Enzymatic and Molecular Identification of Meloidogyne Species in Tomato Orchards in Paraguay
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Collection and Nematode Extraction
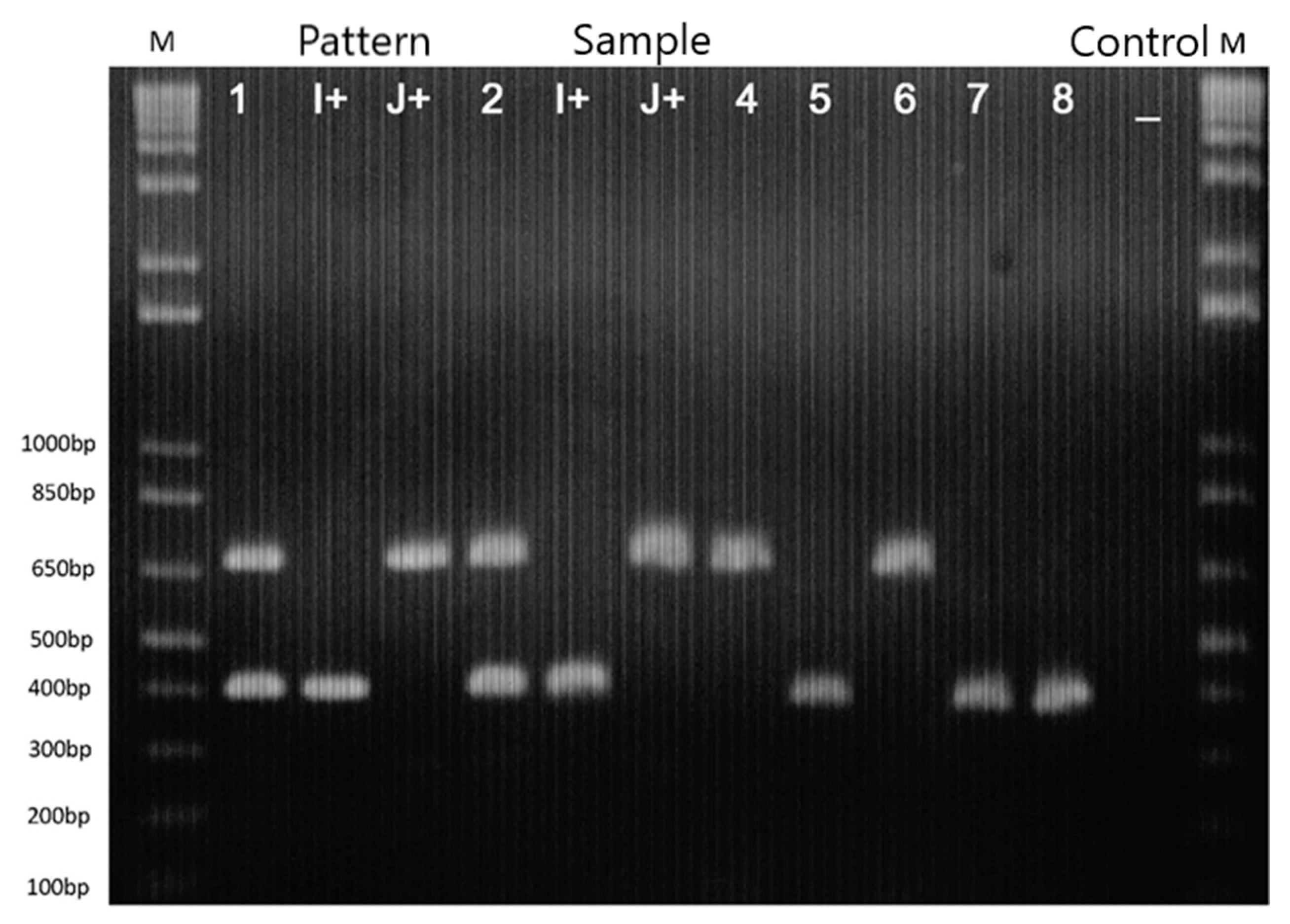
2.2. Morphological, Biochemical, and Molecular Characterization
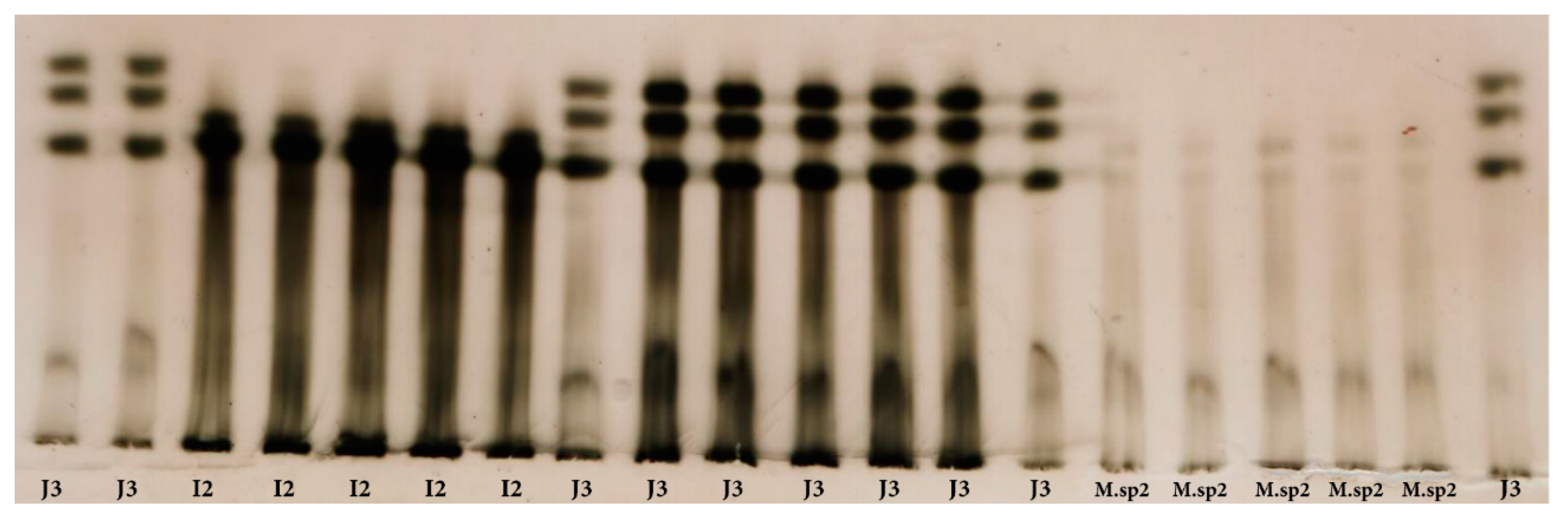
2.3. Identification of Meloidogyne spp. by Esterase Phenotype
2.4. DNA Extraction
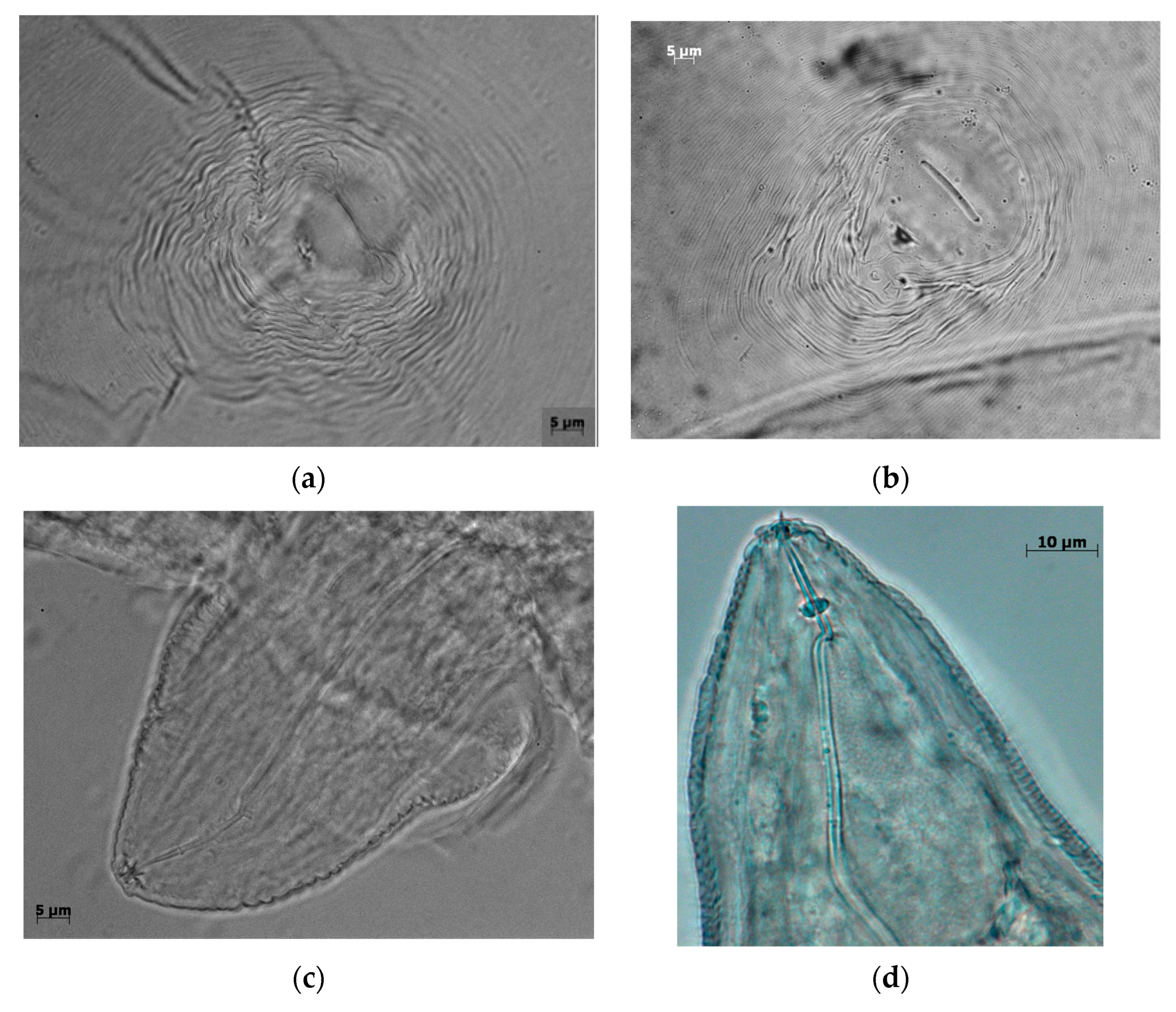
2.5. Perineal Patterns
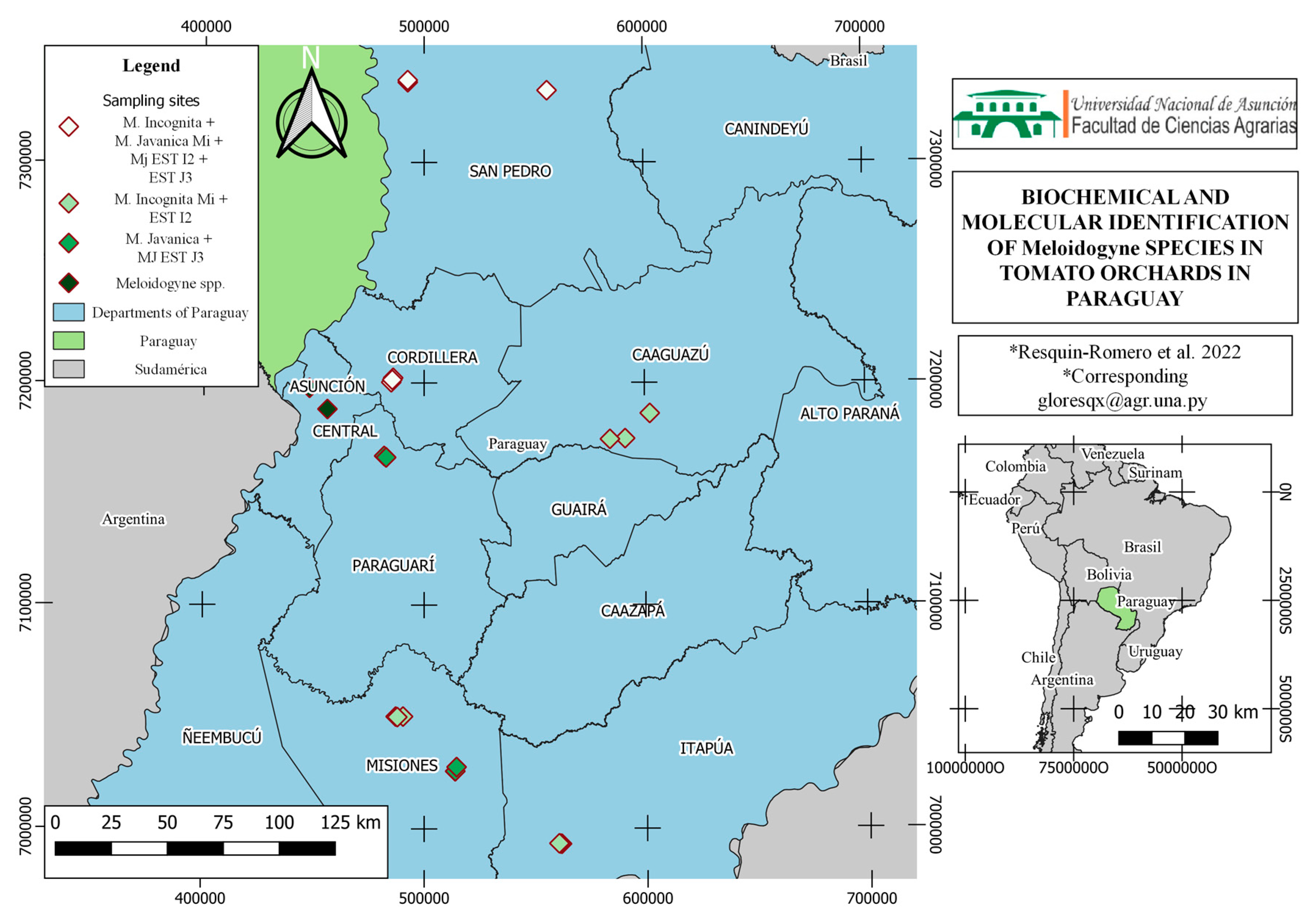
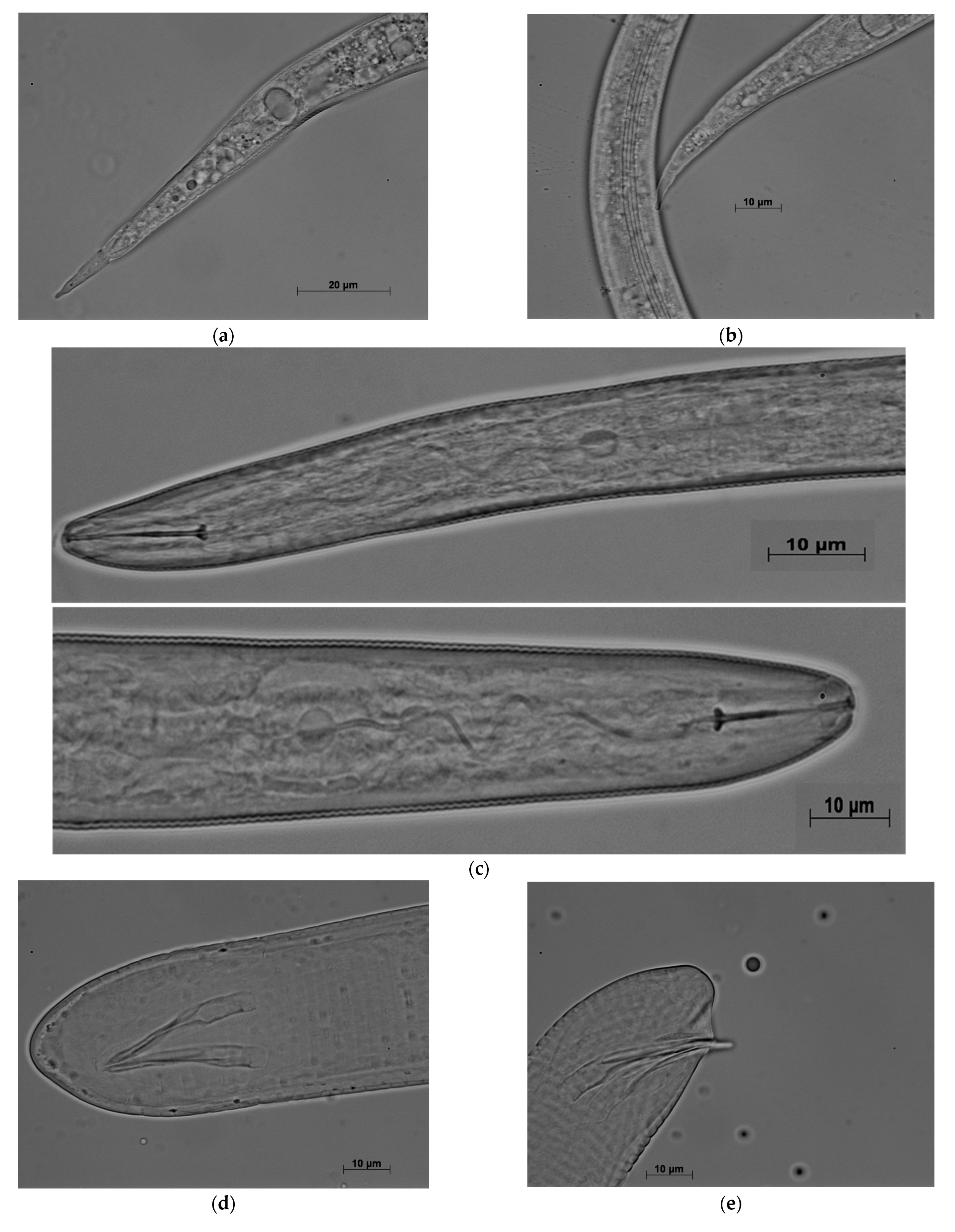
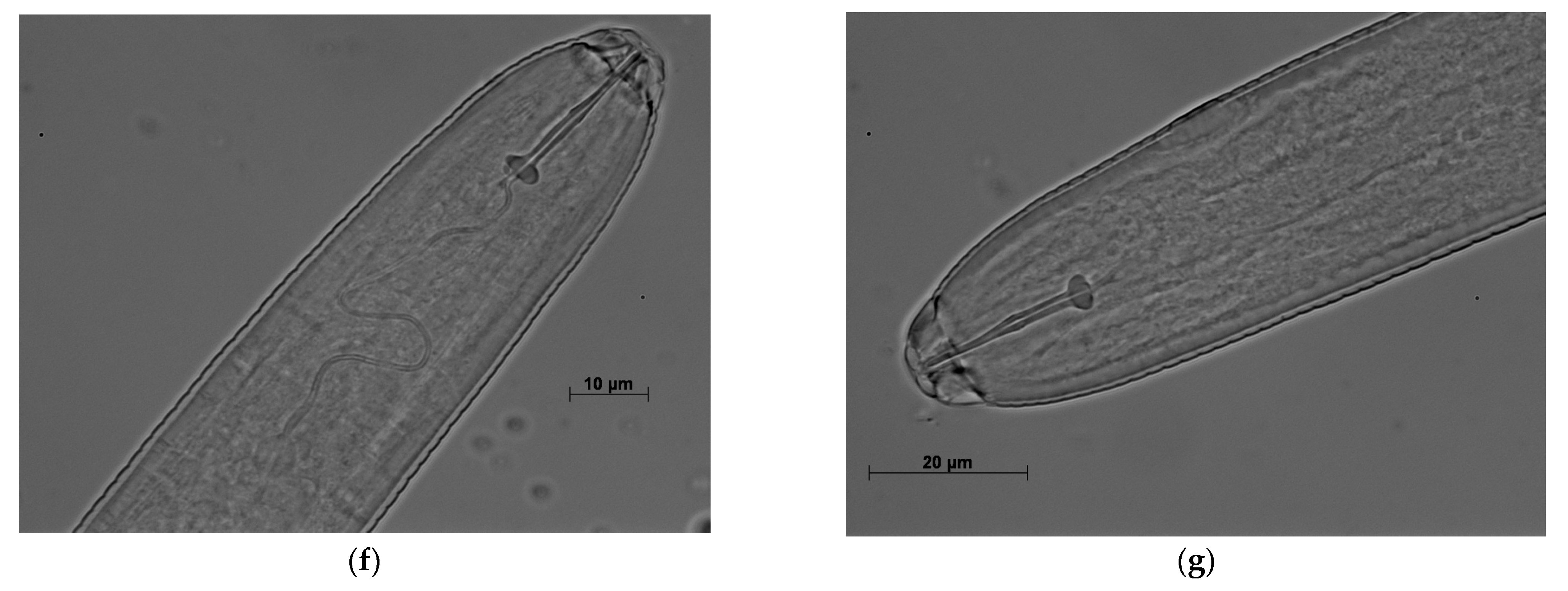
3. Results
3.1. Identification of Meloidogyne spp. by Esterase Phenotype
3.2. Morphological, Biochemical, and Molecular Characterization
3.3. Perineal Patterns
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Direction of Census and Agricultural Statistics—Department of Statistics, 2016/2017, Paraguay. Available online: https://www.academia.edu/41573866/S%C3%8DNTESIS_ESTAD%C3%8DSTICAS_PRODUCCI%C3%93N_AGROPECUARIA_2016_2017 (accessed on 1 January 2022).
- Moens, M.; Perry, R.N.; Starr, J.L. Meloidogyne species—A diverse group of novel and important plant parasites. In Root-Knot Nematodes; Perry, R.N., Moens, M., Starr, J.L., Eds.; CAB International: Wallingford, UK, 2009; pp. 1–17. [Google Scholar]
- Huang, W.K.; Sun, J.H.; Cui, J.K.; Wang, G.F.; Kong, L.A.; Peng, H.; Chen, S.L.; Peng, D.L. Efficacy Evaluation of Fungus Syncephalastrum racemosum and Nematicide Avermectin against the Root-Knot Nematode Meloidogyne incognita on Cucumber. PLoS ONE 2014, 9, e89717. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, R.M.D.G.; Monteiro, J.; Silva, U.C.; Gomes, G. Genero Meloidogyne: Diagnose através de electroforese de isoenzimas e marcadores SCAR. In Diagnose de Fitonematoides; Oliveira, C.M.G., Dos Santos, M.A., Castro, L.H.S., Eds.; Camara Brasileira do livro: Pinheiros, Brazil, 2016; pp. 48–70. [Google Scholar]
- Tapia-Vázquez, I.; Montoya-Martínez, A.C.; De los Santos-Villalobos, S.; Ek-Ramos, M.J.; Montesinos-Matías, R.; Martínez-Anaya, C. Root-knot nematodes (Meloidogyne spp.) a threat to agriculture in Mexico: Biology, current control strategies, and perspectives. J. Microbiol. Biotechnol. 2022, 38, 26. [Google Scholar] [CrossRef] [PubMed]
- Blok, V.C.; Powers, T.O. Biochemical and molecular identification. In Root Knot Nematodes, 1st ed.; Perry, R.N., Moens, M., Star, J., Eds.; CABI International: London, UK, 2009; pp. 98–112. [Google Scholar]
- Hunt, D.; Handoo, Z.A. Taxonomy, identification and principal species. In Root-Knot Nematodes; Perry, R.N., Moens, M., Star, J., Eds.; CABI: Wallingford, UK, 2009; pp. 55–97. [Google Scholar] [CrossRef]
- Gabriel, M.; Kulczynski, M.; Muniz, M.F.B.; Boiteux, L.S.; Carneiro, R.M.D.G. Resistance of ‘Debora Plus’ tomato bearing Mi-1.2 gene/locus against fifteen Meloidogyne species. Plant Pathol. 2020, 69, 944–952. [Google Scholar] [CrossRef]
- Carneiro, R.M.D.G.; Almeida, M.R.A. Técnica de eletroforese usada no estudo de enzimas dos nematoides das galhas para identificação de especies. Nematol. Bras. 2001, 25, 35–44. [Google Scholar]
- Carneiro, R.M.D.G.; Cofcewicz, E.T. Taxonomy of coffee-parasitic root-knot nematodes, Meloidogyne spp. In Plant Parasitic Nematodes of Coffee; Souza, R.M., Ed.; Springer: New York, NY, USA, 2008; pp. 87–122. [Google Scholar] [CrossRef]
- Zijlstra, C.; Donkers-Venne, D.T.H.M.; Fargette, M. Identification of Meloidogyne incognita, M. javanica and M. arenaria using sequence characterised amplified region (SCAR) based PCR assays. Nematology 2000, 2, 847–883. [Google Scholar]
- Randig, O.; Bongiovanni, M.; Carneiro, R.M.D.G.; Castagnone-Sereno, P. Genetic diversity of root knot nematodes from Brazil and development of SCAR markers specific for the coffee-damaging species. Genome 2002, 45, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Tigano, M.; Siqueira, K.; Castagnone-Sereno, P.; Mulet, K.; Queiroz, P.; Santos, M.; Teixeira, C.; Almeida, M.; Silva, J.; Carneiro, R.M.D.G. Genetic diversity of the root-knot nematode Meloidogyne enterolobii and development of a SCAR marker for this guava-damaging species. Plant Pathol. 2010, 59, 1054–1061. [Google Scholar] [CrossRef]
- Correa, V.R.; Santos, M.F.A.; Almeida, M.R.A.; Peixoto, J.R.; Castagnone-Sereno, P.; Carneiro, R.M.D.G. Species-specific DNA markers for identification of two root-knot nematodes of coffee: Meloidogyne arabicida and M. izalcoensis. Eur. J. Plant Pathol. 2013, 137, 305–313. [Google Scholar] [CrossRef]
- Correa, V.R.; Mattos, V.S.; Almeida, M.R.A.; Santos, M.F.A.; Tigano, M.S.; Castagnone-Sereno, P.; Carneiro, R.M.D.G. Genetic diversity of the root-knot nematode Meloidogyne ethiopica and development of a species-specific SCAR marker for its diagnosis. J. Plant Pathol. 2014, 63, 476–483. [Google Scholar] [CrossRef]
- Valiente, A.R. Nematodos de Las Plantas, Morfología (Biología) y Control de Nematodos. Facultad de Ciencias Agrarias de la Universidad Nacional de Asunción (FCA/UNA/JICA); 2010; 978-99953-912-2-5. Available online: https://isbn.cloud/9789995391225/nematodos-de-plantas/ (accessed on 1 January 2022).
- Lordello, R.R.A.; Lordello, A.I.L.; Godoy, I.J. Occurrence of Meloidogyne javanica parasiting roots and nodules of peanuts in Paraguay. Bragantia 1997, 56, 87–89. [Google Scholar] [CrossRef]
- Lopez-Nicora, H.; Pedrozo, L.M.; Grabowski, C.; Orrego Fuente, A.; Villalba, E.H.; Ralston, T. First Report of the Reniform Nematode (Rotylenchulus reniformis) from Soybean in Paraguay. Plant Dis. 2018, 102, 2043. [Google Scholar] [CrossRef] [PubMed]
- Soilan-Duarte, L.C.; Orrego-Fuente, A.L. Fuente. Identificación de la especie del nemátodo de las agallas Meloidogyne en el cultivo de lechuga (Lactuca sativa L.). In III Congreso Nacional de Ciencias Agrarias “Producción Sostenible de Alimentos Para el Desarrollo de Paraguay”; 2014; ISSN/ISBN: 978-9996. Available online: https://www.agr.una.py/descargas/publicaciones/IIICNCA2014.pdf (accessed on 1 January 2022).
- Hartman, K.M.; Sasser, J.N. Identification of Meloidogyne species on the basis of differential host test and perineal pattern morphology. In An Advanced Treatise on Meloidogyne; Barker, K.R., Carter, C.C., Sasser, J.N., Eds.; North Carolina State University Graphics: Raleigh, NC, USA, 1985; pp. 69–77. [Google Scholar]
- Carneiro, R.M.D.G.; Tigano, M.S.; Almeida, M.R.A.; Sarah, J.L. Identification and genetic diversity of Meloidogyne spp. on coffee from Brazil, Central America and Hawaii. Nematology 2004, 6, 287–298. [Google Scholar] [CrossRef]
- Silva, J.G.; Furlanetto, C.; Almeida, M.R.; Rocha, D.B.; Mattos, V.S.; Correa, V.R.; Carneiro, R.M. Occurrence of Meloidogyne spp. in Cerrado vegetations and reaction of native plants to Meloidogyne javanica. J. Phytopathol. 2013, 162, 449–455. [Google Scholar] [CrossRef]
- Lopez-Nicora, H.; Enciso-Maldonado, G.C.; Caballero Mairesse, G.; Sanabria-Velazquez, A.; Armadans-Rojas, A.; Soilan, L.; Grabowski, C.; Resquin-Romero, G.; Colmán, A.; Pedrozo-Fleitas, L.M.; et al. Distribution and abundance of nematodes in horticultural production in Paraguay. Plant Health Prog. 2022, 23, 466–475. [Google Scholar] [CrossRef]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Taisei Kikuchi, J.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.L.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. J. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef]
- Chitwood, D.J. Research on plant-parasitic nematode biology conducted by the United States department of agriculture agricultural research service. Pest Manag. Sci. Former. Pestic. Sci. 2003, 753, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Mesa-Valle, C.M.; Garrido-Cárdenas, J.A.; Cebrián-Carmona, J.; Talavera, M.; Manzano-Agugliaro, F. Investigación global sobre nematodos vegetales. Agronomía 2020, 10, 1148. [Google Scholar] [CrossRef]
- Inés-Vásquez, S.; Aquino-Bolaños, T. Biocontrol y Tolerancia de Meloidogyne incognita en Tomate. Southwest. Entomol. 2021, 45, 957–964. [Google Scholar] [CrossRef]
- Arrúa-Alvarenga, A.; Aquino-Jara, A. Effect of solarization on sclerotia of Sclerotinia sclerotiorum (Lib.) De Bary, and the fluctuation of the population of nematodes present in the soil. Investig. Agrar. 2013, 7, 5–11. [Google Scholar]
- Wesemael, W.M.L.; Viaene, N.; Moens, M. Root-knot nematodes (Meloidogyne spp.) in Europe. Nematology 2011, 13, 3–16. [Google Scholar] [CrossRef]
- Sikandar, A.; Zhang, M.Y.; Wang, Y.Y.; Zhu, X.F.; Liu, X.Y.; Fan, H.Y.; Xuan, Y.H.; Chen, L.J.; Duan, Y.X. Review article: Meloidogyne incognita (root-knot nematode) a risk to agriculture. Appl. Ecol. Environ. Res. 2020, 18, 1679–1690. [Google Scholar] [CrossRef]
| Primer SCAR | Sequence (5′–3′) | Amplification (bp) | Reference | Target Species |
|---|---|---|---|---|
| inc-K14-F inc-K14-R | GGGATGTGTAAATGCTCCTG CCCGCTACACCCTCAACTTC | 399 | [12] | M. incognita |
| Fjav Rjav | GGTGCGCGATTGAACTGAGC CAGGCCCTTCAGTGGAACTATAC | 670 | [11] | M. javanica |
| Origin Department | Samples | Identification of Meloidogyne spp. | |||||
|---|---|---|---|---|---|---|---|
| Code | District | Total, Samples | Species | Identification SCAR/Esterase (EST.) | Perennial Pattern Morphology | ||
| 1 | San Pedro | SPY | 3 | M. incognita + M. javanica | Mi + Mj | EST I2 + EST J3 | M. incognita + M. javanica |
| 2 | Cordillera | TC | 3 | M. incognita + M. javanica | Mi + Mj | EST I2 + EST J3 | M. incognita + M. javanica |
| 3 | Central | JAS | 2 | Meloidogyne sp. | no amplification | Atypical (EST 1.0 and 1.3) | Meloidogyne sp. |
| 4 | Paraguarí | YP | 3 | M. javanica | Mj | EST J3 | M. javanica |
| 5 | Misiones | SJB | 3 | M. incognita | Mi | EST I2 | M. incognita |
| 6 | Misiones | SIM | 3 | M. javanica | Mj | EST J3 | M. javanica |
| 7 | Itapua | SCD | 3 | M. incognita | Mi | EST I2 | M. incognita |
| 8 | Caaguazu | CO | 3 | M. incognita | Mi | EST I2 | M. incognita |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Resquín-Romero, G.; Mattos, V.S.; Monteiro, J.M.S.; Lopez-Nicora, H.D.; Amarilla, S.P.; Chamorro-Diaz, S.; Moral, J.; Carneiro, R.M.D.G. Enzymatic and Molecular Identification of Meloidogyne Species in Tomato Orchards in Paraguay. Agronomy 2023, 13, 670. https://doi.org/10.3390/agronomy13030670
Resquín-Romero G, Mattos VS, Monteiro JMS, Lopez-Nicora HD, Amarilla SP, Chamorro-Diaz S, Moral J, Carneiro RMDG. Enzymatic and Molecular Identification of Meloidogyne Species in Tomato Orchards in Paraguay. Agronomy. 2023; 13(3):670. https://doi.org/10.3390/agronomy13030670
Chicago/Turabian StyleResquín-Romero, Gloria, Vanessa S. Mattos, Jessica M. S. Monteiro, Horacio D. Lopez-Nicora, Shyrley P. Amarilla, Sergio Chamorro-Diaz, Juan Moral, and Regina M. D. G. Carneiro. 2023. "Enzymatic and Molecular Identification of Meloidogyne Species in Tomato Orchards in Paraguay" Agronomy 13, no. 3: 670. https://doi.org/10.3390/agronomy13030670
APA StyleResquín-Romero, G., Mattos, V. S., Monteiro, J. M. S., Lopez-Nicora, H. D., Amarilla, S. P., Chamorro-Diaz, S., Moral, J., & Carneiro, R. M. D. G. (2023). Enzymatic and Molecular Identification of Meloidogyne Species in Tomato Orchards in Paraguay. Agronomy, 13(3), 670. https://doi.org/10.3390/agronomy13030670






