CrossRef]
- Gascuel, Q.; Martinez, Y.; Boniface, M.-C.; Vear, F.; Pichon, M.; Godiard, L. The sunflower downy mildew pathogen Plasmopara halstedii. Mol. Plant Pathol. 2015, 16, 109–122. [Google Scholar] [CrossRef]
- Meliala, C.; Vear, F.; Tourvieille de Labrouhe, D. Relation between date of infection of sunflower downy mildew (Plasmopara halstedii) and symptoms development. Helia 2000, 23, 35–44. [Google Scholar]
- Spring, O. Transition of secondary to systemic infection of sunflower with Plasmopara halstedii—An underestimated factor in the epidemiology of the pathogen. Fungal Ecol. 2009, 2, 75–80. [Google Scholar] [CrossRef]
- Spanu, P.D.; Panstruga, R. Editorial: Biotrophic Plant-Microbe Interactions. Front. Plant Sci. 2017, 8, 192. [Google Scholar] [CrossRef] [PubMed]
- Baldini, M.; Danuso, F.; Turi, M.; Sandra, M.; Raranciuc, S. Main factors influencing downy mildew (Plasmopara halstedii) infection in high-oleic sunflower hybrids in northern Italy. Crop Prot. 2008, 27, 590–599. [Google Scholar] [CrossRef]
- Humann, R.M.; Johnson, K.D.; Wunsch, M.J.; Meyer, S.M.; Jordahl, J.G.; Bauske, E.C.; Halvorson, J.M.; Friskop, A.J.; O’Bryan, K.A.; Gulya, T.J.; et al. Evaluation of Oxathiapiprolin for the Management of Sunflower Downy Mildew. Plant Dis. 2019, 103, 2498–2504. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Kiss, L.; Vajna, L.; Shin, H.-D. Characterization of a Plasmopara species on Ambrosia artemisiifolia, and notes on P. halstedii, based on morphology and multiple gene phylogenies. Mycol. Res. 2009, 113, 1127–1136. [Google Scholar] [CrossRef]
- The European Commission. Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 Establishing a Framework for Community Action to Achieve the Sustainable Use of Pesticides; The European Commission: Brussels, Belgium, 2009; Volume 52, pp. 71–86. [Google Scholar]
- Barzman, M.; Bàrberi, P.; Birch, A.N.E.; Boonekamp, P.; Dachbrodt-Saaydeh, S.; Graf, B.; Hommel, B.; Jensen, J.E.; Kiss, J.; Kudsk, P.; et al. Eight principles of integrated pest management. Agron. Sustain. Dev. 2015, 35, 1199–1215. [Google Scholar] [CrossRef]
- Mouron, P.; Calabrese, C.; Breitenmoser, S.; Spycher, S.; Baur, R. Sustainability Assessment of Plant Protection Strategies in Swiss Winter Wheat and Potato Production. Agriculture 2016, 6, 3. [Google Scholar] [CrossRef]
- Baker, B.P.; Green, T.A.; Loker, A.J. Biological control and integrated pest management in organic and conventional systems. Biol. Control 2020, 140, 104095. [Google Scholar] [CrossRef]
- Germains Seed Technology: Sunflower Seed Treatments for Today’s Field Challenges. Available online: https://germains.com/us/sunflower-seed-treatments/ (accessed on 18 December 2022).
- Allen, R.R.; Hollingsworth, L.D.; Thomas, J.D. Sunflower Planting and Emergence with Coated Seed. Trans. ASAE 1983, 26, 665–668. [Google Scholar] [CrossRef]
- Schwinn, F.J.; Staub, T. Oomycete fungicides. In Modern Selective Fungicides, Properties, Applications, Mechanisms of Action; Lyr, H., Ed.; Jena-Fischer Verlag: New York, NY, USA, 1995; pp. 323–354. [Google Scholar]
- The European Commission. Mission Implementing Regulation (EU) 2020/617 of 5 May 2020 Renewing the Approval of the Active Substance Metalaxyl-M, and Restricting the Use of Seeds Treated with Plant Protection Products Containing It, in Accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council Concerning the Placing of Plant Protection Products on the Market, and Amending the Annex to Commission Implementing Regulation (EU) No 540/2011; The European Commission: Brussels, Belgium, 2020; Volume 143, pp. 6–10. [Google Scholar]
- Spring, O.; Gómez-Zeledón, J. Influence of oxathiapiprolin on preinfectional and early infection stages of Plasmopara halstedii, downy mildew of the sunflower. Plant Protect. Sci. 2020, 56, 83–91. [Google Scholar] [CrossRef]
- Molinero-Ruiz, L. Sustainable and efficient control of sunflower downy mildew by means of genetic resistance: A review. Theor. Appl. Genet. 2022, 135, 3757–3771. [Google Scholar] [CrossRef]
- Agrios, G.N. Genetics of plant disease. Types of plant resistance to pathogens. In Plant Pathology; Agrios, G.N., Ed.; Elsevier, Academic Press: Amsterdam, The Netherlands; Boston, MA, USA, 2005; pp. 134–139. ISBN 978-0-12-044565-3. [Google Scholar]
- Qi, L.L.; Ma, G.J.; Li, X.H.; Seiler, G.J. Diversification of the downy mildew resistance gene pool by introgression of a new gene, Pl35, from wild Helianthus argophyllus into oilseed and confection sunflowers (Helianthus annuus L.). Theor. Appl. Genet. 2019, 132, 2553–2565. [Google Scholar] [CrossRef]
- Heller, A.; Rozynek, B.; Spring, O. Cytological and physiological reasons for the latent type of infection in sunflower caused by Plasmopara halstedii. J. Phytopathol. 1997, 145, 441–445. [Google Scholar] [CrossRef]
- Tarigan, S.I.; Toth, S.; Szalai, M.; Kiss, J.; Turoczi, G.; Toepfer, S. Biological control properties of microbial plant biostimulants. A review. Biocontrol. Sci. Technol. 2022, 32, 1351–1371. [Google Scholar] [CrossRef]
- Ioos, R.; Fourrier, C.; Wilson, V.; Webb, K.; Schereffer, J.-L.; Tourvieille de Labrouhe, D. An Optimized Duplex Real-Time PCR Tool for Sensitive Detection of the Quarantine Oomycete Plasmopara halstedii in Sunflower Seeds. Phytopathology 2012, 102, 908–917. [Google Scholar] [CrossRef]
- Martínez, A.L.; Anderson, F.; Quiroz, F.; Garayalde, A.; Erreguerena, I.; Armando, L.; Huguet, N.; Carrera, A. Methodologies for Plasmopara halstedii Research. Helia 2019, 42, 173–186. [Google Scholar] [CrossRef]
- Duary, S. Seed priming: A comprehensive approach to alter the biotic and abiotic stresses of field crops. Int. J. Chem. Stud. 2020, 8, 1705–1708. [Google Scholar] [CrossRef]
- Catiempo, R.; Photchanachai, S.; Bayogan, E.R.; Wongs-Aree, C. Impact of hydropriming on germination and seedling establishment of sunflower seeds at elevated temperature. Plant Soil. Environ. 2021, 67, 491–498. [Google Scholar] [CrossRef]
- Devika, O.S.; Singh, S.; Sarkar, D.; Barnwal, P.; Suman, J.; Rakshit, A. Seed Priming: A Potential Supplement in Integrated Resource Management Under Fragile Intensive Ecosystems. Front. Sustain. Food Syst. 2021, 5, 654001. [Google Scholar] [CrossRef]
- Debaeke, P.; Bedoussac, L.; Bonnet, C.; Bret-Mestries, E.; Seassau, C.; Gavaland, A.; Raffaillac, D.; Tribouillois, H.; Véricel, G.; Justes, E. Sunflower crop: Environmental-friendly and agroecological. OCL 2017, 24, D304. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Wojciechowski, A.; Bourgeois, C.; Debaeke, P. Genetic Variability for Early Growth Traits in Second Season Sunflower. Front. Agron. 2022, 4, 822456. [Google Scholar] [CrossRef]
- Ma, G.J.; Markell, S.G.; Song, Q.J.; Qi, L.L. Genotyping-by-sequencing targeting of a novel downy mildew resistance gene Pl 20 from wild Helianthus argophyllus for sunflower (Helianthus annuus L.). Theor. Appl. Genet. 2017, 130, 1519–1529. [Google Scholar] [CrossRef]
- Pecrix, Y.; Penouilh-Suzette, C.; Muños, S.; Vear, F.; Godiard, L. Ten Broad Spectrum Resistances to Downy Mildew Physically Mapped on the Sunflower Genome. Front. Plant Sci. 2018, 9, 1780. [Google Scholar] [CrossRef] [PubMed]
- Gontcharov, S.; Goloschapova, N. Evaluation of horizontal resistance of sunflower (Helianthus annuus L.) to downy mildew (Plasmopara halstedii). OCL 2021, 28, 58. [Google Scholar] [CrossRef]
- Rajput, M.; Choudhary, K.; Kumar, M.; Vivekanand, V.; Chawade, A.; Ortiz, R.; Pareek, N. RNA Interference and CRISPR/Cas Gene Editing for Crop Improvement: Paradigm Shift towards Sustainable Agriculture. Plants 2021, 10, 1914. [Google Scholar] [CrossRef]
- Collinge, D.B.; Jensen, D.F.; Rabiey, M.; Sarrocco, S.; Shaw, M.W.; Shaw, R.H. Biological control of plant diseases—What has been achieved and what is the direction? Plant Pathol. 2022, 71, 1024–1047. [Google Scholar] [CrossRef]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- Nagaraju, A.; Sudisha, J.; Murthy, S.M.; Ito, S. Seed priming with Trichoderma harzianum isolates enhances plant growth and induces resistance against Plasmopara halstedii, an incitant of sunflower downy mildew disease. Australas. Plant Pathol. 2012, 41, 609–620. [Google Scholar] [CrossRef]
- Bán, R.; Virányi, F.; Komjáti, H. Benzothiadiazole-induced resistance to Plasmopara halstedii (Farl.) Berl. Et de Toni in sunflower. In Advances in Downy Mildew Research; Spencer-Phillips, P., Jeger, M., Eds.; Kluwer Academic Publishers: Norwell, MA, USA, 2004; Volume 2, pp. 265–273. [Google Scholar]
- Körösi, K.; Bán, R.; Barna, B.; Virányi, F. Biochemical and Molecular Changes in Downy Mildew-infected Sunflower Triggered by Resistance Inducers: Induced Resistance to Sunflower Downy Mildew. J. Phytopathol. 2011, 159, 471–478. [Google Scholar] [CrossRef]
- Miranda-Fuentes, P.; García-Carneros, A.B.; Molinero-Ruiz, L. Updated Characterization of Races of Plasmopara halstedii and Entomopathogenic Fungi as Endophytes of Sunflower Plants in Axenic Culture. Agronomy 2021, 11, 268. [Google Scholar] [CrossRef]
- Bán, R.; Baglyas, G.; Virányi, F.; Barna, B.; Posta, K.; Kiss, J.; Körösi, K. The chemical inducer, BTH (benzothiadiazole) and root colonization by mycorrhizal fungi (Glomus spp.) trigger resistance against white rot (Sclerotinia sclerotiorum) in sunflower. Acta Biol. Hung. 2017, 68, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Bellido, E.; de la Haba, P.; Agüera, E. Physiological Alteration in Sunflower Plants (Helianthus annuus L.) Exposed to High CO2 and Arbuscular Mycorrhizal Fungi. Plants 2021, 10, 937. [Google Scholar] [CrossRef] [PubMed]
- Doshi, P.; Nisha, N.; Yousif, A.I.A.; Körösi, K.; Bán, R.; Turóczi, G. Preliminary Investigation of Effect of Neem-Derived Pesticides on Plasmopara halstedii Pathotype 704 in Sunflower under In Vitro and In Vivo Conditions. Plants 2020, 9, 535. [Google Scholar] [CrossRef] [PubMed]
- Grasse, W.; Zipper, R.; Totska, M.; Spring, O. Plasmopara halstedii virus causes hypovirulence in Plasmopara halstedii, the downy mildew pathogen of the sunflower. Fungal Genet. Biol. 2013, 57, 42–47. [Google Scholar] [CrossRef]
| SWOT ANALYSIS |
|---|
| INTERNAL FACTORS |
| STRENGTHS (+) |
|
| WEAKNESSES (-) |
|
| EXTERNAL FACTORS |
| OPPORTUNITIES (+) |
|
| THREATS (-) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bán, R.; Kiss, J.; Pálinkás, Z.; Körösi, K. Placing Management of Sunflower Downy Mildew (Plasmopara halstedii (Farl.) Berl. et de Toni) under an Integrated Pest Management (IPM) System Approach: Challenges and New Perspectives. Agronomy 2023, 13, 1029. https://doi.org/10.3390/agronomy13041029
Bán R, Kiss J, Pálinkás Z, Körösi K. Placing Management of Sunflower Downy Mildew (Plasmopara halstedii (Farl.) Berl. et de Toni) under an Integrated Pest Management (IPM) System Approach: Challenges and New Perspectives. Agronomy. 2023; 13(4):1029. https://doi.org/10.3390/agronomy13041029
Chicago/Turabian StyleBán, Rita, József Kiss, Zoltán Pálinkás, and Katalin Körösi. 2023. "Placing Management of Sunflower Downy Mildew (Plasmopara halstedii (Farl.) Berl. et de Toni) under an Integrated Pest Management (IPM) System Approach: Challenges and New Perspectives" Agronomy 13, no. 4: 1029. https://doi.org/10.3390/agronomy13041029
APA StyleBán, R., Kiss, J., Pálinkás, Z., & Körösi, K. (2023). Placing Management of Sunflower Downy Mildew (Plasmopara halstedii (Farl.) Berl. et de Toni) under an Integrated Pest Management (IPM) System Approach: Challenges and New Perspectives. Agronomy, 13(4), 1029. https://doi.org/10.3390/agronomy13041029





