Effectiveness of Oregano and Thyme Essential Oils as Alternatives for Sulfur Dioxide in Controlling Decay and Gray Mold and Maintaining Quality of ‘Flame Seedless’ Table Grape (Vitis vinifera L.) during Cold Storage
Abstract
:1. Introduction
2. Materials and Methods
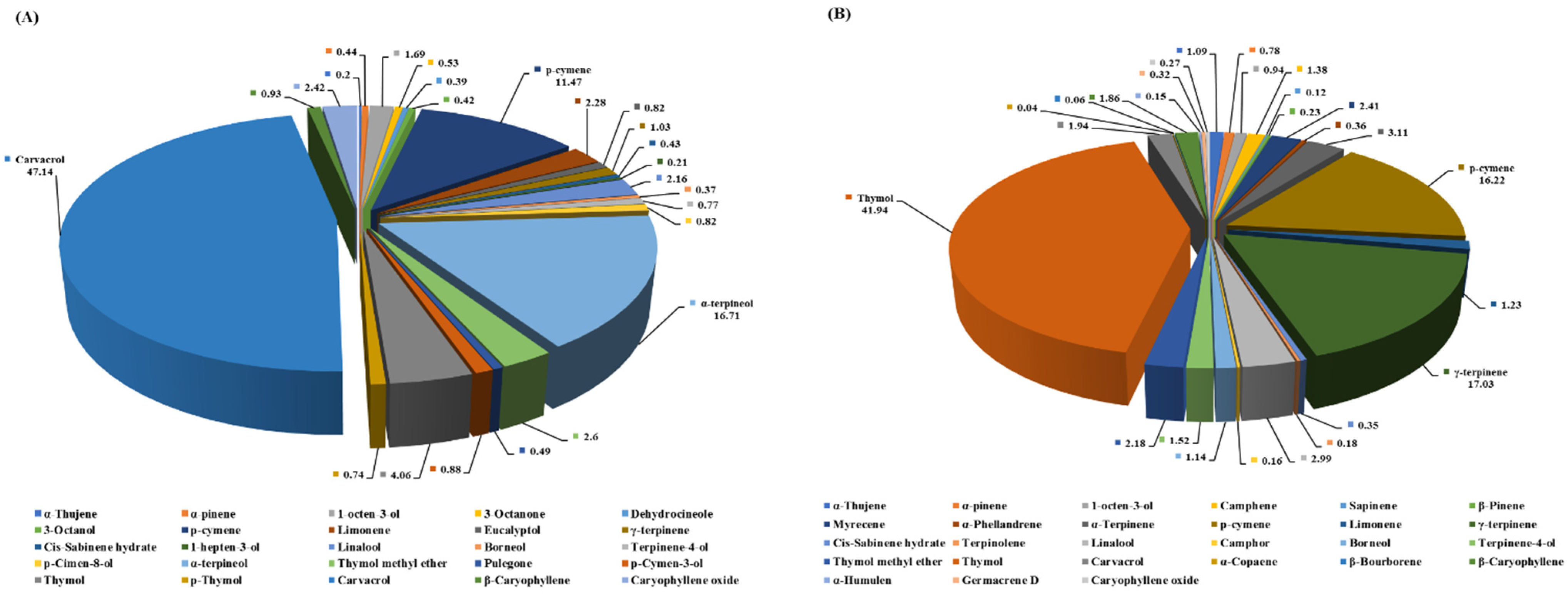
2.1. Source and Analysis of Essential Oils
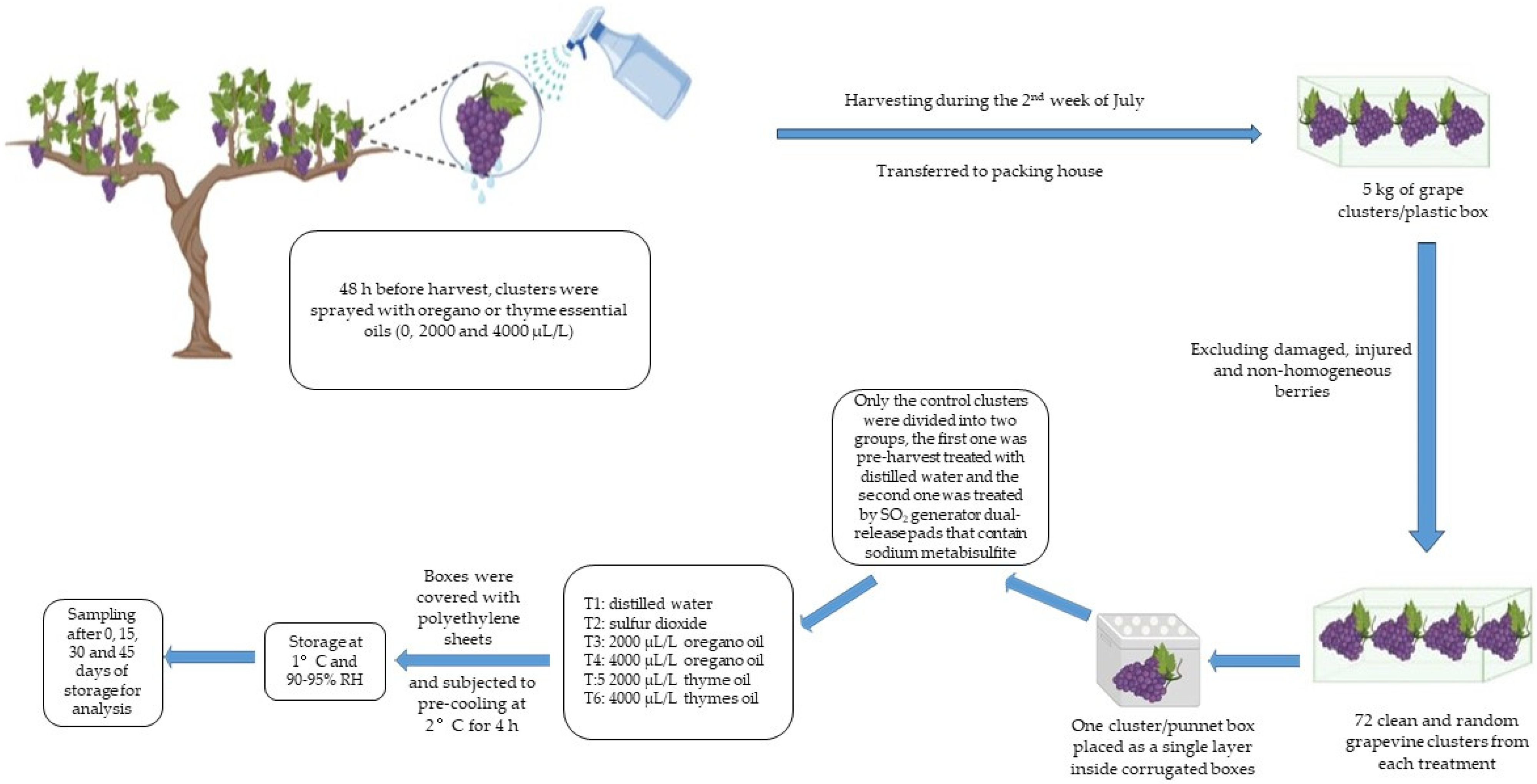
2.2. Plant Materials and Experimental Procedure
2.3. Quality Assessments
2.3.1. Fruit Physical Attributes
2.3.2. Fruit Physio-Biochemical Attributes
Ascorbic Acid (AsA) Content
2.3.3. Enzyme Activities
Peroxidase (POX) Activity (EC 1.11.1.7)
Polyphenol Oxidase (PPO) Activity (EC 1.14.18.1)
Pectin Methylesterase (PME) Activity (EC 3.1.1.11)
2.3.4. Shelf-Life
2.3.5. Economic Viability
2.4. Experimental Design and Statistical Data Analysis
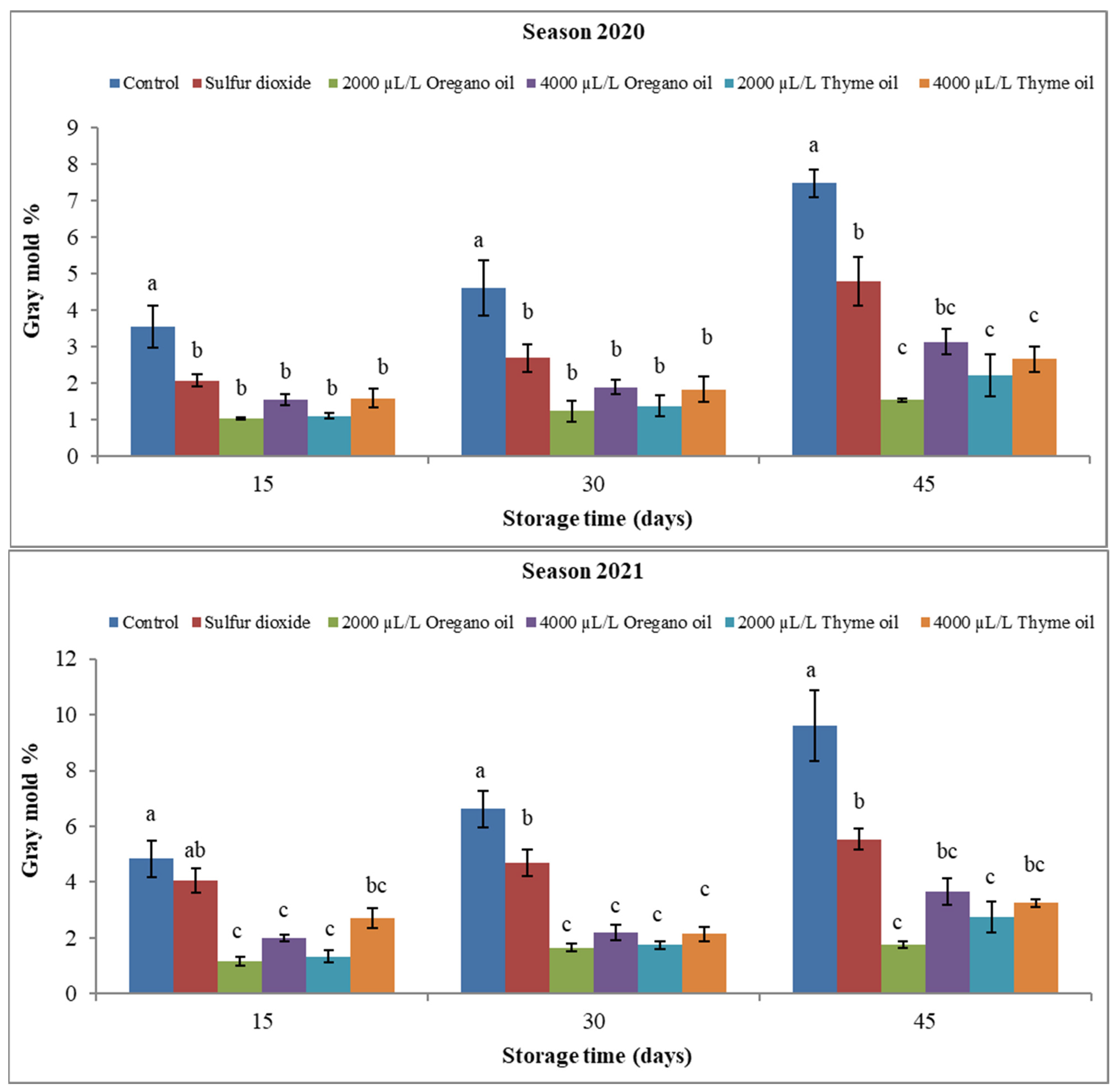
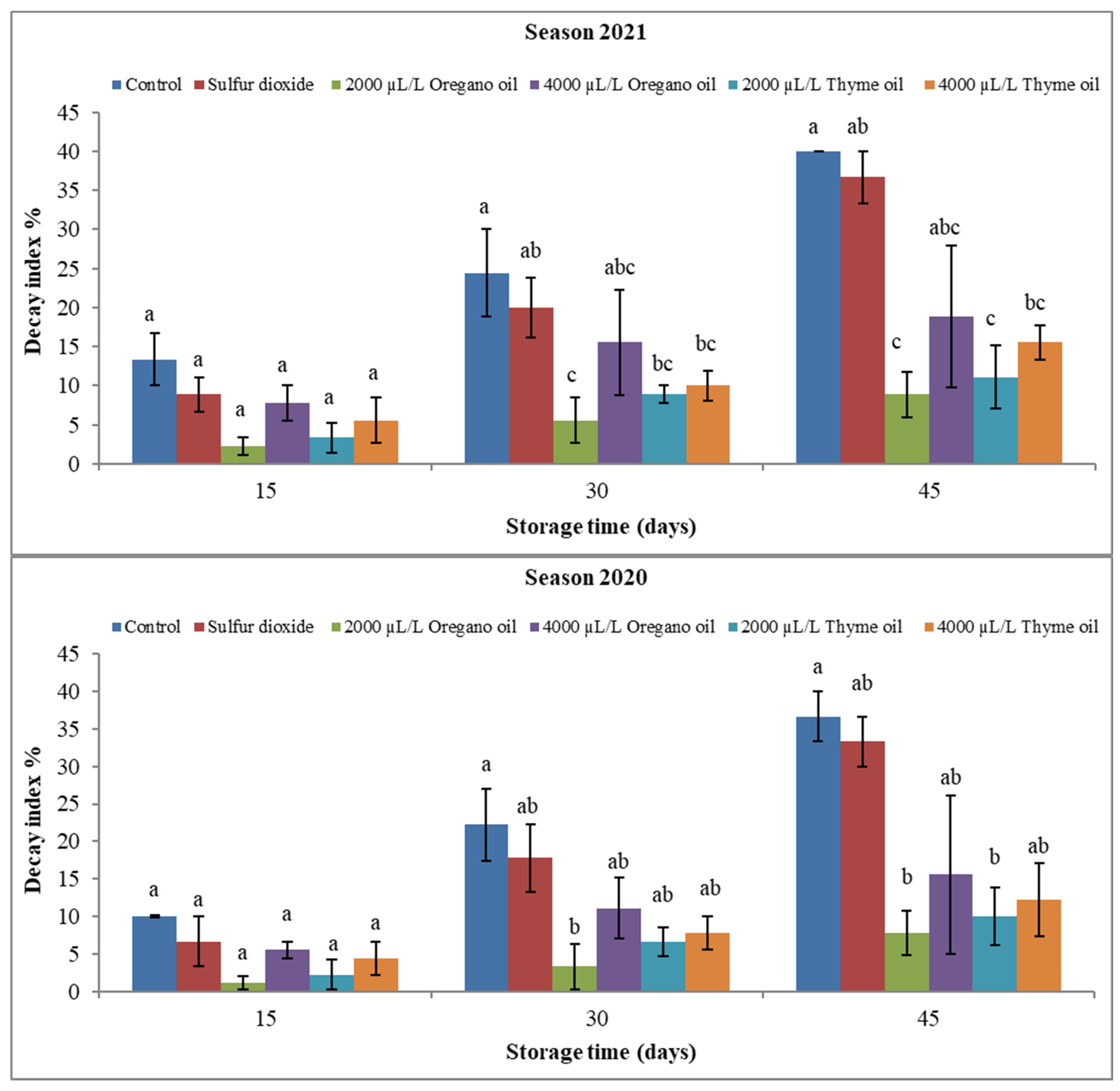
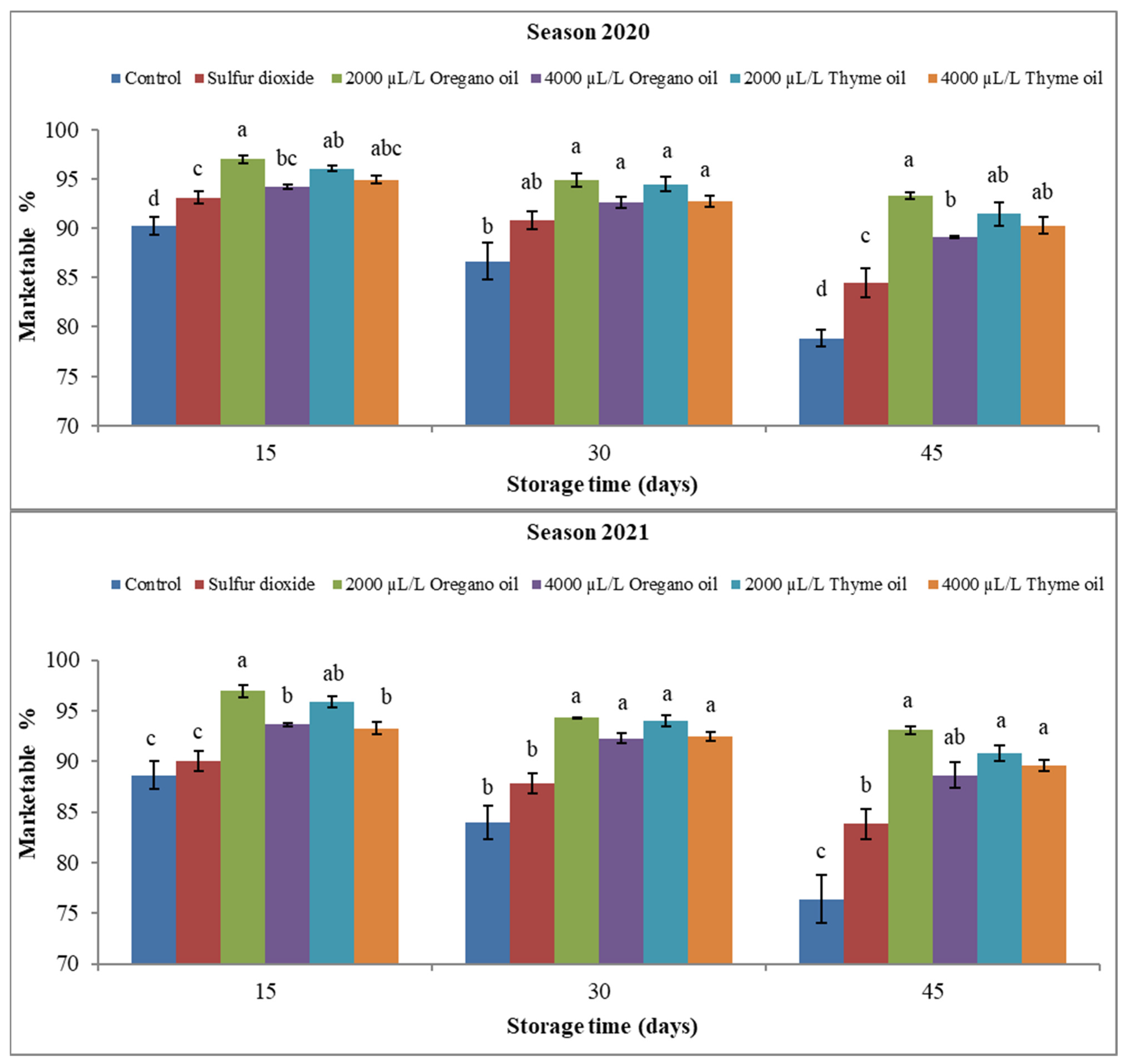
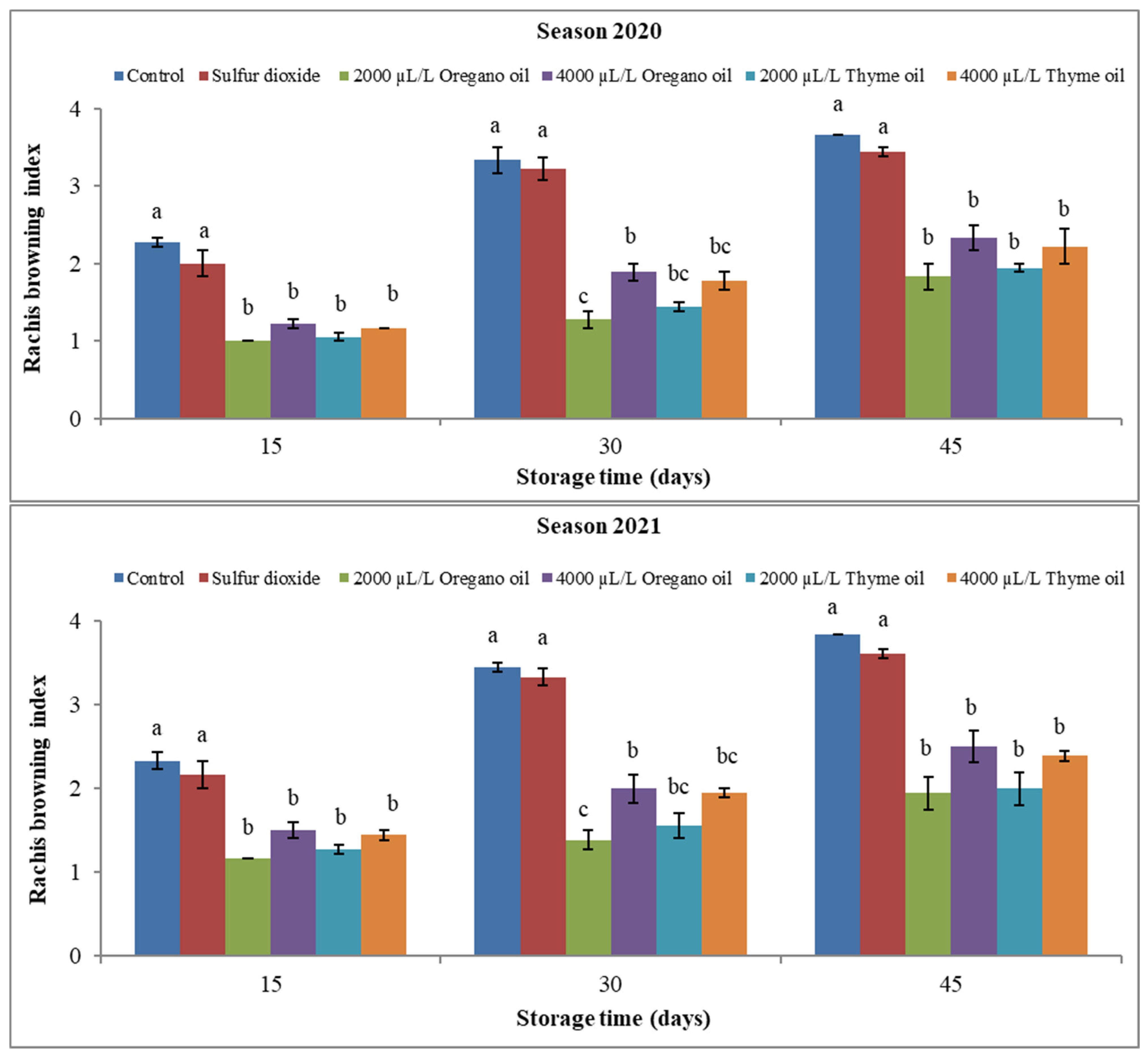
3. Results
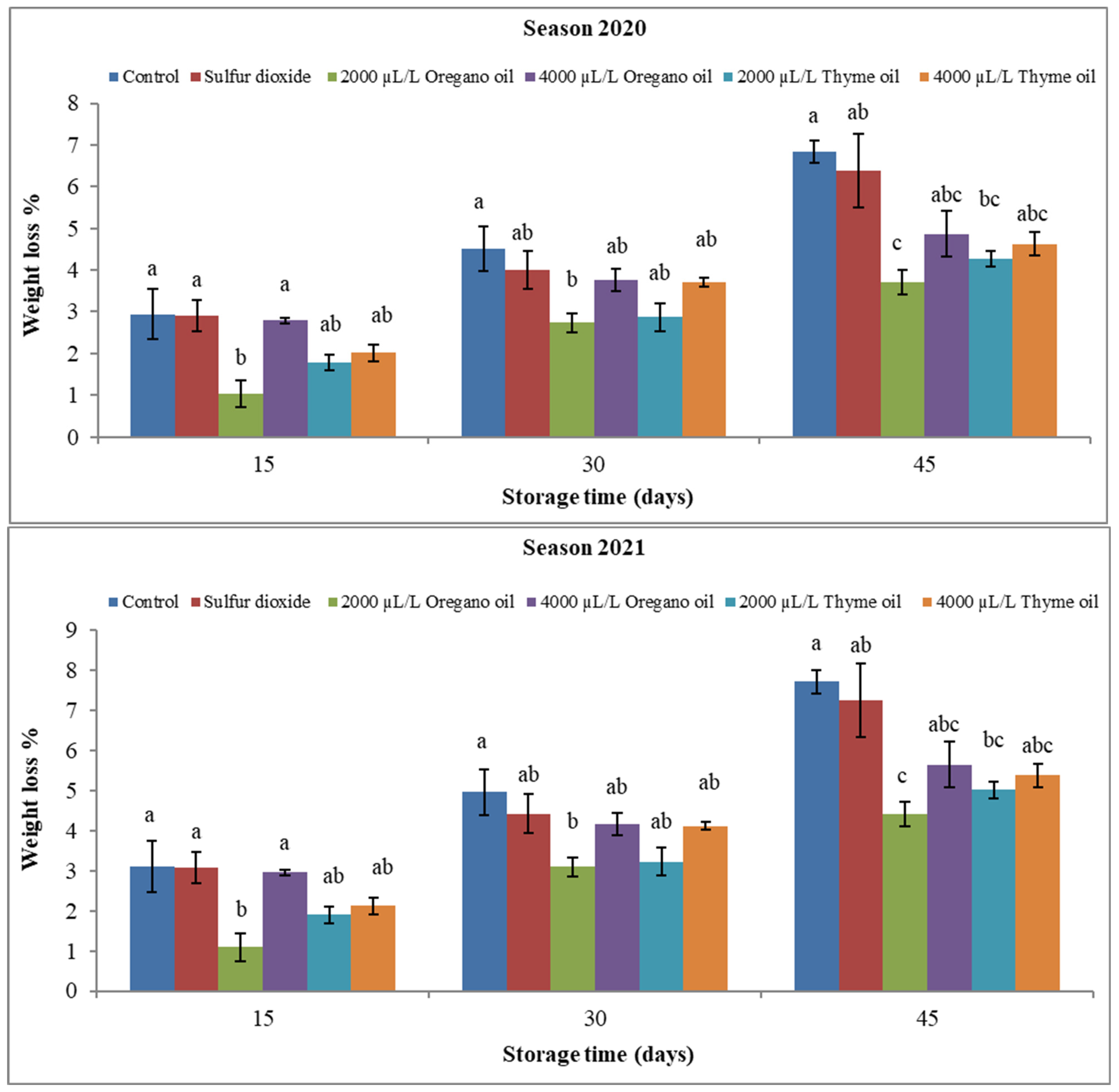
3.1. Effect of Preharvest Applications of Oregano and Thyme Essential Oils on Physical Properties of ‘Flame Seedless’ Grapes during 45 Days of Cold Storage
3.1.1. Fruit Physical Attributes
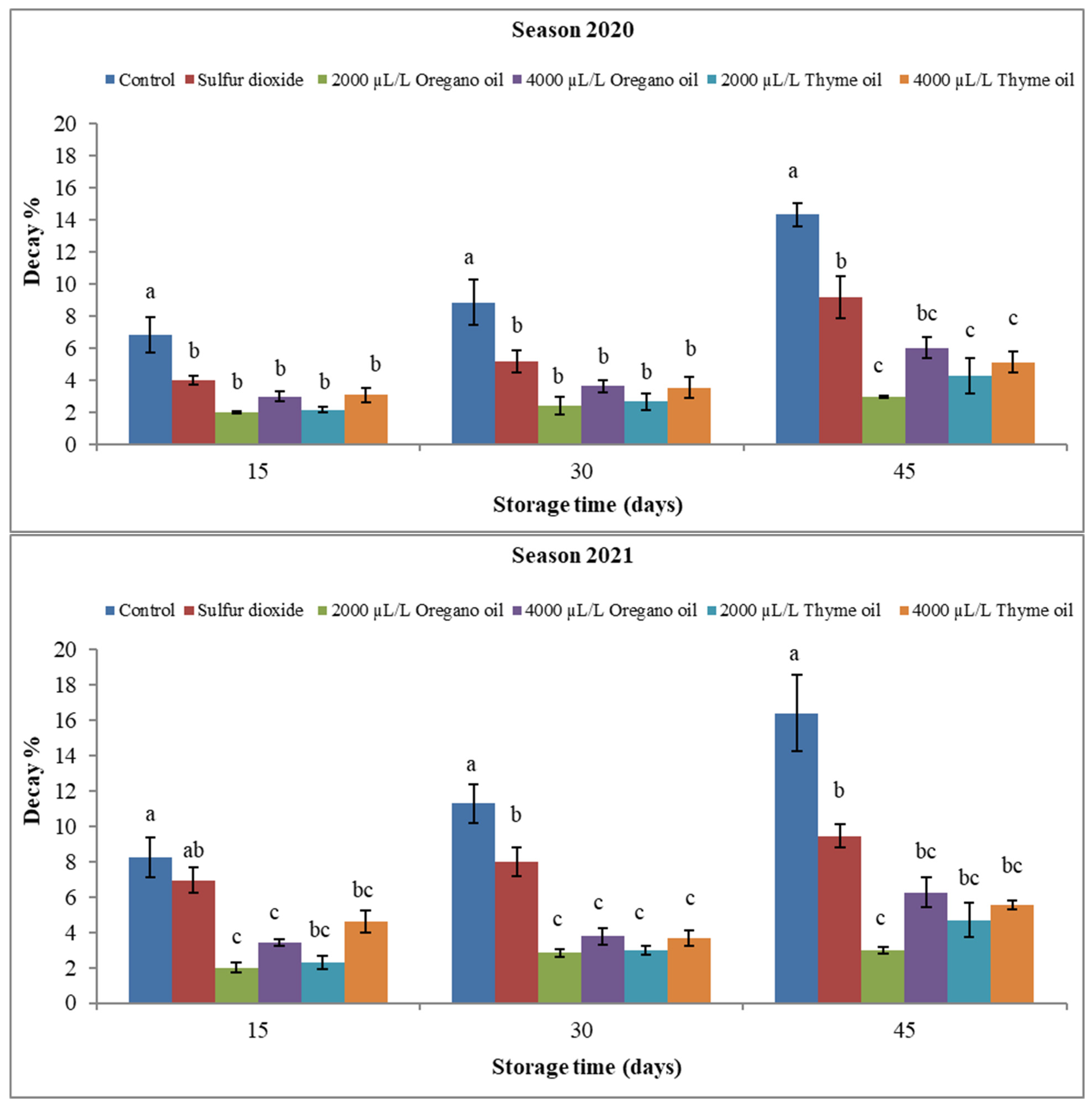
3.1.2. Marketable Fruit Percentage
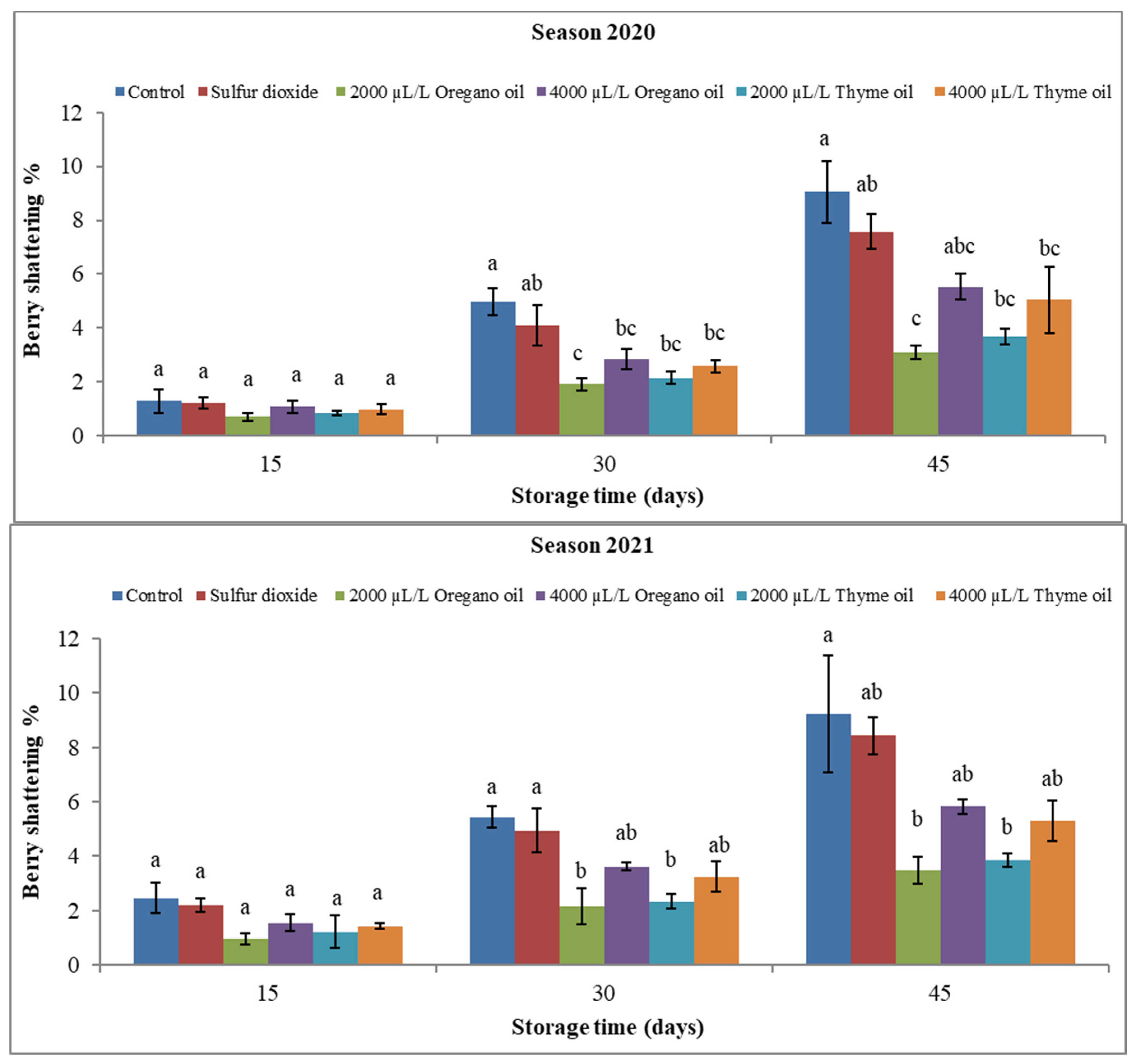
3.1.3. Rachis Browning Index (RBI) and Berry Shattering (BS) Percentage
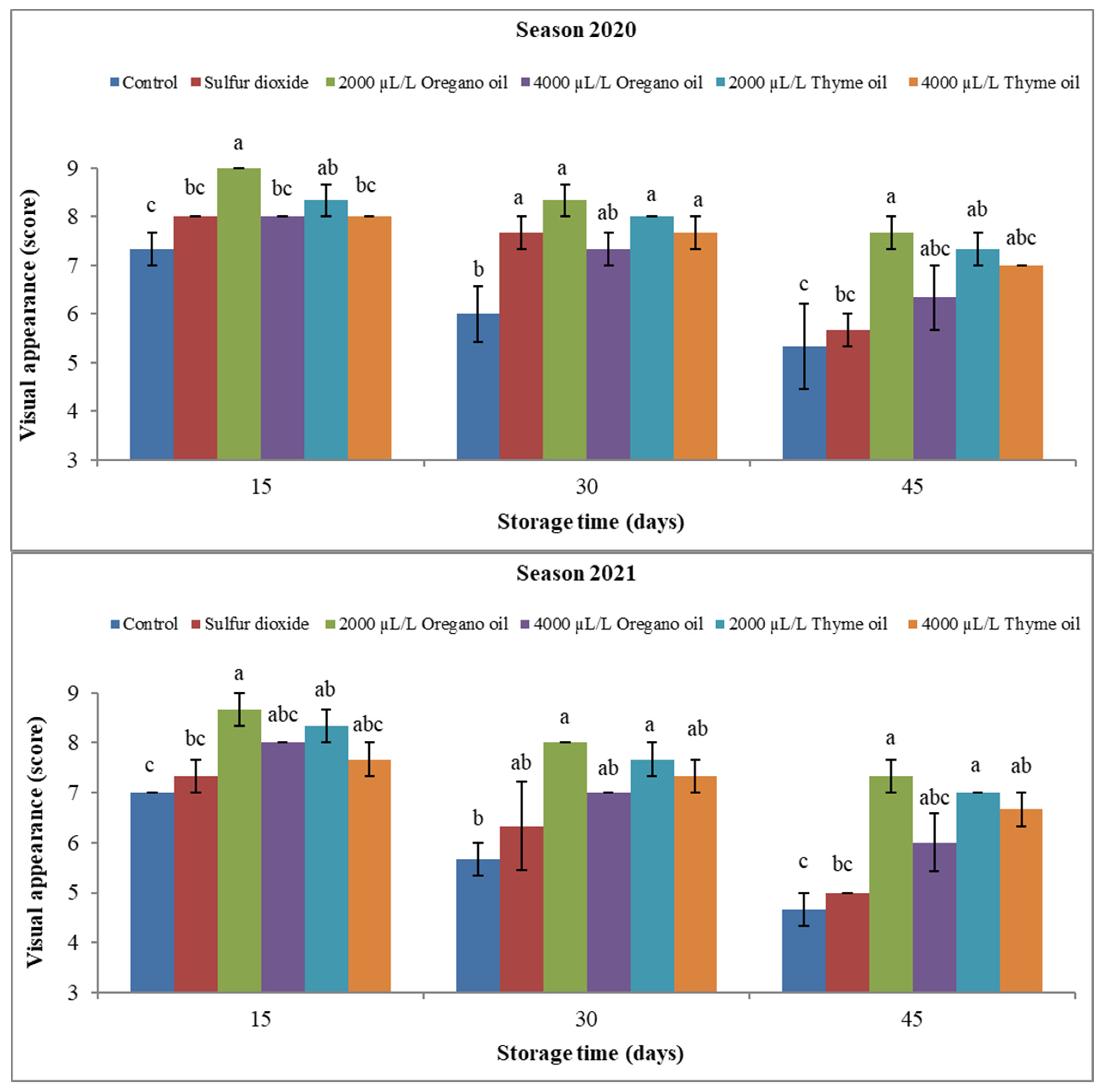
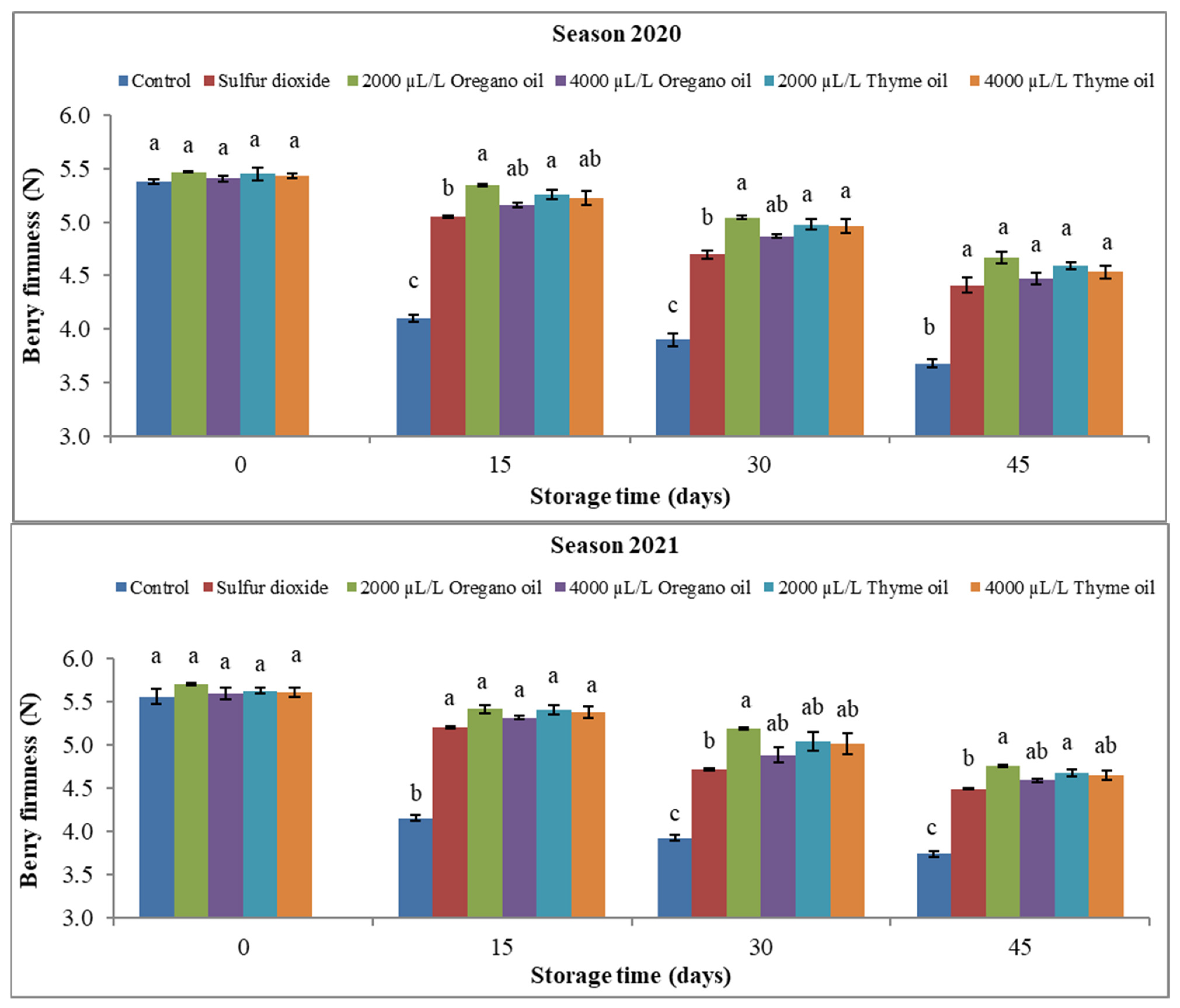
3.1.4. Berry Visual Appearance Score and Firmness
3.2. Effect of Preharvest Applications of Oregano and Thyme Essential Oils on Physio-Biochemical Properties of ‘Flame Seedless’ Grapes during 45 Days of Cold Storage
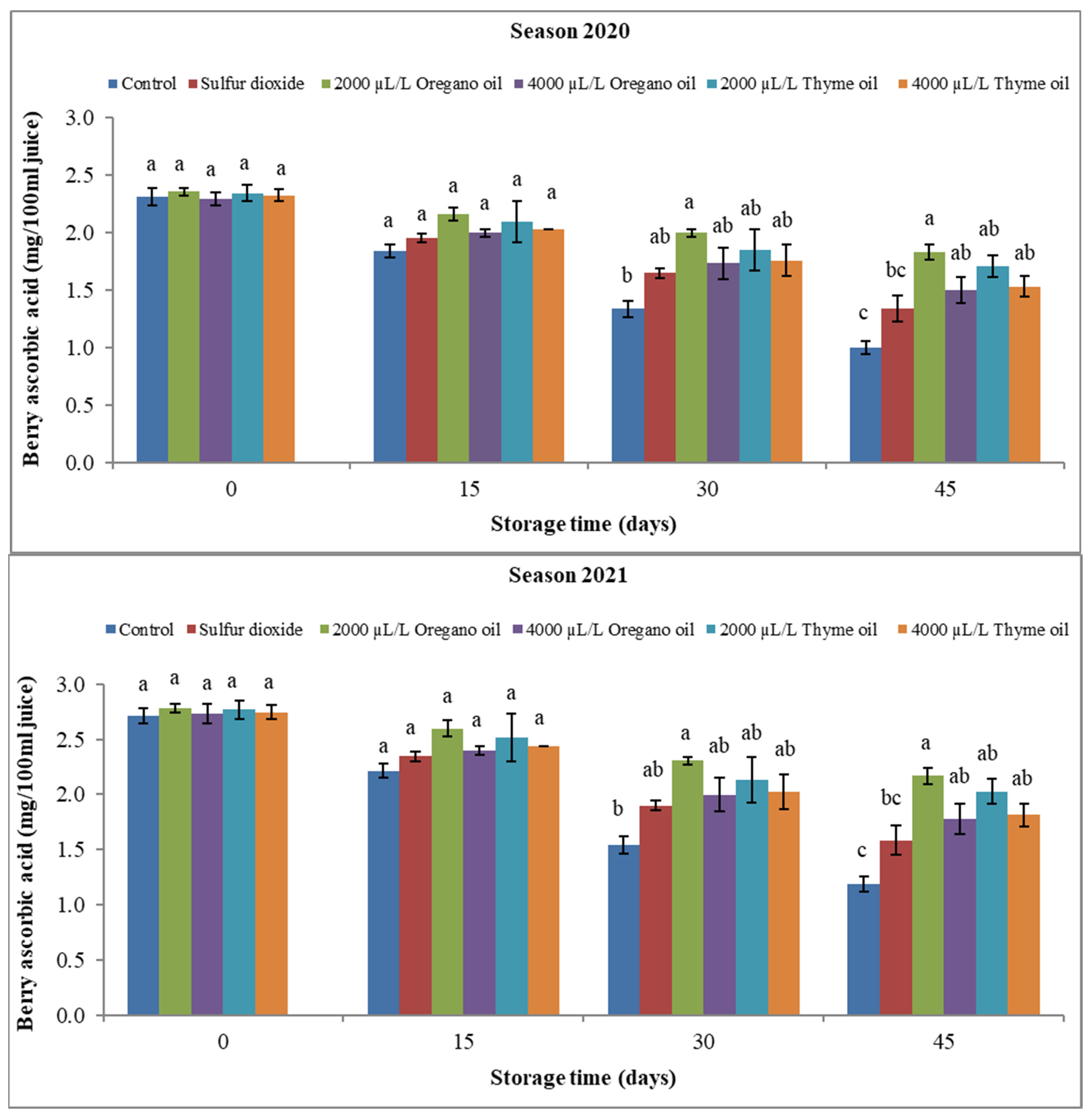
3.2.1. Berry Ascorbic Acid (AsA) Content
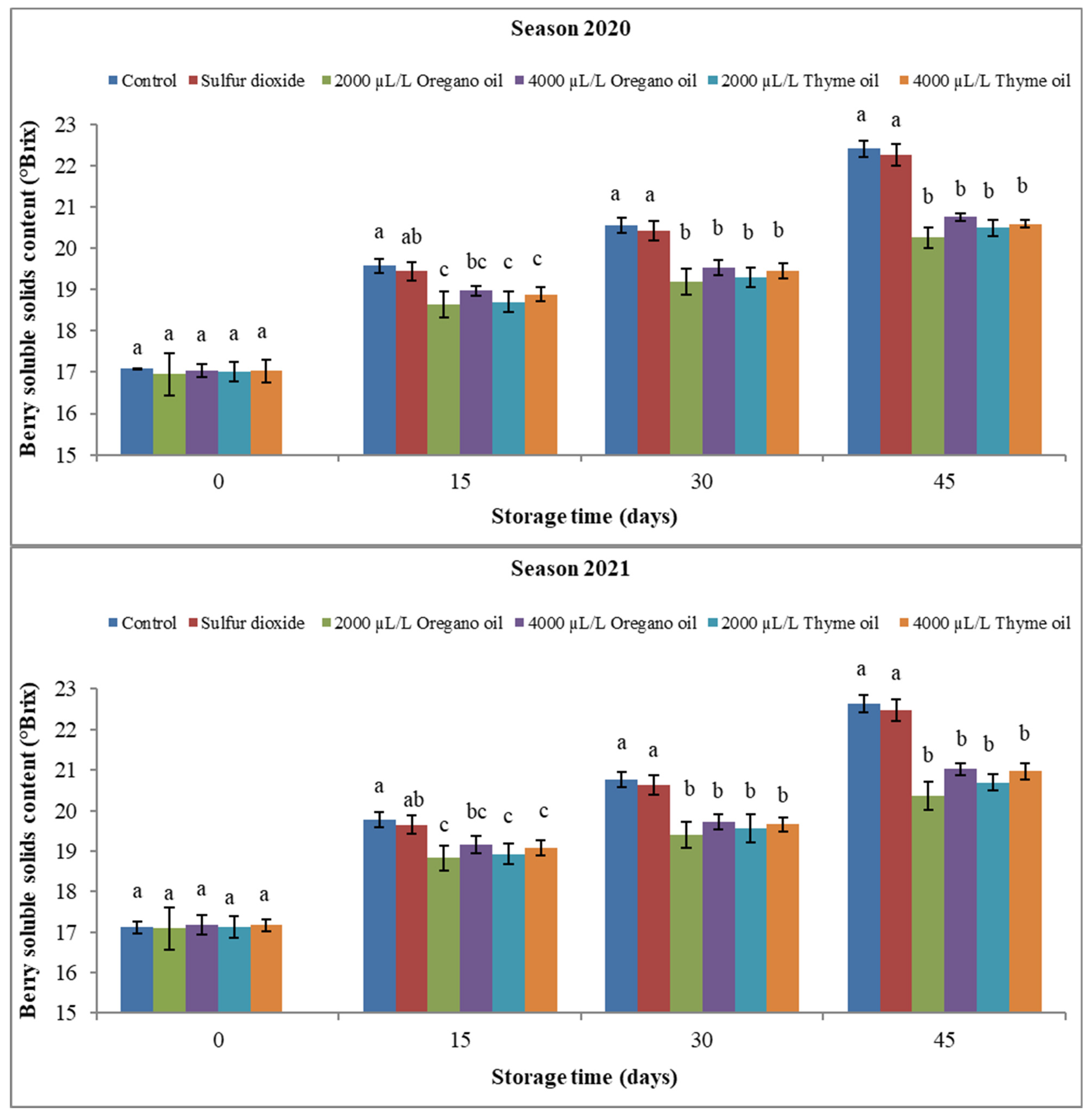
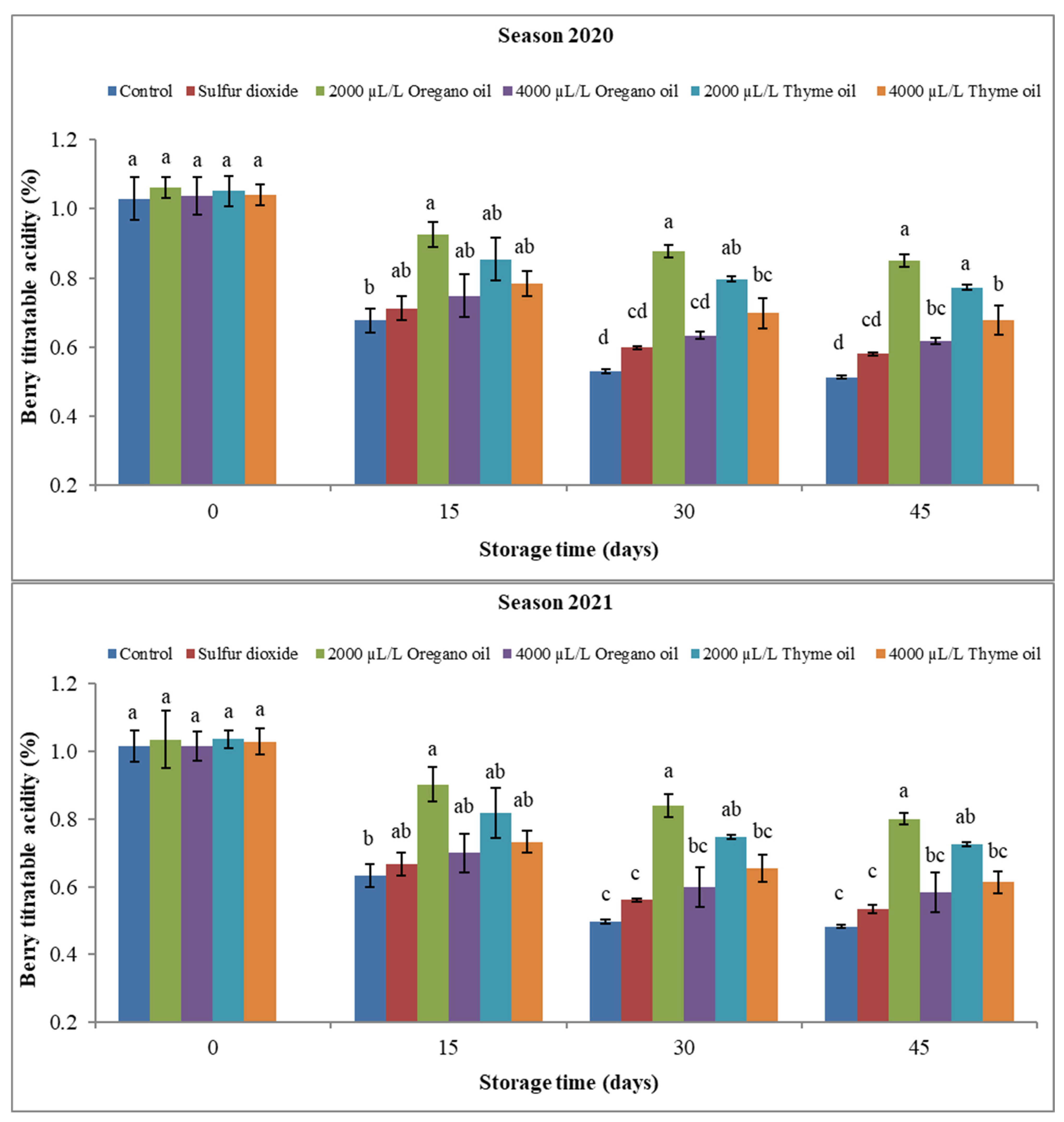
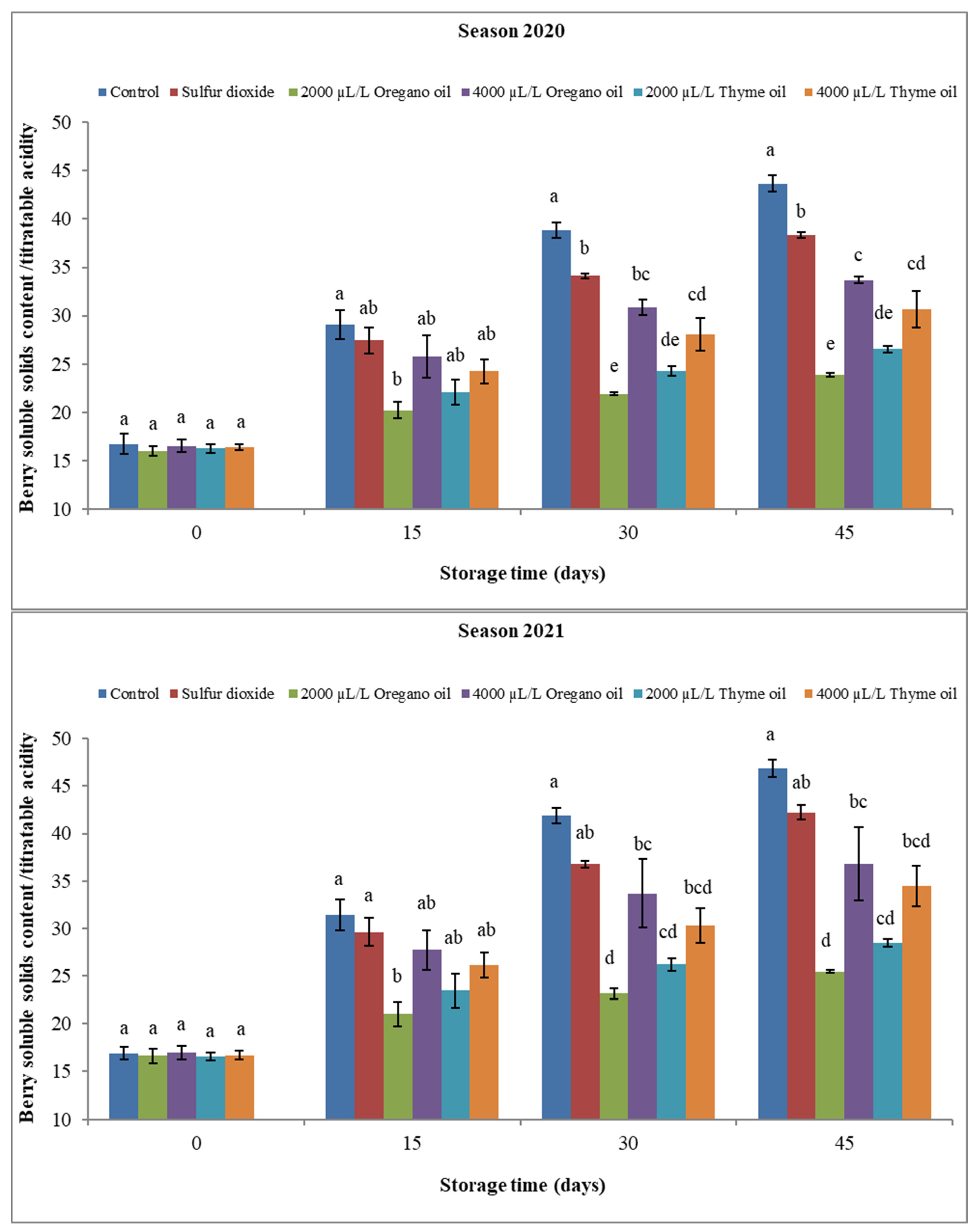
3.2.2. Berry Soluble Solids Content (SSC), Titratable Acidity (TA), and SSC/TA
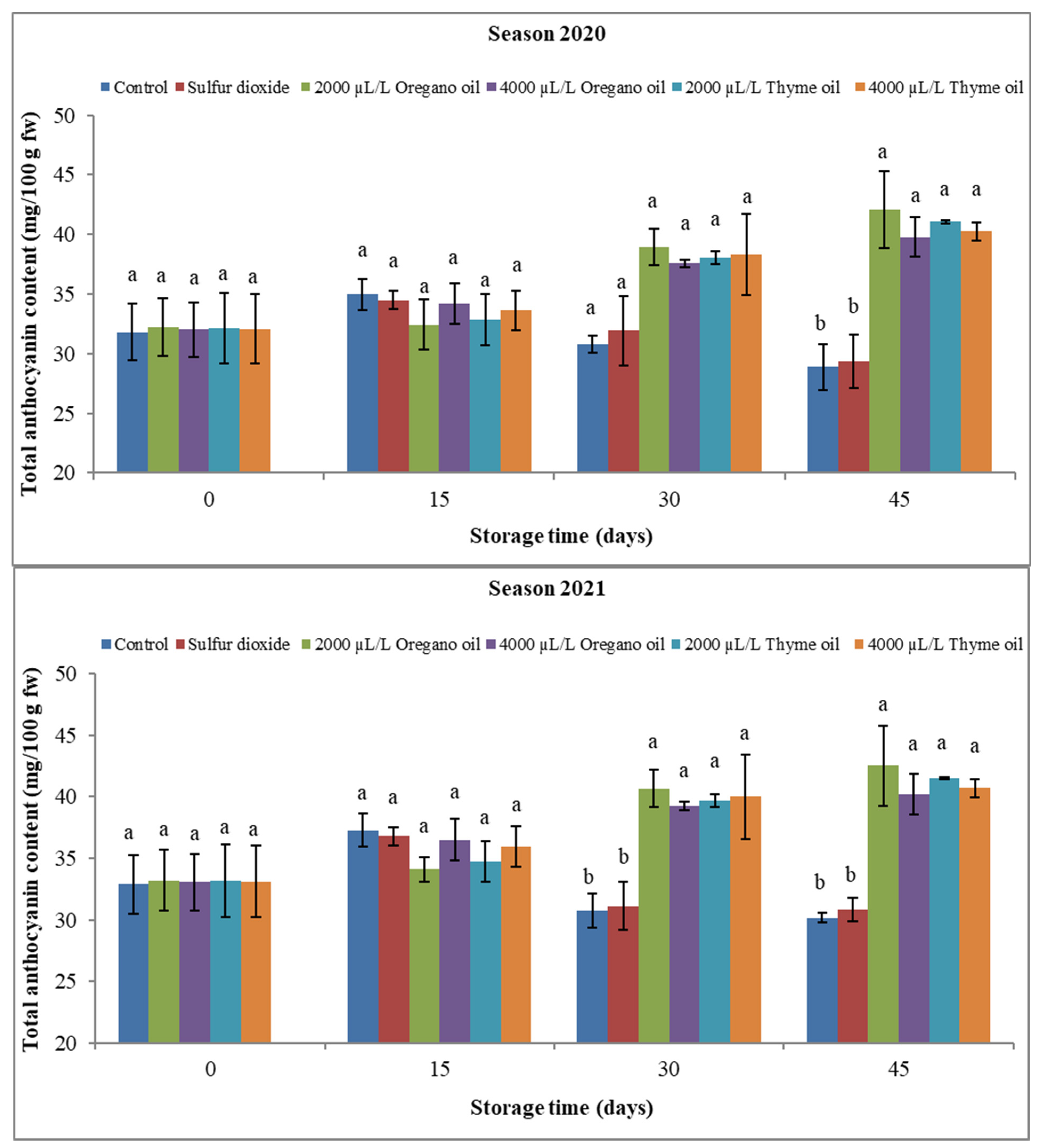
3.2.3. Berry Total Anthocyanin Content
3.3. Effect of Preharvest Applications of Oregano and Thyme Essential Oils on Enzyme Activity of ‘Flame Seedless’ Grapes during 45 Days of Cold Storage
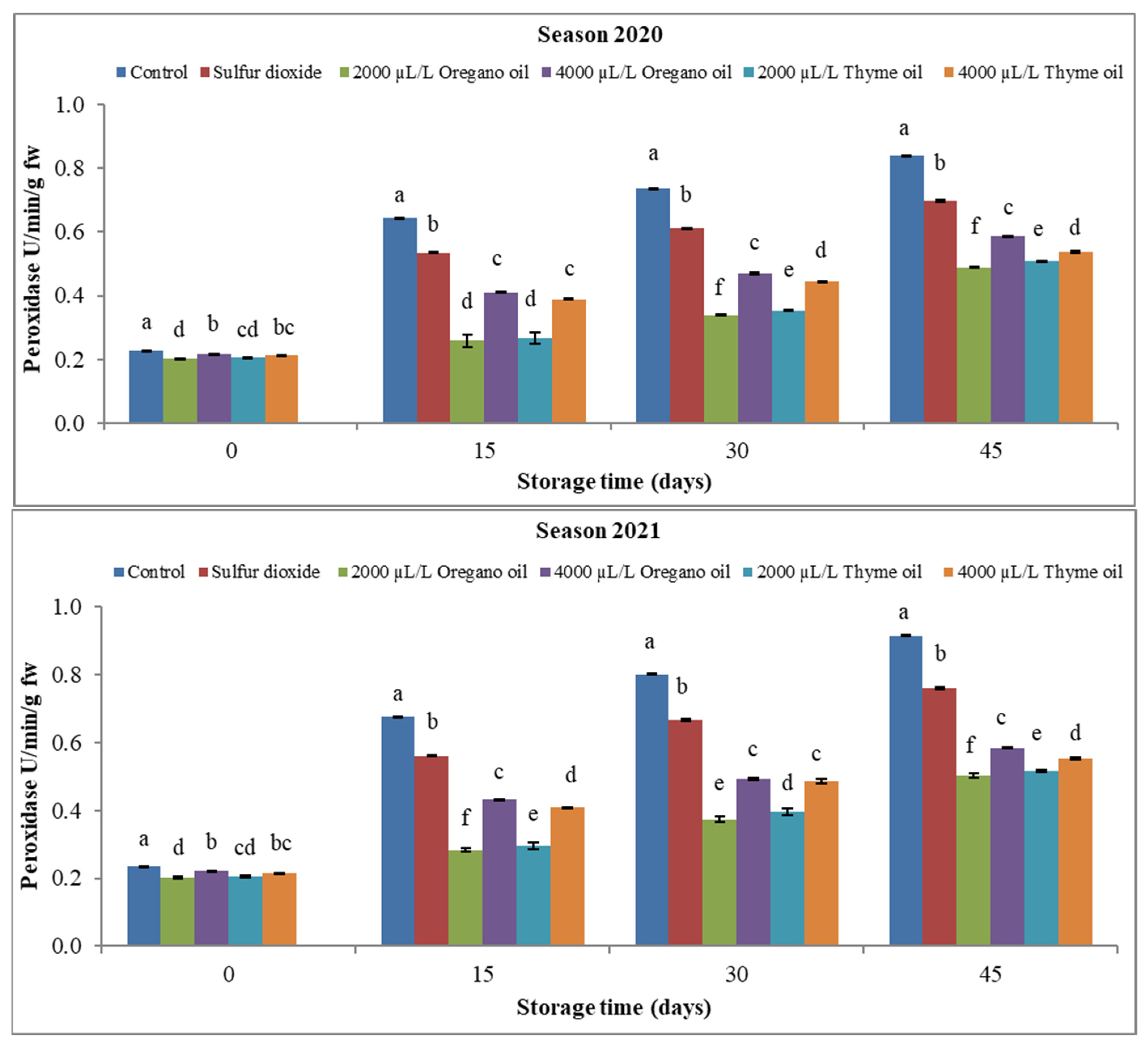
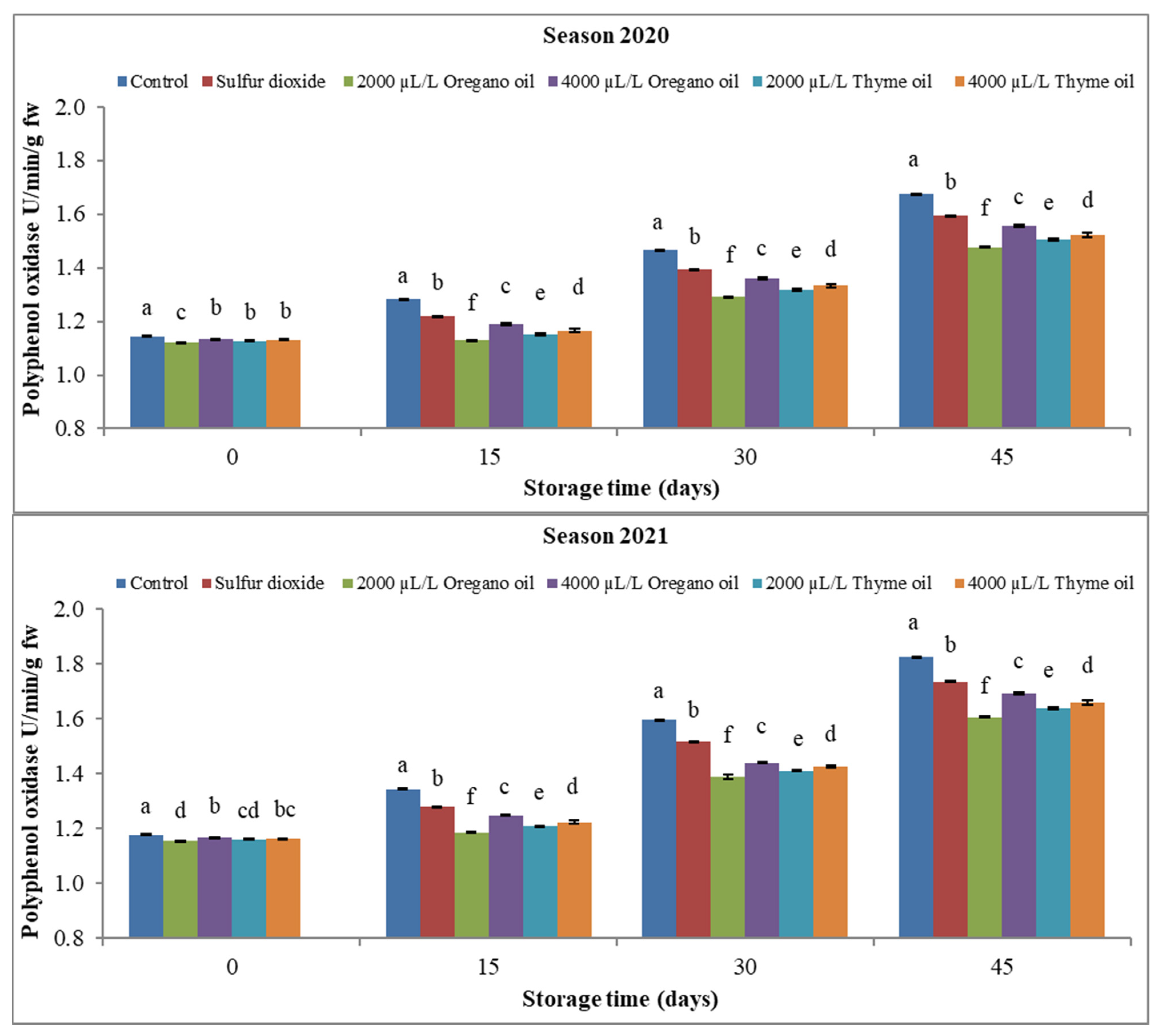
3.3.1. Peroxidase (POX) and Polyphenol Oxidase (PPO)
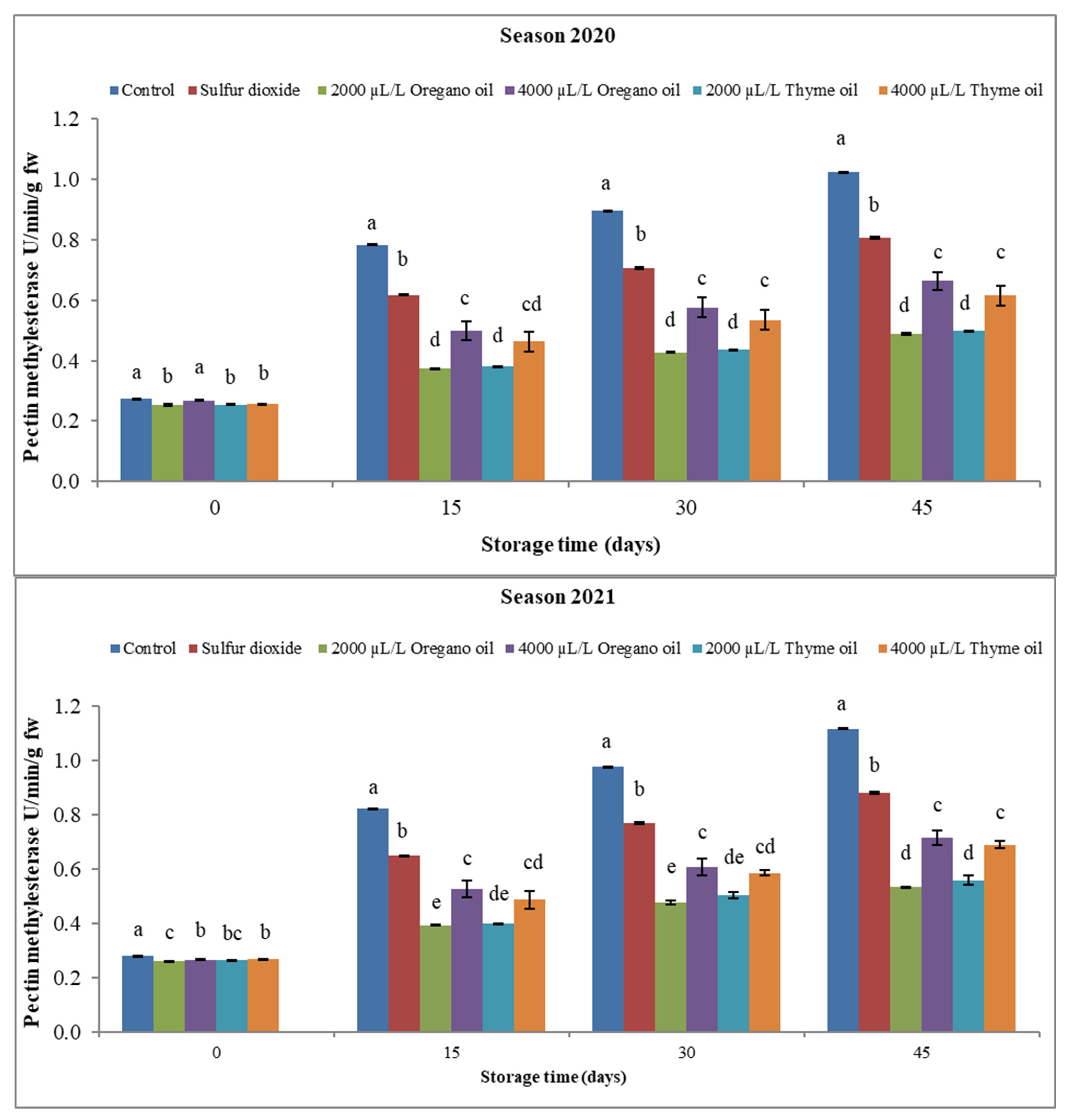
3.3.2. Pectin Methylesterase (PME)
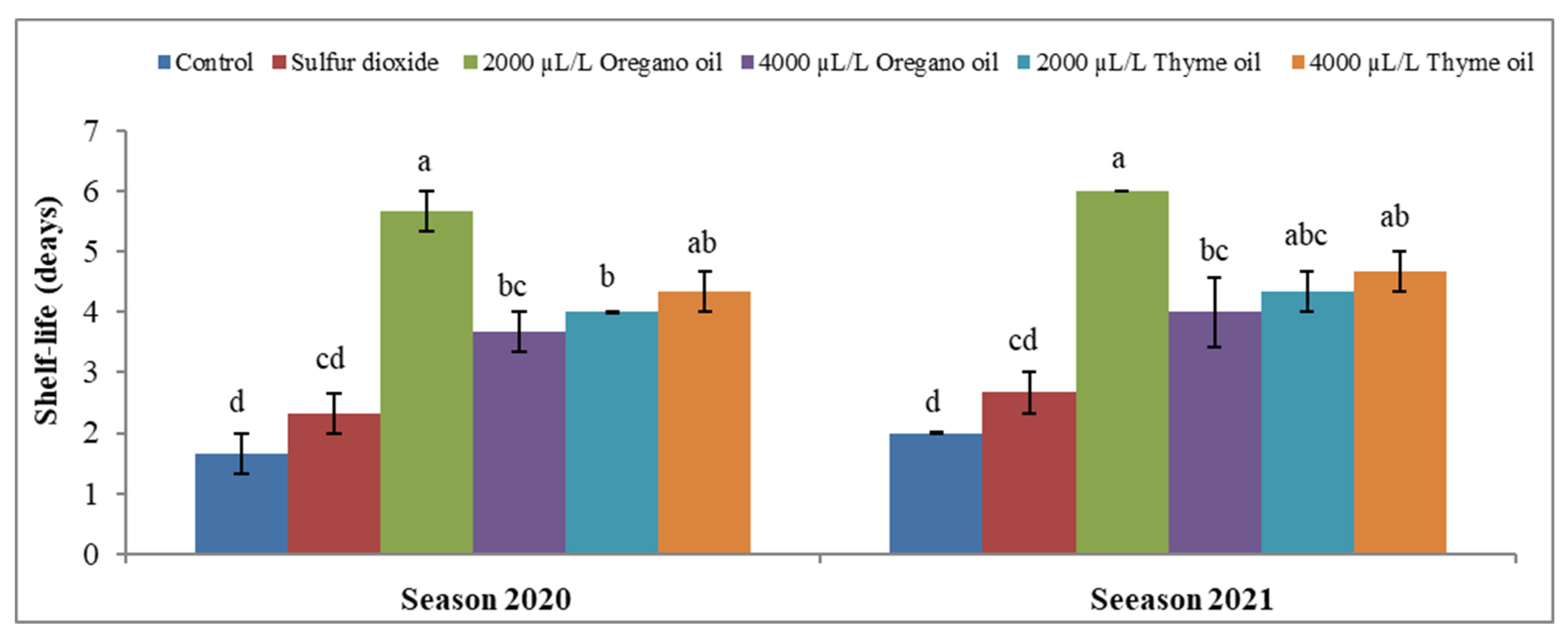
3.4. Effect of Preharvest Applications of Oregano and Thyme Essential Oils on Shelf-Life per Day of ‘Flame Seedless’ Grapes during 45 Days of Cold Storage
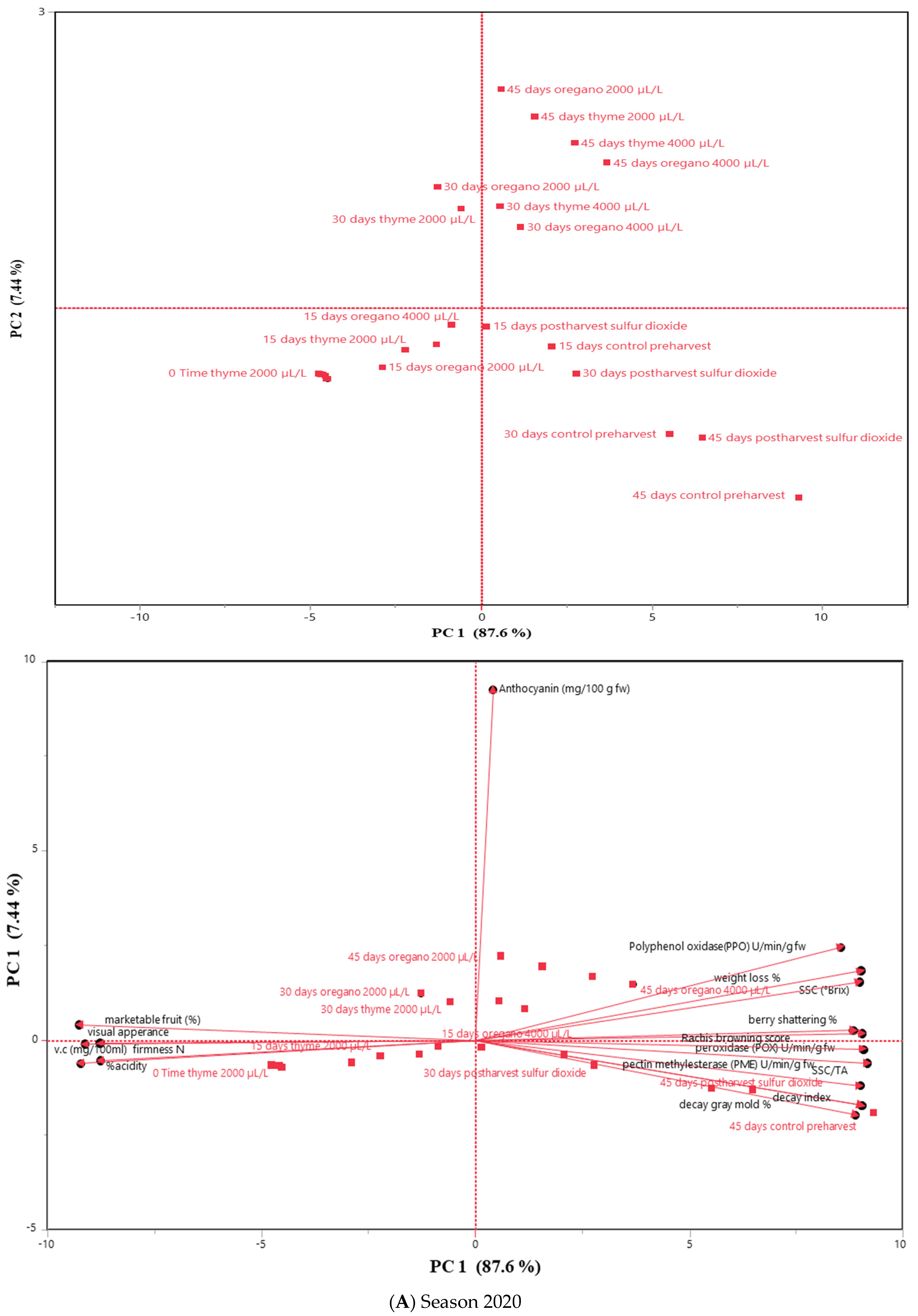
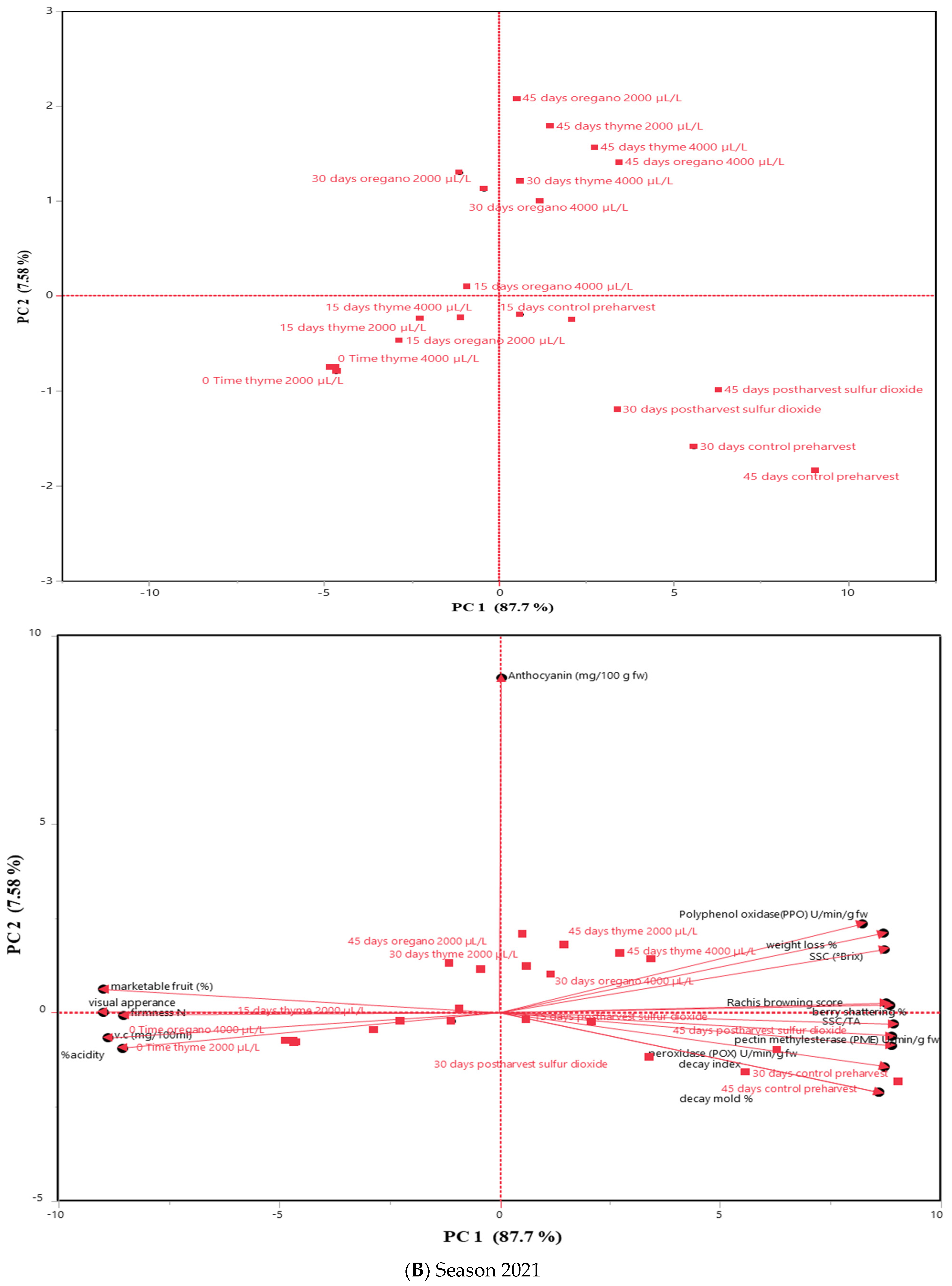
3.5. Principal Component Analysis (PCA)
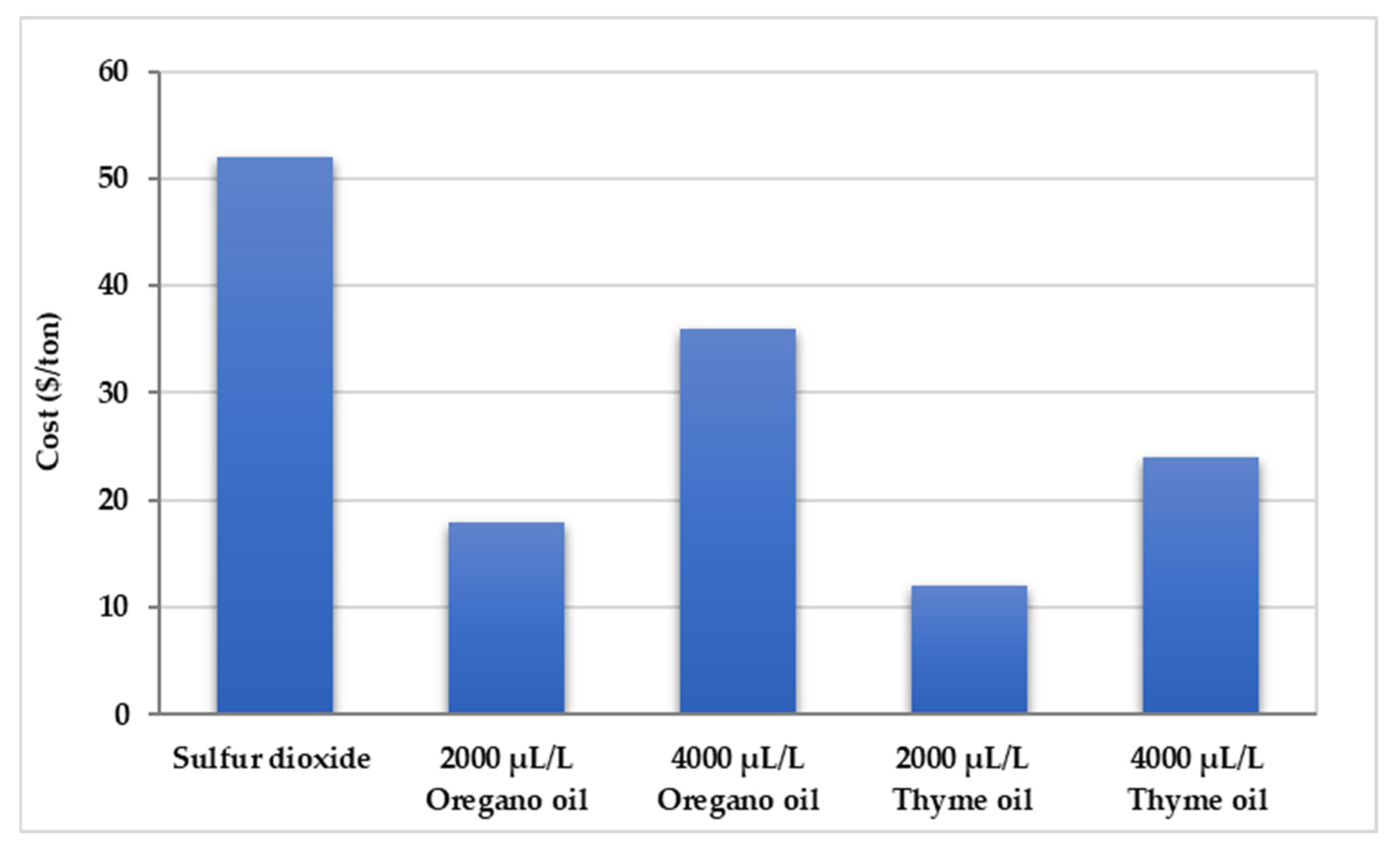
3.6. Economic Viability
4. Discussion
4.1. Effect of Preharvest Applications of Oregano and Thyme Essential Oils (EOs) on Physical Properties of ‘Flame Seedless’ Grapes during 45 Days of Cold Storage
4.2. Effect of Preharvest Applications of Oregano and Thyme Essential Oils on Physio-Biochemical Properties of ‘Flame Seedless’ Grapes during 45 Days of Cold Storage
4.3. Effect of Preharvest Applications of Oregano and Thyme Essential Oils on Enzyme Activity of ‘Flame Seedless’ Grapes during 45 Days of Cold Storage
4.4. Economic Viability
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United States Department of Agriculture (USDA). Fresh Apples, Grapes, and Pears World Markets and Trade; Foreign Agricultural Service/USDA Office of Global Analysis: Washington, DC, USA, 2021; p. 12. Available online: https://downloads.usda.library.cornell.edu/usda-esmis/files/1z40ks800/nc581j510/zw130269m/fruit.pdf (accessed on 26 July 2020).
- Food and Agriculture Organization of the United Nations (FAO). FAO Statistics. Available online: https://www.fao.org/faostat/ar/#data/QCL (accessed on 1 June 2020).
- Bucić-Kojić, A.; Planinić, M.; Tomas, S.; Jakobek, L.; Šeruga, M. Influence of Solvent and Temperature on Extraction of Phenolic Compounds from Grape Seed, Antioxidant Activity, and Color of Extract. Int. J. Food Sci. Technol. 2009, 44, 2394–2401. [Google Scholar] [CrossRef]
- Baiano, A.; Terracone, C. Varietal Differences among the Phenolic Profiles and Antioxidant Activities of Seven Table Grape Cultivars Grown in the South of Italy Based on Chemometrics. J. Agric. Food Chem. 2011, 59, 9815–9826. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wei, J.; Xing, S.; Zhang, Z.; Wu, B.; Guan, J. Sulfur Dioxide (SO2) Accumulation in Postharvest Grape: The Role of Pedicels of Four Different Varieties. Postharvest Biol. Technol. 2022, 190, 111953. [Google Scholar] [CrossRef]
- Agrios, G.N. Significance of Plant Diseases. In Plant Pathology, 4th ed.; Agrios, G.N., Ed.; Academic Press: San Diego, CA, USA, 1997; pp. 25–37. ISBN 0-12-044564-6. [Google Scholar]
- Sonker, N.; Pandey, A.K.; Singh, P. Efficiency of Artemisia nilagirica (Clarke) Pamp. Essential Oil as a Mycotoxicant against Postharvest Mycobiota of Table Grapes. J. Sci. Food Agric. 2015, 95, 1932–1939. [Google Scholar] [CrossRef] [PubMed]
- Sonker, N.; Pandey, A.K.; Singh, P. Strategies to Control Post-Harvest Diseases of Table Grape: A Review. J. Wine Res. 2016, 27, 105–122. [Google Scholar] [CrossRef]
- Hernandez-Montiel, L.G.; Gutierrez-Perez, E.D.; Murillo-Amador, B.; Vero, S.; Chiquito-Contreras, R.G.; Rincon-Enriquez, G. Mechanisms Employed by Debaryomyces Hansenii in Biological Control of Anthracnose Disease on Papaya Fruit. Postharvest Biol. Technol. 2018, 139, 31–37. [Google Scholar] [CrossRef]
- Simone, N.D.; Bernardo, P.; Francesco, G.; Michela, C.; Viwe, T.; Vincenzo, S.; Vittorio, C.; Giancarlo, C.; Giuseppe, S.; Pasquale, R. Botrytis cinerea and Table Grapes: A Review of the Main Physical, Chemical, and Bio-Based Control Treatments in Post-Harvest. Foods 2020, 9, 1138. [Google Scholar] [CrossRef]
- Tripathi, P.; Dubey, N.K. Exploitation of Natural Products as an Alternative Strategy to Control Postharvest Fungal Rotting of Fruit and Vegetables. Postharvest Biol. Technol. 2004, 32, 235–245. [Google Scholar] [CrossRef]
- Brent, K.J.; Hollomon, D.W. Fungicide Resistance: The Assessment of Risk; Global Crop Protection Federation: Brussels, Belgium, 1998; p. 48. [Google Scholar]
- Crisosto, C.H.; Palou, L.; Garner, D.; Armson, D.A. Concentration by Time Product and Gas Penetration after Marine Container Fumigation of Table Grapes with Reduced Doses of Sulfur Dioxide. HortTechnology 2002, 12, 241–245. [Google Scholar] [CrossRef]
- Zoffoli, J.P.; Latorre, B.A.; Naranjo, P. Hairline, A Postharvest Cracking Disorder in Table Grapes Induced by Sulfur Dioxide. Postharvest Biol. Technol. 2008, 47, 90–97. [Google Scholar] [CrossRef]
- Zutahy, Y.; Lichter, A.; Kaplunov, T.; Lurie, S. Extended Storage of ‘Red Globe’ Grapes in Modified SO2 Generating Pads. Postharvest Biol. Technol. 2008, 50, 12–17. [Google Scholar] [CrossRef]
- Lou, T.; Huang, W.; Wu, X.; Wang, M.; Zhou, L.; Lu, B.; Zheng, L.; Hu, Y. Monitoring, Exposure and Risk Assessment of Sulfur Dioxide Residues in Fresh or Dried Fruits and Vegetables in China. Food Addit. Contam. A 2017, 34, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.Y. Sensitivity of Grape to SO2 Injury and Absorption of SO2. Sci. Technol. Food Ind. 2006, 6, 156–159. [Google Scholar]
- Nigro, F.; Schena, L.; Ligorio, A.; Pentimone, I.; Ippolito, A.; Salerno, M.G. Control of Table Grape Storage Rots by Pre-Harvest Applications of Salts. Postharvest Biol. Technol. 2006, 42, 142–149. [Google Scholar] [CrossRef]
- Jacometti, M.A.; Wratten, S.D.; Walter, M. Review: Alternatives to Synthetic Fungicides for Botrytis cinerea Management in Vineyards. Aust. J. Grape Wine Res. 2010, 16, 154–172. [Google Scholar] [CrossRef]
- Calvo-Garrido, C.; Roudet, J.; Aveline, N.; Davidou, L.; Dupin, S.; Fermaud, M. Microbial Antagonism Toward Botrytis Bunch Rot of Grapes in Multiple Field Tests Using One Bacillus ginsengihumi Strain and Formulated Biological Control Products. Front. Plant Sci. 2019, 10, 105. [Google Scholar] [CrossRef]
- González-Barrio, R.; Beltrán, D.; Cantos, E.; Gil, M.I.; Espín, J.C.; Tomás-Barberán, F.A. Comparison of Ozone and UV-C Treatments on the Postharvest Stilbenoid Monomer, Dimer, and Trimer Induction in Var. ‘Superior’ White Table Grapes. J. Agric. Food Chem. 2006, 54, 4222–4228. [Google Scholar] [CrossRef]
- Palou, L.; Smilanick, J.L.; Crisosto, C.H.; Mansour, M. Effect of Gaseous Ozone Exposure on the Development of Green and Blue Molds on Cold Stored Citrus Fruit. Plant Dis. 2001, 85, 632–638. [Google Scholar] [CrossRef]
- Karabulut, O.A.; Gabler, F.M.; Mansour, M.; Smilanick, J.L. Postharvest Ethanol and Hot Water Treatments of Table Grapes to Control Gray Mold. Postharvest Biol. Technol. 2004, 34, 169–177. [Google Scholar] [CrossRef]
- Romero, I.; Sanchez-Ballesta, M.T.; Escribano, M.I.; Merodio, C. Individual Anthocyanins and Their Contribution to Total Antioxidant Capacity in Response to Low Temperature and High CO2 in Stored Cardinal Table Grapes. Postharvest Biol. Technol. 2008, 49, 1–9. [Google Scholar] [CrossRef]
- Youssef, K.; Oliveira, A.G.; Tischer, C.A.; Hussain, I.; Roberto, S.R. Synergistic Effect of a Novel Chitosan/Silica Nanocomposites-Based Formulation against Gray Mold of Table Grapes and Its Possible Mode of Action. Int. J. Biol. Macromol. 2019, 141, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Kalemba, D.; Kunicka, A. Antibacterial and Antifungal Properties of Essential Oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Chebli, B.; Mohamed, A.; Idrissi, H.; Mohamed, H. Chemical Composition and Antifungal Activity of Essential Oils of Seven Moroccan Labiatae against Botrytis cinerea. J. Ethnopharmacol. 2003, 89, 165–169. [Google Scholar]
- Adorjan, B.; Buchbauer, G. Biological Properties of Essential Oils: An Updated Review. Flavour Fragr. J. 2010, 25, 407–426. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I. Investigating the Effects of Plant Essential Oils on Post-Harvest Fruit Decay. In Fungal Pathogenicity; Sultan, S., Ed.; InTechOpen: Rijeka, Croatia, 2016; pp. 83–98. [Google Scholar]
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef]
- Assis, J.S.; Maldonado, R.; Muñoz, T.; Escribano, M.I.; Merodio, C. Effect of High Carbon Dioxide Concentration on PAL Activity and Phenolic Contents in Ripening Cherimoya Fruit. Postharvest Biol. Technol. 2001, 23, 33–39. [Google Scholar] [CrossRef]
- Wang, C.Y. Maintaining Postharvest Quality of Raspberries with Natural Volatile Compounds. Int. J. Food Sci. Technol. 2003, 38, 869–875. [Google Scholar] [CrossRef]
- Marjanlo, A.A.; Mostofi, Y.; Shoeibi, S.; Fattahi, M. Effect of Cumin Essential Oil on Postharvest Decay and Some Quality Factors of Strawberry. J. Med. Plants 2009, 8, 25–43. [Google Scholar]
- Soylu, S.; Yigitbas, H.; Soylu, E.M.; Kurt, Ş. Antifungal Effects of Essential Oils from Oregano and Fennel on Sclerotinia sclerotiorum. J. Appl. Microbiol. 2007, 103, 1021–1030. [Google Scholar] [CrossRef]
- Spadaro, D.; Gullino, M.L. Use of Essential Oils to Control Postharvest Rots on Pome and Stone Fruit. In Post-Harvest Pathology: Plant Pathology in the 21st Century, Contributions to the 10th International Congress, ICPP 2013; Prusky, D., Gullino, M.L., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 101–110. ISBN 978-3-319-07701-7. [Google Scholar]
- Gutiérrez-Pozo, M.; Serna-Escolano, V.; Giménez-Berenguer, M.; Giménez, M.J.; Zapata, P.J. The Preharvest Application of Essential Oils (Carvacrol, Eugenol, and Thymol) Reduces Fungal Decay in Lemons. Agriculture 2023, 13, 1437. [Google Scholar] [CrossRef]
- Rao, A.; Zhang, Y.; Muend, S.; Rao, R. Mechanism of Antifungal Activity of Terpenoid Phenols Resembles Calcium Stress and Inhibition of the TOR Pathway. Antimicrob. Agents Chemother. 2010, 54, 5062–5069. [Google Scholar] [CrossRef] [PubMed]
- Vitoratos, A.; Bilalis, D.; Karkanis, A.; Efthimiadou, A. Antifungal Activity of Plant Essential Oils against Botrytis cinerea, Penicillium italicum and Penicillium digitatum. Not. Bot. Horti. Agrobot. Cluj-Napoca 2013, 41, 86–92. [Google Scholar] [CrossRef]
- Almasaudi, N.M.; Al-Qurashi, A.D.; Elsayed, M.I.; Abo-Elyousr, K.A. Essential Oils of Oregano and Cinnamon as an Alternative Method for Control of Gray Mold Disease of Table Grapes Caused by Botrytis cinerea. J. Plant Pathol. 2022, 104, 317–328. [Google Scholar] [CrossRef]
- Plotto, A.; Roberts, D.D.; Roberts, R.G. Evaluation of Plant Essential Oils as Natural Postharvest Disease Control of Tomato (Lycopersicon esculentum). Acta Hortic. 2003, 628, 737–745. [Google Scholar] [CrossRef]
- Sellamuthu, P.S.; Sivakumar, D.; Soundy, P.; Korsten, L. Essential Oil Vapours Suppress the Development of Anthracnose and Enhance Defence Related and Antioxidant Enzyme Activities in Avocado Fruit. Postharvest Biol. Technol. 2013, 81, 66–72. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.P.; Li, Y.C.; Li, H.Y.; Yu, T.; Zheng, X.D. Antifungal Activity of Thyme Oil against Geotrichum citri-aurantii In Vitro and In Vivo. J. Appl. Microbiol. 2009, 107, 1450–1456. [Google Scholar] [CrossRef]
- Lopez-Reyes, J.G.; Spadaro, D.; Gullino, M.L.; Garibaldi, A. Efficacy of Plant Essential Oils on Postharvest Control of Rot Caused by Fungi on Four Cultivars of Apples In Vivo. Flavour Fragr. J. 2010, 25, 171–177. [Google Scholar] [CrossRef]
- Bosquez-Molina, E.; Jesús, E.R.; Bautista-Baños, S.; Verde-Calvo, J.R.; Morales-López, J. Inhibitory Effect of Essential Oils against Colletotrichum gloeosporioides and Rhizopus stolonifer in Stored Papaya Fruit and Their Possible Application in Coatings. Postharvest Biol. Technol. 2010, 57, 132–137. [Google Scholar] [CrossRef]
- Junior, O.J.C.; Youssef, K.; Koyama, R.; Ahmed, S.; Dominguez, A.R.; Mühlbeier, D.T.; Roberto, S.R. Control of Gray Mold on Clamshell-Packaged ‘Benitaka’ Table Grapes Using Sulphur Dioxide Pads and Perforated Liners. Pathogens 2019, 8, 271. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Fard, J.R. Melatonin Treatment Attenuates Postharvest Decay and Maintains Nutritional Quality of Strawberry Fruit (Fragaria×anannasa cv. Selva) by Enhancing GABA Shunt Activity. Food Chem. 2017, 221, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Watkins, C.; Harman, J. Use of penetrometer to measure flesh firmness of fruit. Orchard. N. Z. 1981, 54, 14–16. [Google Scholar]
- Amerine, M.A.; Pangborn, R.M.; Roessler, E.B. Laboratory studies: Quantity-Quality Evaluation. In Principles of Sensory Evaluation of Food; Academic Press: New York, NY, USA, 1965; pp. 367–375. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemist International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Fuleki, T.; Francis, F.J. Quantitative Methods for Anthocyanins. II. Determination of Total Anthocyanin and Degradation Index for Cranberry Juice. J. Food Sci. 1968, 33, 78–83. [Google Scholar]
- Herzog, V.; Fahimi, H. Determination of the activity of peroxidase. Anal. Biochem. 1973, 55, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.; Harel, E.; Shaul, R. Assay of Catechol Oxidase a Critical Comparison of Methods. Physicochemistry 1965, 5, 783–789. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Austin, P.J. Continuous Spectrophotometric Assay for Plant Pectin Methyl Esterase. J. Agric. Food Chem. 1986, 34, 440–444. [Google Scholar] [CrossRef]
- Mondal, M. (Ed.) Production and Storage of Fruits; BAU Campus: Mymsningh, Bangladesh, 2000; p. 312. (In Bangla) [Google Scholar]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 7th ed.; Iowa State University Press: Ames, IA, USA, 1990; p. 593. [Google Scholar]
- Jolliffe, I. Principal Component Analysis; Lovric, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Deng, Y.; Wu, Y.; Li, Y. Physiological Responses and Quality Attributes of ‘Kyoho’ Grapes to Controlled Atmosphere Storage. LWT Food Sci. Technol. 2006, 39, 584–590. [Google Scholar] [CrossRef]
- Nabifarkhani, N.; Sharifani, M.; Garmakhany, A.D.; Moghadam, E.G.; Shakeri, A. Effect of Nano-Composite and Thyme Oil (Tymus vulgaris L.) Coating on Fruit Quality of Sweet Cherry (Takdaneh cv) during Storage Period. Food Sci. Nutr. 2015, 3, 349–354. [Google Scholar]
- Krasniewska, K.; Gniewosz, M.; Kosakowska, O.; Cis, A. Preservation of Brussels Sprouts by Pullulan Coating Containing Oregano Essential Oil. J. Food Prot. 2016, 79, 493–500. [Google Scholar] [CrossRef]
- Behshti, M.; Jahani, M.; Aminifard, M.H.; Hosseini, S.A. Essential Oils to Control Botrytis cinerea In Vitro and In Vivo on Grape Fruits. J. Hortic. Postharvest Res. 2020, 3, 161–172. [Google Scholar]
- Rattanapitigorn, P.; Arakawa, M.; Tsuro, M. Vanillin Enhances the Antifungal Effect of Plant Essential Oils against Botrytis cinerea. Int. J. Aromather. 2006, 16, 193–198. [Google Scholar] [CrossRef]
- Valero, D.; Valverde, J.M.; Martínez-Romero, D.; Guillén, F.; Castillo, S.; Serrano, M. The Combination of Modified Atmosphere Packaging with Eugenol or Thymol to Maintain Quality, Safety and Functional Properties of Table Grapes. Postharvest Biol. Technol. 2006, 41, 317–327. [Google Scholar] [CrossRef]
- Abdolahi, A.; Hassani, A.; Ghosta, Y.; Bernousi, I.; Meshkatalsadat, M. Study on the Potential Use of Essential Oils for Decay Control and Quality Preservation of Tabarzeh Table Grape. J. Plant Prot. Res. 2010, 50, 45–52. [Google Scholar] [CrossRef]
- Abdollahi, A.; Hassani, A.; Ghosta, Y.; Bernousi, I.; Meshkatalsadat, M.H.; Shabani, R.; Ziaee, S.M. Evaluation of Essential Oils for Maintaining Postharvest Quality of Thompson Seedless Table Grape. Nat. Prod. Res. 2012, 26, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R.; Huang, C.; Zhao, H.; Huang, H.; Ren, L.; Faiq, M.; Hashmi, M.S.; Li, B.; Zheng, D.; Xu, Y.; et al. Antioxidant Activity of Thymol Essential Oil and Inhibition of Polyphenol Oxidase Enzyme: A Case Study on the Enzymatic Browning of Harvested Longan Fruit. Chem. Biol. Technol. Agric. 2021, 8, 61. [Google Scholar] [CrossRef]
- Rienth, M.; Crovadore, J.; Ghaffari, S.; Lefort, F. Oregano Essential Oil Vapour Prevents Plasmopara viticola Infection in Grapevine (Vitis vinifera) and Primes Plant Immunity Mechanisms. PLoS ONE 2019, 14, e0222854. [Google Scholar] [CrossRef]
- Burggraf, A.; Rienth, M. Origanum vulgare Essential Oil Vapour Impedes Botrytis cinerea Development on Grapevine (Vitis vinifera) fruit. Phytopathol. Mediterr. 2020, 59, 331–344. [Google Scholar]
- Banani, H.; Olivieri, L.; Santoro, K.; Garibaldi, A.; Gullino, M.L.; Spadaro, D. Thyme and Savory Essential Oil Efficacy and Induction of Resistance against Botrytis cinerea through Priming of Defense Responses in Apple. Foods 2018, 7, 11. [Google Scholar] [CrossRef]
- Liu, S.; Shao, X.; Wei, Y.; Li, Y.; Xu, F.; Wang, H.S. canadensis L. Essential Oil Vapor Effectively Inhibits Botrytis cinerea Growth and Preserves Postharvest Quality of Strawberry as a Food Model System. Front. Microbiol. 2016, 7, 1179. [Google Scholar] [CrossRef]
- Kedia, A.; Prakash, B.; Mishra, P.K.; Dubey, N.K. Antifungal and Antiaflatoxigenic Properties of Cuminum cyminum (L.) Seed Essential Oil and Its Efficacy as a Preservative in Stored Commodities. Int. J. Food Microbiol. 2014, 168–169, 1–7. [Google Scholar] [CrossRef]
- Svircev, A.M.; Smith, R.J.; Zhou, T.; Hernadez, M.; Liu, W.; Chu, C.L. Effects of Thymol Fumigation on Survival and Ultrastracture of Monilinia fructicola. Postharvest Biol. Technol. 2007, 45, 228–233. [Google Scholar] [CrossRef]
- Abdel-Rahim, I.R.; Abo-Elyousr, K.A.M. Using of Endophytic Saccharomycopsis fibuligera and Thyme Oil for Management of Gray Mold Rot of Guava Fruits. Biol. Control 2017, 110, 124–131. [Google Scholar] [CrossRef]
- Feng, W.; Zheng, X. Essential Oils to Control Alternaria alternata In Vitro and In Vivo. Food Control 2007, 18, 1126–1130. [Google Scholar] [CrossRef]
- Tzortzakis, N.G. Ethanol, vinegar and Origanum vulgare oil vapour suppress the development of anthracnose rot in tomato fruit. Int. J. Food Microbiol. 2010, 142, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Perveen, K.; Bokhari, N.; Al-Rashid, S.; Al-Humaid, L. Chemical Composition of Essential Oil of Ocimum basilicum L. and Its Potential in Managing the Alternaria Rot of Tomato. J. Essent. Oil Bear. Plants 2020, 23, 1428–1437. [Google Scholar] [CrossRef]
- Knaak, N.; Fiuza, L.M. Potential of Essential Plant Oils to Control Insects and Microorganisms. Neotrop. Biol. Conserv. 2010, 5, 120–132. [Google Scholar] [CrossRef]
- Chu, C.; Liu, W.; Zhou, T.; Station, V.; Protection, S.C. Fumigation of Sweet Cherries with Thymol and Acetic Acid to Reduce Postharvest Brown Rot and Blue Mold Rot. Fruits 2001, 56, 123–130. [Google Scholar] [CrossRef]
- Lichter, A.; Gabler, F.M.; Smilanick, J.L. Control of Spoilage in Table Grapes. Stewart Postharvest Rev. 2006, 6, 1–10. [Google Scholar]
- Hassani, A.; Fathi, Z.; Ghosta, Y.; Abdollahi, A.; Meshkatalsadat, M.H.; Marandi, R.J. Evaluation of Plant Essential Oils for Control of Postharvest Brown and Gray Mold Rots on Apricot. J. Food Saf. 2012, 32, 94–101. [Google Scholar] [CrossRef]
- Robledo, N.; López, L.; Bunger, A.; Tapia, C.; Abugoch, L. Effects of Antimicrobial Edible Coating of Thymol Nanoemulsion/Quinoa Protein/Chitosan on the Safety, Sensorial Properties, and Quality of Refrigerated Strawberries (Fragaria ananassa). under commercial storage environment. Food Bioprocess Technol. 2018, 11, 1566–1574. [Google Scholar] [CrossRef]
- Chu, Y.; Gao, C.; Liu, X.; Zhang, N.; Xu, T.; Feng, X. Improvement of Storage Quality of Strawberries by Pullulan Coatings Incorporated with Cinnamon Essential Oil Nanoemulsion. LWT 2020, 122, 109054. [Google Scholar] [CrossRef]
- Yokotsuka, K.; Makino, S.; Singleton, V. Polyphenol Oxidase from Grapes: Precipitation, Re-Solubilization and Characterization. Am. J. Enol. Vitic. 1988, 39, 293–302. [Google Scholar] [CrossRef]
- Rogiers, S.; Hatfield, J.; Jaudzems, V.; White, R.; Keller, M. Grape Berry cv. Shiraz Epicuticular Wax and Transpiration during Ripening and Pre Harvest Weight Loss. Am. J. Enol. Vitic. 2004, 55, 121–127. [Google Scholar] [CrossRef]
- Lydakis, D.; Aked, J. Vapour Heat Treatment of Sultanina Table Grapes. II: Effects on Postharvest Quality. Postharvest Biol. Technol. 2003, 27, 117–126. [Google Scholar] [CrossRef]
- Ge, Y.Q.; Zhang, W.Y.; Chen, Y.; Ye, Q. Effect of Sulfur Dioxide on the Respiration and the Hormone Level of Postharvest Table Grapes. Acta Hortic. Sin. 1997, 24, 120–124. [Google Scholar]
- Gago, C.; Antão, R.; Dores, C.; Guerreiro, A.; Miguel, M.G.; Faleiro, M.L. The Effect of Nanocoatings Enriched with Essential Oils on ‘Rocha’ Pear Long Storage. Foods 2020, 9, 240. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ghosh, A.; Mukherjee, A. Nanoencapsulation-Based Edible Coating of Essential Oils as a Novel Green Strategy against Fungal Spoilage, Mycotoxin Contamination, and Quality Deterioration of Stored Fruits: An Overview. Front. Microbiol. 2021, 12, 768414. [Google Scholar] [CrossRef]
- Deng, Y.; Wu, Y.; Li, Y. Changes in Firmness, Cell Wall Composition and Cell Wall Hydrolases of Grapes Stored in High Oxygen Atmospheres. Food Res. Int. 2005, 38, 769–776. [Google Scholar] [CrossRef]
- Abo-Elyousr, K.A.; Al-Qurashi, A.; Almasoudi, N. Evaluation of the Synergy between Schwanniomyces vanrijiae and Propolis in the Control of Penicillium digitatum on Lemons. Egypt. J. Biol. Pest Control 2021, 31, 66. [Google Scholar] [CrossRef]
- Pinto, L.; Cefola, M.; Bonifacio, M.A.; Cometa, S.; Bocchino, C.; Pace, B.; Giglio, E.D.; Palumbo, M.; Sada, A.; Logrieco, A.F.; et al. Effect of Red Thyme Oil (Thymus vulgaris L.) Vapours on Fungal Decay, Quality Parameters and Shelf-Life of Oranges during Cold Storage. Food Chem. 2021, 336, 127590. [Google Scholar] [CrossRef]
- Sánchez González, L.; Vargas, M.; Gonzalez-Martinez, C.; Chiralt, A.; Cháfer, M. Use of Essential Oils in Bioactive Edible Coatings: A Review. Food Eng. Rev. 2011, 3, 1–16. [Google Scholar] [CrossRef]
- Cháfer, M.; Sánchez González, L.; Gonzalez-Martinez, C.; Chiralt, A. Fungal Decay and Shelf Life of Oranges Coated with Chitosan and Bergamot, Thyme, and Tea Tree Essential Oils. J. Food Sci. 2012, 77, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Maftoonazad, N.; Badii, F. Use of Edible Films and Coatings to Extend the Shelf Life of Food Products. Recent Pat. Food Nutr. Agric. 2009, 1, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Del Caro, A.; Piga, A.; Vacca, V.; Agabbio, M. Changes of flavonoids, vitamin C and Antioxidants Capacity in Minimally Processed Citrus Segments and Juices during Storage. Food Chem. 2004, 84, 99–105. [Google Scholar] [CrossRef]
- Rathore, H.A.; Masud, T.; Sammi, S.; Soomro, H.A. Effect of storage on physico-chemical composition and sensory properties of mango (Mangifera indica L.) variety Dosehari. Pak. J. Nutr. 2007, 6, 143–148. [Google Scholar]
- Davey, M.W.; Van Montagu, M.; Inze, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N. Plant L-ascorbic acid: Chemistry, Function, Metabolism, Bioavailability and Effects of Processing. J. Sci. Food Agric. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Mditshwa, A.; Magwaza, L.S.; Tesfay, S.Z.; Oparac, U.L. Postharvest Factors Affecting Vitamin C Content of Citrus Fruits: A Review. Sci. Hortic. 2017, 218, 95–104. [Google Scholar] [CrossRef]
- El-Abbasy, U.K.; Abd El-Khalek, A.F.; Mohamed, M.I. Postharvest Applications of 1-Methylcyclopropene and Salicylic Acid for Maintaining Quality and Enhancing Antioxidant Enzyme Activity of Apricot Fruits cv. ‘Canino’ during Cold Storage. Egypt. J. Hortic. 2018, 45, 1–23. [Google Scholar]
- Lee, S.-H.; Chang, K.-S.; Su, M.-S.; Huang, Y.-S.; Jang, H.-D. Effects of Some Chinese Medicinal Plant Extracts on Five Different Fungi. Food Control 2007, 18, 1547–1554. [Google Scholar] [CrossRef]
- Martínez, K.; Ortiz, M.; Arrieta, A.R.A.; Castañeda, C.G.; Valencia, M.E.; Tovar, C.D. The Effect of Edible Chitosan Coatings Incorporated with Thymus capitatus Essential Oil on the Shelf-Life of Strawberry (Fragaria ananassa) during Cold Storage. Biomolecules 2018, 8, 155. [Google Scholar] [CrossRef]
- Golding, J.B.; Ekman, J.H.; McGlasson, W.B. Regulation of Fruit Ripening. Stewart Postharvest Review. Postharvest Biol. Technol. 2005, 3, 1–5. [Google Scholar]
- Aloui, H.; Khwaldia, K. Natural Antimicrobial Edible Coatings for Microbial Safety and Food Quality Enhancement. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1080–1103. [Google Scholar] [CrossRef] [PubMed]
- Motelica, L.; Ficai, D.; Oprea, O.C.; Ficai, A.; Andronescu, E. Smart Food Packaging Designed by Nanotechnological and Drug Delivery Approaches. Coatings 2020, 10, 806. [Google Scholar] [CrossRef]
- Marín, I.; Sayas-Barberá, E.; Viuda-Martos, M.; Navarro, C.; Sendra, E. Chemical Composition, Antioxidant and Antimicrobial Activity of Essential Oils from Organic Fennel, Parsley, and Lavender from Spain. Foods 2016, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Shehata, S.A.; Emad, A.A.; Marwa, R.A.; Reda, M.M.; Rwotonen, I.B.; Karima, F.A. Effect of Some Citrus Essential Oils on PostHarvest Shelf Life and Physicochemical Quality of Strawberries during Cold Storage. Agronomy 2020, 10, 1466. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, B.; Wang, Y. Clove Essential Oil Confers Antioxidant Activity and Lifespan Extension in C. elegans via the DAF-16/FOXO Transcription Factor. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 242, 108938. [Google Scholar] [CrossRef] [PubMed]
- Salimi, L.; Arshad, M.; Rahimi, A.; Rokhzadi, A.; Amini, S.; Azizi, M. Effect of Some Essential Oils on Post-Harvest Quality of Grapevine (Vitis vinifera cv Rasha (Siah-e-Sardasht)) during Cold Storage. Int. J. Biosci. 2013, 3, 75–83. [Google Scholar]
- Geransayeh, M.; Sepahvand, S.; Abdossi, V.; Nezhad, R.A. Efect of Thymol Treatment on Decay, Postharvest Life and Quality of Strawberry (Fragaria ananassa) Fruit cv. ‘Gaviota. Int. J. Agron. Agric. Res. 2015, 6, 151–162. [Google Scholar]
- Zauberman, G.; Ronen, R.; Akerman, M.; Weksler, A.; Rot, I.; Fuchs, Y. Post-harvest Retention of the Red Colour of Litchi Fruit Pericarp. Sci. Hortic. 1991, 47, 89–97. [Google Scholar] [CrossRef]
- Jiang, Y.M. Role of anthocyanins, polyphenol oxidase and phenols in lychee pericarp browning. J. Sci. Food Agric. 2000, 80, 305–310. [Google Scholar] [CrossRef]
- González-Neves, G.; Charamelo, D.; Balado, J.; Barreiro, L.; Bochicchio, R.; Gatto, G.; Gil, G.; Tessore, A.; Carbonneau, A.; Moutounet, M. Phenolic Potential of Tannat, Cabernet-Sauvignon and Merlot Grapes and Their Correspondence with Wine Composition. Anal. Chim. Acta 2004, 513, 191–196. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wang, S.Y.; Chen, C. Increasing Antioxidant Activity and Reducing Decay of Blueberries by Essential Oils. J. Agric. Food Chem. 2008, 56, 3587–3592. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Wang, S.Y.; Yin, J.J.; Parry, J.; Yu, L.L. Enhancing Antioxidant, Antiproliferation, and Free Radical Scavenging Activities in Strawberries with Essential Oils. J. Agric. Food Chem. 2007, 55, 6527–6532. [Google Scholar] [CrossRef] [PubMed]
- Youssef, K.; Roberto, S.R.; Tiepo, A.N.; Constantino, L.V.; de Resende, J.T.V.; Abo-Elyousr, K.A.M. Salt Solution Treatments Trigger Antioxidant Defense Response against Gray Mold Disease in Table Grapes. J. Fungi 2020, 6, 179. [Google Scholar] [CrossRef] [PubMed]
- Chisari, M.; Barbagallo, R.N.; Spagna, G. Characterization of Polyphenol Oxidase and Peroxidase and Influence on Browning of Cold Stored Strawberry Fruit. J. Agric. Food Chem. 2007, 55, 3469–3476. [Google Scholar] [CrossRef]
- Ding, C.K.; Chachin, K.; Ueda, Y.; Imahori, Y. Purification and Properties of Polyphenol Oxidase from Loquat Fruit. J. Agric. Food Chem. 1998, 46, 4144–4149. [Google Scholar] [CrossRef]
- Espín, J.; Tomas-Barberan, F. Phenolic Compounds and Related Enzymes as Determinants of Fruits and Vegetables Quality. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar]
- Shao, X.F.; Wang, H.F.; Xu, F.; Cheng, S. Effects and Possible Mechanisms of Tea Tree Oil Vapor Treatment on the Main Disease in Postharvest Strawberry Fruit. Postharvest Biol. Technol. 2013, 77, 94–101. [Google Scholar] [CrossRef]
- Biles, C.L.; Martyn, R.D. Peroxidase Polyphenoloxidase and Shikimate Dehydrogenase Isoenzymes in Relation to Tissue Type, Maturity and Pathogen Induction of Watermelon Seedlings. Plant Physiol. Biochem. 1993, 31, 499–506. [Google Scholar]
- Mejdoub, K.; Mami, I.; Belabbes, R.; Dib, M.; Nassim, D.; Boufeldja, T.; Benyelles, N.; Costa, J.; Muselli, A. Chemical Variability of Atractylis Gummifera Essential Oils at Three Developmental Stages and Investigation of Their Antioxidant. Antifungal and Insecticidal Activities. Curr. Bioact. Compd. 2020, 16, 489–497. [Google Scholar] [CrossRef]
- Jin, P.; Wang, S.Y.; Gao, H.; Chen, H.; Zheng, Y.; Wang, C.Y. Effect of cultural system and essential oil treatment on antioxidant capacity in raspberries. Food Chem. 2012, 132, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Betanzos, C.; Hernández-Sánchez, H.; Quintanar-Guerrero, D.; Galindo-Pérez, M.; Zambrano-Zaragoza, M. Influence of Solid Lipid Nanoparticle/Xanthan Gum Coatings on Compositional and Enzymatic Changes in Guava (Psidium guajava L.) during Ripening. Acta Hortic. 2018, 1194, 289–295. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Abbasy, U.K.; Abdel-Hameed, M.A.; Hatterman-Valenti, H.M.; El-Shereif, A.R.; Abd El-Khalek, A.F. Effectiveness of Oregano and Thyme Essential Oils as Alternatives for Sulfur Dioxide in Controlling Decay and Gray Mold and Maintaining Quality of ‘Flame Seedless’ Table Grape (Vitis vinifera L.) during Cold Storage. Agronomy 2023, 13, 3075. https://doi.org/10.3390/agronomy13123075
El-Abbasy UK, Abdel-Hameed MA, Hatterman-Valenti HM, El-Shereif AR, Abd El-Khalek AF. Effectiveness of Oregano and Thyme Essential Oils as Alternatives for Sulfur Dioxide in Controlling Decay and Gray Mold and Maintaining Quality of ‘Flame Seedless’ Table Grape (Vitis vinifera L.) during Cold Storage. Agronomy. 2023; 13(12):3075. https://doi.org/10.3390/agronomy13123075
Chicago/Turabian StyleEl-Abbasy, Usama K., Mohamed A. Abdel-Hameed, Harlene M. Hatterman-Valenti, Ali R. El-Shereif, and Ahmed F. Abd El-Khalek. 2023. "Effectiveness of Oregano and Thyme Essential Oils as Alternatives for Sulfur Dioxide in Controlling Decay and Gray Mold and Maintaining Quality of ‘Flame Seedless’ Table Grape (Vitis vinifera L.) during Cold Storage" Agronomy 13, no. 12: 3075. https://doi.org/10.3390/agronomy13123075
APA StyleEl-Abbasy, U. K., Abdel-Hameed, M. A., Hatterman-Valenti, H. M., El-Shereif, A. R., & Abd El-Khalek, A. F. (2023). Effectiveness of Oregano and Thyme Essential Oils as Alternatives for Sulfur Dioxide in Controlling Decay and Gray Mold and Maintaining Quality of ‘Flame Seedless’ Table Grape (Vitis vinifera L.) during Cold Storage. Agronomy, 13(12), 3075. https://doi.org/10.3390/agronomy13123075






