Higher Aluminum Tolerance of Lespedeza bicolor Relative to Lespedeza cuneata Is Associated with Saccharide Components of Root Tips
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Monitoring and Energy-Spectrum Analysis of Elements on Root Surface
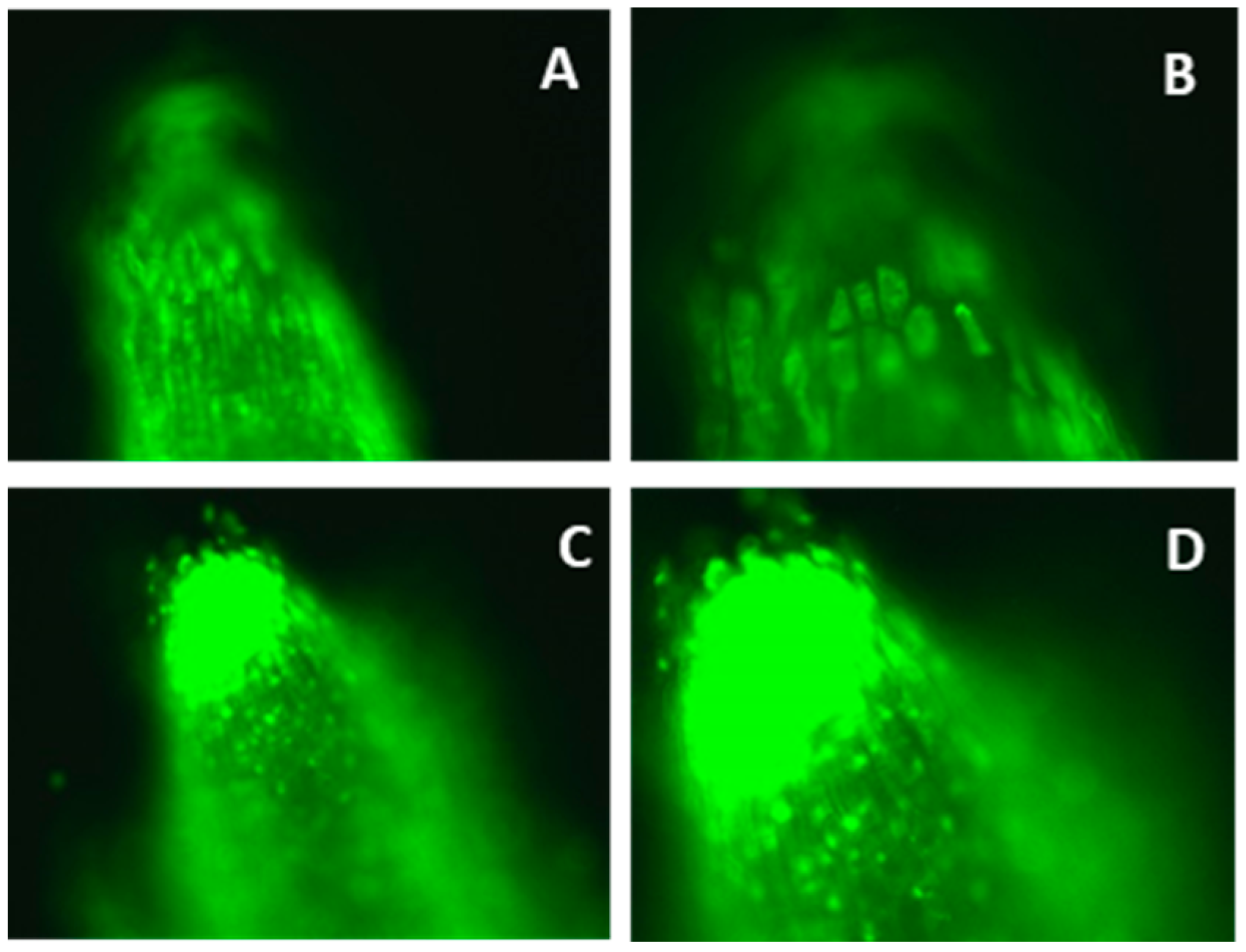
2.3. Morin Staining and Fluorescence Microscopy
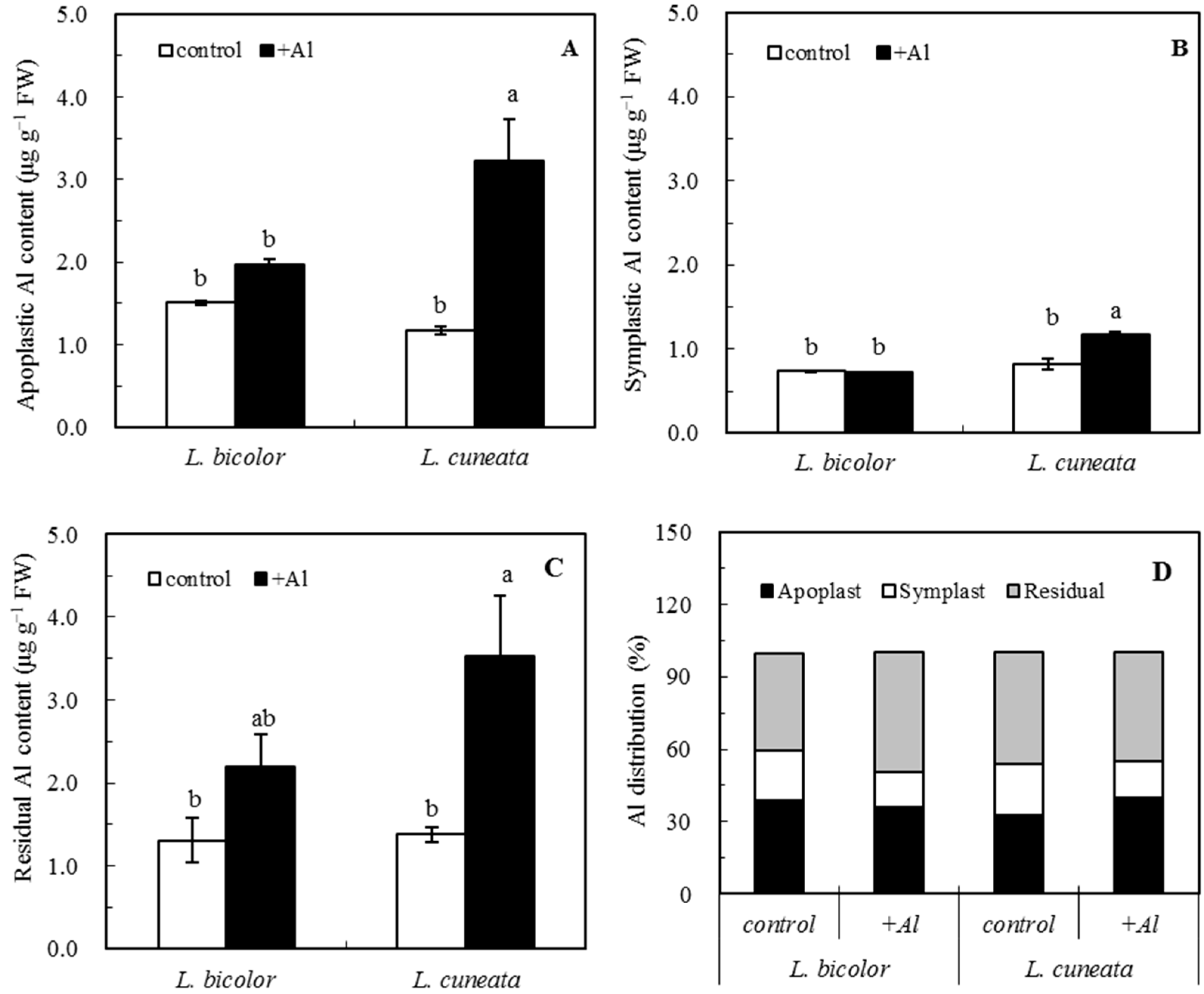
2.4. Al Distribution in Apoplast and Symplast
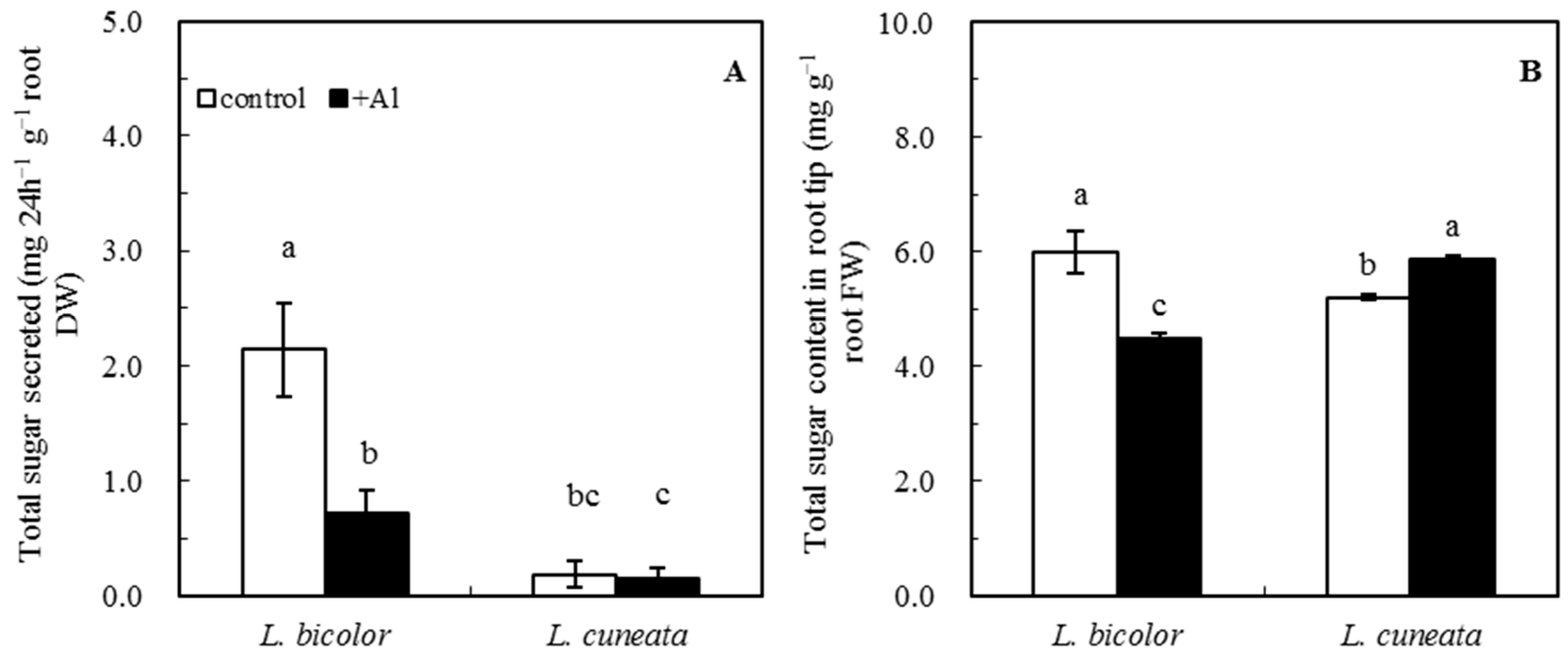
2.5. Determination of Total Sugar Secreted from and within Root Tips
2.6. Cell Wall Extraction
2.7. Measurement of Cell Wall Polysaccharides
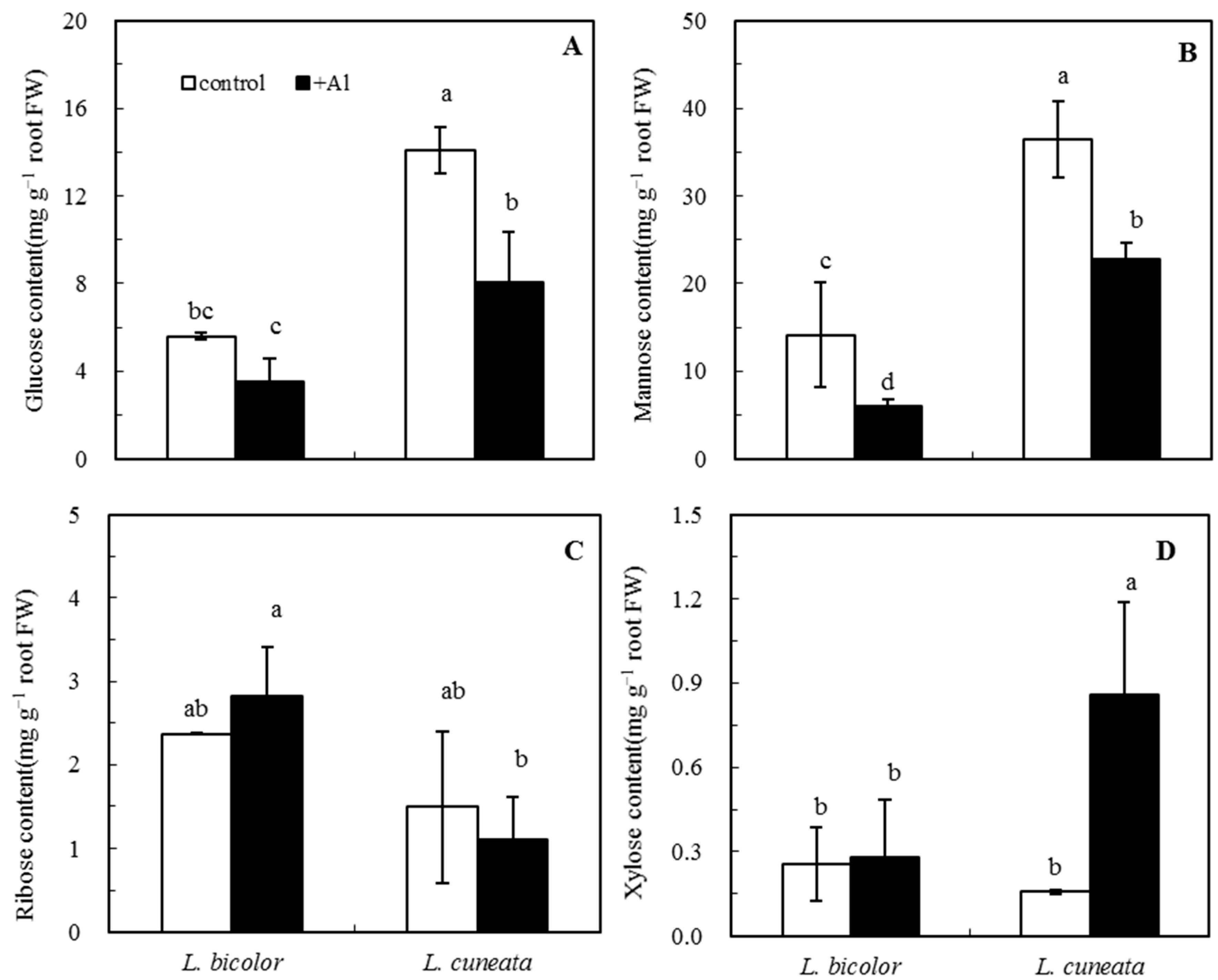
2.8. Pretreatment and Determination of Monosaccharide in Root Tips
2.9. Statistical Analysis
3. Results
3.1. Aluminum-Induced Element Exclusion from Lespedeza Roots
3.2. Aluminum Distribution in Apoplast and Symplast of Roots
3.3. Total Sugar Secreted from and within Root Tips
3.4. Polysaccharides of Root Cell Walls
3.5. Monosaccharides of Root Tips
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hyland, H.L. Comparison of legume growth in different soil types at varying acidity levels. J. Am. Soc. Agron. 1938, 30, 111–121. [Google Scholar] [CrossRef]
- Campbell, T.A.; Nuernberg, N.J.; Foy, C.D. Differential responses of Sericea Lespedeza to aluminum stress. J. Plant Nutr. 1991, 14, 1057–1066. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Chen, R.F.; Shen, R.F. Coadaptation of plants to multiple stresses in acidic soils. Soil Sci. 2014, 179, 503–513. [Google Scholar] [CrossRef]
- Kochian, L.V.; Piñeros, M.A.; Hoekenga, A.O. The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant Soil 2005, 274, 175–195. [Google Scholar] [CrossRef]
- Zhang, X.; Long, Y.; Huang, J.J.; Xia, J.X. Molecular mechanisms for coping with Al toxicity in plants. Int. J. Mol. Sci. 2019, 20, 1551. [Google Scholar] [CrossRef] [PubMed]
- Rahman, R.; Upadhyaya, H. Aluminium toxicity and its tolerance in plant: A review. J. Plant Biol. 2020, 64, 101–121. [Google Scholar] [CrossRef]
- Kochian, L.V.; Piñeros, M.A.; Liu, J.; Magalhaes, J.V. Plant adaptation to acid soils: The molecular basis for crop aluminum resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef]
- Zhu, X.F.; Wan, J.X.; Sun, Y.; Shi, Y.Z.; Braam, J.; Li, G.X.; Zheng, S.J. Xyloglucan endotransglucosylase-hydrolase17 interacts with xyloglucan endotransglucosylase-hydrolase31 to confer xyloglucan endotransglucosylase action and affect aluminum sensitivity in Arabidopsis. Plant Physiol. 2014, 165, 1566–1574. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, R.; Shu, K.; Lv, W.; Wang, S.; Wang, C. Aluminum stress signaling, response, and adaptive mechanisms in plants. Plant Signal Behav. 2022, 17, 2057060. [Google Scholar] [CrossRef]
- Dong, X.Y.; Shen, R.F.; Chen, R.F.; Zhu, Z.L.; Ma, J.F. Secretion of malate and citrate from roots is related to high Al-resistance in Lespedeza bicolor. Plant Soil 2008, 306, 139–147. [Google Scholar] [CrossRef]
- Sun, Q.B.; Shen, R.F.; Zhao, X.Q.; Chen, R.F. P enhances Al tolerance in Al-resistant Lespedeza but not in Al-sensitive Lespedeza. Ann. Bot. 2008, 102, 795–804. [Google Scholar] [CrossRef]
- Chen, Z.C.; Zhao, X.Q.; Shen, R.F. The alleviating effect of ammonium on aluminum toxicity in Lespedeza bicolor results in decreased aluminum-induced malate secretion from roots compared with nitrate. Plant Soil 2010, 337, 389–398. [Google Scholar] [CrossRef]
- Wang, J.Q.; Yu, X.F.; Ding, Z.J.; Ding, Z.J.; Zhang, X.K.; Luo, Y.P.; Xu, X.M.; Xie, Y.; Li, X.X.; Yuan, T.; et al. Structural basis of ALMT1-mediated aluminum resistance in Arabidopsis. Cell Res. 2022, 32, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Hajiboland, R.; Panda, C.K.; Lastochkina, O.; Gavassi, M.A.; Habermann, G.; Pereira, J.F. Aluminum toxicity in plants: Present and future. J. Plant Growth Regul. 2022. [Google Scholar] [CrossRef]
- Yang, Z.B.; Rao, I.M.; Horst, W.J. Interaction of aluminium and drought stress on root growth and crop yield on acid soils. Plant Soil 2013, 372, 3–25. [Google Scholar] [CrossRef]
- Zheng, S.J.; Yang, J.L. Target sites of aluminum phytotoxicity. Biol. Plantarum 2005, 49, 321–331. [Google Scholar] [CrossRef]
- Horst, W.J.; Kollmeier, M.; Schmohl, N.; Sivaguru, M.; Wang, Y.; Felle, H.H.; Hedrich, R.; Schröder, W.; Staß, A. Significance of the root apoplast for aluminium toxicity and resistance of maize. In The Apoplast of Higher Plants: Compartment of Storage, Transport and Reactions 49; Springer: Berlin/Heidelberg, Germany, 2007; pp. 49–66. [Google Scholar]
- He, H.; He, L.; Gu, M. Signal transduction during aluminum-induced secretion of organic acids in plants. Biol. Plant 2015, 59, 601–608. [Google Scholar] [CrossRef]
- Horst, W.J.; Wang, Y.; Eticha, D. The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: A review. Ann. Bot. 2010, 106, 185–197. [Google Scholar] [CrossRef]
- Yang, J.L.; Li, Y.Y.; Zhang, Y.J.; Zhang, S.S.; Wu, Y.R.; Wu, P.; Zheng, S.J. Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiol. 2008, 146, 602–611. [Google Scholar] [CrossRef]
- Yang, J.L.; Zhu, X.F.; Peng, Y.X.; Zheng, C.; Li, G.X.; Liu, Y.; Zheng, S.J. Cell wall hemicellulose contributes significantly to Al adsorption and root growth in Arabidopsis. Plant Physiol. 2011, 155, 1885–1892. [Google Scholar] [CrossRef]
- Ma, B.H.; Gao, L.; Zhang, H.X.; Cui, J.; Shen, Z.G. Aluminum-induced oxidative stress and changes in antioxidant defenses in the roots of rice varieties differing in Al tolerance. Plant Cell Rep. 2012, 31, 687–696. [Google Scholar] [CrossRef]
- Safari, M.; Ghanati, F.; Safarnejad, M.R.; Chashmi, N.A. The contribution of cell wall composition in the expansion of Camellia sinensis seedlings roots in response to aluminum. Planta 2018, 247, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.K.; Yadav, V.; Vaculík, M.; Gassmann, W.; Pike, S.; Arif, N.; Singh, V.P.; Deshmukh, R.; Sahi, S.; Tripathi, D.K. Aluminum toxicity and aluminum stress-induced physiological tolerance responses in higher plants. Crit. Rev. Biotechnol. 2021, 41, 715–730. [Google Scholar] [CrossRef] [PubMed]
- Braybrook, S.A.; Peaucelle, A. Mechano-chemical aspects of organ formation in Arabidopsis thaliana: The relationship between auxin and pectin. PLoS ONE 2013, 8, e57813. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; You, J.F.; Kong, L.N.; Yu, M.; Liu, M.H.; Yang, Z.M. The interaction of salicylic acid and Ca2+ alleviates aluminum toxicity in soybean (Glycine max L.). Plant Physiol. Biochem. 2016, 98, 146–154. [Google Scholar] [CrossRef]
- Tabuchi, A.; Matsumoto, H. Changes in cell-wall properties of wheat (Triticum aestivum L.) roots during aluminum-induced growth inhibition. Biol. Plantarum 2001, 112, 353–358. [Google Scholar] [CrossRef]
- Sun, Q.B.; Zhao, X.Q.; Shen, R.F. Application of three microscopic techniques to research on Al toxicity in plants. Acta Pedol. Sin. 2009, 46, 1026–1032. [Google Scholar]
- Tice, K.R.; Parker, D.R.; DeMason, D.A. Operationally defined apoplastic and symplastic aluminum fractions in root tips of aluminum-intoxicated wheat. Plant Physiol. 1992, 100, 309–318. [Google Scholar] [CrossRef]
- Bloom, P.R.; Weaver, R.M.; McBride, M.B. The spectrophotometric and fluorometric determination of aluminum with 8-hydroxyquinoline and butyl acetate extraction. Soil Sci. Soc. Am. J. 1978, 42, 713–716. [Google Scholar] [CrossRef]
- Heim, D.R.; Skomp, J.R.; Waldron, C.; Larrinua, I.M. Differential response to isoxaben of cellulose biosynthesis by wild-type and resistant strains of Arabidopsis thaliana. Pest. Biochem. Physiol. 1991, 39, 93–99. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Buchana, B.B.; Gruissem, W.; Jones, R.L. The cell wall. In Biochemistry and Molecular Biology of Plants 52; Elsevier: New York, NY, USA, 2000; pp. 52–98. [Google Scholar]
- Mariano, E.D.; Pinheiro, A.S.; Garcia, E.E.; Keltjens, W.G.; Jorge, R.A.; Menossi, M. Differential aluminium-impaired nutrient uptake along the root axis of two maize genotypes contrasting in resistance to aluminium. Plant Soil 2015, 388, 323–335. [Google Scholar] [CrossRef]
- De Souza, L.C.; Nogueira, D.C.S.; Machado, L.C.; Costa, T.C.; Martins, T.S.; Mendes, C.A.P.; Pires, N.M.C.; Neto, C.F.O.; Conceição, S.S.; Brito, A.E.A. Nitrogen compounds, proteins and amino acids in corn subjected to doses of aluminium. Afr. J. Agric. Res. 2016, 11, 1519–1524. [Google Scholar]
- De Rosmaninho, C.L.B.; Dias, A.S.; da Silva, M.F.; de Vasconcelos, A.; Santos, W.O.; Perez, C.E.A.; Vergutz, L.; Cardoso, L.G. Performance of Crambe submitted to aluminum stress: An important oilseed plant. J. Agric. Sci. 2019, 11, 454–463. [Google Scholar] [CrossRef]
- Sun, Q.B.; Shen, R.F.; Yin, C.Q.; Zhao, X.Q. Analysis of variations and factors affecting callose formation in response to Al stress in Lespedeza root tips. Acta Ecol. Sin. 2016, 36, 1073–1082. [Google Scholar]
- Brito, D.S.; Neri-Silva, R.; Ribeiro, K.V.G.; Peixoto, P.H.P.; Ribeiro, C. Effects of aluminum on the external morphology of root tips in rice. Braz. J. Bot. 2020, 43, 413–418. [Google Scholar] [CrossRef]
- Grignon, C.; Sentenac, H. pH and ionic conditions in the apoplast. Ann. Rev. Plant Physiol. Mol. Biol. 1991, 42, 103–128. [Google Scholar] [CrossRef]
- Hossain, A.K.M.Z.; Hossain, M.A.; Asgar, M.A.; Tosaki, T.; Koyama, H.; Hara, T. Changes in cell wall polysaccharides and hydroxycinnamates in wheat roots by aluminum stress at higher calcium supply. J. Plant Nutr. 2006, 29, 601–613. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, X.Q.; Chen, R.F.; Dong, X.Y.; Lan, P.; Ma, J.F.; Shen, R.F. Altered cell wall properties are responsible for ammonium-reduced aluminum accumulation in rice roots. Plant Cell Environ. 2015, 38, 1382–1390. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Q.-B.; Yin, C.-Q.; Zheng, H.; Dong, X.-Y.; Shen, R.-F.; Zhao, X.-Q. Higher Aluminum Tolerance of Lespedeza bicolor Relative to Lespedeza cuneata Is Associated with Saccharide Components of Root Tips. Agronomy 2023, 13, 629. https://doi.org/10.3390/agronomy13030629
Sun Q-B, Yin C-Q, Zheng H, Dong X-Y, Shen R-F, Zhao X-Q. Higher Aluminum Tolerance of Lespedeza bicolor Relative to Lespedeza cuneata Is Associated with Saccharide Components of Root Tips. Agronomy. 2023; 13(3):629. https://doi.org/10.3390/agronomy13030629
Chicago/Turabian StyleSun, Qing-Bin, Chun-Qin Yin, Han Zheng, Xiao-Ying Dong, Ren-Fang Shen, and Xue-Qiang Zhao. 2023. "Higher Aluminum Tolerance of Lespedeza bicolor Relative to Lespedeza cuneata Is Associated with Saccharide Components of Root Tips" Agronomy 13, no. 3: 629. https://doi.org/10.3390/agronomy13030629
APA StyleSun, Q.-B., Yin, C.-Q., Zheng, H., Dong, X.-Y., Shen, R.-F., & Zhao, X.-Q. (2023). Higher Aluminum Tolerance of Lespedeza bicolor Relative to Lespedeza cuneata Is Associated with Saccharide Components of Root Tips. Agronomy, 13(3), 629. https://doi.org/10.3390/agronomy13030629






