Can Basic Soil Quality Indicators and Topography Explain the Spatial Variability in Agricultural Fields Observed from Drone Orthomosaics?
Abstract
1. Introduction
2. Materials and Methods
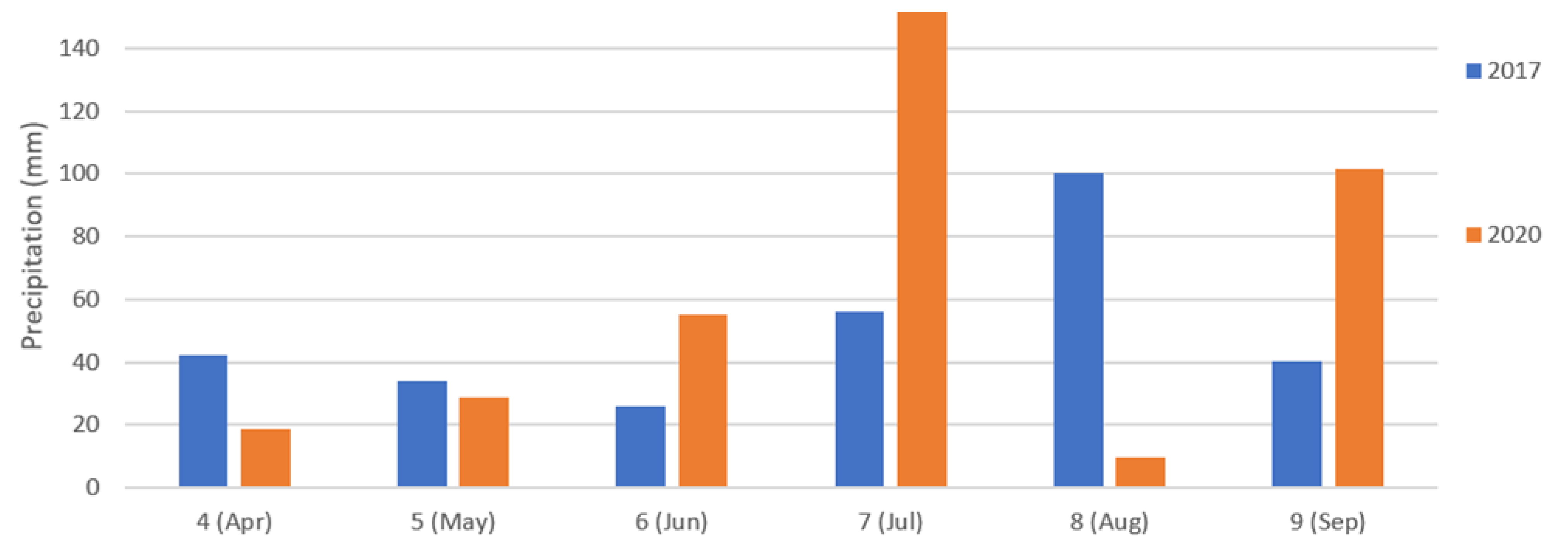
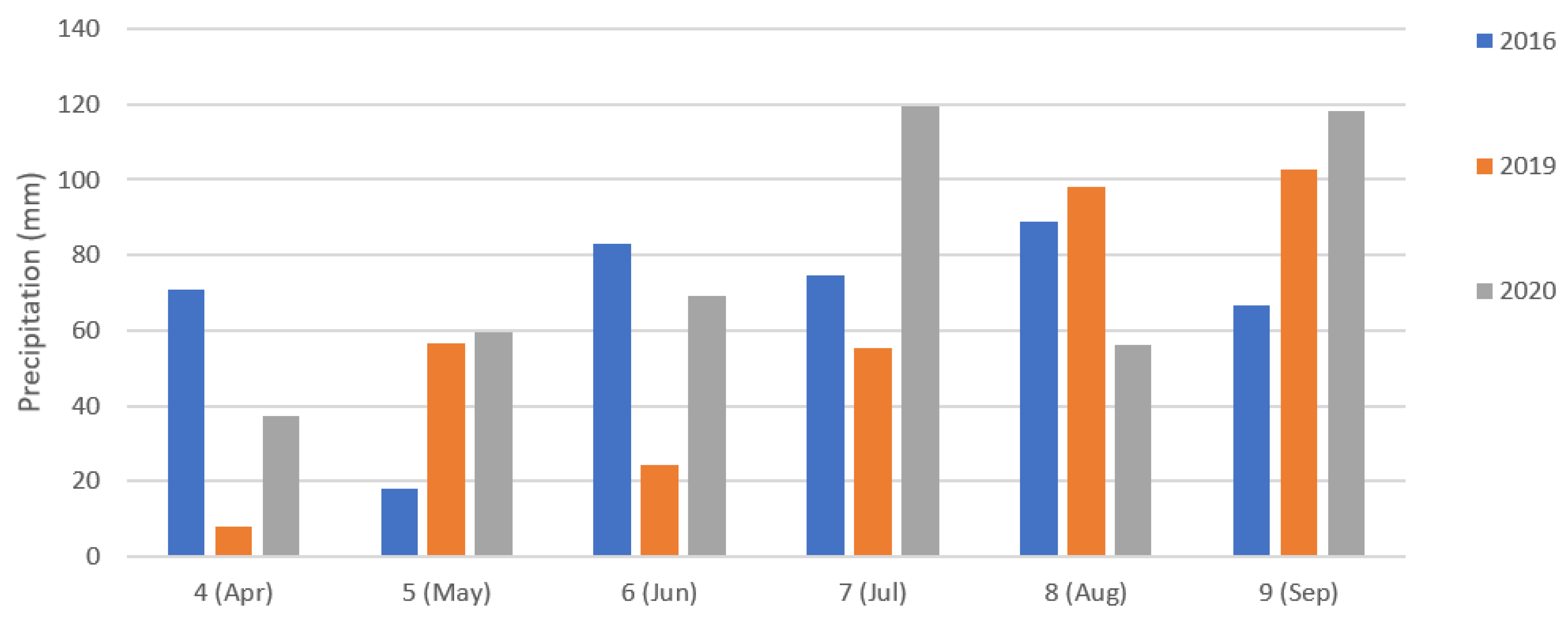
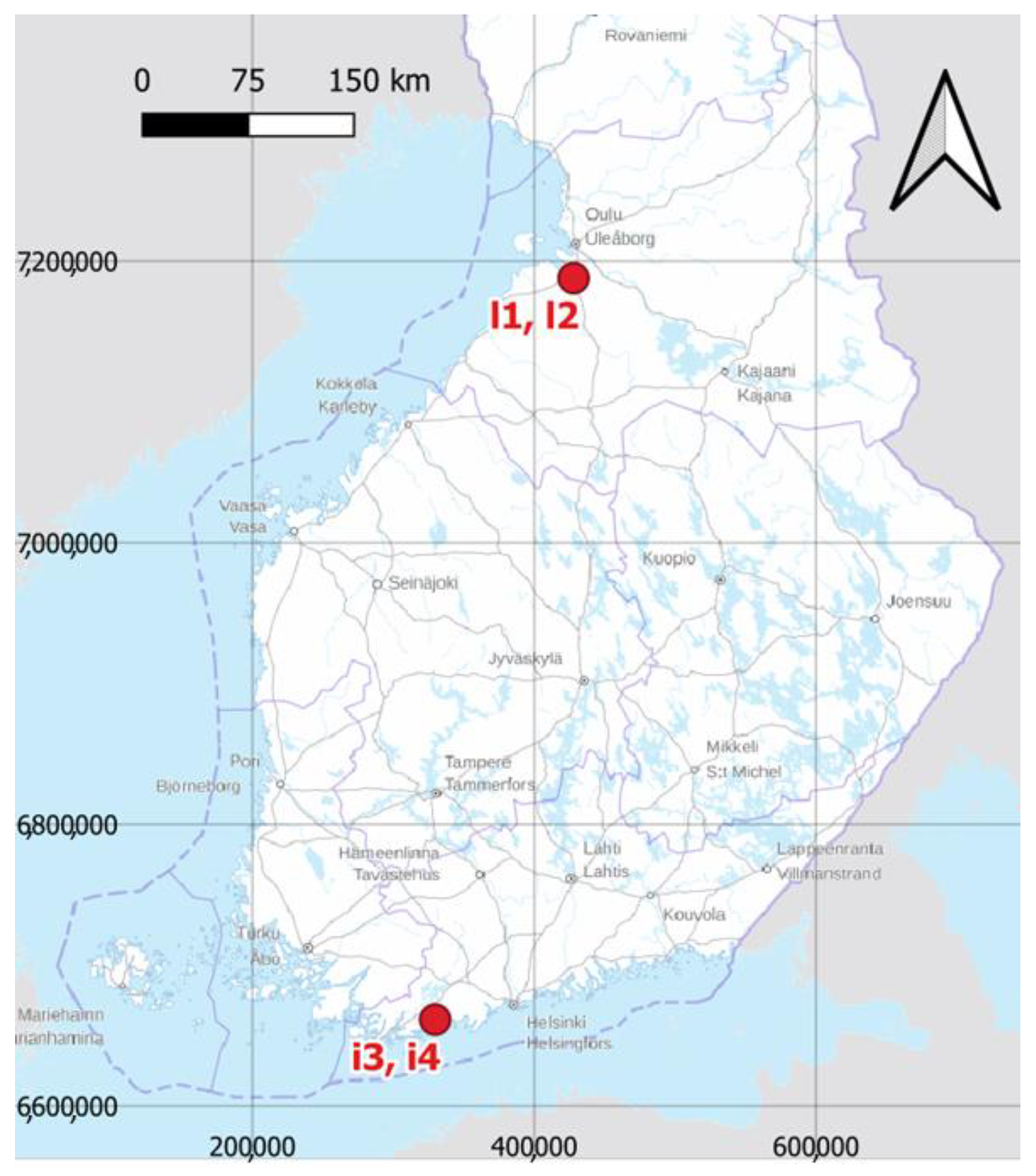
2.1. Experimental Fields
2.2. Measurements and Determinations
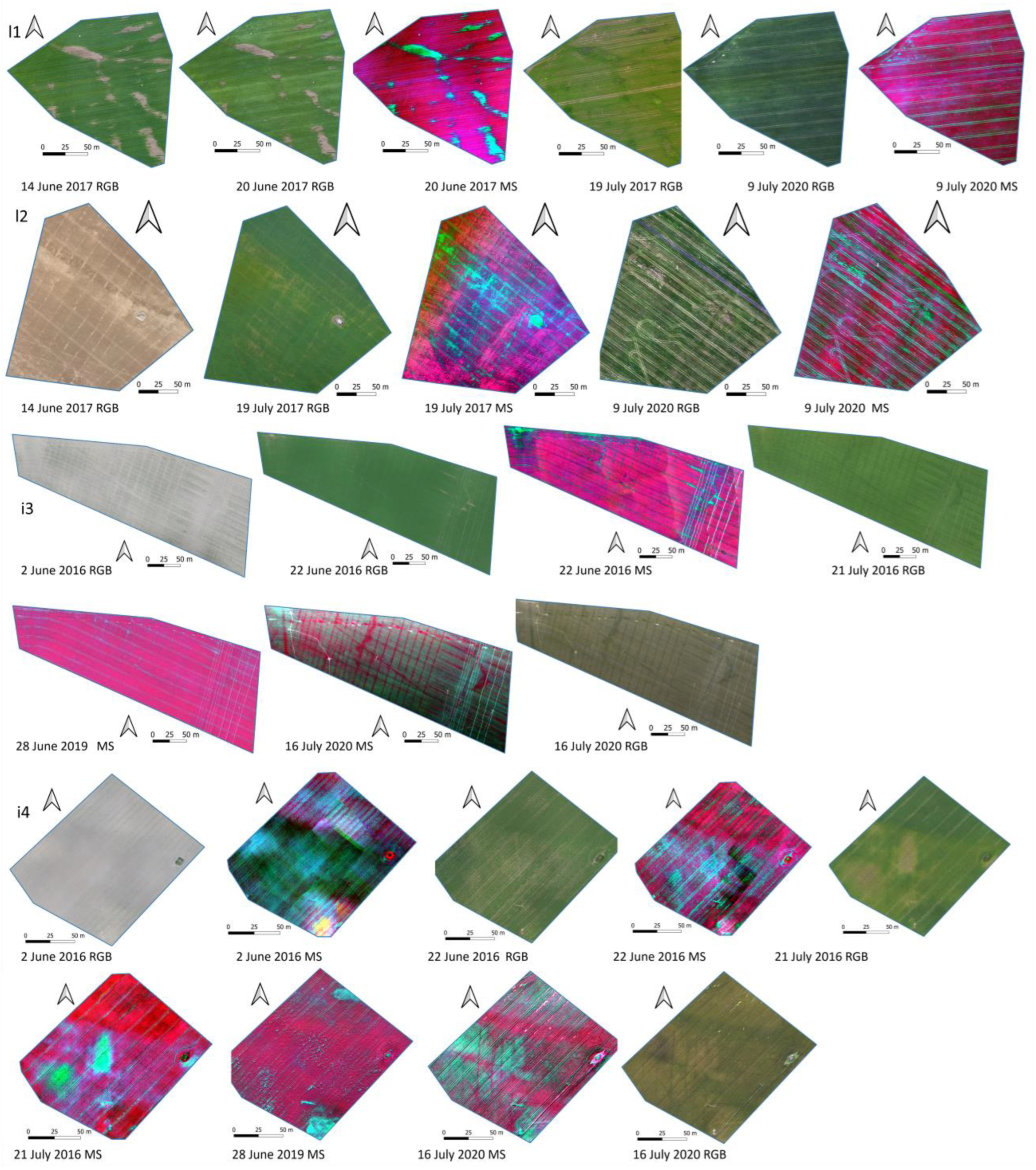
2.2.1. Drone Data Collection
2.2.2. Drone Data Processing
2.2.3. Soil and Topography Data Collection
2.2.4. Soil and Field Indicator Determinations
2.2.5. Data Preprocessing
2.2.6. Statistical Analysis and Machine Learning
3. Results
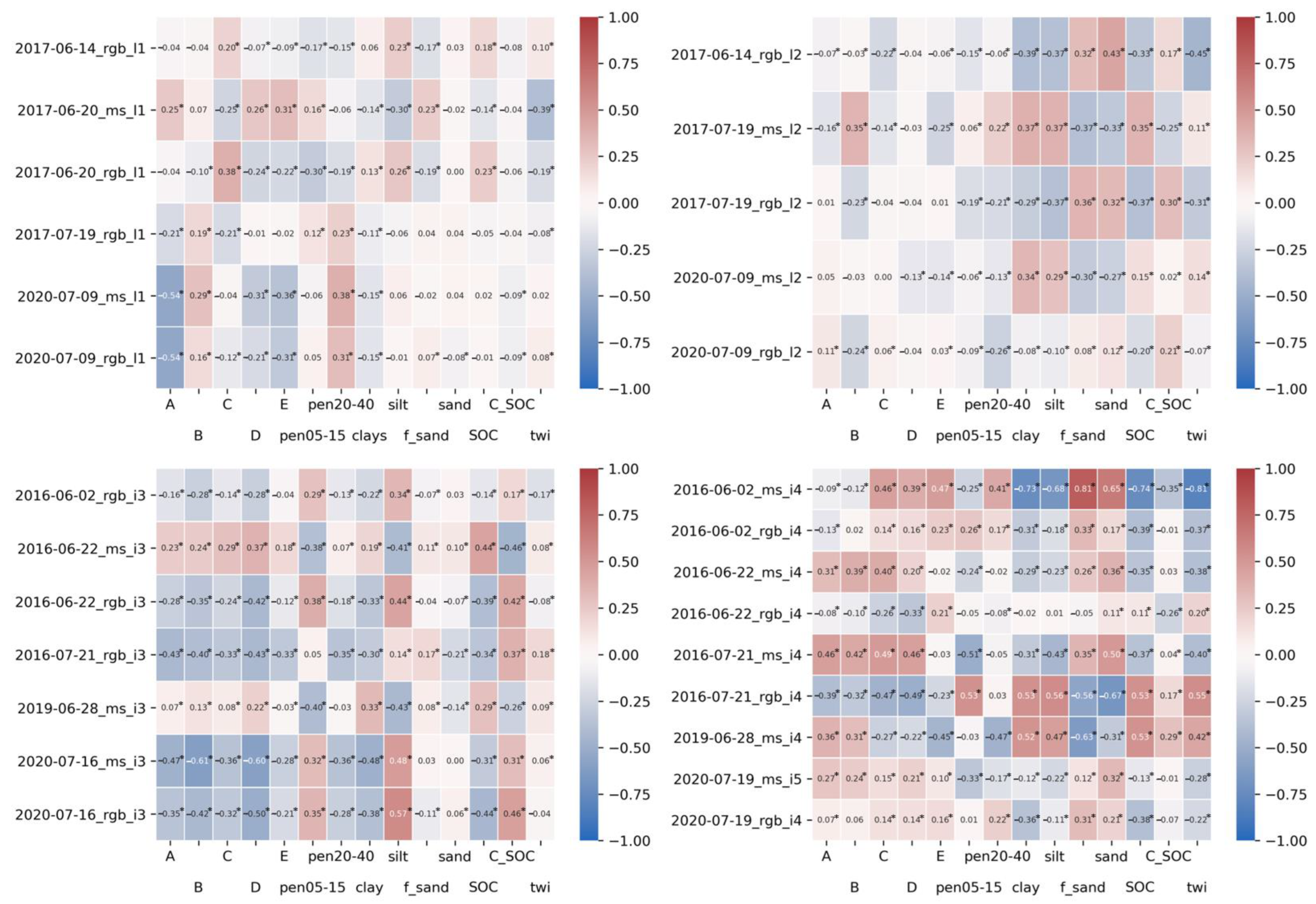
3.1. Correlation Analysis of Single Variables
3.2. Multi-Parameter Non-Linear Models
4. Discussion
4.1. Assessment of the Correlations and Multi-Parameter Models
4.2. Explainability of Individual Topography and Soil Indicators in Study Fields Based on Drone Image Data
4.3. Outlook and Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Leroux, C.; Tisseyre, B. How to Measure and Report Within-Field Variability: A Review of Common Indicators and Their Sensitivity. Precis. Agric. 2019, 20, 562–590. [Google Scholar] [CrossRef]
- Lark, R.; Catt, J.; Stafford, J. Towards the Explanation of Within-Field Variability of Yield of Winter Barley: Soil Series Differences. J. Agric. Sci. 1998, 131, 409–416. [Google Scholar] [CrossRef]
- Raun, W.R.; Solie, J.B.; Stone, M.L. Independence of Yield Potential and Crop Nitrogen Response. Precis. Agric. 2011, 12, 508–518. [Google Scholar] [CrossRef]
- Keller, T.; Sutter, J.A.; Nissen, K.; Rydberg, T. Using Field Measurement of Saturated Soil Hydraulic Conductivity to Detect Low-Yielding Zones in Three Swedish Fields. Soil Tillage Res. 2012, 124, 68–77. [Google Scholar] [CrossRef]
- Diaz-Gonzalez, F.A.; Vuelvas, J.; Correa, C.A.; Vallejo, V.E.; Patino, D. Machine Learning and Remote Sensing Techniques Applied to Estimate Soil Indicators–Review. Ecol. Indic. 2022, 135, 108517. [Google Scholar] [CrossRef]
- Karlen, D.L.; Mausbach, M.; Doran, J.W.; Cline, R.; Harris, R.; Schuman, G. Soil Quality: A Concept, Definition, and Framework for Evaluation (a Guest Editorial). Soil Sci. Soc. Am. J. 1997, 61, 4–10. [Google Scholar] [CrossRef]
- Nykänen, A.; Jauhiainen, L.; Kemppainen, J.; Lindström, K. Field-Scale Spatial Variation in Yields and Nitrogen Fixation of Clover-Grass Leys and in Soil Nutrients. Agric. Food Sci. 2008, 17, 376–393. [Google Scholar] [CrossRef]
- Hakojärvi, M.; Hautala, M.; Ristolainen, A.; Alakukku, L. Yield Variation of Spring Cereals in Relation to Selected Soil Physical Properties on Three Clay Soil Fields. Eur. J. Agron. 2013, 49, 1–11. [Google Scholar] [CrossRef]
- Juhos, K.; Szabó, S.; Ladányi, M. Explore the Influence of Soil Quality on Crop Yield Using Statistically-Derived Pedological Indicators. Ecol. Indic. 2016, 63, 366–373. [Google Scholar] [CrossRef]
- Lipiec, J.; Usowicz, B. Spatial Relationships among Cereal Yields and Selected Soil Physical and Chemical Properties. Sci. Total Environ. 2018, 633, 1579–1590. [Google Scholar] [CrossRef]
- Weiss, M.; Jacob, F.; Duveiller, G. Remote Sensing for Agricultural Applications: A Meta-Review. Remote Sens. Environ. 2020, 236, 111402. [Google Scholar] [CrossRef]
- Berni, J.A.; Zarco-Tejada, P.J.; Suárez, L.; Fereres, E. Thermal and Narrowband Multispectral Remote Sensing for Vegetation Monitoring from an Unmanned Aerial Vehicle. IEEE Trans. Geosci. Remote Sens. 2009, 47, 722–738. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Y.; Wang, M.; Fan, Q.; Tian, H.; Qiao, X.; Li, Y. Applications of UAS in Crop Biomass Monitoring: A Review. Front. Plant Sci. 2021, 12, 616689. [Google Scholar] [CrossRef]
- Geipel, J.; Link, J.; Wirwahn, J.A.; Claupein, W. A Programmable Aerial Multispectral Camera System for In-Season Crop Biomass and Nitrogen Content Estimation. Agriculture 2016, 6, 4. [Google Scholar] [CrossRef]
- Alves Oliveira, R.; Marcato Junior, J.; Soares Costa, C.; Näsi, R.; Koivumäki, N.; Niemeläinen, O.; Kaivosoja, J.; Nyholm, L.; Pistori, H.; Honkavaara, E. Silage Grass Sward Nitrogen Concentration and Dry Matter Yield Estimation Using Deep Regression and RGB Images Captured by UAV. Agronomy 2022, 12, 1352. [Google Scholar] [CrossRef]
- Gopp, N.; Savenkov, O. Relationships between the NDVI, Yield of Spring Wheat, and Properties of the Plow Horizon of Eluviated Clay-Illuvial Chernozems and Dark Gray Soils. Eurasian Soil Sci. 2019, 52, 339–347. [Google Scholar] [CrossRef]
- Van Klompenburg, T.; Kassahun, A.; Catal, C. Crop Yield Prediction Using Machine Learning: A Systematic Literature Review. Comput. Electron. Agric. 2020, 177, 105709. [Google Scholar] [CrossRef]
- Nevavuori, P.; Narra, N.; Lipping, T. Crop Yield Prediction with Deep Convolutional Neural Networks. Comput. Electron. Agric. 2019, 163, 104859. [Google Scholar] [CrossRef]
- Mohidem, N.A.; Che’Ya, N.N.; Juraimi, A.S.; Fazlil Ilahi, W.F.; Mohd Roslim, M.H.; Sulaiman, N.; Saberioon, M.; Mohd Noor, N. How Can Unmanned Aerial Vehicles Be Used for Detecting Weeds in Agricultural Fields? Agriculture 2021, 11, 1004. [Google Scholar] [CrossRef]
- Peña, J.M.; Torres-Sánchez, J.; de Castro, A.I.; Kelly, M.; López-Granados, F. Weed Mapping in Early-Season Maize Fields Using Object-Based Analysis of Unmanned Aerial Vehicle (UAV) Images. PLoS ONE 2013, 8, e77151. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; González-Dugo, V.; Berni, J.A. Fluorescence, Temperature and Narrow-Band Indices Acquired from a UAV Platform for Water Stress Detection Using a Micro-Hyperspectral Imager and a Thermal Camera. Remote Sens. Environ. 2012, 117, 322–337. [Google Scholar] [CrossRef]
- Manfreda, S.; McCabe, M.F.; Miller, P.E.; Lucas, R.; Pajuelo Madrigal, V.; Mallinis, G.; Ben Dor, E.; Helman, D.; Estes, L.; Ciraolo, G. On the Use of Unmanned Aerial Systems for Environmental Monitoring. Remote Sens. 2018, 10, 641. [Google Scholar] [CrossRef]
- Khanal, S.; Kc, K.; Fulton, J.P.; Shearer, S.; Ozkan, E. Remote Sensing in Agriculture—Accomplishments, Limitations, and Opportunities. Remote Sens. 2020, 12, 3783. [Google Scholar] [CrossRef]
- Hu, J.; Peng, J.; Zhou, Y.; Xu, D.; Zhao, R.; Jiang, Q.; Fu, T.; Wang, F.; Shi, Z. Quantitative Estimation of Soil Salinity Using UAV-Borne Hyperspectral and Satellite Multispectral Images. Remote Sens. 2019, 11, 736. [Google Scholar] [CrossRef]
- Žížala, D.; Minařík, R.; Zádorová, T. Soil Organic Carbon Mapping Using Multispectral Remote Sensing Data: Prediction Ability of Data with Different Spatial and Spectral Resolutions. Remote Sens. 2019, 11, 2947. [Google Scholar] [CrossRef]
- Aldana-Jague, E.; Heckrath, G.; Macdonald, A.; van Wesemael, B.; Van Oost, K. UAS-Based Soil Carbon Mapping Using VIS-NIR (480–1000 Nm) Multi-Spectral Imaging: Potential and Limitations. Geoderma 2016, 275, 55–66. [Google Scholar] [CrossRef]
- Khanal, S.; Fulton, J.; Klopfenstein, A.; Douridas, N.; Shearer, S. Integration of High Resolution Remotely Sensed Data and Machine Learning Techniques for Spatial Prediction of Soil Properties and Corn Yield. Comput. Electron. Agric. 2018, 153, 213–225. [Google Scholar] [CrossRef]
- ProAgria 2022. Peltomaan Laatutesti. 2022. Available online: https://www.proagria.fi/uploads/archive/attachment/peltomaan_laatutesti_havaintojen_ja_mittausten_teko-ohjeet.pdf (accessed on 23 February 2023).
- Finnish Meteorological Institute. Suomen Ilmastovyöhykkeet. 2023. Available online: https://www.ilmatieteenlaitos.fi/suomen-ilmastovyohykkeet (accessed on 23 February 2023). (In Finnish).
- Finnish Meteorological Institute. Valitse Oikea Kasvi Oikealle Kasvuvyöhykkeelle. 2023. Available online: https://www.ilmatieteenlaitos.fi/kasvuvyohykkeet (accessed on 23 February 2023). (In Finnish).
- Mäkynen, J.; Holmlund, C.; Saari, H.; Ojala, K.; Antila, T. Unmanned Aerial Vehicle (UAV) Operated Megapixel Spectral Camera; SPIE: Bellingham, WA, USA, 2011; Volume 8186, pp. 295–303. [Google Scholar]
- Honkavaara, E.; Saari, H.; Kaivosoja, J.; Pölönen, I.; Hakala, T.; Litkey, P.; Mäkynen, J.; Pesonen, L. Processing and Assessment of Spectrometric, Stereoscopic Imagery Collected Using a Lightweight UAV Spectral Camera for Precision Agriculture. Remote Sens. 2013, 5, 5006–5039. [Google Scholar] [CrossRef]
- Hakala, T.; Markelin, L.; Honkavaara, E.; Scott, B.; Theocharous, T.; Nevalainen, O.; Näsi, R.; Suomalainen, J.; Viljanen, N.; Greenwell, C. Direct Reflectance Measurements from Drones: Sensor Absolute Radiometric Calibration and System Tests for Forest Reflectance Characterization. Sensors 2018, 18, 1417. [Google Scholar] [CrossRef]
- Viljanen, N.; Honkavaara, E.; Näsi, R.; Hakala, T.; Niemeläinen, O.; Kaivosoja, J. A Novel Machine Learning Method for Estimating Biomass of Grass Swards Using a Photogrammetric Canopy Height Model, Images and Vegetation Indices Captured by a Drone. Agriculture 2018, 8, 70. [Google Scholar] [CrossRef]
- Wu, Y.; Lim, J.; Yang, M.-H. Online Object Tracking: A Benchmark. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, New York, NY, USA, 23–28 June 2013; pp. 2411–2418. [Google Scholar]
- Näsi, R.; Honkavaara, E.; Lyytikäinen-Saarenmaa, P.; Blomqvist, M.; Litkey, P.; Hakala, T.; Viljanen, N.; Kantola, T.; Tanhuanpää, T.; Holopainen, M. Using UAV-Based Photogrammetry and Hyperspectral Imaging for Mapping Bark Beetle Damage at Tree-Level. Remote Sens. 2015, 7, 15467–15493. [Google Scholar] [CrossRef]
- Holman, F.H.; Riche, A.B.; Castle, M.; Wooster, M.J.; Hawkesford, M.J. Radiometric Calibration of ‘Commercial off the Shelf’Cameras for UAV-Based High-Resolution Temporal Crop Phenotyping of Reflectance and NDVI. Remote Sens. 2019, 11, 1657. [Google Scholar] [CrossRef]
- Burggraaff, O.; Schmidt, N.; Zamorano, J.; Pauly, K.; Pascual, S.; Tapia, C.; Spyrakos, E.; Snik, F. Standardized Spectral and Radiometric Calibration of Consumer Cameras. Opt. Express 2019, 27, 19075–19101. [Google Scholar] [CrossRef] [PubMed]
- Mattivi, P.; Franci, F.; Lambertini, A.; Bitelli, G. TWI Computation: A Comparison of Different Open Source GISs. Open Geospat. Data Softw. Stand. 2019, 4, 6. [Google Scholar] [CrossRef]
- Alakukku, L. Properties of Compacted Fine-Textured Soils as Affected by Crop Rotation and Reduced Tillage. Soil Tillage Res. 1998, 47, 83–89. [Google Scholar] [CrossRef]
- Pietola, L. Effect of Soil Compactness on the Growth and Quality of Carrot. Agric. Food Sci. 1995, 4, 139–237. [Google Scholar] [CrossRef]
- Li, J.; Heap, A.D. Spatial Interpolation Methods Applied in the Environmental Sciences: A Review. Environ. Model. Softw. 2014, 53, 173–189. [Google Scholar] [CrossRef]
- Shepard, D. A Two-Dimensional Interpolation Function for Irregularly-Spaced Data. In Proceedings of the 1968 23rd ACM National Conference, New York, NY, USA, 27–29 August 1968; pp. 517–524. [Google Scholar]
- Bărbulescu, A.; Șerban, C.; Indrecan, M.-L. Computing the Beta Parameter in IDW Interpolation by Using a Genetic Algorithm. Water 2021, 13, 863. [Google Scholar] [CrossRef]
- Radočaj, D.; Jug, I.; Vukadinović, V.; Jurišić, M.; Gašparović, M. The Effect of Soil Sampling Density and Spatial Autocorrelation on Interpolation Accuracy of Chemical Soil Properties in Arable Cropland. Agronomy 2021, 11, 2430. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Belgiu, M.; Drăguţ, L. Random Forest in Remote Sensing: A Review of Applications and Future Directions. ISPRS J. Photogramm. Remote Sens. 2016, 114, 24–31. [Google Scholar] [CrossRef]
- Maestrini, B.; Basso, B. Drivers of Within-Field Spatial and Temporal Variability of Crop Yield across the US Midwest. Sci. Rep. 2018, 8, 14833. [Google Scholar] [CrossRef] [PubMed]
- Peltonen-Sainio, P.; Jauhiainen, L.; Hakala, K. Crop Responses to Temperature and Precipitation According to Long-Term Multi-Location Trials at High-Latitude Conditions. J. Agric. Sci. 2011, 149, 49–62. [Google Scholar] [CrossRef]
- Peltonen-Sainio, P.; Venäläinen, A.; Mäkelä, H.M.; Pirinen, P.; Laapas, M.; Jauhiainen, L.; Kaseva, J.; Ojanen, H.; Korhonen, P.; Huusela-Veistola, E. Harmfulness of Weather Events and the Adaptive Capacity of Farmers at High Latitudes of Europe. Clim. Res. 2016, 67, 221–240. [Google Scholar] [CrossRef]
- Peltonen-Sainio, P.; Jauhiainen, L.; Trnka, M.; Olesen, J.E.; Calanca, P.; Eckersten, H.; Eitzinger, J.; Gobin, A.; Kersebaum, K.C.; Kozyra, J. Coincidence of Variation in Yield and Climate in Europe. Agric. Ecosyst. Environ. 2010, 139, 483–489. [Google Scholar] [CrossRef]
- Poley, L.G.; McDermid, G.J. A Systematic Review of the Factors Influencing the Estimation of Vegetation Aboveground Biomass Using Unmanned Aerial Systems. Remote Sens. 2020, 12, 1052. [Google Scholar] [CrossRef]
- Karila, K.; Alves Oliveira, R.; Ek, J.; Kaivosoja, J.; Koivumäki, N.; Korhonen, P.; Niemeläinen, O.; Nyholm, L.; Näsi, R.; Pölönen, I. Estimating Grass Sward Quality and Quantity Parameters Using Drone Remote Sensing with Deep Neural Networks. Remote Sens. 2022, 14, 2692. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Viña, A.; Ciganda, V.; Rundquist, D.C.; Arkebauer, T.J. Remote Estimation of Canopy Chlorophyll Content in Crops. Geophys. Res. Lett. 2005, 32. [Google Scholar] [CrossRef]
- Da Silva, J.M.; Silva, L.L. Evaluation of the Relationship between Maize Yield Spatial and Temporal Variability and Different Topographic Attributes. Biosyst. Eng. 2008, 101, 183–190. [Google Scholar] [CrossRef]
- Kumhalova, J.; Matějková, Š. Yield Variability Prediction by Remote Sensing Sensors with Different Spatial Resolution. Int. Agrophys. 2017, 31, 195. [Google Scholar] [CrossRef]
- Green, T.R.; Erskine, R.H. Measurement, Scaling, and Topographic Analyses of Spatial Crop Yield and Soil Water Content. Hydrol. Process. 2004, 18, 1447–1465. [Google Scholar] [CrossRef]
- Gomez, C.; Rossel, R.A.V.; McBratney, A.B. Soil Organic Carbon Prediction by Hyperspectral Remote Sensing and Field Vis-NIR Spectroscopy: An Australian Case Study. Geoderma 2008, 146, 403–411. [Google Scholar] [CrossRef]
- Oldfield, E.E.; Bradford, M.A.; Wood, S.A. Global Meta-Analysis of the Relationship between Soil Organic Matter and Crop Yields. Soil 2019, 5, 15–32. [Google Scholar] [CrossRef]
- Kulkarni, S.; Bajwa, S.; Huitink, G. Investigation of the Effects of Soil Compaction in Cotton. Trans. ASABE 2010, 53, 667–674. [Google Scholar] [CrossRef]
- Nevavuori, P.; Narra, N.; Linna, P.; Lipping, T. Assessment of Crop Yield Prediction Capabilities of CNN Using Multisource Data. In New Developments and Environmental Applications of Drones; Springer: Berlin/Heidelberg, Germany, 2022; pp. 173–186. [Google Scholar]
- Änäkkälä, M.; Lajunen, A.; Hakojärvi, M.; Alakukku, L. Evaluation of the Influence of Field Conditions on Aerial Multispectral Images and Vegetation Indices. Remote Sens. 2022, 14, 4792. [Google Scholar] [CrossRef]
- Suomalainen, J.; Hakala, T.; Alves de Oliveira, R.; Markelin, L.; Viljanen, N.; Näsi, R.; Honkavaara, E. A Novel Tilt Correction Technique for Irradiance Sensors and Spectrometers On-Board Unmanned Aerial Vehicles. Remote Sens. 2018, 10, 2068. [Google Scholar] [CrossRef]
- Suomalainen, J.; Oliveira, R.A.; Hakala, T.; Koivumäki, N.; Markelin, L.; Näsi, R.; Honkavaara, E. Tilt correction of onboard drone irradiance measurements–evaluation of hyperspectral methods. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2022, 43, 67–72. [Google Scholar] [CrossRef]
- Saiz-Rubio, V.; Rovira-Más, F. From Smart Farming towards Agriculture 5.0: A Review on Crop Data Management. Agronomy 2020, 10, 207. [Google Scholar] [CrossRef]
- Peltonen-Sainio, P.; Jauhiainen, L.; Laurila, H.; Sorvali, J.; Honkavaara, E.; Wittke, S.; Karjalainen, M.; Puttonen, E. Land Use Optimization Tool for Sustainable Intensification of High-Latitude Agricultural Systems. Land Use Policy 2019, 88, 104104. [Google Scholar] [CrossRef]
- Trevisan, R.; Bullock, D.; Martin, N. Spatial Variability of Crop Responses to Agronomic Inputs in On-Farm Precision Experimentation. Precis. Agric. 2021, 22, 342–363. [Google Scholar] [CrossRef]
- Peltonen-Sainio, P.; Jauhiainen, L. Risk of Low Productivity Is Dependent on Farm Characteristics: How to Turn Poor Performance into an Advantage. Sustainability 2019, 11, 5504. [Google Scholar] [CrossRef]
- Rikkonen, P.; Lahnamäki-Kivelä, S.; Leppänen, J.; Hänninen, H. Pellonomistajat Ja Maatalouden Tilusrakenteen Kehittäminen 2020-Luvulla. 2022. Available online: https://jukuri.luke.fi/handle/10024/551740 (accessed on 23 February 2023). (In Finnish).

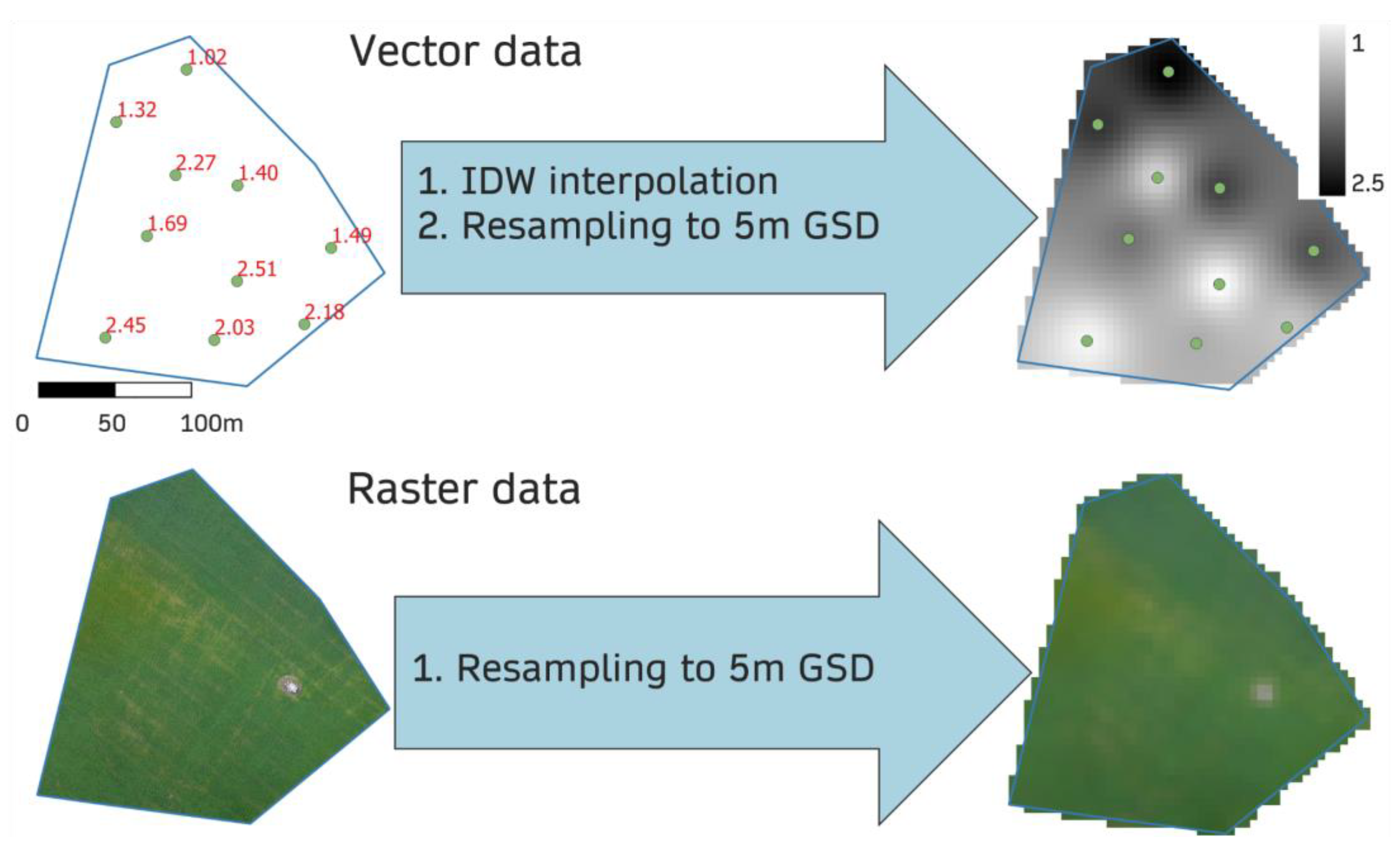
| Field l1 | Field l2 | Field i3 | Field i4 | |
|---|---|---|---|---|
| Surface area (ha) | 2.1 | 3.8 | 4.4 | 2.0 |
| Minimum altitude (m) | 3.2 | 4.5 | 12.0 | 14.3 |
| Maximum altitude (m) | 4.3 | 5.3 | 13.3 | 20.0 |
| Growing zone in Finland | V | V | I | I |
| Date | Sensor Type | Sensor Model | Drone | GSD (cm) | Crop |
|---|---|---|---|---|---|
| l1 | |||||
| 14 June 2017 | RGB | Phantom RGB | DJI Phantom 4 | 5.5 | Grass |
| 20 June 2017 | RGB | Phantom RGB | DJI Phantom 4 | 5.5 | Grass |
| 20 June 2017 | MS | Parrot Sequoia | DJI Phantom 4 | 14 | Grass |
| 19 July 2017 | RGB | Phantom RGB | DJI Phantom 4 | 5.5 | Grass |
| 9 July 2020 | RGB | Sony a7 | FGI Quadro | 2 | Barley |
| 9 July 2020 | MS | Micasense Altum | FGI Quadro | 6 | Barley |
| l2 | |||||
| 14 June 2017 | RGB | Phantom RGB | DJI Phantom 4 | 5.5 | Barley |
| 19 July 2017 | RGB | Phantom RGB | DJI Phantom 4 | 5.5 | Barley |
| 19 July 2017 | MS | Phantom RGB | DJI Phantom 4 | 14 | Barley |
| 9 July 2020 | RGB | Sony a7 | FGI Quadro | 2 | Grass |
| 9 July 2020 | MS | Micasense Altum | FGI Quadro | 6 | Grass |
| i3 | |||||
| 2 June 2016 | RGB | Samsung NX500 | FGI Hexa | 4 | Rapeseed |
| 22 June 2016 | RGB | Samsung NX500 | FGI Hexa | 4 | Rapeseed |
| 22 June 2016 | MS * | FGI2012b FPI | FGI Hexa | 14 | Rapeseed |
| 21 July 2016 | RGB | Samsung NX500 | FGI Hexa | 4 | Rapeseed |
| 28 June 2019 | MS | Micasense Altum | FGI Quadro | 5 | Wheat |
| 16 July 2020 | RGB | Sony a7 | FGI Quadro | 2 | Barley |
| 16 July 2020 | MS * | Rikola | FGI Quadro | 10 | Barley |
| i4 | |||||
| 2 June 2016 | RGB | Samsung NX500 | FGI Hexa | 4 | Pea |
| 2 June 2016 | MS * | FGI2012b FPI | FGI Hexa | 14 | Pea |
| 22 June 2016 | RGB | Samsung NX500 | FGI Hexa | 4 | Pea |
| 22 June 2016 | MS * | FGI2012b FPI | FGI Hexa | 14 | Pea |
| 21 July 2016 | RGB | Samsung NX500 | FGI Hexa | 4 | Pea |
| 21 July 2016 | MS * | FGI2012b FPI | FGI Hexa | 14 | Pea |
| 28 June 2019 | MS | Micasense Altum | FGI Quadro | 5 | Barley |
| 16 July 2020 | RGB | Sony a7 | FGI Quadro | 2 | Barley |
| 16 July 2020 | MS * | Rikola | FGI Quadro | 10 | Barley |
| Soil/Field Indicator | Acronym | Field l1 | Field l2 | Field i3 | Field i4 |
|---|---|---|---|---|---|
| Texture (%), particle size (mm) | |||||
| Clay, <0.002 | clay | 11.5 (2.6) | 6.9 (1.8) | 40.3 (1.8) | 33.2 (12.7) |
| Silt, 0.002–0.019 | silt | 27.8 (10.2) | 20.8 (4.5) | 29.7 (1.9) | 26.8 (9.4) |
| Fine sand, 0.02–0.19 | f_sand | 53.8 (8.2) | 66.1 (7.4) | 26.2 (2.3) | 33.0 (14.1) |
| Sand, 0.2–2 | sand | 6.9 (5.4) | 6.2 (4.2) | 3.8 (0.6) | 7.4 (5.9) |
| SOC (%) | SOC | 2.40 (0.86) | 2.40 (0.51) | 2.35 (0.57) | 3.23 (1.11) |
| Clay/SOC (ratio) | C_SOC | 5.1 (1.2) | 2.9 (0.8) | 18.4 (6.3) | 10.2 (1.8) |
| Penetrometer resistance (MPa) | |||||
| 5–15 cm | pen05_15 | 1.8 (0.5) | 1.4 (0.4) | 1.6 (0.6) | 1.5 (0.3) |
| 20–40 cm | pen20_40 | 3.7 (0.9) | 4.3 (0.9) | 2.1 (0.3) | 2.5 (0.8) |
| Soil quality properties | |||||
| A | A | 4.9 (1.4) | 5.2 (2.4) | 7.3 (1.4) | 7.2 (1.9) |
| B | B | 2.6 (2.0) | 0.8 (1.3) | 5.8 (2.3) | 6.1 (2.2) |
| C | C | 8.1 (2.9) | 6.6 (3.9) | 7.8 (3.6) | 5.3 (5.1) |
| D | D | 8.9 (1.3) | 8.0 (3.5) | 5.8 (2.5) | 6.0 (1.7) |
| E | E | 8.8 (1.8) | 8.4 (3.3) | 6.5 (2.4) | 4.6 (3.6) |
| Topography | |||||
| Topographic wetness index | TWI | 4.1 (0.3) | 6.6 (0.4) | 8.8 (0.6) | 8.4 (2.9) |
| Dataset | CV | R2 | r | Three Most Important Features | ||
|---|---|---|---|---|---|---|
| 2017-06-14_RGB_l1 | 0.854 | 0.401 | 0.638 | silt | TWI | C_SOC |
| 2017-06-20_MS_l1 | 0.072 | 0.656 | 0.815 | TWI | E | C |
| 2017-06-20_RGB_l1 | 0.587 | 0.553 | 0.746 | C | TWI | sand |
| 2017-07-19_RGB_l1 | 0.514 | 0.457 | 0.678 | B | TWI | C_SOC |
| 2020-07-09_MS_l1 | 0.060 | 0.841 | 0.918 | A | silt | TWI |
| 2020-07-09_RGB_l1 | 0.055 | 0.732 | 0.857 | A | f_sand | pen20–40 |
| 2017-06-14_RGB_l2 | 0.843 | 0.572 | 0.763 | clay | TWI | C_SOC |
| 2017-07-19_MS_l2 | 0.115 | 0.619 | 0.790 | E | TWI | silt |
| 2017-07-19_RGB_l2 | 0.240 | 0.220 | 0.519 | SOC | TWI | clay |
| 2020-07-09_MS_l2 | 0.071 | 0.441 | 0.668 | clay | TWI | f_sand |
| 2020-07-09_RGB_l2 | 0.233 | 0.189 | 0.459 | TWI | pen_20–40 | C_SOC |
| 2016-06-02_RGB_i3 | 0.108 | 0.883 | 0.941 | silt | sand | B |
| 2016-06-22_MS_i3 | 0.104 | 0.774 | 0.882 | pen20–40 | pen05–15 | D |
| 2016-06-22_RGB_i3 | 0.081 | 0.894 | 0.947 | D | silt | pen05–15 |
| 2016-07-21_RGB_i3 | 0.070 | 0.681 | 0.827 | D | sand | silt |
| 2019-06-28_MS_i3 | 0.063 | 0.496 | 0.711 | pen05–15 | silt | TWI |
| 2020-07-16_MS_i3 | 0.178 | 0.949 | 0.975 | pen05–15 | D | B |
| 2020-07-16_RGB_i3 | 0.174 | 0.868 | 0.932 | pen05–15 | pen20–40 | silt |
| 2016-06-02_MS_i4 | 0.204 | 0.897 | 0.947 | TWI | SOC | C/SOC |
| 2016-06-02_RGB_i4 | 0.086 | 0.825 | 0.913 | pen05–15 | TWI | E |
| 2016-06-22_MS_i4 | 0.097 | 0.654 | 0.817 | B | TWI | sand |
| 2016-06-22_RGB_i4 | 0.107 | 0.738 | 0.861 | D | pen05–15 | C |
| 2016-07-21_MS_i4 | 0.100 | 0.746 | 0.874 | pen05–15 | silt | A |
| 2016-07-21_RGB_i4 | 0.139 | 0.768 | 0.877 | pen05–15 | SOC | TWI |
| 2019-06-28_MS_i4 | 0.099 | 0.849 | 0.923 | TWI | A | B |
| 2020-07-19_MS_i4 | 0.080 | 0.544 | 0.743 | TWI | A | pen05–15 |
| 2020-07-19_RGB_i4 | 0.062 | 0.323 | 0.573 | SOC | silt | TWI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Näsi, R.; Mikkola, H.; Honkavaara, E.; Koivumäki, N.; Oliveira, R.A.; Peltonen-Sainio, P.; Keijälä, N.-S.; Änäkkälä, M.; Arkkola, L.; Alakukku, L. Can Basic Soil Quality Indicators and Topography Explain the Spatial Variability in Agricultural Fields Observed from Drone Orthomosaics? Agronomy 2023, 13, 669. https://doi.org/10.3390/agronomy13030669
Näsi R, Mikkola H, Honkavaara E, Koivumäki N, Oliveira RA, Peltonen-Sainio P, Keijälä N-S, Änäkkälä M, Arkkola L, Alakukku L. Can Basic Soil Quality Indicators and Topography Explain the Spatial Variability in Agricultural Fields Observed from Drone Orthomosaics? Agronomy. 2023; 13(3):669. https://doi.org/10.3390/agronomy13030669
Chicago/Turabian StyleNäsi, Roope, Hannu Mikkola, Eija Honkavaara, Niko Koivumäki, Raquel A. Oliveira, Pirjo Peltonen-Sainio, Niila-Sakari Keijälä, Mikael Änäkkälä, Lauri Arkkola, and Laura Alakukku. 2023. "Can Basic Soil Quality Indicators and Topography Explain the Spatial Variability in Agricultural Fields Observed from Drone Orthomosaics?" Agronomy 13, no. 3: 669. https://doi.org/10.3390/agronomy13030669
APA StyleNäsi, R., Mikkola, H., Honkavaara, E., Koivumäki, N., Oliveira, R. A., Peltonen-Sainio, P., Keijälä, N.-S., Änäkkälä, M., Arkkola, L., & Alakukku, L. (2023). Can Basic Soil Quality Indicators and Topography Explain the Spatial Variability in Agricultural Fields Observed from Drone Orthomosaics? Agronomy, 13(3), 669. https://doi.org/10.3390/agronomy13030669






