1. Introduction
Avocado (
Persea americana Mill.) is an important evergreen tropical fruit tree native to Mexico and Central America that belongs to the Lauraceae family [
1]. It is grown commercially in several tropical and subtropical regions, with a global production of more than 8 million tons in 2020 [
2]. The Americas, including the Caribbean, is the dominant producing region, at about 72.2% of total production, followed by Africa (13.3%), Asia (11.7%), Oceania (1.4%), and Europe (1.4%). Mexico is the major avocado-producing country, with nearly 2.4 million tons in 2020 [
2]. The avocado cultivation area worldwide has been significantly extended in the last decades due to increased consumer demand, especially in North America and Europe, because of Avocado’s nutritional benefits [
3].
Spain is the largest producer of avocado in Europe and the seventeenth largest in the world, with a production area covering more than 15,850 ha, a total production of about 99,125 tons and a production value of approximately EUR 220.5 million in 2020 [
4]. Most of the Spanish avocado production is in Andalusia (84.2%) and the Canary Islands (12.2%), with ‘Hass’ being the most extended cultivar, as it occupies 90% of the cultivated avocado area [
4]. Most of the production is exported to other European Union countries such as France (38.6%), The Netherlands (14.6%), and Germany (11%) [
5].
Avocado trees in Spain were free from important pests until the discovery of the Persea mite,
Oligonychus perseae Tuttle, Baker & Abattiello (Acari: Tetranychidae) in the avocado production areas of Andalusia (provinces of Málaga and Granada) in 2004 [
6,
7], and subsequently in the Canary Islands in 2006 [
8,
9]. Since its arrival, this exotic pest has rapidly spread to all Spanish avocado-growing regions, becoming the most damaging foliar pest of the susceptible cultivar ‘Hass’ [
8,
10]. The Persea mite is an important pest of avocados native to Mesoamerica [
11], from where it has spread and established in other avocado-growing areas worldwide, probably through the trade of plant material [
12]. This pest has been reported to cause significant damage to avocados in Mexico [
13], the USA (California, Florida, Hawaii) [
13,
14], Costa Rica [
15,
16], Israel [
17], mainland Spain [
7,
10], Portugal (mainland and Madeira) [
18], the Canary Islands [
8,
9], Italy [
19], and Morocco [
20]. In addition to avocado,
O. perseae is an oligophagous species that can develop on a wide range of fruit species, ornamentals, and weeds [
21].
Motile stages of the Persea mite feed and establish colonies on the underside of avocado leaves, building dense silken nests, mainly along the midrib and main nerves, where they develop and reproduce [
13,
22]. Among other functions, nests protect mites against attack from some species of natural enemies and against adverse environmental conditions [
23]. Feeding damage produces characteristic circular brown necrotic spots (of about 1–5 mm
2) that can extend to up to 90% of the leaf area, affecting the photosynthesis and transpiration efficiency of the tree and, consequently, the fruit yield [
13]. In some growing regions such as California or the Canary Islands, high mite populations (>100–500 mites per leaf) and subsequent feeding can cause partial or total tree defoliation [
8,
9,
13,
24], which opens the canopy, increasing the risk of sunburn to young fruit, premature fruit drop, and yield losses [
24,
25]. Chemical control has been shown to be the only tool available to Spanish growers to prevent high mite populations and the risk of tree defoliation [
7,
8,
9,
10], sulphur being the most common active ingredient used in the Canary Islands [
8,
9]. However, in Andalusia, this damages is less dramatic, and there is generally no such evidence of tree defoliation with high mite populations [
7,
10].
Since the appearance of
O. perseae in Spain, several strategies have been developed according to the principles of Integrated Pest Management (IPM) [
8,
9,
10] established in the current European Union regulations for the sustainable use of pesticides (European Directive, 2009/128/EEC). These principles include the establishment of threshold values [i.e., economic injury level (EIL) and economic threshold (ET)] [
26] as the basis for pest control decision-making. EIL has been defined as the lowest pest density at which the cost of reducing the pest population equals the economic loss prevented by implementing control measures [
27,
28]. ET, which is a direct function of the EIL, is the pest population at which control measures should be applied to prevent an increasing pest population from reaching EIL [
29]. Effective decision-making in IPM also relies on understanding the relationships between pest numbers, plant responses to injury, and the resultant economic losses [
30]. There are very few studies dealing with the relationship between leaf damage produced by herbivores and fruit yield reduction, though negative relationships between
Tetranychus urticae Koch (Acari: Tetranychidae) density and yield and fruit damage was shown in citrus [
31]. With respect to
O. perseae, an ET of 50–100 motile
O. perseae per leaf in Hass avocados is used for timing management decisions in Israel [
32]. In California, an ET of 50 mites per leaf throughout the period of the growing season (i.e., April–September in California) is the amount mentioned by Lara and Hoddle [
33] to be monitored for potential control. However, accurate estimates of pest-induced losses on avocado crops in Spain are not available. Determining the production losses and economic impact caused by
O. perseae in the Spanish avocado crops is necessary for pest control decision making.
The objective of the present study was to determine the incidence of O. perseae on avocado tree development as well as to estimate the damage, production losses and economic impact of this pest in the main avocado-producing areas of Spain.
2. Materials and Methods
2.1. Study Site and Experimental Design
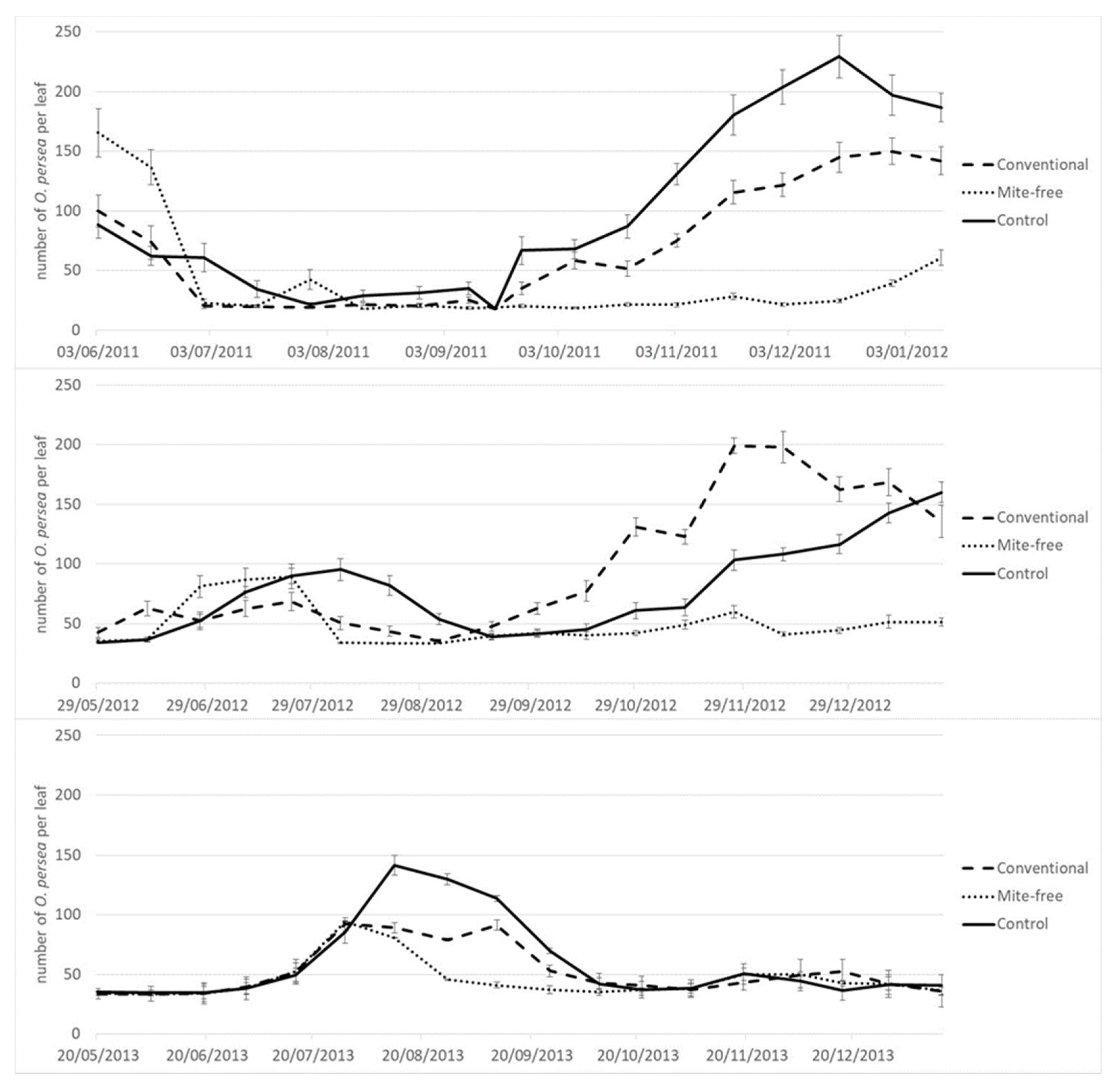
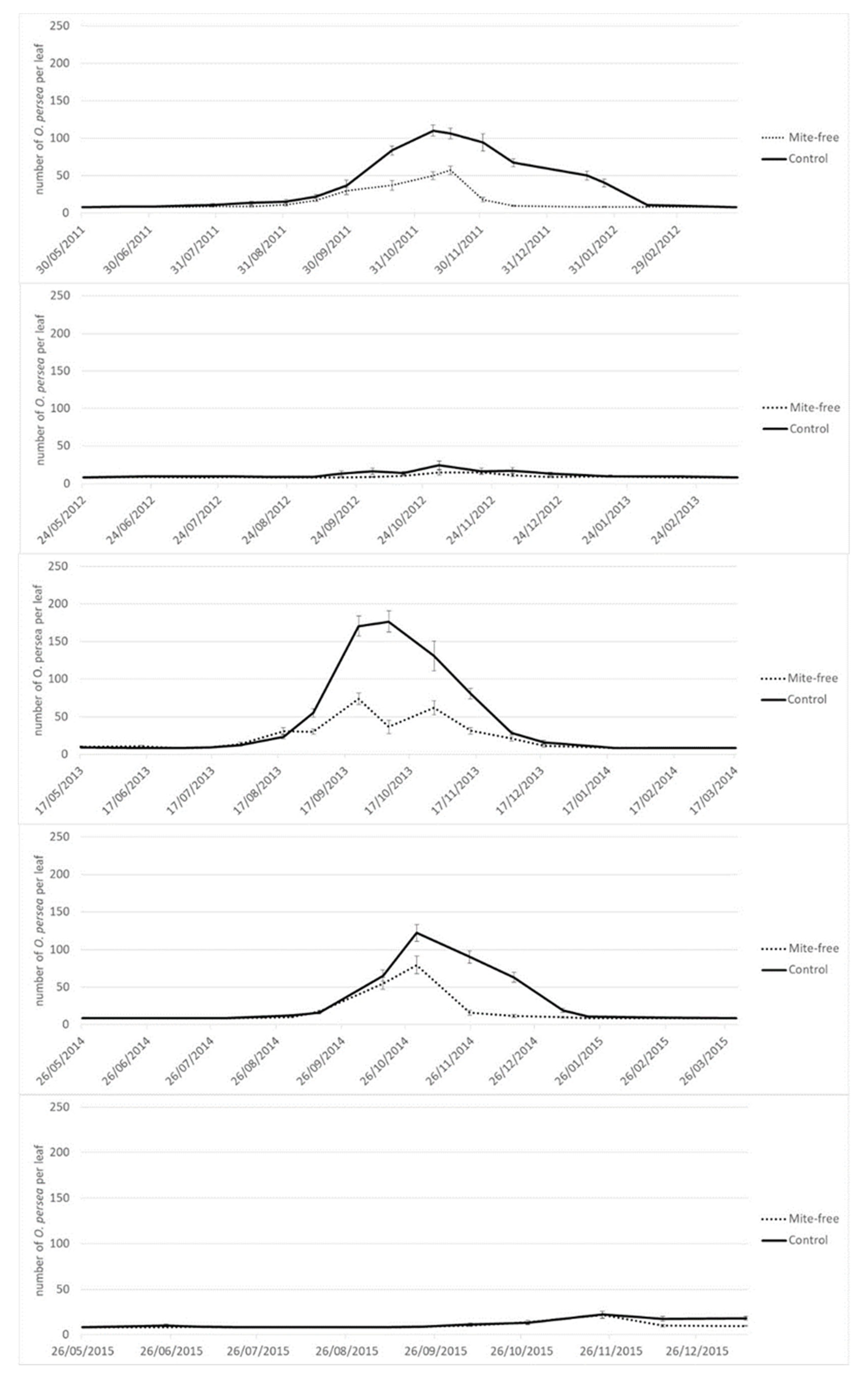
The experiment was conducted in two avocado orchards located in the two main growing areas of Spain. The first one was in Tenerife, Canary Islands (Coord x: 352433.32; Coord y: 3142194.07) on 3.5-year-old West Indian rootstock grafted with “Hass”. The second orchard was in Málaga, Andalusia (Coord x: 339644.0146; Coord y: 4053847.47) on 5-year-old clonal “Duke 7” rootstock grafted with “Hass”. Mite sampling was conducted between the years 2011–2013 in the first orchard (Tenerife), and between the years 2011–2015 in the second orchard (Málaga). Both avocado orchards were drip irrigated. Trees were maintained following the cultural practices routinely employed in these avocado cultivation areas.
In Tenerife, the experimental design consisted of a plot with 12 rows, each with 17 avocado trees. Three treatments were applied in the plot, one to each grouping of four rows: (i) Mite-free treatment: this consisted in spraying the trees to down the pest population below a level of 50 mites per leaf (
Table 1); (ii) Conventional treatment: the trees were sprayed to get between 50–150 mites per leaf (
Table 1); and (iii) Control treatment, which involved the absence of spraying and letting the Persea mite populations develop freely. To obtain different levels of mite damage, the trees were sprayed with Sulphur 80% WG (Azufre Flow SC, Cheminova Agro, S.A., Madrid, Spain; 1.0 g/L) when mite levels exceeded the designated levels, in agreement to the different treatments and the published pest management guidelines [
26]. In each grouping of four rows, only the two central ones were sampled for each treatment.
In Málaga, the experimental design consisted of a plot of six rows, each with 17 avocado trees. Two treatments were applied in the plot, one to each grouping of three rows, where only the middle row was sampled: (i) Mite-free treatment; and (ii) Control treatment, both as described above. To obtain different levels of mite damage, the mite-free treatment was sprayed with Abamectin 1.8% EC (Vertimec, Syngenta España S.A., Madrid, Spain; 1.2 l/ha), Fenpyroximate 6.24% + Hexythiazox 3.12% SC (Mitacid Plus, Sipcam Iberia, Valencia, Spain; 1.2 l/ha) or Tebufenpyrad 20% WP (Shirudo, Certis Belchim B.V., Utrecht, The Netherlands; 0.5 kg/ha).
In both orchards, spraying was carried out using a skid-mounted sprayer at the mean rate of 3.5 L per tree to ensure complete coverage of all parts of the tree. No treatments were applied against other arthropod pests during the sampling period. The number of treatments applied was different from one year to another in each orchard, because the population dynamics were influenced by climate (
Table 1). Of each row of 17 trees, the trees at the end were not sampled due to the border effect.
2.2. Sampling Method for Oligonychus perseae Populations
In both orchards, pest mite populations were monitored fortnightly, taking a random sample of six leaves from the outer canopy of the tree, from waist to eye level, around the tree (15 replicates × 6 leaves = 90 leaves per treatment). Pest mites were counted in the laboratory under a stereoscopic microscope using the fast field counting method developed by Machlitt [
34]. All motile stages adjacent to the distal side of the second vein to the left of the midrib (UML2) were counted. More recently, Lara et al. [
35] proposed an exponential equation to predict the mite population in the leaves. As that equation was optimized for California’s conditions, we sampled 101 leaves of 20 trees in 2017 from the same experimental field in Tenerife and 394 leaves in Málaga. All motile stages were counted, in addition to those along vein UML2. According to the exponential equation proposed by Lara et al. [
35], we adjusted the parameters α and β for our conditions, and the resultant equations for Tenerife (1) and Málaga (2) were:
where
y is the expected mean of total
O. perseae counts on a leaf given the presence of random effects and
xi is the partial count of
O. perseae along vein UML2. In Tenerife, the mean counted mites were 112.92 ± 69.49 (Mean ± SD), whereas the mean predicted mites were 111.86 ± 41.72. A Pearson’s
r data analysis revealed a significant (
p < 0.001) moderate positive correlation,
r = 0.56. In Málaga, the mean counted mites were 26.19 ± 48.51, whereas the mean predicted mites was 26.17 ± 35.43. A Pearson’s
r data analysis revealed a significant (
p < 0.001) strong positive correlation,
r = 0.73. Mite-days (of all motile stages) for two consecutive counts were calculated by multiplying the mean number of mites in two sampling dates by the number of days between counts [
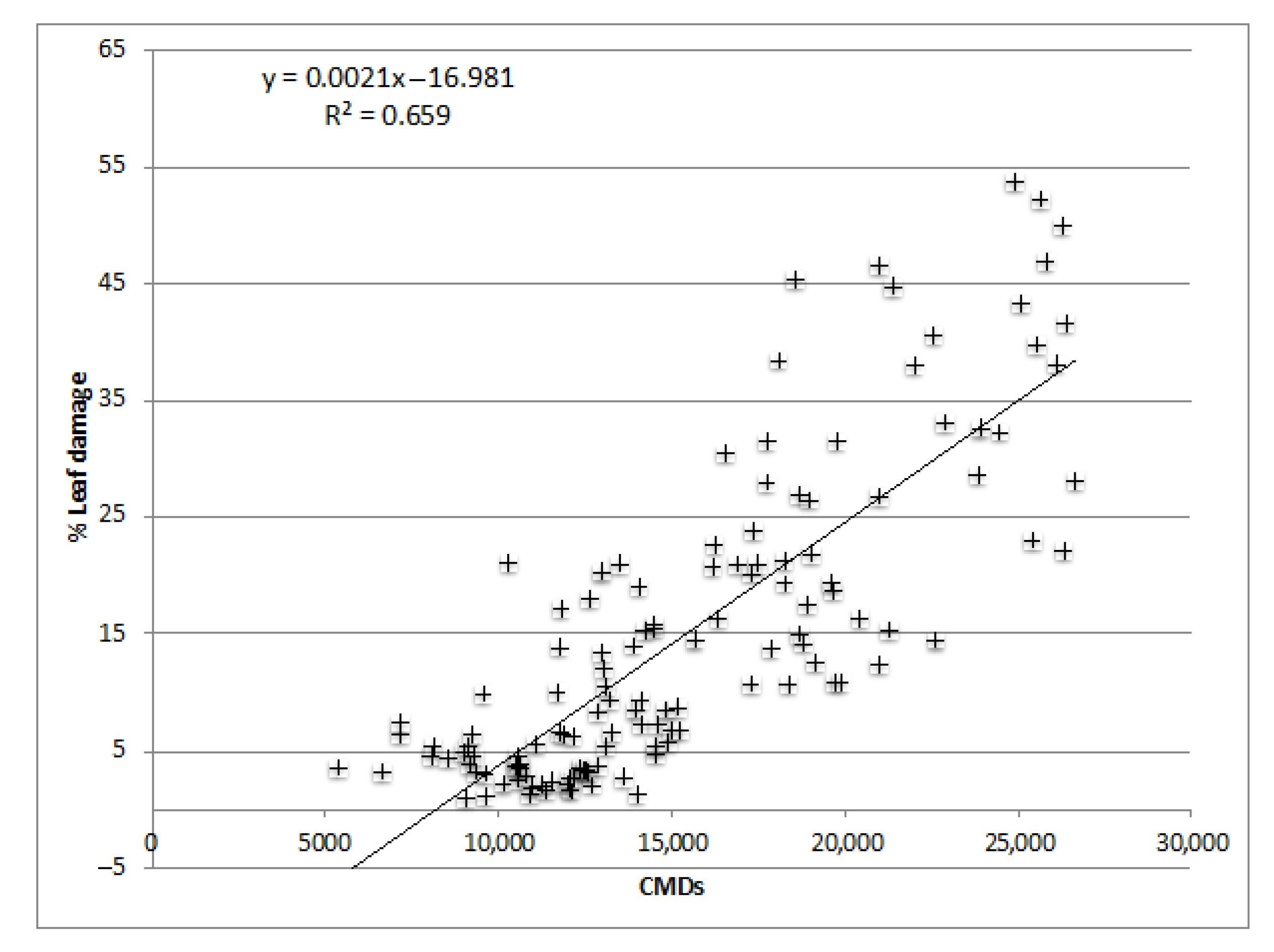
36]. Summing these mites’ days over a season gave the cumulative mite-days (CMDs).
2.3. Estimating Foliar Damage
To assess foliar damage, 90 leaves were sampled at harvest from each treatment. The samples were taken at the beginning of the trial (2011) and after the harvest (2012, 2013 and 2014). All leaves were scanned using a flatbed colour scanner (HP Scanjet 7400c; resolution 150 dpi) and saved as TIFF format files. Image analysis software (WinFOLIATM 2014, Régent Instruments Inc., Quebec, Canada) was used to quantify total and damaged leaf areas. With the total area and the damaged area of each leaf, the percentage of leaf area damaged (PLAD) was calculated.
To calculate the prediction of CMDs on PLAD, the Nash-Sutcliffe Efficiency (NSE) index was used, which ranges from 1 (perfect model), 0 (a performance no better than simply using the mean) to negative values (a worse performance) [
36,
37].
2.4. Estimation of Economic Losses Caused by O. perseae
Direct economic losses were estimated by sampling the fruits of each plant of the different treatments. The number of fruits per plant was counted, and the total yield per plant was obtained. In the Tenerife orchard, a hundred fruits were randomly taken from each treatment to determine the polar and equatorial diameter, the average weight of the fruit, and the fruit gauge. In the Málaga orchard, 30 fruits per tree were measured and weighed.
2.5. Estimated Costs of Control
The costs derived from
O. perseae control on avocado were estimated following the established methodology [
38,
39]. To calculate the acaricide cost, the prices of the phytosanitary products were obtained from local distributors. The application rates and the frequency of treatments were those recommended by the manufacturer on avocado cultivation. As the implementation costs are variable, we calculated the total cost as a function of the price of the acaricide, the dose applied, the volume of each treatment, and the labour costs (€/h and €/ha) (
Table 2).
2.6. Statistical Analysis
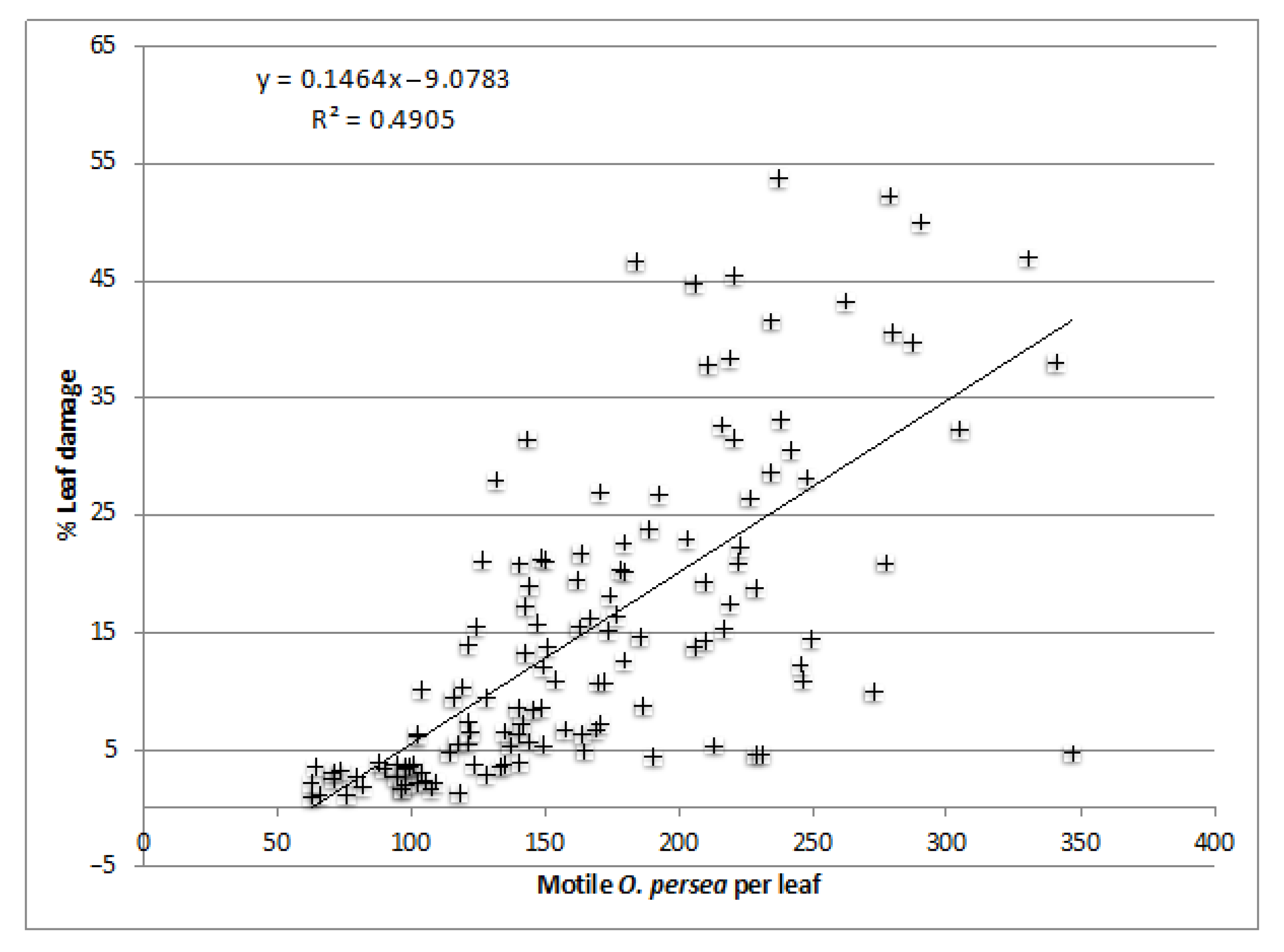
Data were summarized as mean ± SE. The normality and homoscedastic hypotheses were tested and considered significant when p < 0.05. Data were analyzed with SPSS 20 (SPSS, Chicago, IL, USA) using a two-way repeated measures ANOVA to compare treatments and years for CMDs, and a two-way ANOVA for the rest of the measured variables, i.e., leaf damage, fruits per tree, and yield. Means separation (of years, treatments, or treatment × years) was performed by Tukey’s HSD multiple range test (p < 0.05) when data followed a normal distribution and were homoscedastic, and a Dunnett’s test (p < 0.05) when data was normal but not homoscedastic. When normality was not fulfilled, even with transformations of the parameters, the Kruskal-Wallis test was used. Linear regression was used to evaluate the effect of CMDs on foliar damage using the average of each tree for three years and to compare foliar damage with the maximum estimation of motile O. perseae per leaf.
4. Discussion
There is a striking difference in Persea mite abundance between populations from northern Tenerife and the coast of Málaga, being higher in Tenerife than in Málaga. Northern Tenerife, where the Canary Islands experimental plot is situated, is affected by trade winds, which produce a higher humidity during the summer months as compared to the orchards in southern Spain, where the climate is characterized by high summer temperatures and low humidity. The average daily maximum temperature is 29.8 °C, and the average relative humidity (RH) is 61.3% (in Málaga Airport). By contrast, in northern Tenerife, the average maximum temperature is 25.1 °C, with an RH of 69.6% [
40]. This means that summer periods are less favorable for
O. perseae in Málaga because of the high temperatures and low humidity, conditions which are a well-established mortality factor for the pest [
41]. In fact, the number of Persea mites per leaf was demonstrated to be significantly affected by the minimum temperature and average relative humidity in Tenerife [
9]. Furthermore, mild weather conditions in northern Tenerife cause the avocado trees to produce larger size fruits than in Málaga, as factors such as temperature, relative humidity, light intensity, and plant water potential directly influence elementary processes within the leaf, such as the assimilation of CO
2 into carbohydrates and other metabolites, storage as starch, and transport to other parts of the growing tree, including fruit [
42].
There is a correlation between mite feeding damage and photosynthesis inhibition. Mite feeding implies removing the leaf cells content and therefore a reduction in leaf chlorophyll [
43,
44]. For example,
Oligonychus yothersi (McGregor) feeding on the upper surface of avocado leaves can cause up to a 30% reduction in the photosynthetic activity of leaves, many of which often fall to the ground within 45–60 days after infestation, thus average populations of 245 mites per day can lead to a yield reduction of 36.45% [
45]. The high number of mites producing this damage over time can reduce the yield [
46,
47,
48]. As the leaf area damaged is cumulative over time, it increases as the local mite population grows. Hence, the PLAD recorded in the present study correlated with CMDs.
Because of the higher Persea mite populations in Tenerife, we have here reported values of PLAD ranging from 11.73 to 34.98, whilst in Málaga, values ranged from 8.93 to 13.98. High PLAD in Tenerife may explain why both the number of fruits per tree and the yield (in 2012 and 2013) were significantly higher in the mite-free treatment, in which acaricide applications depleted Persea mite populations. In Málaga, no increase was recorded either in fruits per tree or in the yield under mite-free treatment with respect to the control. This was likely due to the harsher environmental conditions in Málaga which were less suitable for Persea mite survival. In addition, the trees in Málaga, being older than in Tenerife, were more resistant to mite infestation.
A significant linear relationship was found between PLAD and CMDs, but there was no linear correlation between CMDs and yield. The Persea mite is a very important factor negatively affecting yield, but there are likely other factors involved, such as climatic conditions, alternative bearing, stress, etc. Therefore, mite infestation cannot be considered the only cause responsible for yield reduction [
25]. However, we have estimated for the Northern Tenerife orchard an economic injury level (EIL) of 17 PLAD, which is the damage that should not be exceeded. This means a maximum of 178 motile forms of Persea mite per leaf. In Israel, Maoz et al. [
25] established an EIL of ca. 15 PLAD, or 50–100 mites per leaf, which is a lower threshold than that in Tenerife.
Relative to the individual characteristics of fruit sizing, i.e., polar and equatorial diameters and weight, in Tenerife only small differences were found between control and mite-free treatments in the equatorial diameter (2012), but there were no differences in weight. On the contrary, the avocados from Málaga were heavier in the control trees than in the mite-free treatment. This fact was counteracted by a somewhat insignificant lesser number of fruits in the control than in the treatment mite-free (from 2012 to 2015) group. Finally, the yield in Málaga was nearly the same in the mite-free treatment and in the control. The lesser individual fruit weight in the mite-free treatment, i.e., in trees subjected to acaricide applications, may be a result of a lesser mite infestation, but may also be due to a negative impact of the chemical compounds applied.
In the Tenerife orchard, the yield was significantly higher in mite-free treatment than in the control or conventional treatments in the last two years of the study. This means that high populations of the Persea mite are responsible for a significant production loss, and that control measures are necessary on the island. Similar results were observed in the state of Guerrero, Mexico, where the size and weight of avocados from treatments with Persea mite populations below the control threshold were larger and showed significant differences compared to the control [
49].
During the first season (2011), the results may have been obscured due to the resilience of the trees to the treatments, i.e., the trees need time to incorporate the changes produced by the treatments to their physiology, in this case to the yield. However, in 2012 and 2013, the yield changed in the order of mite-free treatment > conventional > control. In this way, an increased yield of 372 kg/ha is enough to compensate for acaricide treatments, and between 558–1115 kg/ha is sufficient to compensate for the treatments necessary to achieve a mite population below EIL.
The yield loss observed in Tenerife could be related to the defoliation observed in the control treatment during the summer season and during flowering (spring). Processes such as photosynthesis and transpiration are negatively affected as the percentage of damaged leaf area increases [
42]. Therefore, the loss of leaf from the tree canopy implies a total reduction of the photosynthetic area of the plant, thus decreasing CO
2 assimilation. In addition, the processes carried out in the leaf depend on the age of the leaf [
43], and this is different at the times when leaf fall is observed, affecting carbon assimilation and storage.