Methane Uptake and Nitrous Oxide Emission in Saline Soil Showed High Sensitivity to Nitrogen Fertilization Addition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Gas Sample Collection and Determination
2.3. Soil Collection and Analysis
2.4. Data Processing and Plotting
3. Results
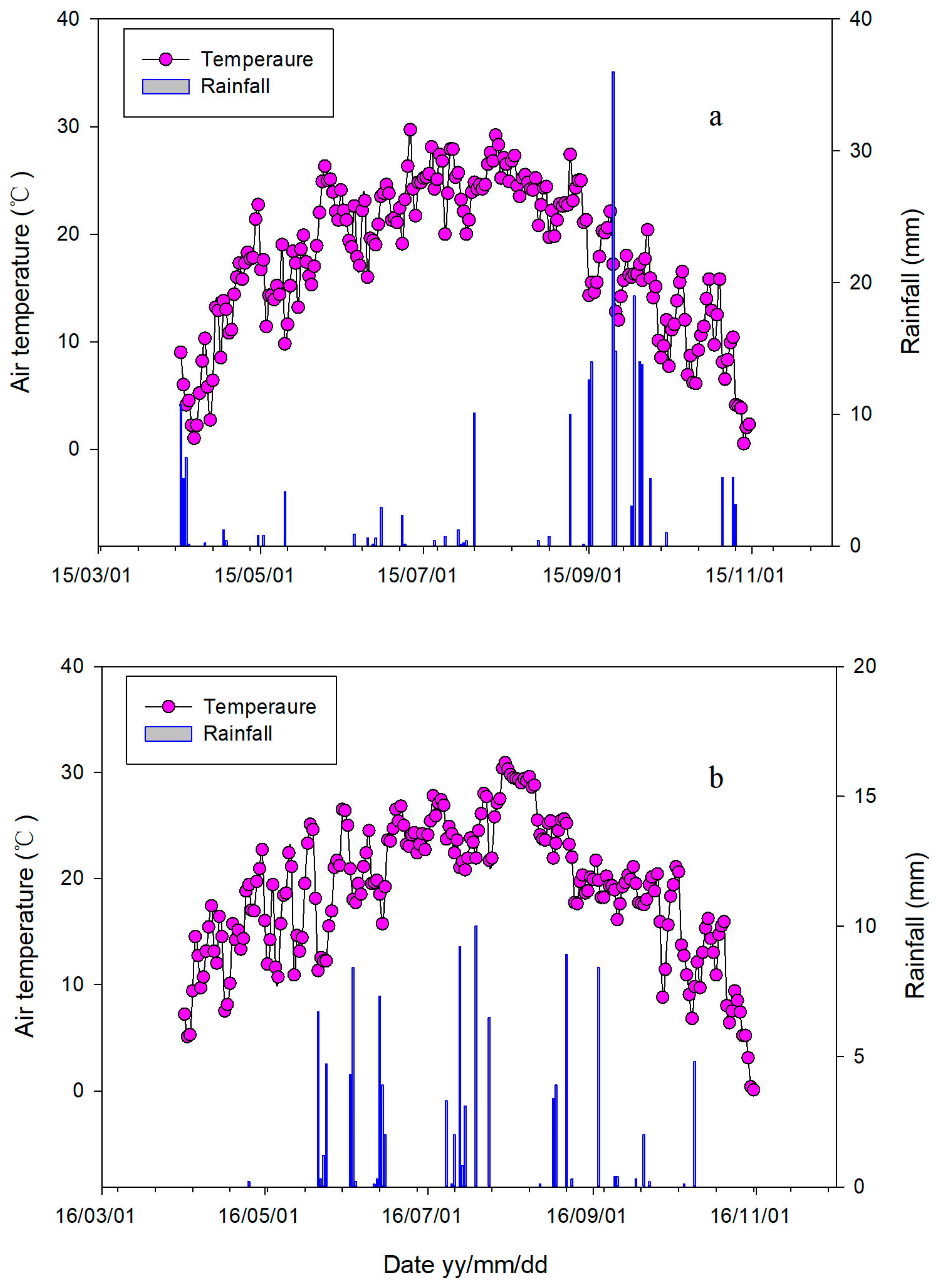
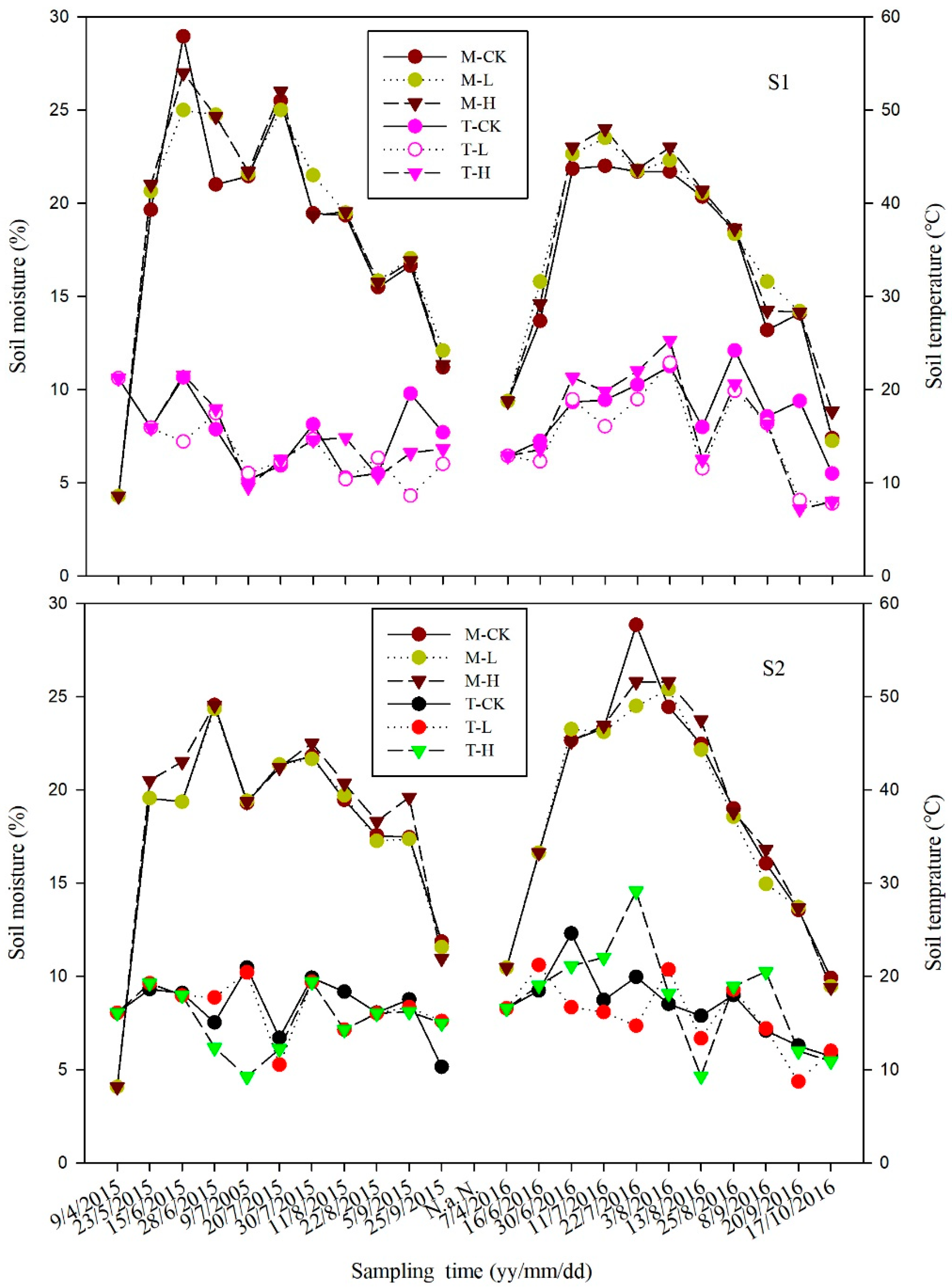
3.1. Effects of Temperature and Moisture in the Soil on Seasonal Variation in CH4 and N2O Fluxes
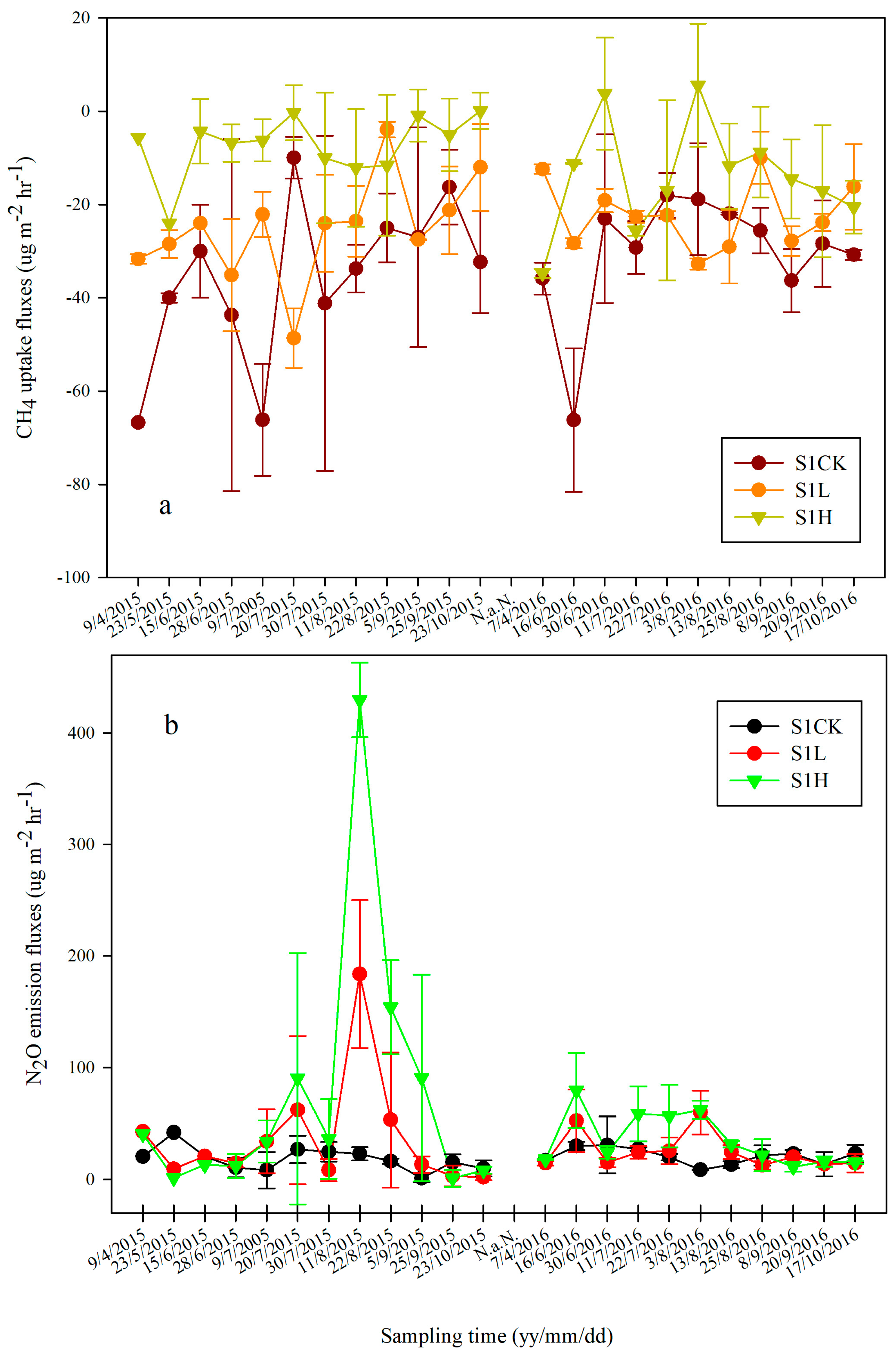
3.2. Cumulative N2O Emission and CH4 Uptake
4. Discussion
4.1. Fertilization Reduced CH4 Uptake in Soils under Different Salinity Conditions
4.2. Effect of Nitrogen Fertilization on N2O Emission in Saline Soils under Different Salinity Conditions
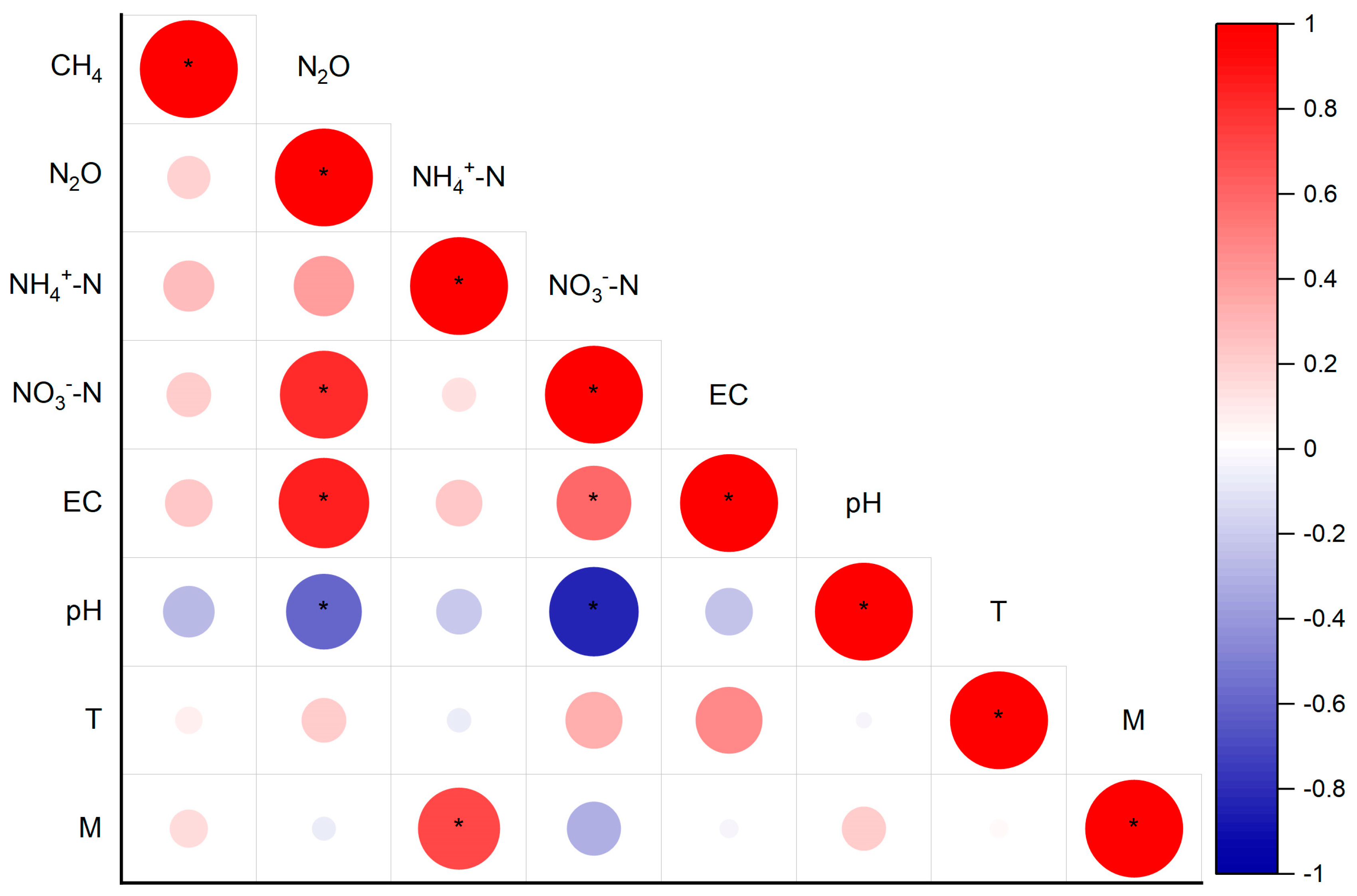
4.3. The Effects of Interactions from Fertilization and EC on the Cumulative CH4 Uptake and N2O Emissions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2013: The Physical Science Basis, Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- US-EPA. Global Anthropogenic Non-CO2 Greenhouse Gas Emissions, 1990–2020; EPA 430-R-06-003; United States Environmental Protection Agency: Washington, DC, USA, 2006.
- IPCC. Agriculture. In Climate Change 2007, Mitigation, Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Metz, B., Davidson, O.R., Bosch, P.R., Dave, R., Meyer, L.A., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- Liu, S.A.; Wang, J.J.; Tian, Z.; Wang, X.D.; Harrison, S. Ammonia and greenhouse gas emissions from a subtropical wheat field under different nitrogen fertilization strategies. J. Environ. Sci. 2017, 57, 196–210. [Google Scholar] [CrossRef]
- Lam, S.K.; Suter, H.; Davies, R.; Bai, M.; Mosier, A.R.; Sun, J.; Chen, D. Direct and indirect greenhouse gas emissions from two intensive vegetable farms applied with a nitrification inhibitor. Soil Biol. Biochem. 2018, 116, 48–51. [Google Scholar] [CrossRef]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Pathak, H.; Rao, D.L.N. Carbon and Nitrogen Mineralization from added Organic Matter in Saline and Alkali Soils; Central Soil Salinity Research Institute: India, Haryana, 1998. [Google Scholar]
- Fagodiya, R.K.; Malyan, S.K.; Singh, D.; Kumar, A.; Yadav, R.K.; Sharma, P.C.; Pathak, H. Greenhouse Gas Emissions from Salt-Affected Soils: Mechanistic Understanding of Interplay Factors and Reclamation Approaches. Sustainability 2022, 14, 11876. [Google Scholar] [CrossRef]
- Haj-Amor, Z.; Araya, T.; Kim, D.G.; Bouri, S.; Lee, J.; Ghiloufi, W.; Yang, Y.; Kang, H.; Kumar Jhariya, M.; Banerjee, A.; et al. Soil salinity and its associated effects on soil microorganisms, greenhouse gas emissions, crop yield, biodiversity and desertification: A review. Sci. Total Environ. 2022, 843, 156946. [Google Scholar] [CrossRef] [PubMed]
- Rengasamy, P. Soil processes affecting crop production in salt-affected soils. Funct. Plant Biol. 2010, 37, 613–620. [Google Scholar] [CrossRef]
- Meena, M.D.; Yadav, R.K.; Narjary, B.; Yadav, G.; Jat, H.S.; Sheoran, P.; Meena, M.K.; Antil, R.S.; Meena, B.L.; Singh, H.V.; et al. Municipal solid waste (MSW): Strategies to improve salt affected soil sustainability: A review. Waste Manage. 2019, 84, 38–53. [Google Scholar] [CrossRef]
- Yang, T.T.; Hu, C.Y.; Ding, G.D.; Wang, H.J. Types and recognizing system of saline-alkaline land in Hetao irrigation district. J. Inner Mongolia Agr. Univ. 2005, 26, 44–49. [Google Scholar]
- Bohm, J.; Messerer, M.; Muller, H.M.; Scholz-Starke, J.; Gradogna, A.; Scherzer, S.; Maierhofer, T.; Bazihizina, N.; Zhang, H.; Stigloher, C.; et al. Understanding the molecular basis of salts equestration in epidermal bladder cells of Chenopodium quinoa. Curr. Biol. 2018, 28, 3075–3805. [Google Scholar] [CrossRef]
- Wang, R.S.; Wan, S.Q.; Sun, J.X.; Xiao, H.J. Soilsalinity, sodicity and cotton yield parameters under different drip irrigation regimes during saline wasteland reclamation. Agr. Water Manage. 2018, 209, 20–31. [Google Scholar] [CrossRef]
- Yang, W.Z.; Jiao, Y.; Yang, M.D.; Wen, H.Y.; Liu, L.J. Absorbed carbon dioxide in saline soil from northwest China. CATENA 2021, 207, 105677. [Google Scholar] [CrossRef]
- Yang, W.Z.; Jiao, Y.; Yang, M.D.; Wen, H.Y. Methane uptake by saline–alkaline soils with varying electrical conductivity in the Hetao Irrigation District of Inner Mongolia, China. Nutr. Cycl. Agroecosys. 2018, 112, 265–276. [Google Scholar] [CrossRef]
- Pisinaras, V.; Tsihrintzis, V.A.; Petalas, C.; Ouzounis, K. Soil salinization in the agricultural lands of Rhodope District, northeastern Greece. Environ. Monit. Assess. 2010, 166, 79–94. [Google Scholar] [CrossRef]
- Easley, R.A.; Patsavas, M.C.; Byrne, R.H.; Liu, X.; Feely, R.A. Spectrophotometric measurement of calcium carbonate saturation states in seawater. Environ. Sci. Technol. 2013, 47, 1468–1477. [Google Scholar] [CrossRef]
- Bocanegra-Garcia, G.; Carrillo-Chavez, A. Hydro-geochemical behavior of bicarbonate and sulfate ions leaching from a sulfide-poor silver mine in Central Mexico: Potential indicator of acid mine drainage. B Environ. Contam. Tox. 2003, 71, 1222–1229. [Google Scholar] [CrossRef]
- King, G.M.; Schnell, S. Effects of ammonium and non-ammonium salt additions on methane oxidation by Methylosinustrichosporium OB3b and Maine forest soils. Appl. Environ. Microb. 1998, 64, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Silva, N.; Valenzuela-Encinas, C.; Marsch, R.; Dendooven, L.; Alcántara-Hernández, R.J. Changes in methane oxidation activity and methanotrophic community composition in saline-alkaline soils. Extremophiles 2014, 18, 561–571. [Google Scholar] [CrossRef]
- Zhang, J.F.; Li, Z.J.; Ning, T.Y.; Gu, S.B. Methane uptake in salt-affected soils shows low sensitivity to salt addition. Soil Biol. Biochem. 2011, 43, 1434–1439. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, L.M.; Zheng, Y.M.; Di, H.; He, J.Z. Abundance and community composition of methanotrophs in a Chinese paddy soil under long-term fertilization practices. J. Soil Sediment 2008, 8, 406–414. [Google Scholar] [CrossRef]
- LeMer, J.; Roger, P. Production, xidation, emission and consumption of methane by soils: A review. Eur. J. Soil Biol. 2001, 7, 25–35. [Google Scholar] [CrossRef]
- Valenzuela-Encinas, C.; Alcantara-Hernandez, R.J.; Estrada-Alvarado, I.; Zavala-Díaz de la Serna, F.J.; Dendooven, L.; Marsch, R. The archaeal diversity and population in a drained alkaline saline soil of the former lake Texcoco (Mexico). Geomicrobiol. J. 2012, 29, 18–22. [Google Scholar] [CrossRef]
- Unteregelsbacher, S.; Gasche, R.; Lipp, L.; Sun, W.; Kreyling, O.; Geitlinger, H.; Kgel-Knabner, I.; Papen, H.; Kiese, R.; Schmid, H.P.; et al. Increased methane uptake but unchanged nitrous oxide flux in montane grasslands under simulated climate change conditions. Eur. J. Soil Sci. 2013, 64, 586–596. [Google Scholar] [CrossRef]
- Grassmann, C.S.; Mariano, E.; Rocha, K.F.; Gilli, B.R.; Rosolem, C.A. Effect of tropical grass and nitrogen fertilization on nitrous oxide, methane, and ammonia emissions of maize-based rotation systems. Atmos. Environ. 2020, 234, 117571. [Google Scholar] [CrossRef]
- Powlson, S.D.; Goulding, W.T.K.; Willison, W.T.; Websterb, P.C.; Hütsch, B.W. The effect of agriculture on methane oxidation in soil. Nutr. Cycl. Agroecosys. 1997, 49, 59–70. [Google Scholar] [CrossRef]
- Horz, H.P.; Yimga, M.T.; Liesack, W. Detection of methanotroph diversity on roots of submerged rice plants by molecu larretrieval of pmoA, mmoX, mxaF, and 16SrRNA and ribosomal DNA, including profiling. Appl. Environ. Microb. 2001, 67, 4177–4185. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Fan, L.; He, P.J.; Shao, L.M. Response of methanotrophs and methane oxidation on ammonium application in landfill soils. Appl. Microbiol. Biot. 2011, 92, 1073–1082. [Google Scholar] [CrossRef]
- Sjogersten, S.; Wookey, P.A. Spatio-temporal variability and environmental controls of methane fluxes at the forest-tundra ecotone in the fennoscandian mountains. Global Change Biol. 2002, 8, 885–894. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, G.; Li, Q.; Liao, N.L.; Guo, H.; Min, W.; Ma, L.; Ye, J.; Hou, Z. Saline water irrigation stimulate N2O emission from a drip-irrigated cotton field. Acta Agr. Scand. B S. P. 2016, 66, 141–152. [Google Scholar] [CrossRef]
- Ghosh, U.; Thapa, R.; Desutter, T.; He, Y.; Chatterjee, A. Saline-sodic soils: Potential sources of nitrous oxide and carbon dioxide emissions? Pedosphere 2017, 27, 65–75. [Google Scholar] [CrossRef]
- Zhou, M.H.; Butterbach-Bahl, K.; Vereecken, H.; Bruggemann, N. Ameta-analysis of soil salinization effects on nitrogen pools, cycles and fluxes in coastal ecosystems. Global Change Biol. 2017, 23, 1338–1352. [Google Scholar] [CrossRef]
- Reddy, N.; Crohn, D.M. Effects of soils alinity and carbon availability from organic amendments on nitrous oxide emissions. Geoderma 2014, 235–236, 363–371. [Google Scholar] [CrossRef]
- Wang, C.; Tong, C.; Chambers, L.G.; Liu, X.T. Identifying the salinity thresholds that impact greenhouse gas production in subtropical tidal fresh water marsh soils. Wetlands 2017, 37, 559–571. [Google Scholar] [CrossRef]
- Wu, Q.F.; Wu, X.P.; Li, Y.K.; Wu, H.J.; Yan, P.; Zhang, Y.C.; Li, R.N.; Wang, L.Y.; Wang, X.B.; Cai, D.X. Studies on the fluxes of nitrous oxide from greenhouse vegetable soil. J. Plant Nutr. Fert. 2011, 17, 942–948. [Google Scholar]
- Hou, A.X.; Tsuruta, H. Nitrous oxide and nitric oxide fluxes from an upland field in Japan: Effect of urea type, placement, and crop residues. Nutr. Cycl. Agroecosys. 2003, 65, 191–200. [Google Scholar] [CrossRef]
- Zhao, C.S.; Hu, C.X.; Sun, X.C.; Huang, W. Influence of temperature and moisture on nitrogen mineralization in vegetable fields of central China. Chinese J. Eco-Agr. 2012, 20, 861–866. [Google Scholar] [CrossRef]
- Laura, R.D. Salinity and nitrogen mineralization in soil. Soil Biol. Biochem. 1977, 9, 333–336. [Google Scholar] [CrossRef]
- Gandhi, A.P.; Paliwal, K.V. Mineralization and gaseous losses of nitrogen from urea and ammonium sulphate in salt-affected soils. Plant Soil 1976, 45, 247–255. [Google Scholar] [CrossRef]
- Yang, W.Z.; Yang, M.D.; Wen, H.Y.; Jiao, Y. Global Warming Potential of CH4 uptake and N2O emissions in saline–alkaline soils. Atmos. Environ. 2018, 191, 172–180. [Google Scholar] [CrossRef]
- Gu, J.X.; Nicoullaud, B.; Rochette, P.; Grossel, A.; Hénault, C.; Cellier, P.; Richard, G. Aregional experiment suggests that soil texture is a major control of N2O emissions from tile-drained winterwheat fields during the fertilization period. Soil Biol. Biochem. 2013, 60, 134–141. [Google Scholar] [CrossRef]
- Zhou, F.; Shang, Z.Y.; Ciais, P.; Tao, S.; Piao, S.L.; Raymond, P.; He, C.F.; Li, B.G.; Wang, R.H.; Wang, X.H.; et al. A new high-resolution N2O emission inventory for China in 2008. Environ. Sci. Technol. 2014, 48, 8538–8547. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Q.L.; Chen, M.; Xu, K.; Tang, J.Y.; Saikawa, E.; Lu, Y.Y.; Melillo, J.M.; Prinn, R.G.; McGuire, A.D. Response of global soil consumption of atmospheric methane to changes in atmospheric climate and nitrogen deposition. Global Biogeochem. Cy. 2013, 27, 650–663. [Google Scholar] [CrossRef]
- Zhou, X.B.; Zhang, Y.M.; Tao, Y.; Wu, L. Effects of fluxes of nitrous oxide, methane and carbon dioxide and their responses to increasing nitrogen deposition in the Gurbantünggüt Desert of Xinjiang, China. Chinese J. Plant Ecology 2017, 41, 290–300. [Google Scholar]


| Soils | K+/(%) | Na+/(%) | Ca2+/(%) | Mg2+/(%) | SO42−/(%) | CO32−/(%) | HCO3−/(%) | Cl−/(%) | Total Salt Content/(%) |
|---|---|---|---|---|---|---|---|---|---|
| S1 | 0.002 | 0.009 | 0.014 | 0.0056 | 0.013 | 0.000 | 0.064 | 0.010 | 0.120 |
| S2 | 0.006 | 0.120 | 0.083 | 0.045 | 0.390 | 0.000 | 0.048 | 0.140 | 0.830 |
| Time Treatments | Soil Salt Content (%) | Saline Type | ||||
|---|---|---|---|---|---|---|
| Non Salinization | Low | Medium | High | Saline Soil | ||
| Coastal and semi- humid, semi-arid and arid Regions | <0.1 | 0.1–0.2 | 0.2–0.4 | 0.4–0.6 (1.0) | >0.6 (1.0) | HCO3−+CO32−, Cl−, Cl−-SO42−, SO42−-Cl- |
| Semi desert and desert area | <0.2 | 0.2–0.3 (0.4) | 0.3–0.5 (0.6) | 0.5(0.6)–1.0 (2.0) | 1.10 (2.0) | SO42−, Cl−-SO42−, SO42−-Cl− |
| Soils | Texture | pH | OC (g kg−1) | TN/ (mg kg−1) | TP/ (mg kg−1) | BS/ (%) | CEC (cmol kg−1) | ||
|---|---|---|---|---|---|---|---|---|---|
| Clay (%) | Silt (%) | Sand (%) | |||||||
| S1 | 33.7 | 38.2 | 28.1 | 8.11 | 9.6 | 32.8 | 0.55 | 61.7 | 10.5 |
| S2 | 34.1 | 40.5 | 25.4 | 8.79 | 8.3 | 22.1 | 0.85 | 70.3 | 10.1 |
| Time | Treatments | NH4+-N (mg kg−1) | NO3−-N (mg kg−1) | Inorganic Nitrogen (mg kg−1) | pH |
|---|---|---|---|---|---|
| 2015 | S1CK | 2.0 ± 0.18 a | 33.0 ± 1.91 b | 35.1 ± 1.81 b | 8.0 ± 0.11 a |
| S1L | 4.1 ± 0.26 b | 82.8 ± 4.20 c | 86.9 ± 4.46 d | 8.1 ± 0.09 a | |
| S1H | 6.6 ± 0.07 c | 150.5 ± 19.53 d | 157.2 ± 19.60 e | 8.2 ± 0.06 a | |
| S2CK | 4.4 ± 0.96 b | 19.4 ± 0.98 a | 23.8 ± 1.94 a | 8.7 ± 0.07 b | |
| S2L | 6.6 ± 0.10 c | 75.0 ± 3.34 c | 81.6 ± 3.44 d | 8.8 ± 0.12 b | |
| S2H | 7.6 ± 0.16 d | 125.0 ± 12.21 d | 132.7 ± 12.37 e | 8.8 ± 0.09 b | |
| 2016 | S1CK | 7.0 ± 0.13 d | 16.2 ± 2.85 a | 23.2 ± 2.98 a | 8.1 ± 0.14 a |
| S1L | 9.5 ± 0.21 e | 33.3 ± 4.56 b | 42.7 ± 4.77 b | 8.2 ± 0.11 a | |
| S1H | 10.0 ± 0.32 e | 41.5 ± 0.78 b | 51.5 ± 1.01 c | 8.1 ± 0.10 a | |
| S2CK | 8.0 ± 0.19 d | 14.1 ± 1.59 a | 22.2 ± 1.78 a | 8.7 ± 0.96 b | |
| S2L | 10.2 ± 0.35 e | 21.2 ± 0.98 a | 31.4 ± 1.33 b | 8.7 ± 0.93 b | |
| S2H | 14.0 ± 0.39 f | 30.7 ± 1.32 b | 44.7 ± 1.71 b | 8.8 ± 0.81 b |
| Factors | Cumulative N2O Amount | Cumulative CH4 Amount | ||||
|---|---|---|---|---|---|---|
| F | p | Significance | F | p | Significance | |
| Nitrogen fertilization | 14.572 | 0.005 | *** | 2.18 | 0.05 | * |
| Saline soil | 5.610 | 0.05 | * | 5.515 | 0.05 | * |
| Nitrogen fertilization * saline soil | 2.508 | 0.05 | * | 28.163 | 0.001 | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Hu, Y.; Yang, M.; Wen, H.; Jiao, Y. Methane Uptake and Nitrous Oxide Emission in Saline Soil Showed High Sensitivity to Nitrogen Fertilization Addition. Agronomy 2023, 13, 473. https://doi.org/10.3390/agronomy13020473
Yang W, Hu Y, Yang M, Wen H, Jiao Y. Methane Uptake and Nitrous Oxide Emission in Saline Soil Showed High Sensitivity to Nitrogen Fertilization Addition. Agronomy. 2023; 13(2):473. https://doi.org/10.3390/agronomy13020473
Chicago/Turabian StyleYang, Wenzhu, Youlin Hu, Mingde Yang, Huiyang Wen, and Yan Jiao. 2023. "Methane Uptake and Nitrous Oxide Emission in Saline Soil Showed High Sensitivity to Nitrogen Fertilization Addition" Agronomy 13, no. 2: 473. https://doi.org/10.3390/agronomy13020473
APA StyleYang, W., Hu, Y., Yang, M., Wen, H., & Jiao, Y. (2023). Methane Uptake and Nitrous Oxide Emission in Saline Soil Showed High Sensitivity to Nitrogen Fertilization Addition. Agronomy, 13(2), 473. https://doi.org/10.3390/agronomy13020473





