Investigation of Soil Microbial Communities Involved in N Cycling as Affected by the Long-Term Use of the N Stabilizers DMPP and NBPT
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Collection Site Description
2.2. N2O Emissions In Situ Measurements
2.3. Potential Nitrification Rates Assay (PNR)
2.4. DNA Extraction
2.5. Illumina MiSeq Sequencing and Bioinformatics Analysis
2.6. Statistical Analysis
3. Results
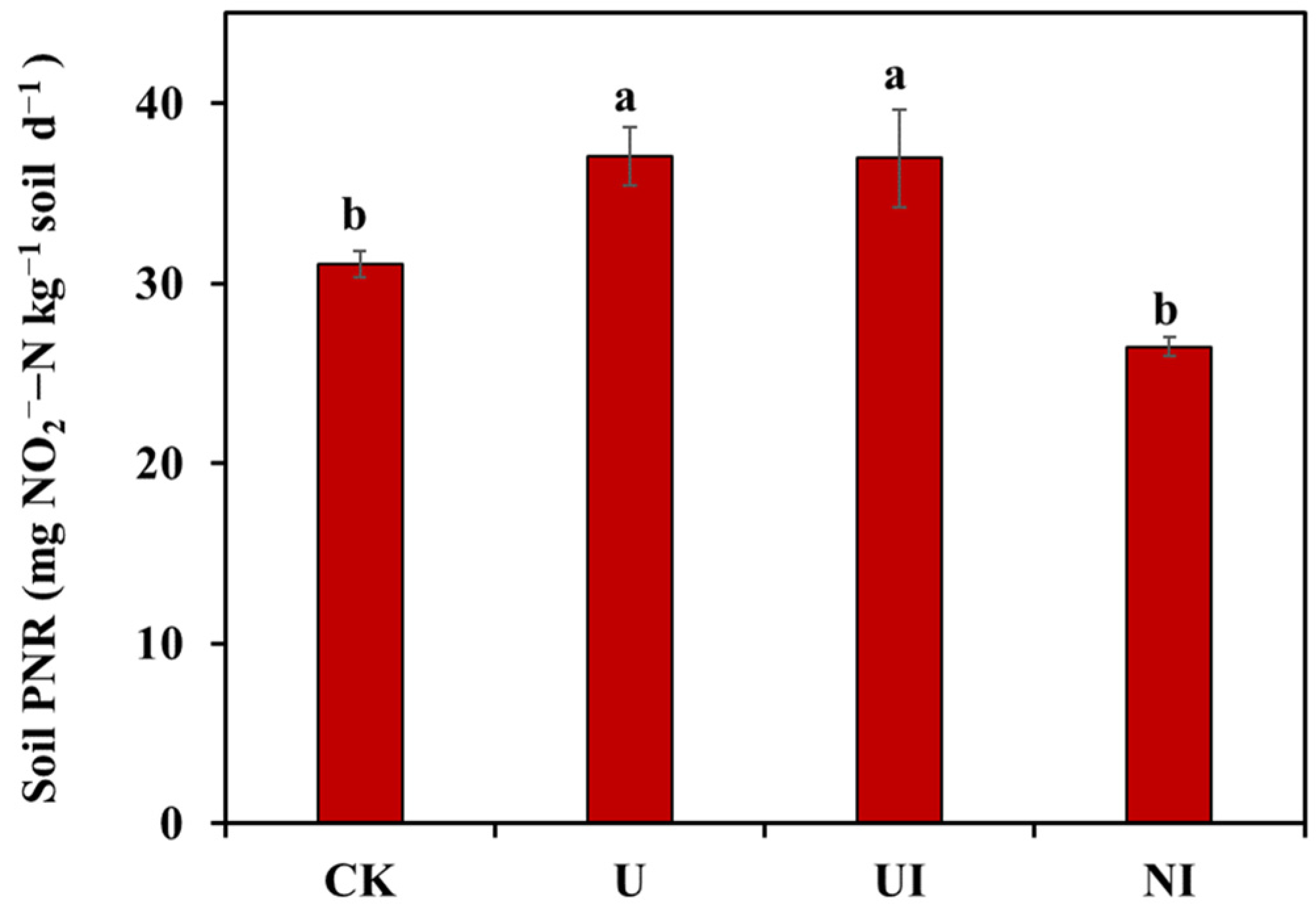
3.1. Soil PNR and Inter-Annual Variation of N2O
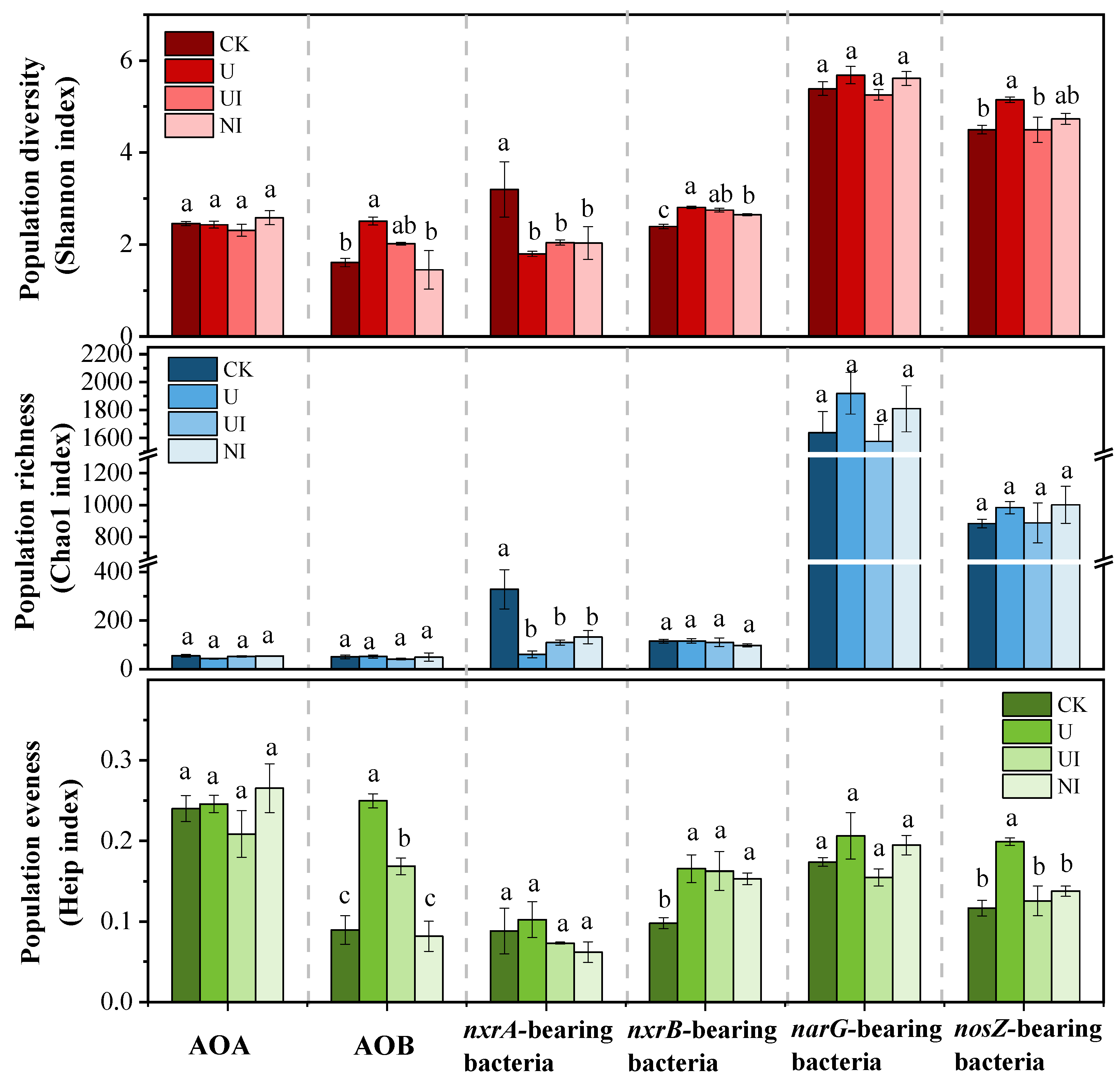
3.2. The Response of α-Diversity of Nitrifiers and Denitrifiers to Long-Term Application of Urea with N Stabilizer
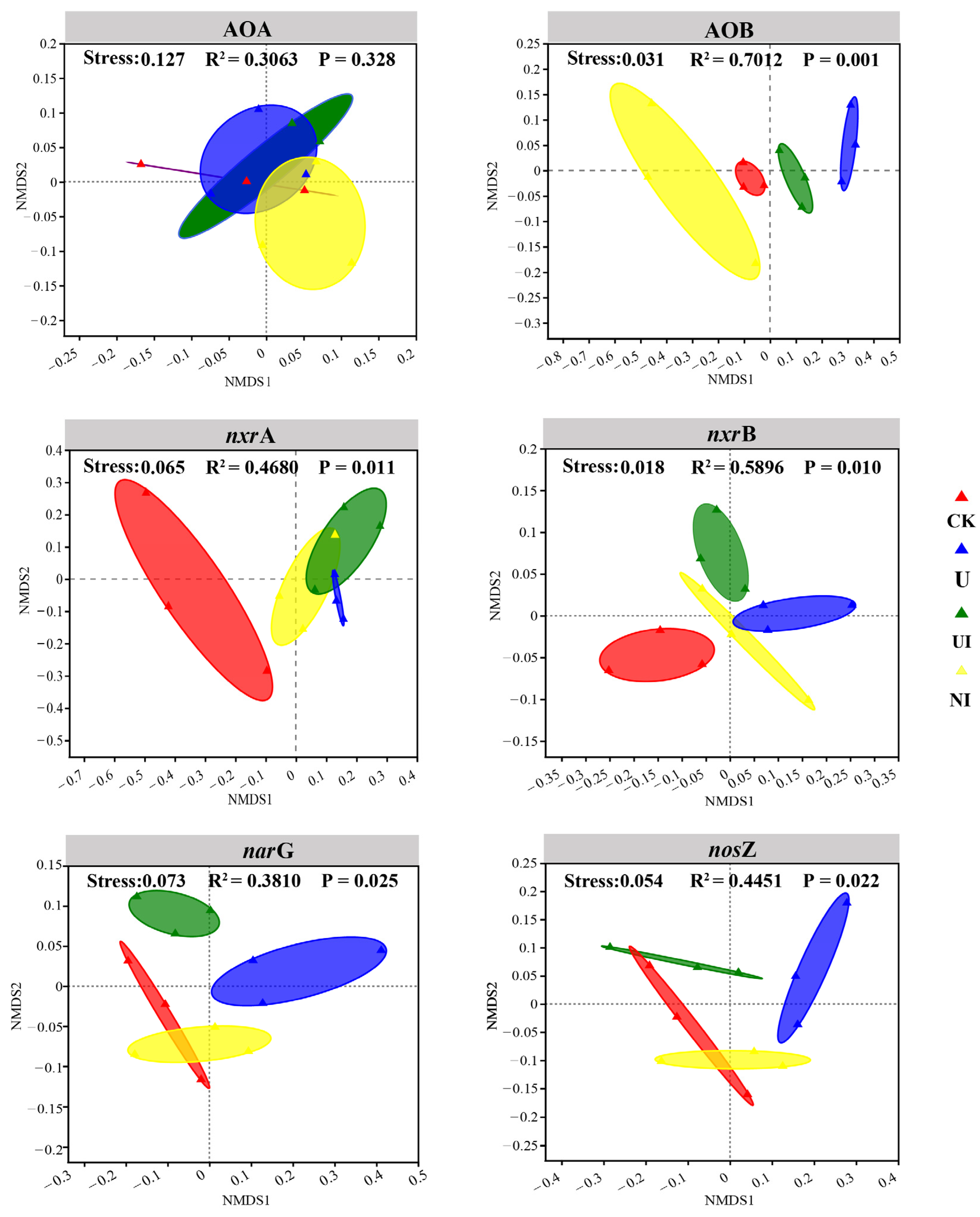
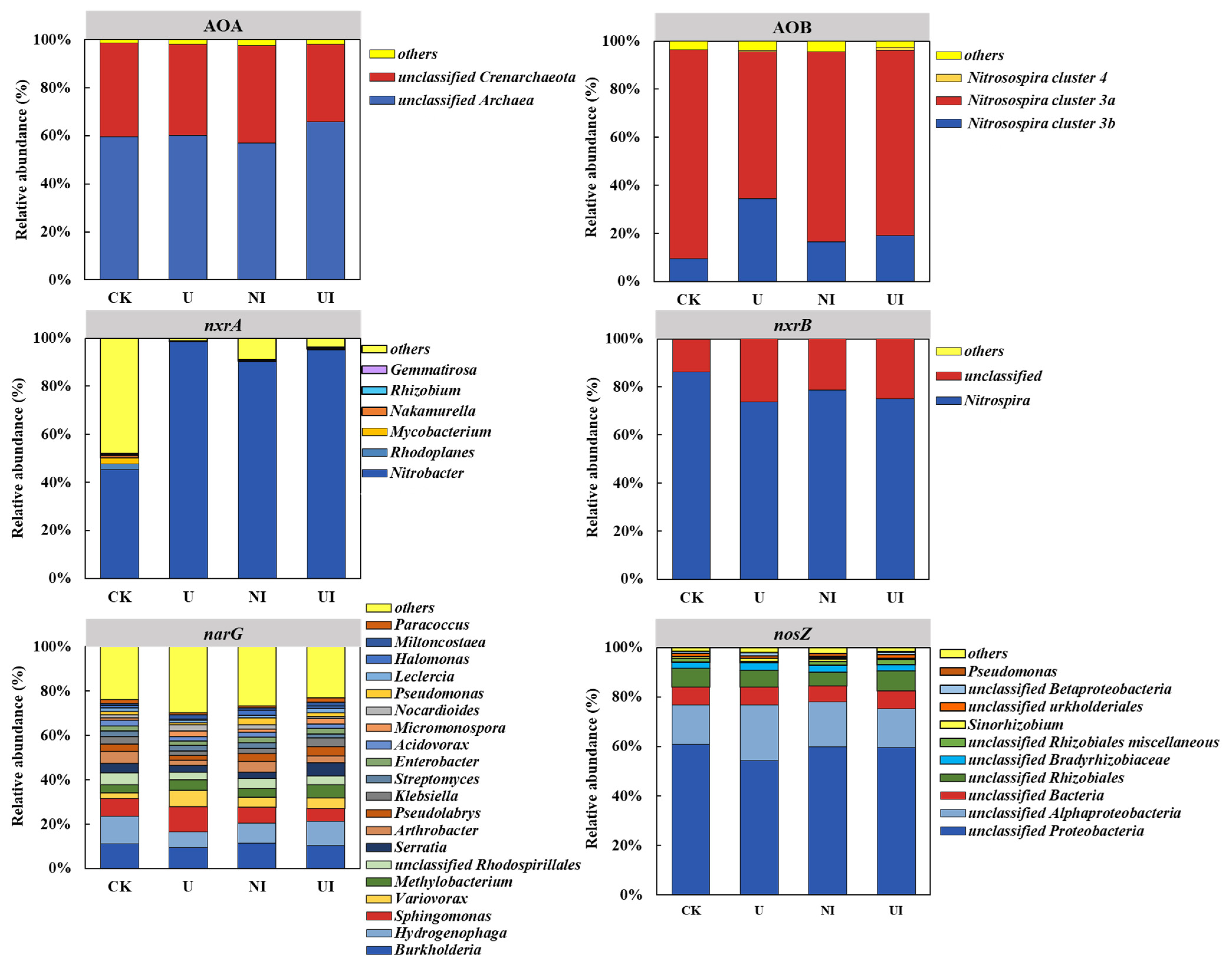
3.3. Variations in Nitrifier and Denitrifier Population Composition
3.4. Linkage between Microbial Population and Soil Properties
4. Discussion
4.1. Effects of DMPP and NBPT on Ammonia Oxidizers Population
4.2. Effects of DMPP and NBPT on Nitrite-Oxidizing Bacteria Population
4.3. Effects of DMPP and NBPT on Denitrifier Populations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Devaraju, N.; Prudhomme, R.; Lungarska, A.; Wang, X.; Yin, Z.; de Noblet-Ducoudré, N.; Chakir, R.; Jayet, P.A.; Brunelle, T.; Viovy, N.; et al. Quantifying the benefits of reducing synthetic nitrogen application policy on ecosystem carbon sequestration and biodiversity. Sci. Rep. 2022, 12, 20715. [Google Scholar] [CrossRef]
- Angus, J.F.; Grace, P.R. Nitrogen balance in Australia and nitrogen use efficiency on Australian farms. Soil Res. 2017, 55, 435–450. [Google Scholar] [CrossRef]
- Giuseppe, C.; Colombani, N.; Elisa, S.; Fabio, V.; Anna, F.E.; Micol, M. Reactive nitrogen losses via denitrification assessed in saturated agricultural soils. Geoderma 2019, 37, 91–98. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014. Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Edenhofer, O., Pichs-Madruga, R., Sokona, Y., Farahani, E., Kadner, S., Seyboth, K., Adler, A., Baum, I., Brunner, S., Eickemeier, P., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 1281–1304. [Google Scholar]
- United States Environmental Protection Agency. Methane and Nitrous Oxide Emissions from Natural Sources. Available online: https://www.researchgate.net/publication/290824404_Methane_and_nitrous_oxide_emissions_from_natural_sources (accessed on 15 December 2022).
- Chen, J.H.; Liu, X.Y.; Li, L.Q.; Zheng, J.W.; Qu, J.J.; Zheng, J.F.; Zhang, X.H.; Pan, G.X. Consistent increase in abundance and diversity but variable change in community composition of bacteria in topsoil of rice paddy under short term biochar treatment across three sites from south China. Appl. Soil Ecol. 2015, 91, 68–79. [Google Scholar] [CrossRef]
- Martins, C.S.C.; Nazaries, L.; Macdonald, C.A.; Anderson, I.C.; Singh, B.K. Water availability and abundance of microbial groups are key determinants of greenhouse gas fluxes in a dryland forest ecosystem. Soil Biol. Biochem. 2015, 86, 5–16. [Google Scholar] [CrossRef]
- Tosi, M.; Brown, S.; Ferrari Machado, P.V.; Wagner-Riddle, C.; Dunfield, K. Short-term response of soil N-cycling genes and transcripts to fertilization with nitrification and urease inhibitors, and relationship with field-scale N2O emissions. Soil Biol. Biochem. 2020, 142, 107703. [Google Scholar] [CrossRef]
- Duff, A.M.; Forrestal, P.; Ikoyi, I.; Brennan, F. Assessing the long-term impact of urease and nitrification inhibitor use on microbial community composition, diversity and function in grassland soil. Soil Biol. Biochem. 2022, 170, 108709. [Google Scholar] [CrossRef]
- Marcel, M.M.K.; Hannah, K.M.; Boran, K. The Microbial Nitrogen-Cycling Network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Miao, L.; Yang, G.Q.; Tao, T.; Peng, Y.Z. Recent advances in nitrogen removal from landfill leachate using biological treatments—A review. J. Environ. Manag. 2019, 235, 178–185. [Google Scholar] [CrossRef]
- Han, S.; Luo, X.S.; Liao, H.; Nie, H.L.; Huang, Q.Y. Nitrospira are more sensitive than Nitrobacter to land management in acid, fertilized soils of a rapeseed-rice rotation field trial. Sci. Total Environ. 2017, 599–600, 135. [Google Scholar] [CrossRef]
- Shrewsbury, L.H.; Smith, J.L.; Huggins, D.R.; Carpenter-Boggs, L.; Reardon, C.L. Denitrifier abundance has a greater influence on denitrification rates at larger landscape scales but is a lesser driver than environmental variables. Soil Biol. Biochem. 2016, 103, 221–231. [Google Scholar] [CrossRef]
- Philippot, L.; Hallin, S.; Schloter, M. Ecology of denitrifying prokaryotes in agricultural soil. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2007; Volume 96, pp. 249–305. [Google Scholar]
- Kleineidam, K.; Kosmrlj, K.; Kublik, S.; Palmer, I.; Pfab, H.; Ruser, R.; Fiedler, S.; Schloter, M. Influence of the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) on ammonia-oxidizing bacteria and archaea in rhizosphere and bulk soil. Chemosphere 2011, 84, 182–186. [Google Scholar] [CrossRef]
- Barrena, I.; Sergio, M.; Correa-Galeote, D.; Vega-Mas, I.; Estavillo, J.M. Soil water content modulates the effect of the nitrification inhibitor 3,4-Ddimethylpyrazole phosphate (DMPP) on nitrifying and denitrifying bacteria. Geoderma 2017, 303, 1–8. [Google Scholar] [CrossRef]
- Diego, A.; Simon, J.; Alberto, S.; Guillermo, G.; Antonio, V. Meta-analysis of the effect of urease and nitrification inhibitors on crop productivity and nitrogen use efficiency. Agric. Ecosyst. Environ. 2014, 189, 136–144. [Google Scholar] [CrossRef]
- Li, T.Y.; Zhang, W.F.; Yin, J.; Chadwick, D.; Norse, D.; Lu, Y.L.; Liu, X.J.; Chen, X.P.; Zhang, F.S.; Powlson, D.; et al. Enhanced-efficiency fertilizers are not a panacea for resolving the nitrogen problem. Glob. Chang. Biol. 2018, 24, e511–e521. [Google Scholar] [CrossRef]
- Lei, J.L.; Fan, Q.Y.; Yu, J.Y.; Ma, Y.; Yin, J.H.; Liu, R. A Meta-analysis to examine whether nitrification inhibitors work through selectively inhibiting ammonia-oxidizing bacteria. Front. Microbiol. 2022, 13, 962146. [Google Scholar] [CrossRef] [PubMed]
- Harty, M.A.; Forrestal, P.J.; Watson, C.J.; Mcgeough, K.L.; Carolan, R.; Elliot, C.; Krol, D.; Laughlin, R.J.; Richards, K.G.; Lanigan, G.J. Reducing nitrous oxide emissions by changing N fertiliser use from calcium ammonium nitrate (CAN) to urea-based formulations. Sci. Total Environ. 2016, 563–564, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Cantarella, H.; Otto, R.; Soares, J.R.; Silva, A.G.D.B. Agronomic efficiency of NBPT as a urease inhibitor: A review. J. Adv. Res. 2018, 13, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Shu, K.L.; Chen, D.; Wang, J.; Quan, T.; Yan, X. Can knowledge-based N management produce more staple grain with lower greenhouse gas emission and reactive nitrogen pollution? A Meta-analysis. Glob. Chang. Biol. 2017, 23, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Hayden, H.; Suter, H.; He, J.Z.; Chen, D.L. The effect of nitrification inhibitors in reducing nitrification and the ammonia oxidizer population in three contrasting soils. J. Soils Sediments 2015, 15, 1113–1118. [Google Scholar] [CrossRef]
- Chen, D.L.; Suter, H.C.; Islam, A.; Edis, R. Influence of nitrification inhibitors on nitrification and nitrous oxide (N2O) emission from a clay loam soil fertilized with urea. Soil Biol. Biochem. 2010, 42, 660–664. [Google Scholar] [CrossRef]
- Weiske, A.; Benckiser, G.; Herbert, T.; Ottow, J. Influence of the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) in comparison to dicyandiamide (DCD) on nitrous oxide emissions, carbon dioxide fluxes and methane oxidation during 3 years of repeated application in field experiments. Biol. Fertil. Soils 2001, 34, 109–117. [Google Scholar] [CrossRef]
- Dong, D.; Yang, W.; Sun, H.; Kong, S.; Xu, H. Nitrous oxide emissions in response to long-term application of the nitrification inhibitor DMPP in an acidic luvisol. Appl. Soil Ecol. 2021, 159, 103861. [Google Scholar] [CrossRef]
- Shu, K.L.; Suter, H.; Mosier, A.R.; Chen, D.L. Using nitrification inhibitors to mitigate agricultural N2O emission: A double-edged sword? Glob. Chang. Biol. 2017, 23, 485–489. [Google Scholar] [CrossRef]
- Weller, S.; Fischer, A.; Willibald, G.; Navé, B.; Kiese, R. N2O emissions from maize production in south-west Germany and evaluation of N2O mitigation potential under single and combined inhibitor application. Agric. Ecosyst. Environ. 2019, 269, 215–223. [Google Scholar] [CrossRef]
- Gong, P.; Zhang, L.L.; Wu, Z.J.; Chen, Z.H.; Chen, L.J. Responses of ammonia-oxidizing bacteria and archaea in two agricultural soils to nitrification inhibitors DCD and DMPP: A pot experiment. Pedosphere 2013, 23, 729–739. [Google Scholar] [CrossRef]
- Shi, X.Z.; Hu, H.W.; He, J.Z.; Chen, D.L.; Suter, H.C. Effects of 3,4-dimethylpyrazole phosphate (DMPP) on nitrification and the abundance and community composition of soil ammonia oxidizers in three land uses. Biol. Fertil. Soils 2016, 52, 927–939. [Google Scholar] [CrossRef]
- Tao, R.; Li, J.; Hu, B.W.; Chu, G.X. Mitigating N2O emission by synthetic inhibitors mixed with urea and cattle manure application via inhibiting ammonia-oxidizing bacteria, but not archaea, in a calcareous soil. Environ. Pollut. 2021, 273, 116478. [Google Scholar] [CrossRef]
- Luchibia, A.O.; Lam, S.K.; Suter, H.; Chen, Q.; O’Mara, B.; He, J.-Z. Effects of repeated applications of urea with DMPP on ammonia oxidizers, denitrifiers, and non-targeted microbial communities of an agricultural soil in queensland, australia. Appl. Soil Ecol. 2020, 147, 103392. [Google Scholar] [CrossRef]
- Luchibia, A.O.; Suter, H.; Hu, H.W.; Lam, S.K.; He, J.Z. Responses of ureolytic and nitrifying microbes to urease and nitrification inhibitors in selected agricultural soils in Victoria, Australia. J. Soils Sediments 2020, 20, 1309–1322. [Google Scholar] [CrossRef]
- Tao, R.; Li, J.; Hu, B.W.; Chu, G.X. Ammonia-oxidizing bacteria are sensitive and not resilient to organic amendment and Nitrapyrin disturbances, but ammonia-oxidizing archaea are resistant. Goederma 2021, 384, 114814. [Google Scholar] [CrossRef]
- Bachtsevani, E.; Papazlatani, C.V.; Rousidou, C.; Lampronikou, E.; Menkissoglu-Spiroudi, U.; Nicol, G.W.; Karpouzas, D.G.; Papadopoulou, E.S. Effects of the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) on the activity and diversity of the soil microbial community under contrasting soil pH. Biol. Fertil. Soils 2021, 5, 1117–1135. [Google Scholar] [CrossRef]
- Corrochano-Monsalve, M.; González-Murua, C.; Estavillo, J.M.; Estonba, A.; Zarraonaindia, I. Impact of dimethylpyrazole-based nitrification inhibitors on soil-borne bacteria. Sci. Total Environ. 2021, 792, 148374. [Google Scholar] [CrossRef]
- Liu, C.R.; Zhang, Y.S.; Liu, H.R.; Liu, X.Q.; Ren, D.Y.; Wang, L.G.; Guan, D.H.; Li, Z.H.; Zhang, M.C. Fertilizer stabilizers reduce nitrous oxide emissions from agricultural soil by targeting microbial nitrogen transformations. Sci. Total Environ. 2022, 806, 151225. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.B.; Jiang, S.J.; Assemien, F.; Qin, M.S.; Ma, B.B.; Xie, Z.; Liu, Y.J.; Feng, H.Y.; Du, G.Z.; Ma, X.J.; et al. Response of microbial functional groups involved in soil N cycle to N, P and NP fertilization in Tibetan alpine meadows. Soil Biol. Biochem. 2016, 101, 195–206. [Google Scholar] [CrossRef]
- Liu, C.R.; Liu, H.R.; Liu, X.Q.; Zhang, Y.S.; Wang, L.G.; Guan, D.H.; Al-Kaisi, M.M.; Li, Z.H.; Zhang, M.C. Nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) reduces N2O emissions by altering the soil microbial community in a wheat–maize rotation on the North China Plain. Eur. J. Soil Sci. 2021, 72, 1270–1291. [Google Scholar] [CrossRef]
- Tedeschi, A.; Marco, A.D.; Polimeno, F.; Tommasi, P.D.; Maglione, G.; Ottaiano, L.; Arena, C.; Magliulo, V.; Vitale, L. Effects of the fertilizer added with DMPP on soil nitrous oxide emissions and microbial functional diversity. Agriculture 2021, 11, 12. [Google Scholar] [CrossRef]
- Kong, X.W.; Duan, Y.F.; Schramm, A.; Eriksen, J.; Petersen, S.O. 3,4-dimethylpyrazole phosphate (DMPP) reduces activity of ammonia oxidizers without adverse effects on non-target soil microorganisms and functions. Appl. Soil Ecol. 2016, 105, 67–75. [Google Scholar] [CrossRef]
- Shi, X.Z.; Hu, H.W.; Kelly, K.; Chen, D.L.; He, J.Z.; Suter, H. Response of ammonia oxidizers and denitrifiers to repeated applications of a nitrification inhibitor and a urease inhibitor in two pasture soils. J. Soils Sediments 2017, 17, 974–984. [Google Scholar] [CrossRef]
- Castellano-Hinojosa, A.; González-López, J.; Vallejo, A.; Bedmar, E.J. Effect of urease and nitrification inhibitors on ammonia volatilization and abundance of n-cycling genes in an agricultural soil. J. Plant Nutr. Soil Sci. 2020, 183, 99–109. [Google Scholar] [CrossRef]
- Fu, Q.L.; Abadie, M.; Blaud, A.; Carswell, A.; Misselbrook, T.H.; Clark, I.M.; Hirsch, P.R. Effects of urease and nitrification inhibitors on soil N, nitrifier abundance and activity in a sandy loam soil. Biol. Fertil. Soils 2020, 56, 185–194. [Google Scholar] [CrossRef]
- Meng, X.T.; Li, Y.Y.; Yao, H.Y.; Wang, J.; Dai, F.; Wu, Y.P.; Chapman, S. Nitrification and urease inhibitors improve rice nitrogen uptake and prevent denitrification in alkaline paddy soil. Appl. Soil Ecol. 2020, 154, 103665. [Google Scholar] [CrossRef]
- Schmidt, R.; Wang, X.B.; Garbeva, P.; Yergeau, É. The Nitrification inhibitor nitrapyrin has non-target effects on the soil microbial community structure, composition, and functions. Appl. Soil Ecol. 2022, 171, 104350. [Google Scholar] [CrossRef]
- Fan, X.P.; Yin, C.; Yan, G.C.; Cui, P.Y.; Shen, Q.; Wang, Q.; Chen, H.; Zhang, N.; Ye, M.J.; Zhao, Y.H.; et al. The contrasting effects of N-(n-butyl) thiophosphoric triamide (NBPT) on N2O emissions in arable soils differing in pH are underlain by complex microbial mechanisms. Sci. Total Environ. 2018, 642, 155–167. [Google Scholar] [CrossRef]
- Xu, C.; Han, X.; Ru, S.; Cardenas, L.; Rees, R.M.; Wu, D.; Wu, W.; Meng, F. Crop straw incorporation interacts with N fertilizer on N2O emissions in an intensively cropped farmland. Geoderma 2019, 341, 129–137. [Google Scholar] [CrossRef]
- Shi, Y.F.; Wu, W.L.; Meng, F.Q.; Zhang, Z.H.; Wang, D.P. Integrated management practices significantly affect N2O emissions and wheat–maize production at field scale in the North China Plain. Nutr. Cycl. Agroecosyst. 2013, 95, 203–218. [Google Scholar] [CrossRef]
- Zhao, Z.C.; Wu, D.; Bol, R.; Shi, Y.F.; Guo, Y.B.; Meng, F.Q.; Wu, W.L. Nitrification inhibitor’s effect on mitigating N2O emissions was weakened by urease inhibitor in calcareous soils. Atmos. Environ. 2017, 166, 142–150. [Google Scholar] [CrossRef]
- Pasda, G.; Hähndel, R.; Zerulla, W. Effect of fertilizers with the new nitrification inhibitor DMPP (3,4-dimethylpyrazole phosphate) on yield and quality of agricultural and horticultural crops. Biol. Fertil. Soils 2001, 34, 85–97. [Google Scholar] [CrossRef]
- Serna, M.D.; Baňuls, J.; Quiňones, A.; Primo-Millo, E.; Legaz, F. Evaluation of 3,4 dimethylpyrazole phosphate as a nitrification inhibitor in a citrus-cultivated soil. Biol Fertil. Soils 2000, 32, 41–46. [Google Scholar] [CrossRef]
- Alberto, S.; Laura, S.; Lourdes, G.; Antonio, V. Gaseous emissions of N2O and NO and NO3− leaching from urea applied with urease and nitrification inhibitors to a maize (Zea mays) crop. Agric. Ecosyst. Environ. 2012, 149, 64–73. [Google Scholar] [CrossRef]
- Tan, Y.C.; Wu, D.; Bol, R.; Wu, W.L.; Meng, F.Q. Conservation farming practices in winter wheat–summer maize cropping reduce GHG emissions and maintain high yields. Agric. Ecosyst. Environ. 2019, 272, 266–275. [Google Scholar] [CrossRef]
- Hu, X.K.; Su, F.; Ju, X.T.; Gao, B.; Oenema, O.; Christie, P.; Huang, B.X.; Jiang, R.F.; Zhang, F.S. Greenhouse gas emissions from a wheat–maize double cropping system with different nitrogen fertilization regimes. Environ. Pollut. 2013, 176, 198–207. [Google Scholar] [CrossRef]
- Dong, D.; Kou, Y.; Yang, W.; Chen, G.; Xu, H. Effects of urease and nitrification inhibitors on nitrous oxide emissions and nitrifying/denitrifying microbial communities in a rainfed maize soil: A 6-year field observation. Soil Tillage Res. 2018, 180, 82–90. [Google Scholar] [CrossRef]
- Hu, H.W.; Macdonald, C.A.; Trivedi, P.; Holmes, B.; Bodrossy, L.; He, J.Z.; Singh, B.K. Water addition regulates the metabolic activity of ammonia oxidizers responding to environmental perturbations in dry subhumid ecosystems. Environ. Microbiol. 2015, 17, 444–461. [Google Scholar] [CrossRef]
- Chen, S.F.; Zhou, Y.Q.; Chen, Y.R.; Gu, J. fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Cold Spring Harb. Lab. 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiefje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 623–656. [Google Scholar] [CrossRef]
- Chao, A. Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- He, Y.; Zhou, B.J.; Deng, G.H.; Jiang, X.T.; Zhang, H.; Zhou, H.W. Comparison of microbial diversity determined with the same variable tag sequence extracted from two different PCR amplicons. BMC Microbiol. 2013, 13, 208. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Trivedi, P.; Osanai, Y.; Liu, Y.R.; Hamonts, K.; Jeffries, T.C.; Singh, B.K. Carbon content and climate variability drive global soil bacterial diversity patterns. Ecol. Monogr. 2016, 86, 373–390. [Google Scholar] [CrossRef]
- Avrahami, S.; Conrad, R.; Braker, G. Effect of soil ammonium concentration on N2O release and on the community structure of ammonia oxidizers and denitrifiers. Appl. Environ. Microbiol. 2002, 68, 5685–5692. [Google Scholar] [CrossRef]
- Pester, M.; Rattei, T.; Flechl, S.; Grngrft, A.; Wagner, M. amoA-based consensus phylogeny of ammonia-oxidizing archaea and deep sequencing of amoA genes from soils of four different geographic regions. Environ. Microbiol. 2012, 14, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, L.; Sun, R.; Dai, T.; Tian, J.; Wen, D. Ammonia-oxidizing bacteria and archaea in wastewater treatment plant sludge and nearby coastal sediment in an industrial area in China. Appl. Microbiol. Biotechnol. 2015, 99, 4495–4507. [Google Scholar] [CrossRef] [PubMed]
- Di, H.J.; Cameron, K.C.; Shen, J.P.; Winefield, C.S.; O’Callaghan, M.; Bowatte, S.; He, J.Z. Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nat. Geosci. 2009, 2, 621–624. [Google Scholar] [CrossRef]
- Hink, L.; Gubry-Rangin, C.; Nicol, G.W.; Prosser, J.I. The consequences of niche and physiological differentiation of archaeal and bacterial ammonia oxidisers for nitrous oxide emissions. ISME J. 2018, 12, 1084–1093. [Google Scholar] [CrossRef]
- Fan, X.P.; Yin, C.; Chen, H.; Ye, M.J.; Zhao, Y.H. The efficacy of 3,4-dimethylpyrazole phosphate on N2O emissions is linked to niche differentiation of ammonia oxidizing archaea and bacteria across four arable soils -sciencedirect. Soil Biol. Biochem. 2019, 130, 82–93. [Google Scholar] [CrossRef]
- Maria, T.; Thomas, E.F.; James, I.P. Stable isotope probing analysis of interactions between ammonia oxidizers. Appl. Environ. Microbiol. 2010, 76, 2468–2477. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, X.J.; Song, L.; Lin, X.G.; Zhang, H.Y.; Shen, C.C.; Chu, H.Y. Nitrogen fertilization directly affects soil bacterial diversity and indirectly affects bacterial community composition. Soil Biol. Biochem. 2016, 92, 41–49. [Google Scholar] [CrossRef]
- Schramm, A.; de Beer, D.; van den Heuvel, J.C.; Ottengraf, S.; Amann, R. Microscale distribution of populations and activities of Nitrosospira and Nitrospira spp. along a macroscale gradient in a nitrifying bioreactor: Quantification by in situ hybridization and the use of microsensors. Appl. Environ. Microbiol. 1999, 65, 3690–3696. [Google Scholar] [CrossRef]
- Le Roux, X.; Bouskill, N.J.; Niboyet, A.; Barthes, L.; Dijkstra, P.; Field, C.B.; Hungate, B.A.; Lerondelle, C.; Pommier, T.; Tang, J.; et al. Predicting the responses of soil nitrite-oxidizers to multi-factorial global change: A trait-based approach. Front. Microbiol. 2016, 7, 628. [Google Scholar] [CrossRef]
- Luo, X.S.; Han, S.; Lai, S.S.; Huang, Q.Y.; Chen, W.L. Long-term straw returning affects Nitrospira-like nitrite oxidizing bacterial community in a rapeseed-rice rotation soil. J. Basic Microbiol. 2017, 57, 309–315. [Google Scholar] [CrossRef]
- Wu, D.; Zhao, Z.C.; Han, X.; Meng, F.Q.; Wu, W.L.; Zhou, M.; Brüggemann, N.; Bol, R. Potential dual effect of nitrification inhibitor 3,4-dimethylpyrazole phosphate on nitrifier denitrification in the mitigation of peak N2O emission events in North China Plain cropping systems. Soil Biol. Biochem. 2018, 121, 147–153. [Google Scholar] [CrossRef]
- Cao, W.C.; Liu, S.; Qu, Z.; Song, H.; Qin, W.; Guo, J.H.; Chen, Q.; Lin, S.; Wang, J.G. Contribution and driving mechanism of N2O emission bursts in a Chinese vegetable greenhouse after manure application and irrigation. Sustainability 2019, 11, 1624. [Google Scholar] [CrossRef]
- Ma, L.; Shan, J.; Yan, X.Y. Nitrite behavior accounts for the nitrous oxide peaks following fertilization in a fluvo-aquic soil. Biol. Fertil. Soils 2015, 51, 563–572. [Google Scholar] [CrossRef]
- Ramotowski, D.; Shi, W. Nitrapyrin-based nitrification inhibitors shaped the soil microbial community via controls on soil pH and inorganic N composition. Appl. Soil Ecol. 2022, 170, 104295. [Google Scholar] [CrossRef]
- Burgin, A.J.; Groffman, P.M. Soil O2 controls denitrification rates and N2O yield in a riparian wetland. J. Geophys. Res. Biogeosci. 2012, 117, G01010. [Google Scholar] [CrossRef]
- Philippot, L.; Mirleau, P.; Mazurier, S.; Siblot, S.; Hartmann, A.; Lemanceau, P.; Germon, J.C. Characterization and transcriptional analysis of pseudomonas fluorescens denitrifying clusters containing the nar, nir, nor and nos genes. Biochim. Biophys. Acta BBA Gene Struct. Expr. 2001, 1517, 436–440. [Google Scholar] [CrossRef]
- Francis, C.; Roberts, K.; Beman, J.; Santoro, A.; Oakley, B. Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc. Natl. Acad. Sci. USA 2005, 102, 14683–14688. [Google Scholar] [CrossRef]
- Rotthauwe, J.; Witzel, K.; Liesack, W. The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ. Microbiol. 1997, 63, 4704–4712. [Google Scholar] [CrossRef]
- Wertz, S.; Poly, F.; Le Roux, X.; Degrange, V. Development and application of a PCR-denaturing gradient gel electrophoresis tool to study the diversity of Nitrobacter-like nxrA sequences in soil. FEMS Microbiol. Ecol. 2008, 63, 261. [Google Scholar] [CrossRef] [PubMed]
- Pester, M.; Maixner, F.; Berry, D.; Rattei, T.; Koch, H.; Lücker, S.; Nowka, B.; Richter, A.; Spieck, E.; Lebedeva, E.; et al. nxrB encoding the beta subunit of nitrite oxidoreductase as functional and phylogenetic marker for nitrite-oxidizing Nitrospira. Environ. Microbiol. 2014, 16, 3055–3071. [Google Scholar] [CrossRef] [PubMed]
- Bru, D.; Sarr, A.; Philippot, L. Relative abundances of proteobacterial membrane-bound and periplasmic nitrate reductases in selected environments. Appl. Environ. Microbiol. 2007, 73, 5971–5974. [Google Scholar] [CrossRef] [PubMed]
- Kloos, K.; Mergel, A.; Rosch, C.; Bothe, H. Denitrification within the genus Azospirillim and other associative bacteria. Aust. J. Plant. Physiol. 2001, 28, 991–998. [Google Scholar] [CrossRef]

| Year | Treatment | Cumulative N2O Emissions | EF | N2O Mitigation |
|---|---|---|---|---|
| kg N ha−1 | % | % | ||
| 2012–2013 | CK | 1.03 ± 0.06 d | ||
| U | 3.20 ± 0.06 a | 0.36 | ||
| NI | 1.65 ± 0.09 c | 0.10 | 71.0 | |
| UI | 2.42 ± 0.07 b | 0.23 | 35.3 | |
| 2013–2014 | CK | 1.07 ± 0.06 d | ||
| U | 3.39 ± 0.12 a | 0.40 | ||
| NI | 1.76 ± 0.09 c | 0.13 | 70.9 | |
| UI | 2.63 ± 0.22 b | 0.27 | 33.7 | |
| 2014–2015 | CK | 0.75 ± 0.06 d | ||
| U | 3.92 ± 0.16 a | 0.53 | ||
| NI | 1.43 ± 0.08 c | 0.11 | 79.0 | |
| UI | 2.65 ± 0.07 b | 0.32 | 39.7 | |
| 2015–2016 | CK | 1.16 ± 0.14 c | ||
| U | 4.09 ± 0.05 a | 0.49 | ||
| NI | 1.47 ± 0.03 c | 0.05 | 89.9 | |
| UI | 2.55 ± 0.20 b | 0.23 | 52.6 |
| Factors | Cumulative N2O Emissions kg N ha−1 | |||||
|---|---|---|---|---|---|---|
| NI | UI | |||||
| df | F | p-Value | df | F | p-Value | |
| Year | 3 | 4.413 | <0.05 | 3 | 5.723 | <0.01 |
| Inhibitor | 1 | 841.398 | <0.01 | 1 | 123.708 | <0.01 |
| Year × inhibitor | 3 | 14.201 | <0.01 | 3 | 3.349 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Ma, Y.; Yang, X.; Xu, X.; Ni, B.; Liu, R.; Meng, F. Investigation of Soil Microbial Communities Involved in N Cycling as Affected by the Long-Term Use of the N Stabilizers DMPP and NBPT. Agronomy 2023, 13, 659. https://doi.org/10.3390/agronomy13030659
Zhang W, Ma Y, Yang X, Xu X, Ni B, Liu R, Meng F. Investigation of Soil Microbial Communities Involved in N Cycling as Affected by the Long-Term Use of the N Stabilizers DMPP and NBPT. Agronomy. 2023; 13(3):659. https://doi.org/10.3390/agronomy13030659
Chicago/Turabian StyleZhang, Wei, Yan Ma, Xuan Yang, Xiuchun Xu, Bang Ni, Rui Liu, and Fanqiao Meng. 2023. "Investigation of Soil Microbial Communities Involved in N Cycling as Affected by the Long-Term Use of the N Stabilizers DMPP and NBPT" Agronomy 13, no. 3: 659. https://doi.org/10.3390/agronomy13030659
APA StyleZhang, W., Ma, Y., Yang, X., Xu, X., Ni, B., Liu, R., & Meng, F. (2023). Investigation of Soil Microbial Communities Involved in N Cycling as Affected by the Long-Term Use of the N Stabilizers DMPP and NBPT. Agronomy, 13(3), 659. https://doi.org/10.3390/agronomy13030659





