Allelopathic Effects of Cannabis sativa L. Aqueous Leaf Extracts on Seed Germination and Seedling Growth in Durum Wheat and Barley
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Leaf Extract
2.3. Germination Tests
2.4. Radicle and Shoot Measurements
2.5. Total Phenolic Content Measurement on Leaf Extract
2.6. Data Analysis
3. Results
3.1. Total Phenolic Content (TPC)
3.2. Germination Course
3.3. FGP, MGT, t50, GI
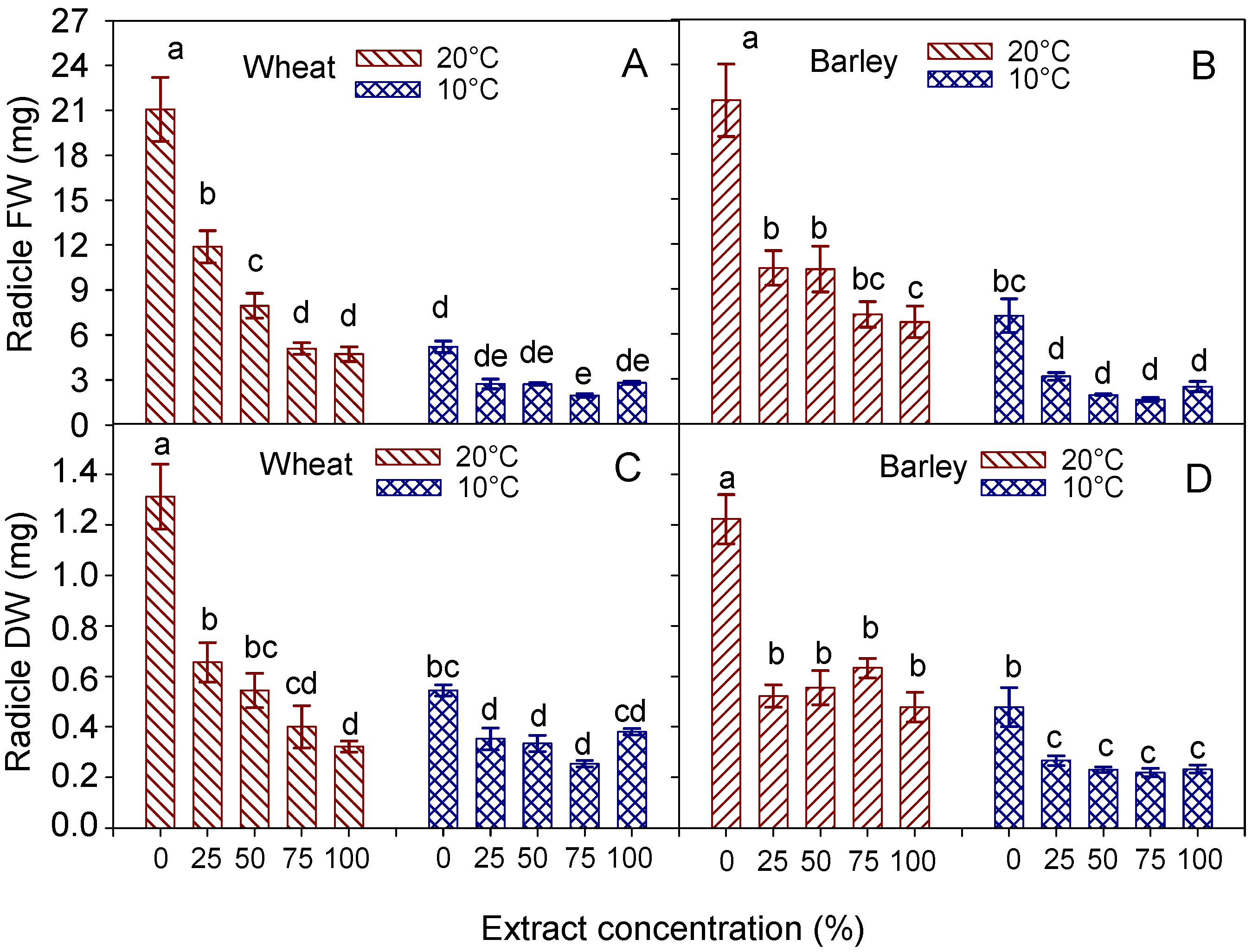
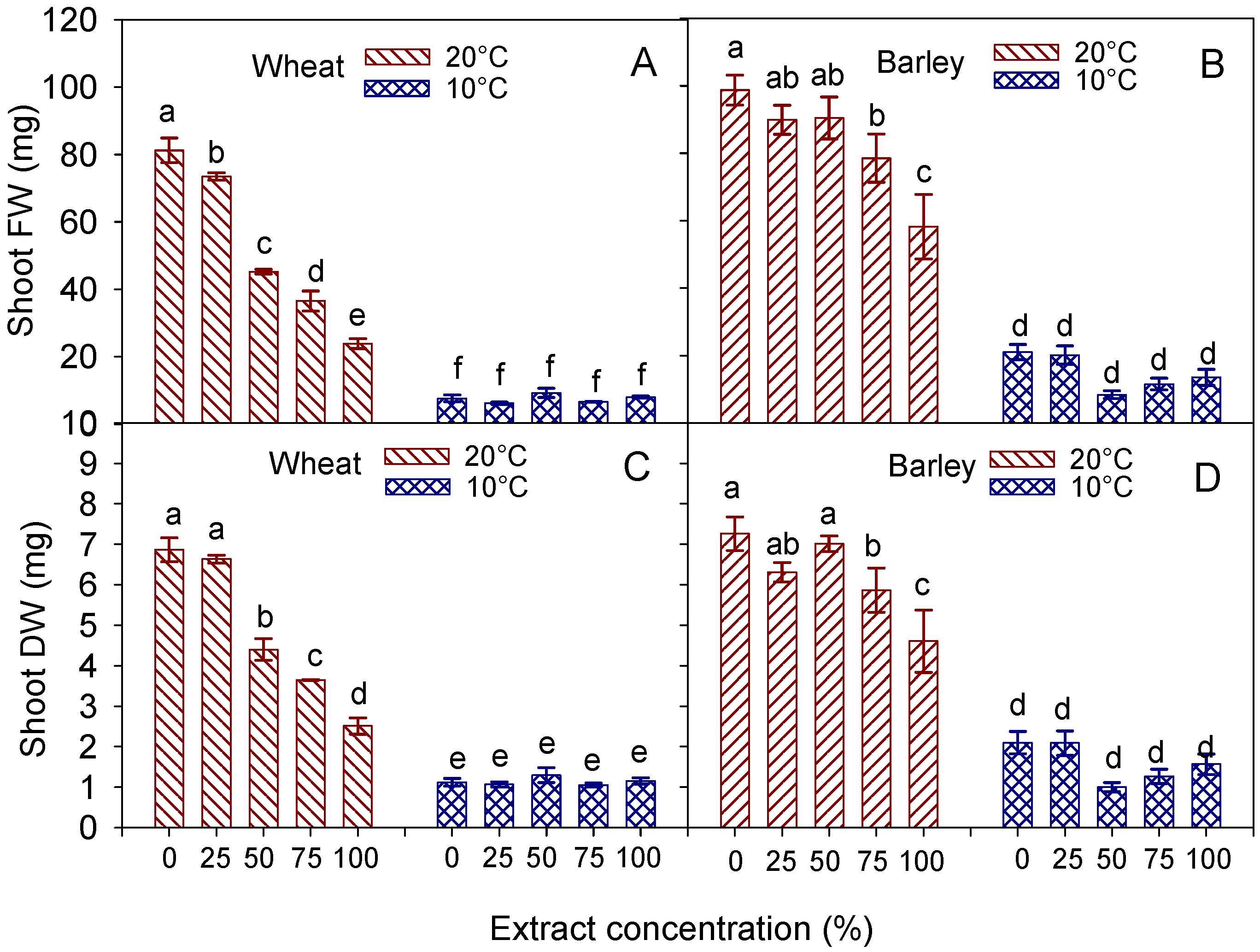
3.4. Radicle and Shoot Traits
3.5. Germination Index (GI) and Vigor Index (VI)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Einhellig, F. Interactions involving allelopathy in cropping systems. Agron. J. 1996, 893, 886–893. [Google Scholar] [CrossRef]
- Sangeetha, C.; Baskar, P. Allelopathy in weed management: A critical review. Afr. J. Agric Res. 2015, 10, 1004–1015. [Google Scholar]
- Gulzar, A.; Siddiqui, M.B. Allelopathic effect of Calotropis procera (Ait.) R. Br. on growth and antioxidant activity of Brassica oleracea var. botrytis. J. Saudi Soc. Agric. Sci. 2017, 16, 375–382. [Google Scholar] [CrossRef]
- Madany, M.M.Y.; Saleh, A.M. Phytotoxicity of Euphorbia helioscopia L. on Triticum aestivum L. and Pisum sativum L. Annals Agric. Sci. 2015, 60, 141–151. [Google Scholar] [CrossRef]
- Kohli, R.K.; Batish, D.; Singh, H.P. Allelopathy and its implications in Agroecosystems. J. Crop. Prod. 1998, 1, 169–202. [Google Scholar] [CrossRef]
- Khan, A.U.; Ullah, F.; Mehmood, S.; Irshad, M.; Khan, F.U. Allelopathic effects of Jatropha curcas L. leaf aqueous extract on early seedling growth of Parthenium hysterophorus. Pakistan J. Agric. Res. 2017, 30, 45–54. [Google Scholar]
- Golisz, A.; Lata, B.; Gawronski, S.W.; Fujii, Y. Specific and total activities of the allelochemicals identified in buckwheat. Weed Biol. Manag. 2007, 7, 164–171. [Google Scholar] [CrossRef]
- Kalinova, J.; Vrchotova, N.; Triska, J. Exudation of allelopathic substances in buckwheat (Fagopyrum esculentum Moench.). J. Agric. Food Chem. 2007, 55, 6453–6459. [Google Scholar] [CrossRef]
- Szwed, M.; Mitrus, J.; Wiczkowski, W.; Dębski, H.; Horbowicz, M. If phenolic compounds in the soil with buckwheat residues affect the emergence and growth of weed seedlings? Acta Physiol. Plant. 2020, 42, 154. [Google Scholar] [CrossRef]
- Ziaebrahimi, L.; Khavari-Nejad, R.A.; Fahimi, H.; Nejadsatari, T. Effects of aqueous eucalyptus extracts on seed germination, seedling growth and activities of peroxidase and polyphenoloxidase in three wheat cultivar seedlings (Triticum aestivum L.). Pak. J. Biol. Sci. 2007, 10, 3415–3419. [Google Scholar] [CrossRef]
- Sarkar, E.; Chatterjee, S.N.; Chakraborty, P. Allelopathic effect of Cassia tora on seed germination and growth of mustard. Turk. J. Bot. 2012, 36, 488–494. [Google Scholar] [CrossRef]
- Sahu, A.; Devkota, A. Allelopathic effects of aqueous extract of leaves of Mikania micrantha H.B.K. on seed germination and seedling growth of Oryza sativa l. and Raphanus sativus L. Sci. World 2013, 11, 90–93. [Google Scholar] [CrossRef]
- Leoni, M.; Musio, S.; Croci, M.; Tang, K.; Magagnini, G.M.; Thouminot, C.; Müssig, J.; Amaducci, S. The effect of agronomic management of hemp (Cannabis sativa L.) on stem processing and fibre quality. Ind. Crops Prod. 2022, 188, 115520. [Google Scholar] [CrossRef]
- Höppner, F.; Menge-Hartmann, U. Yield and quality of fibre and oil of fourteen hemp cultivars in Northern Germany at two harvest dates. Landbauforsch. Volkenrode 2007, 57, 219–232. [Google Scholar]
- Benelli, G.; Pavela, R.; Petrelli, R.; Cappellacci, L.; Santini, G.; Fiorini, D.; Sutf, S.; Dall’Acqua, S.; Canale, A.; Maggi, F. The essential oil from industrial hemp (Cannabis sativa L.) by-products as an effective tool for insect pest management in organic crops. Ind. Crops Prod. 2018, 122, 308–315. [Google Scholar] [CrossRef]
- Mahmoodzadeh, H.; Ghasem, M.; Zanganeh, H. Allelopathic effect of medicinal plant Cannabis sativa L. on Lactuca sativa L. seed germination. Acta Agric. Slov. 2015, 105, 233–239. [Google Scholar] [CrossRef]
- Pudełko, K.; Majchrzak, L.; Narożna, D. Allelopathic effect of fibre hemp (Cannabis sativa L.) on monocot and dicot plant species. Ind. Crops Prod. 2014, 56, 191–199. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Testa, G.; Scordia, D.; Copani, V. Sowing time and prediction of flowering of different hemp (Cannabis sativa L.) genotypes in southern Europe. Ind. Crops Prod. 2012, 37, 20–33. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Riggi, E.; Testa, G.; Scordia, D.; Copani, V. Evaluation of European developed fibre hemp genotypes (Cannabis sativa L.) in semi-arid Mediterranean environment. Ind. Crops Prod. 2013, 50, 312–324. [Google Scholar] [CrossRef]
- Tanveer, A.; Rehman, A.; Javaid, M.M.; Abbas, R.N.; Sibtain, M.; Ahmad, A.U.; Ibin-I-Zamir, M.S.; Chaudhary, K.M.; Aziz, A. Allelopathic potential of Euphorbia helioscopia L. against wheat (Triticum aestivum L.), chickpea (Cicer arietinum L.) and lentil (Lens culinaris Medic.). Turk. J. Agric. Forest. 2010, 34, 75–81. [Google Scholar] [CrossRef]
- Jabran, K. Manipulation of allelopathic crops for weed control. In SpringerBriefs in Plant Science; Springer: Berlin/Heidelberg, Germany, 2017; p. 87. [Google Scholar]
- Abdul-Baki, A.A.; Anderson, J.D. Vigor Determination in Soybean Seed by Multiple Criteria. Crop. Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Patanè, C.; Cavallaro, V.; Cosentino, S.L. Germination and radicle growth in unprimed and primed seeds of sweet sorghum as affected by reduced water potential in NaCl at different temperatures. Ind. Crops Prod. 2009, 30, 1–8. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Sanzone, E.; Testa, G.; Patanè, C.; Anastasi, U.; Scordia, D. Does post-anthesis heat stress affect plant phenology, physiology, grain yield and protein content of durum wheat in a semi-arid Mediterranean environment? J. Agron. Crop. Sci. 2019, 205, 309–323. [Google Scholar] [CrossRef]
- Guo, C.; Shen, Y.; Shi, F. Effect of temperature, light, and storage time on the seed germination of Pinus bungeana Zucc. ex Endl.: The role of seed-covering layers and abscisic acid changes. Forests 2020, 11, 300. [Google Scholar] [CrossRef]
- Chaves, N.; Sosa, T.; Escudero, J.C. Plant growth inhibiting fl avonoids in exudate of Cistus ladanifer and in associated soils. J. Chem. Ecol. 2001, 27, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef]
- Tawaha, A.M.; Turk, M.A. Allelopathic effects of black musard (Brassica nigra) on germination and growth of wild barley (Hordeum spontaneum). J. Agron. Crop. Sci. 2003, 189, 298–303. [Google Scholar] [CrossRef]
- Chandler, P.M.; Zucar, J.A.; Jacobson, J.V.; Higgins, T.J.V.; Inglis, A.S. The effect of gibberellic acid and abscisic acid on α-amylase mRNA levels in barley aleurone layers studies using an α-amylase c DNA clone. Plant Mol. Biol. 1984, 3, 407–408. [Google Scholar] [CrossRef]
- Knox, J.A.I.; Jaggi, D.; Paul, M.S. Allelopathic effect of selected weeds on biochemical activity of Parthenium hysterophorus. Curr. Res. J. Biol. Sci. 2014, 2, 238–240. [Google Scholar]
- Nishida, N.; Tamotsu, S.; Negata, N.; Saito, C.; Sakai, A. Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla: Inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. J. Chem. Ecol. 2005, 31, 1187–1203. [Google Scholar] [CrossRef] [PubMed]
- Mondal, F.M.; Asaduzzaman, M.; Asao, T. Adverse effects of allelopathy from legume crops and its possible avoidance. Am. J. Plant Sci. 2015, 6, 804–810. [Google Scholar] [CrossRef]
- Troyjack, C.; Pimentel, J.; Dubai Padilha, I.; Veliz Escalera, R.; Acosta Jaque, L.; Koch, F.; Monteiro, M.; Demari, G.; Szareski, V.; Carvalho, I.; et al. Nitrogen fertilization on maize sowing: Plant growth and seed vigor. Am. J. Plant Sci. 2018, 9, 83–97. [Google Scholar] [CrossRef]
- Foy, C.L.; Inderjit. Understanding the role of allelopathy in weed interference and declining plant diversity. Weed Technol. 2001, 15, 837–878. [Google Scholar] [CrossRef]
- Abdalla, M. The potential of Moringa oleifera extract as a biostimulant in enhancing the growth, biochemical and hormonal contents in rocket (Eruca vesicaria subsp. Sativa) plants. Int. J. Plant Physiol. Biochem. 2013, 5, 42–49. [Google Scholar]
- Pannacci, E.; Baratta, S.; Falcinelli, B.; Farneselli, M.; Tei, F. Mugwort (Artemisia vulgaris L.). Aqueous extract: Hormesis and biostimulant activity for seed germination and seedling growth in vegetable crops. Agriculture 2022, 12, 1329. [Google Scholar] [CrossRef]
- Pannacci, E.; Masi, M.; Farneselli, M.; Tei, F. Evaluation of Mugwort (Artemisia vulgaris L.) Aqueous extract as a potential bioherbicide to control Amaranthus retroflexus L. in maize. Agriculture 2020, 10, 642. [Google Scholar] [CrossRef]
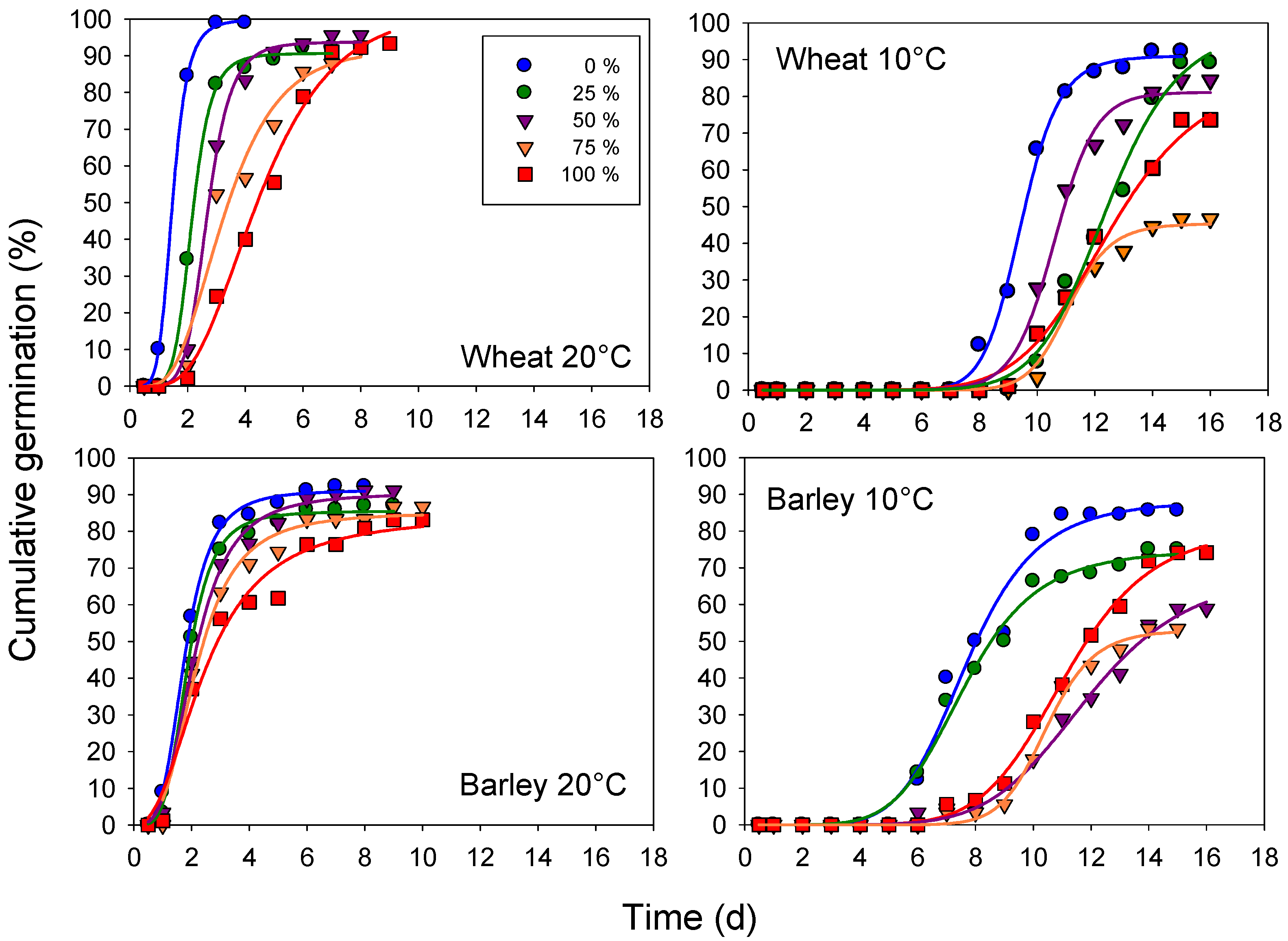
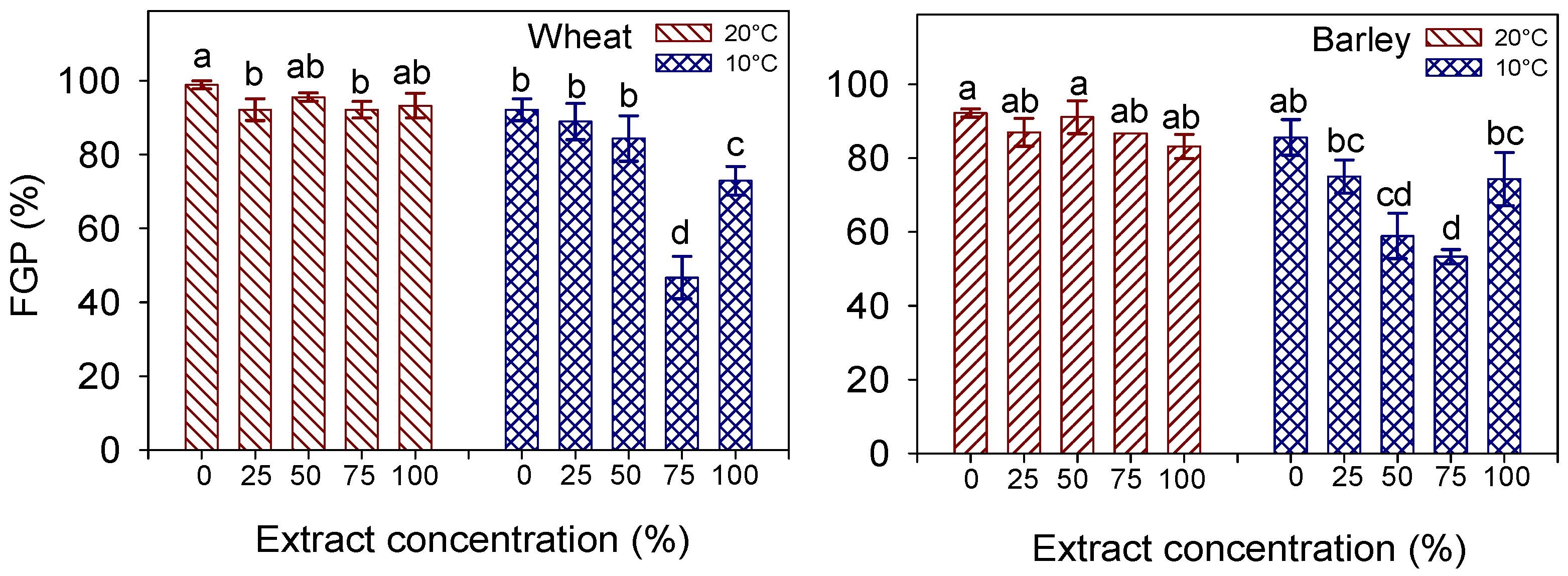
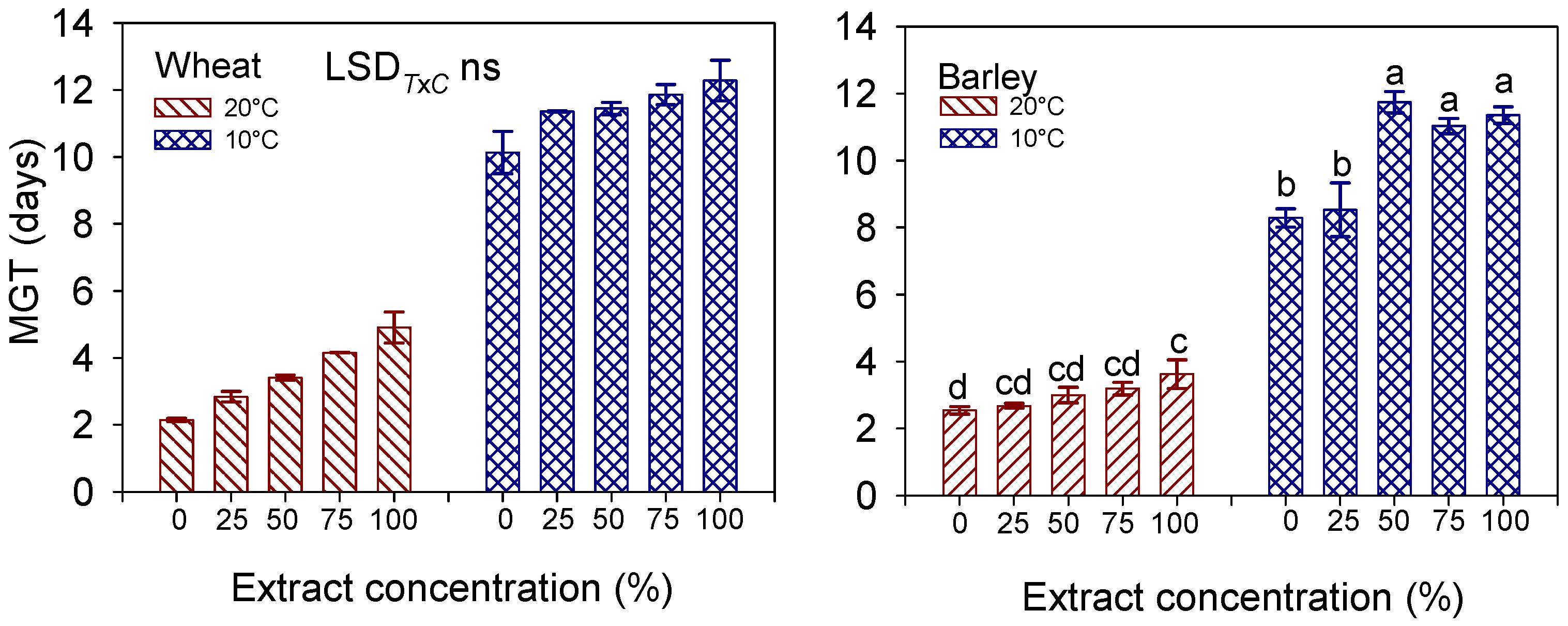
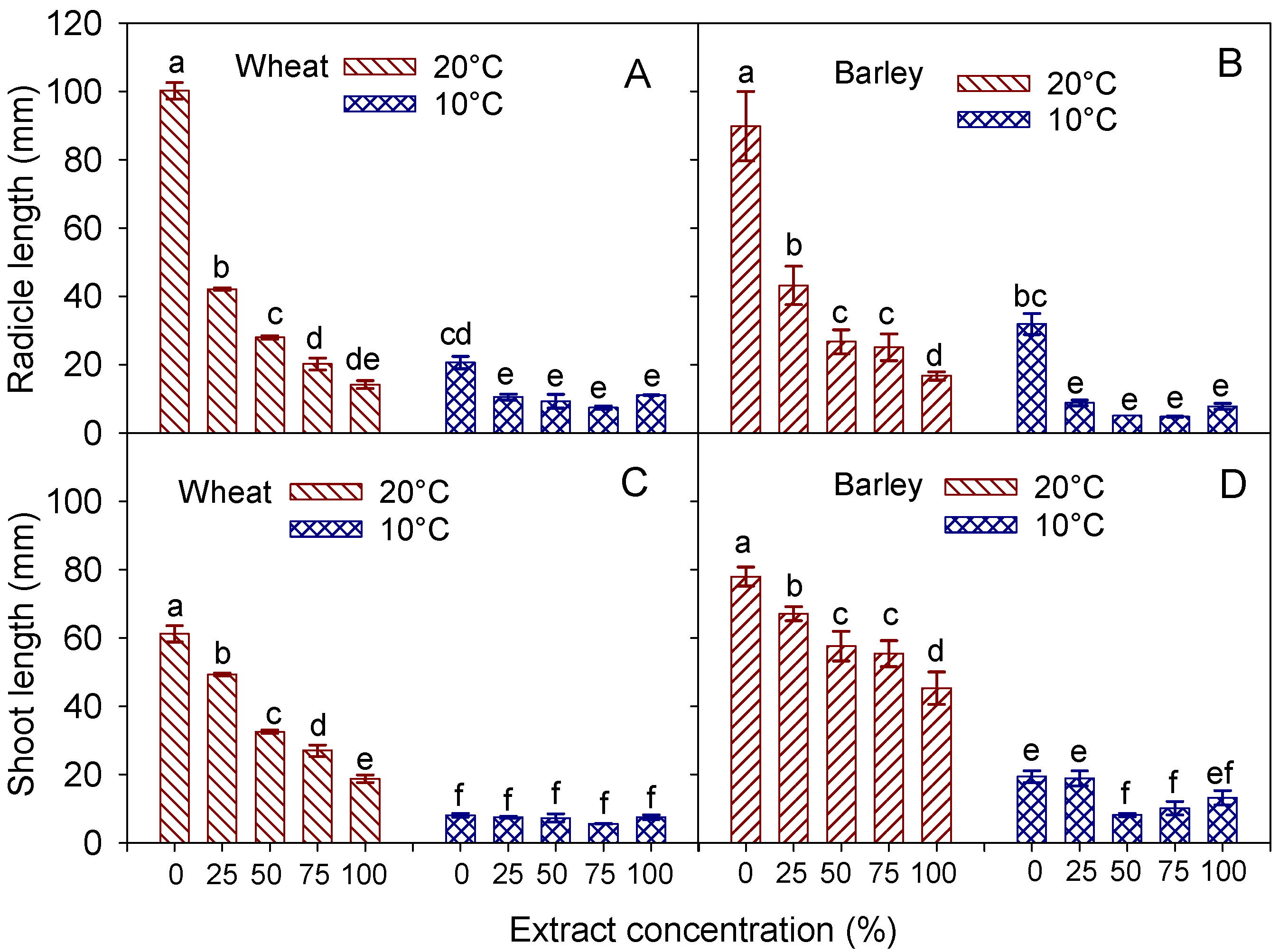
| Extract Concentration (%) | Aqueous Extract (g L−1) | TPC (mg GAE g−1 DW) |
|---|---|---|
| 0 (distilled water) | 0.0 | 0 e |
| 25 | 5.0 | 3.81 ± 0.04 d |
| 50 | 10.0 | 6.87 ± 0.09 c |
| 75 | 15.0 | 9.85 ± 0.07 b |
| 100 | 20.0 | 13.14 ± 0.20 a |
| Extract Concentration (%) | Wheat (cv. Mongibello) | |||||||
|---|---|---|---|---|---|---|---|---|
| 20 °C | 10 °C | |||||||
| a | R2 | x0 | Σx2 | a | R2 | x0 | Σx2 | |
| 0 | 99.9 ± 0.61 | 0.99 | 1.48 ± 0.02 | 1.1 | 91.0 ± 1.14 | 0.99 | 9.45 ± 0.51 | 53.3 |
| 25 | 90.7 ± 0.95 | 0.99 | 2.15 ± 0.03 | 14.3 | 99.3 ± 5.12 | 0.99 | 12.42 ± 0.20 | 133.7 |
| 50 | 93.8 ± 1.23 | 0.99 | 2.68 ± 0.05 | 33.7 | 82.1 ± 1.86 | 0.99 | 10.60 ± 0.09 | 130.1 |
| 75 | 94.2 ± 8.83 | 0.97 | 3.28 ± 0.31 | 304.2 | 45.4 ± 1.38 | 0.99 | 11.11 ± 0.12 | 63.8 |
| 100 | 95.4 ± 3.85 | 0.99 | 4.38 ± 0.14 | 77.4 | 78.4 ± 3.06 | 0.99 | 11.90 ± 0.17 | 55.3 |
| Extract Concentration (%) | Barley (cv. Alamo) | |||||||
|---|---|---|---|---|---|---|---|---|
| 20 °C | 10 °C | |||||||
| a | R2 | x0 | Σx2 | a | R2 | x0 | Σx2 | |
| 0 | 91.2 ± 0.97 | 0.99 | 1.76 ± 0.04 | 19.5 | 88.3 ± 3.40 | 0.99 | 7.70 ± 0.20 | 310.9 |
| 25 | 85.5 ± 0.93 | 0.99 | 1.85 ± 0.04 | 27.5 | 75.0 ± 2.08 | 0.99 | 7.61 ± 0.15 | 111.0 |
| 50 | 90.5 ± 1.84 | 0.99 | 2.08 ± 0.08 | 63.0 | 69.0 ± 4.68 | 0.99 | 11.88 ± 0.33 | 65.9 |
| 75 | 85.2 ± 2.33 | 0.98 | 2.17 ± 0.11 | 111.5 | 53.1 ± 1.28 | 0.99 | 10.46 ± 0.09 | 35.5 |
| 100 | 84.4 ± 4.96 | 0.99 | 2.47 ± 0.23 | 220.6 | 81.2 ± 2.47 | 0.99 | 11.12 ± 0.14 | 45.3 |
| FGP (%) | MGT (Days) | t50 | ||
|---|---|---|---|---|
| Temperature (T) | 20 °C | 94.4 ± 1.11 a | 3.49 ± 0.27 b | 2.85 |
| 10 °C | 77.1 ± 4.78 b | 11.41 ± 0.25 a | 12.39 | |
| Extract concentration (C) | 0% (control) | 95.6 ± 2.05 a | 6.14 ± 1.81 c | 5.52 |
| 25% | 90.6 ± 2.65 b | 7.09 ± 1.90 bc | 7.34 | |
| 50% | 90.0 ± 3.75 b | 7.43 ± 1.80 b | 6.84 | |
| 75% | 69.5 ± 10.55 c | 8.01 ± 1.73 ab | 9.71 | |
| 100% | 83.1 ± 5.10 bc | 8.59 ± 1.68 a | 8.76 | |
| Significance | T | *** | *** | |
| C | *** | *** | ||
| T × C | * | ns |
| FGP (%) | MGT (Days) | t50 | ||
|---|---|---|---|---|
| Temperature (T) | 20 °C | 88.0 ± 1.43 a | 3.01 ± 0.14 b | 2.28 |
| 10 °C | 69.4 ± 3.69 b | 10.19 ± 0.43 a | 11.02 | |
| Extract concentration (C) | 0% (control) | 88.9 ± 2.67 a | 5.41 ± 1.29 b | 4.95 |
| 25% | 81.0 ± 3.74 ab | 5.61 ± 1.36 b | 5.26 | |
| 50% | 75.0 ± 7.97 ab | 7.37 ± 1.96 a | 7.96 | |
| 75% | 70.0 ± 7.50 b | 7.11 ± 1.76 a | 7.70 | |
| 100% | 78.7 ± 4.05 ab | 7.49 ± 1.75 a | 7.38 | |
| Significance | T | *** | *** | |
| C | * | *** | ||
| T × C | * | *** |
| Radicle (mm) | Shoot (mm) | Radicle FW (mg) | Radicle DW (mg) | Shoot FW (mg) | Shoot DW (mg) | ||
|---|---|---|---|---|---|---|---|
| Temperature (T) | 20 °C | 40.9 ± 8.4 a | 37.8 ± 4.2 a | 10.15 ± 1.7 a | 0.65 ± 0.09 a | 52.06 ± 5.9 a | 4.81 ± 0.46 a |
| 10 °C | 11.8 ± 1.3 b | 7.2 ± 0.3 b | 3.09 ± 0.3 b | 0.37 ± 0.03 b | 7.55 ± 0.4 b | 1.14 ± 0.05 b | |
| Extract concentration (C) | 0% (control) | 60.4 ± 18.1 a | 34.7 ± 11.9 a | 13.14 ± 3.7 a | 0.93 ± 0.18 a | 44.42 ± 16.5 a | 3.99 ± 1.29 a |
| 25% | 26.3 ± 7.1 b | 28.4 ± 9.3 b | 7.32 ± 2.1 b | 0.50 ± 0.08 b | 39.86 ± 15.0 a | 3.85 ± 1.25 a | |
| 50% | 18.7 ± 4.4 bc | 20.0 ± 5.7 c | 5.35 ± 1.2 bc | 0.44 ± 0.06 b | 27.25 ± 8.1 b | 2.85 ± 0.71 b | |
| 75% | 13.8 ± 2.9 c | 16.3 ± 4.8 d | 3.78 ± 0.7 c | 0.33 ± 0.05 b | 21.57 ± 6.8 c | 2.35 ± 0.58 c | |
| 100% | 12.7 ± 1.0 c | 13.1 ± 2.6 d | 3.53 ± 0.5 c | 0.35 ± 0.02 b | 15.93 ± 3.6 d | 1.83 ± 0.32 d | |
| Significance | T | *** | *** | *** | *** | *** | *** |
| C | *** | *** | *** | *** | *** | *** | |
| T × C | *** | *** | *** | *** | *** | *** |
| Radicle (mm) | Shoot (mm) | Radicle FW (mg) | Radicle DW (mg) | Shoot FW (mg) | Shoot DW (mg) | ||
|---|---|---|---|---|---|---|---|
| Temperature (T) | 20 °C | 40.1 ± 7.3 a | 60.6 ± 3.3 a | 11.28 ± 1.5 a | 0.68 ± 0.01 a | 83.28 ± 4.5 a | 6.21 ± 0.31 a |
| 10 °C | 11.5 ± 2.8 b | 14.0 ± 1.4 b | 3.28 ± 0.6 b | 0.29 ± 0.03 b | 14.92 ± 1.5 b | 1.60 ± 0.15 b | |
| Extract concentration (C) | 0% (control) | 60.8 ± 13.8 a | 48.7 ± 13.2 a | 14.41 ± 3.4 a | 0.85 ± 0.17 a | 59.88 ± 17.5 a | 4.68 ± 1.17 a |
| 25% | 25.8 ± 8.1 b | 42.9 ± 10.9 a | 6.78 ± 1.7 b | 0.40 ± 0.06 b | 55.03 ± 15.8 a | 4.20 ± 0.96 ab | |
| 50% | 15.8 ± 5.1 bc | 32.8 ± 11.2 b | 6.13 ± 2.0 b | 0.39 ± 0.08 b | 49.43 ± 18.6 b | 4.01 ± 1.35 abc | |
| 75% | 15.0 ± 4.9 bc | 32.8 ± 10.3 b | 4.46 ± 1.3 b | 0.43 ± 0.09 b | 45.05 ± 15.3 c | 3.57 ± 1.06 bc | |
| 100% | 11.5 ± 2.4 c | 29.3 ± 7.5 b | 4.64 ± 1.1 b | 0.36 ± 0.06 b | 35.87 ± 10.9 d | 3.09 ± 0.77 c | |
| Significance | T | *** | *** | *** | *** | *** | *** |
| C | *** | *** | *** | *** | ** | ** | |
| T × C | *** | ** | ** | *** | * | * |
| Extract Concentration (C) | Temperature (T) | |||||
|---|---|---|---|---|---|---|
| Germination Index | Vigor Index | |||||
| 20 °C | 10 °C | Average (T) | 20 °C | 10 °C | Average (T) | |
| 0% (control) | 1.56 ± 0.04 | 0.19 ± 0.02 | 0.88 a | 605.0 ± 25.9 | 75.1 ± 7.3 | 340.1 a |
| 25% | 1.18 ± 0.07 | 0.15 ± 0.02 | 0.67 b | 454.9 ± 13.7 | 66.6 ± 3.0 | 260.8 b |
| 50% | 1.01 ± 0.01 | 0.15 ± 0.02 | 0.58 bc | 311.1 ± 5.6 | 60.6 ± 6.9 | 185.8 c |
| 75% | 0.85 ± 0.03 | 0.08 ± 0.02 | 0.47 cd | 248.7 ± 13.9 | 26.2 ± 3.5 | 137.5 d |
| 100% | 0.71 ± 0.09 | 0.12 ± 0.02 | 0.42 d | 175.4 ± 13.1 | 55.2 ± 7.6 | 115.3 d |
| Average (C) | 1.06 a | 0.14 b | 359.0 a | 56.7 b | ||
| LSDT×C(0.05) | 0.13 | 35.3 | ||||
| Extract Concentration (C) | Temperature (T) | |||||
|---|---|---|---|---|---|---|
| Germination Index | Vigor Index | |||||
| 20 °C | 10 °C | Average (T) | 20 °C | 10 °C | Average (T) | |
| 0% (control) | 1.44 ± 0.06 | 0.22 ± 0.01 | 0.83 a | 719.3 ± 27.0 | 165.0 ± 9.5 | 442.2 a |
| 25% | 1.28 ± 0.07 | 0.19 ± 0.02 | 0.74 ab | 584.8 ± 40.5 | 142.1 ± 19.8 | 363.5 b |
| 50% | 1.22 ± 0.13 | 0.11 ± 0.01 | 0.67 ab | 521.2 ± 22.7 | 49.0 ± 7.7 | 285.1 c |
| 75% | 1.08 ± 0.05 | 0.10 ± 0.004 | 0.59 b | 480.5 ± 33.0 | 53.3 ± 8.7 | 266.9 c |
| Average (C) | 1.20 a | 0.15 b | 534.0 a | 102.0 b | ||
| LSDT×C(0.05) | ns | 70.3 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patanè, C.; Pellegrino, A.; Cosentino, S.L.; Testa, G. Allelopathic Effects of Cannabis sativa L. Aqueous Leaf Extracts on Seed Germination and Seedling Growth in Durum Wheat and Barley. Agronomy 2023, 13, 454. https://doi.org/10.3390/agronomy13020454
Patanè C, Pellegrino A, Cosentino SL, Testa G. Allelopathic Effects of Cannabis sativa L. Aqueous Leaf Extracts on Seed Germination and Seedling Growth in Durum Wheat and Barley. Agronomy. 2023; 13(2):454. https://doi.org/10.3390/agronomy13020454
Chicago/Turabian StylePatanè, Cristina, Alessandra Pellegrino, Salvatore L. Cosentino, and Giorgio Testa. 2023. "Allelopathic Effects of Cannabis sativa L. Aqueous Leaf Extracts on Seed Germination and Seedling Growth in Durum Wheat and Barley" Agronomy 13, no. 2: 454. https://doi.org/10.3390/agronomy13020454
APA StylePatanè, C., Pellegrino, A., Cosentino, S. L., & Testa, G. (2023). Allelopathic Effects of Cannabis sativa L. Aqueous Leaf Extracts on Seed Germination and Seedling Growth in Durum Wheat and Barley. Agronomy, 13(2), 454. https://doi.org/10.3390/agronomy13020454








