Changes in Nutrient-Regulated Soil Microbial Communities in Soils Concomitant with Grassland Restoration in the Alpine Mining Region of the Qilian Mountains
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Site, Experimental Design, and Soil Sampling
2.2. Soil of Physical and Chemical Properties
2.3. Soil DNA Extraction, PCR and Sequencing
2.4. Soil Bioinformatics Analysis
2.5. Statistical Analysis
3. Results
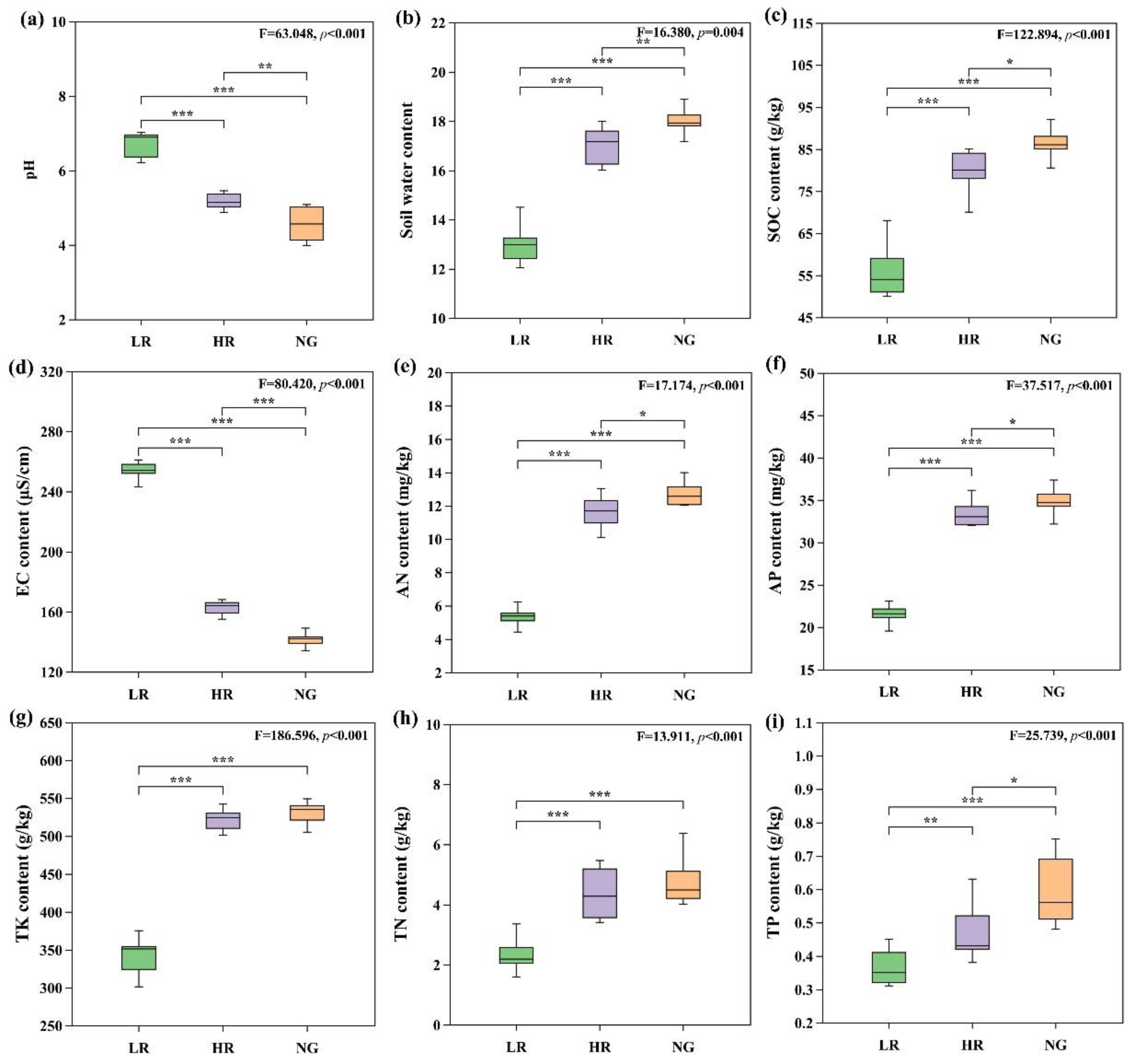
3.1. Changes in Vegetation Characteristics and Soil Properties across Different Restoration Levels
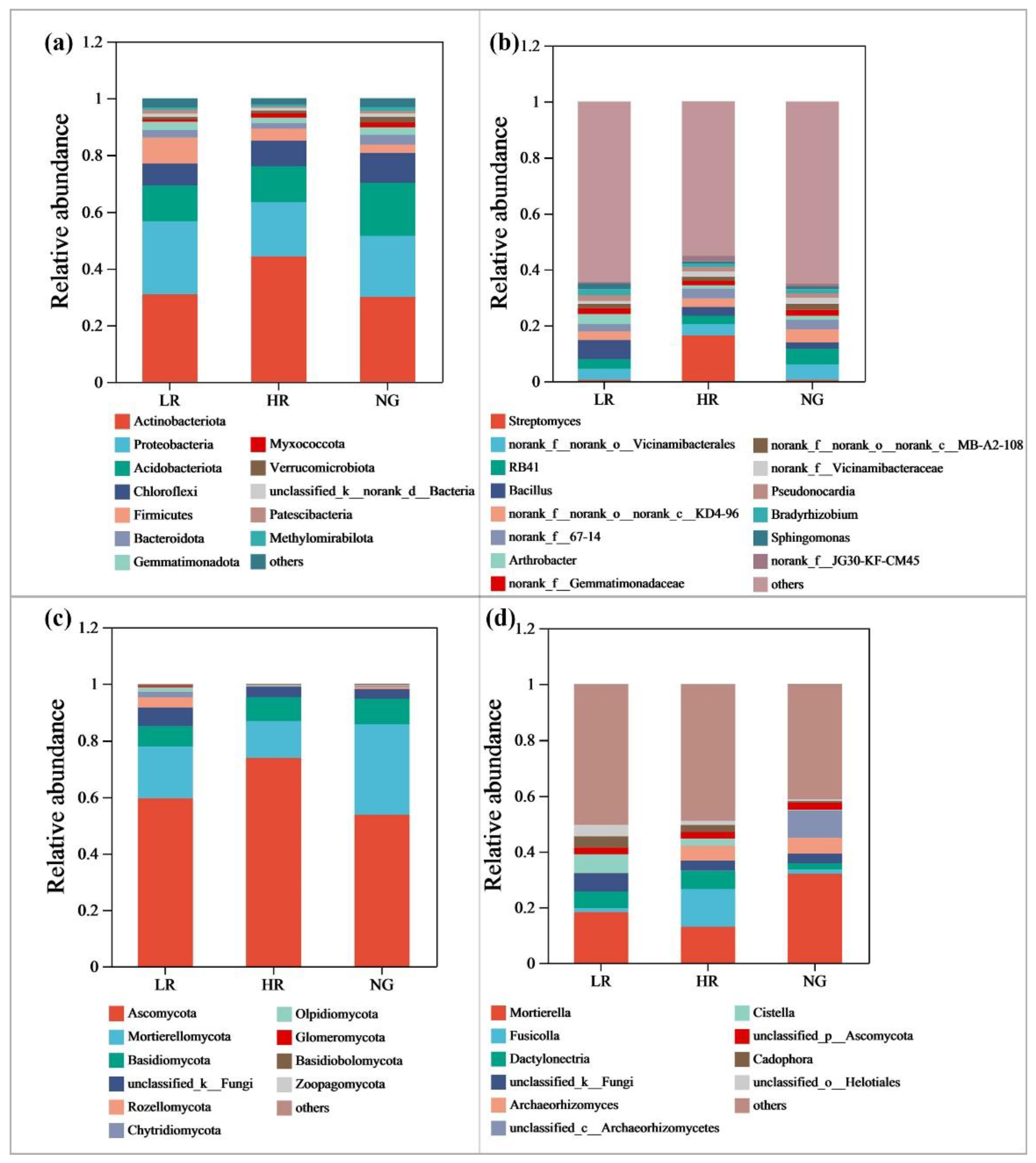
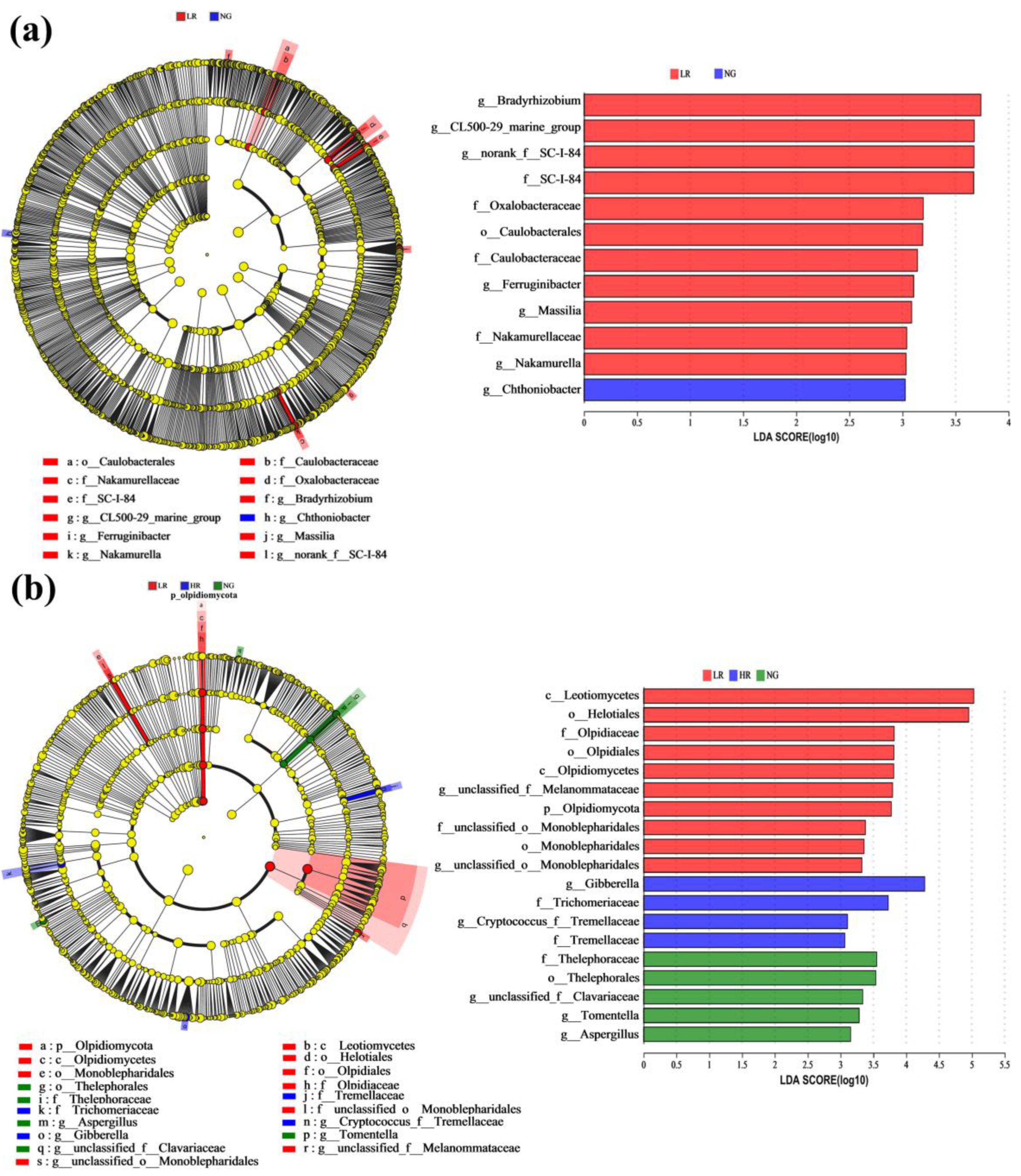
3.2. Soil Microbial Community Composition for Grassland Restoration in Alpine Mining Areas
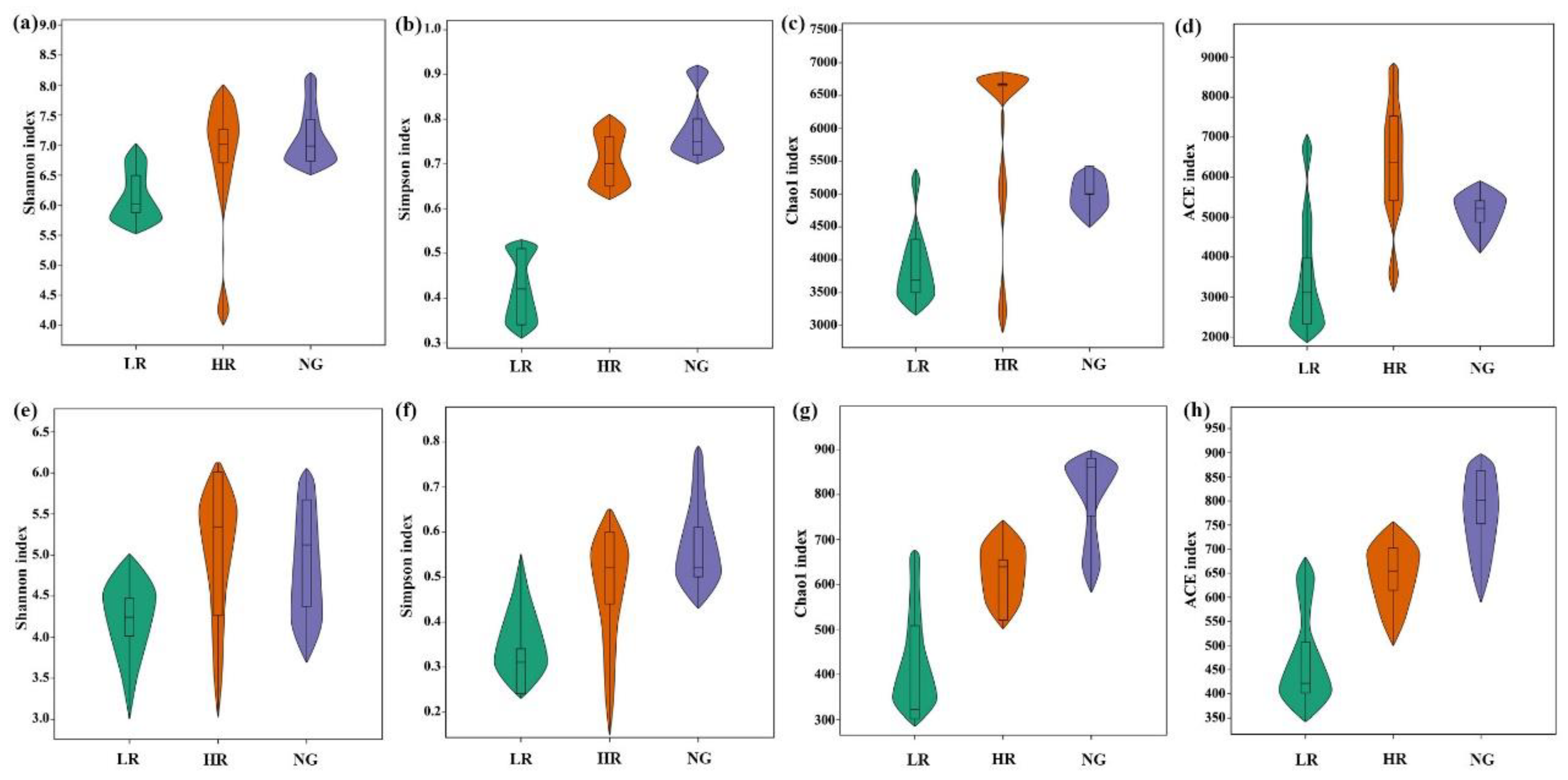
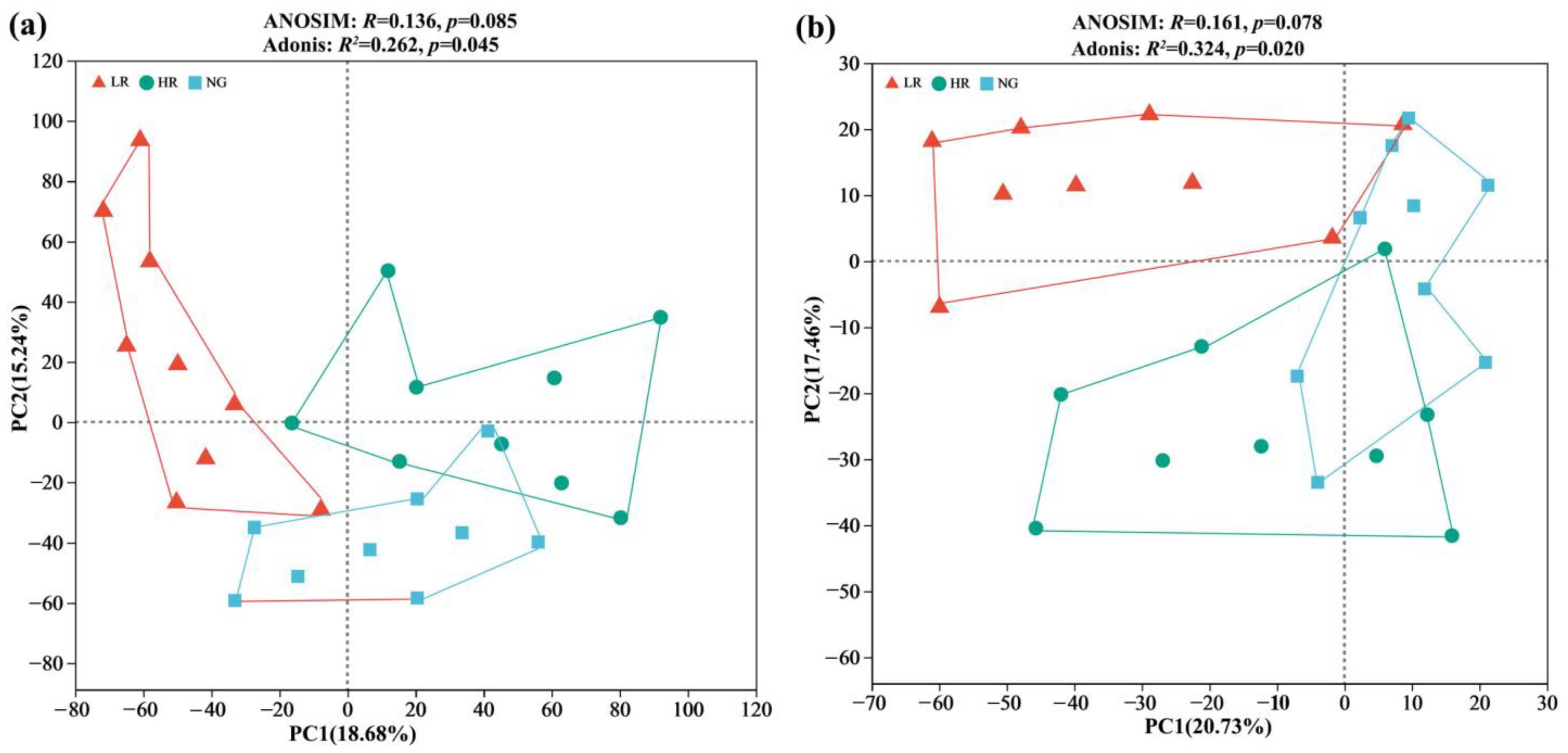
3.3. Soil Microbial Community Alpha and Beta Diversity for Grassland Restoration in Alpine Mining Areas
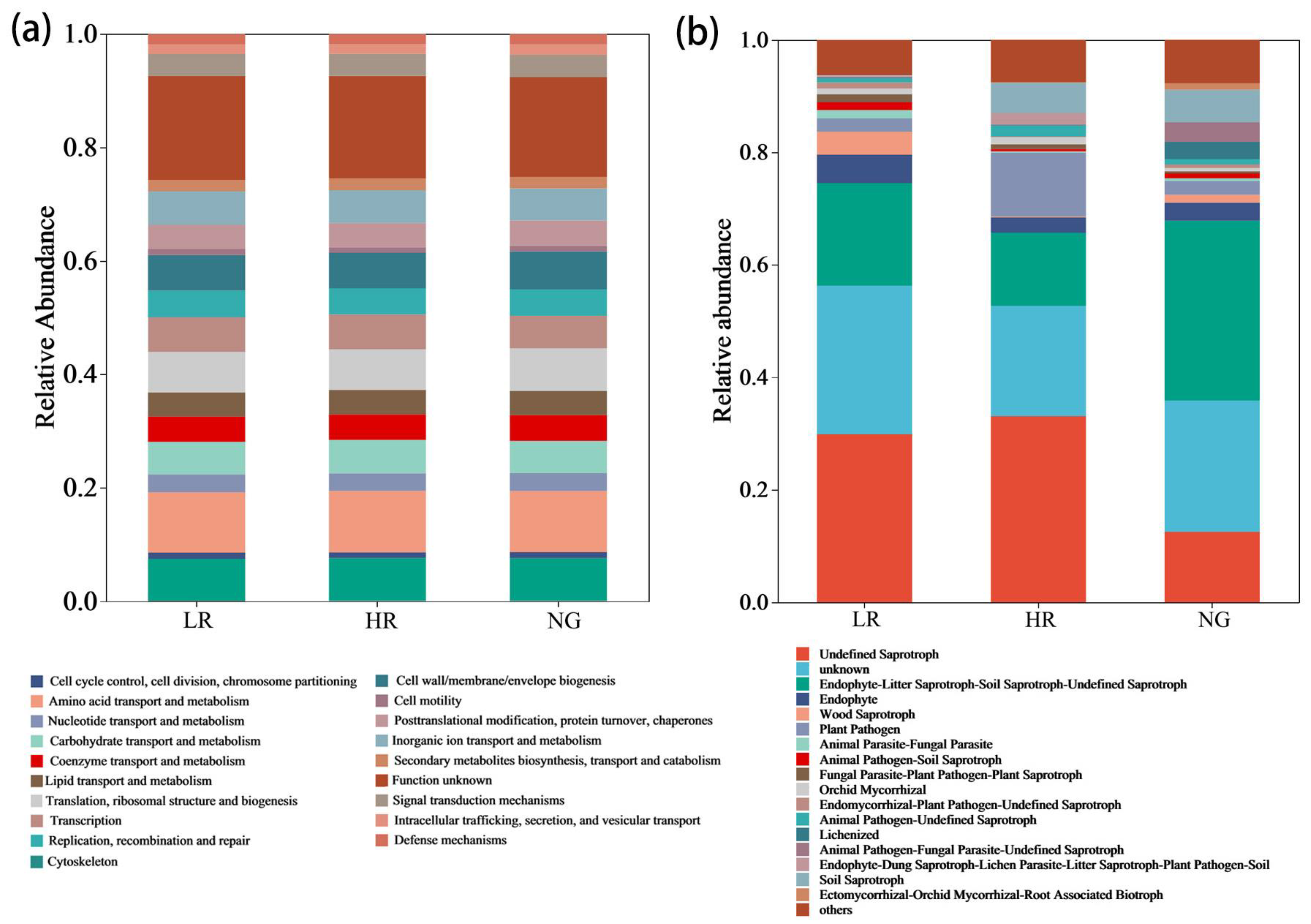
3.4. Functional Profiles of Soil Microorganisms for Grassland Restoration in Alpine Mining Areas
3.5. The Correlation between Soil Microbial Community and Environmental Factors for Grassland Restoration in Alpine Mining Areas
4. Discussion
4.1. Effects on Vegetation and Soil Physicochemical Properties in High-Altitude Mining Area Grassland Restoration
4.2. Effects of Grassland Restoration on Soil Microbial Communities in Alpine Grassland Mining Areas
4.3. Impact of Vegetation and Soil Environmental Factors on Soil Microbial Communities in Alpine Grassland Mining Areas
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cao, J.; Wang, H.; Holden, N.M.; Adamowski, J.F.; Biswas, A.; Zhang, X.; Feng, Q. Soil properties and microbiome of annual and perennial cultivated grasslands on the Qinghai–Tibetan Plateau. Land Degrad. Dev. 2021, 32, 5306–5321. [Google Scholar] [CrossRef]
- Lei, T.; Feng, J.; Lv, J.; Wang, J.; Song, H.; Gao, X. Net primary productivity loss under different drought levels in different grassland ecosystems. J. Environ. Manag. 2020, 274, 111144. [Google Scholar] [CrossRef]
- Comer, J.; Perkins, L. Resistance of the soil microbial community to land-surface disturbances of high-intensity winter grazing and wildfire. J. Environ. Manag. 2021, 279, 111596. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.L.; Wang, L.; Liu, J.S.; Zhu, H.; Zhong, Z.W. Grassland ecology in China: Perspectives and challenges. Front. Agric. Sci. Eng. 2018, 5, 24–43. [Google Scholar] [CrossRef]
- He, F.; Yang, J.; Dong, S.; Zhi, Y.; Hao, X.; Shen, H.; Xiao, J.; Kwaku, E.A.; Zhang, R.; Shi, H.; et al. Short-term grazing changed temporal productivity stability of alpine grassland on Qinghai-Tibetan Plateau via response species richness and functional groups asynchrony. Ecol. Indicat. 2023, 146, 109800. [Google Scholar] [CrossRef]
- Deng, J.; Bai, X.; Zhou, Y.; Zhu, W.; Yin, Y. Variations of soil microbial communities accompanied by different vegetation restoration in an open-cut iron mining area. Sci. Total Environ. 2020, 704, 135243. [Google Scholar] [CrossRef]
- Qin, G.X.; Wu, J.; Li, C.B.; Qin, A.N.; Yao, X.Q. Grassland vegetation phenology change and its response to climate changes in North China. Chin. J. Appl. Ecol. 2019, 30, 4099–4107. [Google Scholar]
- Wu, Y.; Xu, N.; Wang, H.; Li, J.; Zhong, H.; Dong, H.; Zeng, Z.; Zong, C. Variations in the diversity of the soil microbial community and structure under various categories of degraded wetland in Sanjiang Plain, northeastern China. Land Degrad. Dev. 2021, 32, 2143–2156. [Google Scholar] [CrossRef]
- Lorite, J.; Ballesteros, M.; García-Robles, H.; Cañadas, E.M. Economic evaluation of ecological restoration options in gypsum habitats after mining. J. Nat. Conserv. 2021, 59, 125935. [Google Scholar] [CrossRef]
- Kong, J.Q.; He, Z.B.; Chen, L.F.; Yang, R.; Du, J. Efficiency of biochar, nitrogen addition, and microbial agent amendments in remediation of soil properties and microbial community in Qilian Mountains mine soils. Ecol. Evol. 2021, 11, 9318–9331. [Google Scholar] [CrossRef]
- Dong, S.K.; Shang, Z.H.; Gao, J.X.; Boone, R.B. Enhancing sustainability of grassland ecosystem through ecological restoration and grazing management in an era of climate change on Qinghai-Tibetan Plateau. Agric. Ecosyst. Environ. 2020, 287, 106684. [Google Scholar] [CrossRef]
- Baranova, A.; Schickhoff, U.; Wang, S.L.; Jin, M. Mountain pastures of Qilian Shan: Plant communities, grazing impact and degradation status (Gansu province, NW China). Hacquetia 2016, 15, 21–35. [Google Scholar] [CrossRef]
- Siebert, F.; Van Staden, N.; Komape, D.M.; Swemmer, A.M.; Siebert, S.J. Effects of land-use change on herbaceous vegetation in a semi-arid Mopaneveld savanna. Bothalia 2021, 51, a8. [Google Scholar] [CrossRef]
- Jing, L.H.; Mipam, T.D.; Ai, Y.; Jiang, A.; Gan, T.; Zhang, S.; Liu, J.; Tian, L. Grazing intensity alters soil microbial diversity and network complexity in alpine meadow on the Qinghai-Tibet Plateau. Agric. Ecosyst. Environ. 2023, 353, 108541. [Google Scholar] [CrossRef]
- Wei, Z.F.; Huang, Q.Y.; Zhang, R. Dynamics of vegetation coverage and response to climate change in China-South Asia-Southeast Asia during 1982–2013. Appl. Ecol. Environ. Res. 2019, 17, 2865–2879. [Google Scholar] [CrossRef]
- Li, P.; Zhang, X.; Hao, M.; Cui, Y.; Zhu, S.; Zhang, Y. Effects of vegetation restoration on soil bacterial communities, enzyme activities, and nutrients of reconstructed soil in a mining area on the Loess Plateau, China. Sustainability 2019, 11, 2295. [Google Scholar] [CrossRef]
- Che, R.X.; Wang, Y.F.; Li, K.X.; Xu, Z.H.; Hu, J.M.; Wang, F.; Rui, Y.C.; Li, L.F.; Pang, Z.; Cui, X.Y. Degraded patch formation significantly changed microbial community composition in alpine meadow soils. Soil Tillage Res. 2019, 195, 104426. [Google Scholar] [CrossRef]
- Li, Y.; Nie, C.; Liu, Y.H.; Du, W.; He, P. Soilmicrobial community composition closely associates with specific enzyme activities and soil carbon chemistry in a long-term nitrogen fertilized grassland. Sci. Total Environ. 2019, 654, 264–274. [Google Scholar] [CrossRef]
- Huang, Y.; Cao, Y.; Zhou, W.; Kuang, X.; Wang, F.; Bai, Z. Effects of straw biochar on the growth of medicago falcata in the reconstructed soil of grassland mining area. Acta Ecol. Sin. 2021, 41, 588–602. [Google Scholar]
- Muneer, M.A.; Huang, X.; Hou, W.; Zhang, Y.; Cai, Y.; Munir, M.Z.; Wu, L.; Zheng, C. Response of fungal diversity, community composition, and functions to nutrients management in red soil. J. Fungi 2021, 7, 554. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Z.; Niu, S.; Tian, D.; Wu, Q.; Gao, X.; Schellenberg, M.P.; Han, G. Diversity of plant and soil microbes mediates the response of ecosystem multifunctionality to grazing disturbance. Sci. Total Environ. 2021, 776, 145730. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Wang, S.P.; Jiang, L.L.; Zhang, L.R.; Cui, S.J.; Meng, F.D.; Wang, Q.; Li, X.N.; Zhou, Y. Changes of soil microbial community under different degraded gradients of alpine meadow. Agric. Ecosyst. Environ. 2016, 222, 213–222. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Luo, Y. Meta-analysis of the impacts of global change factors on soil microbial diversity and functionality. Nat. Commun. 2020, 11, 3072. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Zhao, H.; Chen, J.; Zhang, T.; Cai, Z.; Zhou, G.; Li, Z.; Qiu, Z.; Wu, Z. Soil microbial community dynamics mediate the priming effects caused by in situ decomposition of fresh plant residues. Sci. Total Environ. 2020, 737, 139708. [Google Scholar] [CrossRef]
- Su, J.; Ji, W.; Sun, X.; Wang, H.; Kang, Y.; Yao, B. Effects of different management practices on soil microbial community structure and function in alpine grassland. J. Environ. Manag. 2023, 327, 116859. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; He, Z.; Zhao, W.; Liu, J.; Zhou, H.; Li, J.; Meng, Y.; Wang, L. Soil structure and nutrient supply drive changes in soil microbial communities during conversion of virgin desert soil to irrigated cropland. Eur. J. Soil Sci. 2020, 71, 768–781. [Google Scholar] [CrossRef]
- Jing, Z.B.; Cheng, J.M.; Su, J.S.; Bai, Y.; Jin, J.W. Changes in plant community composition and soil properties under 3-decade grazing exclusion in semiarid grassland. Ecol. Eng. 2014, 64, 171–178. [Google Scholar] [CrossRef]
- Pei, L.; Xiao, J.; Sun, L. The effects of reclaimed water irrigation on the soil characteristics and microbial populations of plant rhizosphere. Environ. Sci. Pollut. Res. 2021, 29, 17570–17579. [Google Scholar]
- Liu, S.B.; Zamanian, K.; Schleuss, P.M.; Zarebanadkouki, M.; Kuzyakov, Y. Degradation of Tibetan grasslands: Consequences for carbon and nutrient cycles. Agric. Ecosyst. Environ. 2018, 252, 93–104. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Dudley, N.; Eufemia, L.; Fleckenstein, M.; Periago, M.E.; Petersen, I.; Timmers, J.F. Grasslands and savannahs in the UN decade on ecosystem restoration. Restor. Ecol. 2020, 28, 1313–1317. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, G.B.; Xue, S.; Wang, G.L. Soil bacterial community dynamics reflect changes in plant community and soil properties during the secondary succession of abandoned farmland in the Loess Plateau. Soil Biol. Biochem. 2016, 97, 40–49. [Google Scholar] [CrossRef]
- Zhou, H.L.; Zhou, G.S.; Song, X.Y.; Geng, J.J.; He, Q.J. Evolving soil water limitation changes maize production potential and biomass accumulation but not its relationship with grain yield. Agronomy 2023, 13, 2637. [Google Scholar] [CrossRef]
- Li, H.Y.; Qiu, Y.Z.; Yao, T.; Han, D.R.; Gao, Y.M.; Zhang, J.G.; Ma, Y.C.; Zhang, H.R.; Yang, X.L. Nutrients available in the soil regulate the changes of soil microbial community alongside degradation of alpine meadows in the northeast of the Qinghai-Tibet Plateau. Sci. Total Environ. 2021, 792, 148363. [Google Scholar] [CrossRef]
- Yang, X.M.; Feng, Q.; Liu, W.; Xia, H.H.; Zhang, J.T.; Yang, L.S.; Zhang, C.Q.; Wang, Z.Y.; Feng, Y.L. Community structure and plant diversity under different degrees of restored grassland in mining areas of the Qilian Mountains, Northwestern China. Front. Environ. Sci. 2023, 11, 1191599. [Google Scholar] [CrossRef]
- Fu, B.J.; Liu, S.L.; Chen, L.D.; Lü, Y.H.; Qiu, J. Soil quality regime in relation to land cover and slope position across a highly modified slope landscape. Ecol. Res. 2004, 19, 111–118. [Google Scholar] [CrossRef]
- Xie, H.T.; Yang, X.M.; Drury, C.F.; Yang, J.Y.; Zhang, X.D. Predicting soil organic carbon and total nitrogen using mid- and near-infrared spectra for Brookston clay loam soil in Southwestern Ontario, Canada. Can. J. Soil Sci. 2011, 91, 53–63. [Google Scholar] [CrossRef]
- Xiong, W.; Zhao, Q.; Zhao, J.; Xun, W.; Li, R.; Zhang, R.; Wu, H.; Shen, Q. Different continuous cropping spans signifcantly affect microbial community membership and structure in a vanilla-grown soil as revealed by deep pyrosequencing. Microb. Ecol. 2015, 70, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Z.; Wu, L.Y.; Deng, Y.; Zhi, X.Y.; Jiang, Y.H.; Tu, Q.C.; Xie, J.P.; Van Nostrand, J.D.; He, Z.L.; Yang, Y.F. Reproducibility and quantitation of amplicon sequencing-based detection. ISME J. 2011, 5, 1303–1313. [Google Scholar] [CrossRef]
- Miao, L.; Wang, S.Y.; Li, B.K.; Cao, T.H.; Zhang, F.Z.; Wang, Z.; Peng, Y.Z. Effect of carbon source type on intracellular stored polymers during endogenous denitritation (ED) treating landfill leachate. Water Res. 2016, 100, 405–412. [Google Scholar] [CrossRef]
- Han, X.; Li, Y.; Du, X.; Li, Y.; Wang, Z.; Jiang, S.; Li, Q. Effect of grassland degradation on soil quality and soil biotic community in a semi-arid temperate steppe. Ecol. Process. 2020, 9, 63. [Google Scholar] [CrossRef]
- Yang, W.J.; Wang, Y.H.; Webb, A.A.; Li, Z.Y.; Tian, X.; Han, Z.T.; Wang, S.L.; Yu, P.T. Influence of climatic and geographic factors on the spatial distribution of Qinghai spruce forests in the dryland Qilian Mountains of northwest China. Sci. Total Environ. 2018, 612, 1007–1017. [Google Scholar] [CrossRef]
- Stokes, A.; Angeles, G.; Anthelme, F.; Aranda-Delgado, F.; Barois, I.; Bounous, M.; Cruz-Maldonado, N.; Decaens, T.; Fourtier, S.; Freschet, G.T.; et al. Shifts in soil and plant functional diversity along an altitudinal gradient in the French Alps. BMC Res. 2021, 14, 54. [Google Scholar] [CrossRef]
- Gao, Y.; An, Y.; Qi, B.; Liu, J.; Yu, H.; Wang, D. Grazing exclusion mediates the trade-off between plant diversity and productivity in Leymus chinensis meadows along a chronosequence on the Songnen Plain, China. Ecol. Indic. 2021, 126, 107655. [Google Scholar] [CrossRef]
- Zhang, C.; Dong, Q.; Chu, H.; Shi, J.; Li, S.; Wang, Y.; Yang, X. Grassland community composition response to grazing intensity under different grazing regimes. Rangel. Ecol. Manag. 2018, 71, 196–204. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wei, M.; Wu, B.D.; Wang, S.; Jiang, K. Alpine grassland degradation reduced plant species diversity and stability of plant communities in the Northern Tibet Plateau. Acta Oecologica 2019, 98, 25–29. [Google Scholar] [CrossRef]
- Wang, L.; Ali, A. Climate regulates the functional traits aboveground biomass relationships at a community-level in forests: A global meta-analysis. Sci. Total Environ. 2021, 761, 143238. [Google Scholar] [CrossRef]
- Jochum, M.; Fischer, M.; Isbell, F.; Roscher, C.; van der Plas, F.; Boch, S.; Boenisch, G.; Buchmann, N.; Catford, J.A.; Cavender-Bares, J.; et al. The results of biodiversity-ecosystem functioning experiments are realistic. Nat. Ecol. Evol. 2020, 4, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Mota, J.F.; Martínez-Hernández, F.; Salmerón-Sánchez, E.; Mendoza-Fernández, A.J.; Pérez-García, F.J.; Merlo, M.E. Spontaneous primary succession and vascular plant recovery in the Iberian gypsum quarries: Insights for ecological restoration in an EU priority habitat. Plants 2023, 12, 1162. [Google Scholar] [CrossRef]
- Ling, N.; Chen, D.; Guo, H.; Wei, J.; Bai, Y.; Shen, Q.; Hu, S. Differential responses of soil bacterial communities to long-term N and P inputs in a semi-arid steppe. Geoderma 2017, 292, 25–33. [Google Scholar] [CrossRef]
- Oksana, C.; Gerlinde, B.D.; Martine, V.D.P. Soil microbiota as game-changers in restoration of degraded lands. Science 2022, 375, 6584. [Google Scholar]
- Wang, J.; Li, W.; Cao, W.; Abalori, T.A.; Liu, Y.; Xin, Y.; Wang, S.; Zhang, D. Soil bacterial community responses to short-term grazing exclusion in a degraded alpine shrubland-grassland ecotone. Ecol. Indicat. 2021, 130, 108043. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, J.J.; Banerjee, S.; White, J.F.; Zhou, N.; Zhao, Z.Y.; Zhang, K.; Hu, M.-F.; Kinglsey, K.; Tian, C.-Y. Not by salinity alone: How environmental factors shape fungal communities in saline soils. Soil Sci. Soc. Am. J. 2019, 83, 1387–1398. [Google Scholar] [CrossRef]
- Araya, Y.N.; Bartelheimer, M.; Valle, C.J.; Crujeiras, R.M.; García-Baquero, G. Does functional soil microbial diversity contribute to explain within-site plant β-diversity in an alpine grassland and a dehesa meadow in Spain? J. Veg. Sci. 2017, 28, 1018–1027. [Google Scholar] [CrossRef]
- Calderón, K.; Spor, A.; Breuil, M.C.; Bru, D.; Bizouard, F.; Violle, C.; Barnard, R.L.; Philippot, L. Effectiveness of ecological rescue for altered soil microbial communities and functions. ISME J. 2017, 11, 272–283. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, F.; Wang, X.; Lu, N. Effects of meteorology and soil on the herb species diversity in plantations in a reclamation area of coal mine after 6 years. Environ. Sci. Pollut. Res. 2020, 27, 24231–24241. [Google Scholar] [CrossRef]
- Bao, G.; Qin, Z.H.; Bao, Y.H.; Zhou, Y.; Li, W.J.; Sanjjav, A. NDVI-based long-term vegetation dynamics and its response to climatic change in the Mongolian Plateau. Remote Sens. 2014, 6, 8337–8358. [Google Scholar] [CrossRef]
- An, H.; Xu, K. The effect of grazing disturbance on soil properties in desert steppe. Acta Pratacult. Sin. 2013, 22, 35–42. [Google Scholar]
- Zeng, Q.C.; An, S.S.; Liu, Y. Soil bacterial community response to vegetation succession after fencing in the grassland of China. Sci. Total Environ. 2017, 609, 2–10. [Google Scholar] [CrossRef]
- Zhang, Q.D.; Wei, W.; Chen, L.D.; Yang, L.; Luo, Y.Q.; Cai, A.D. Plant traits in influencing soil moisture in semiarid grasslands of the Loess Plateau, China. Sci. Total Environ. 2020, 718, 137355. [Google Scholar] [CrossRef]
- Dong, Q.M.; Zhao, X.Q.; Wu, G.L.; Shi, J.J.; Ren, G.H. A review of formation mechanism and restoration measures of “black-soil-type” degraded grassland in the Qinghai-Tibetan Plateau. Environ. Earth Sci. 2013, 70, 2359–2370. [Google Scholar] [CrossRef]
- Eilers, K.G.; Lauber, C.L.; Knight, R.; Fierer, N. Shifts in bacterial community structure associated with inputs of low molecular weight carbon compounds to soil. Soil Biol. Biochem. 2010, 42, 896–903. [Google Scholar] [CrossRef]
- Prober, S.M.; Leff, J.W.; Bates, S.T.; Borer, E.T.; Firn, J.; Harpole, W.S. Plant diversity predicts beta but not alpha diversity of soil microbes across grasslands worldwide. Ecol. Lett. 2014, 18, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Zhang, Z.C.; Yu, Z.Q.; Shen, G.F.; Cheng, H.F.; Tao, S. Composition and diversity of soil microbial communities in the alpine wetland and alpine forest ecosystems on the Tibetan Plateau. Sci. Total Environ. 2020, 747, 141358. [Google Scholar] [CrossRef] [PubMed]
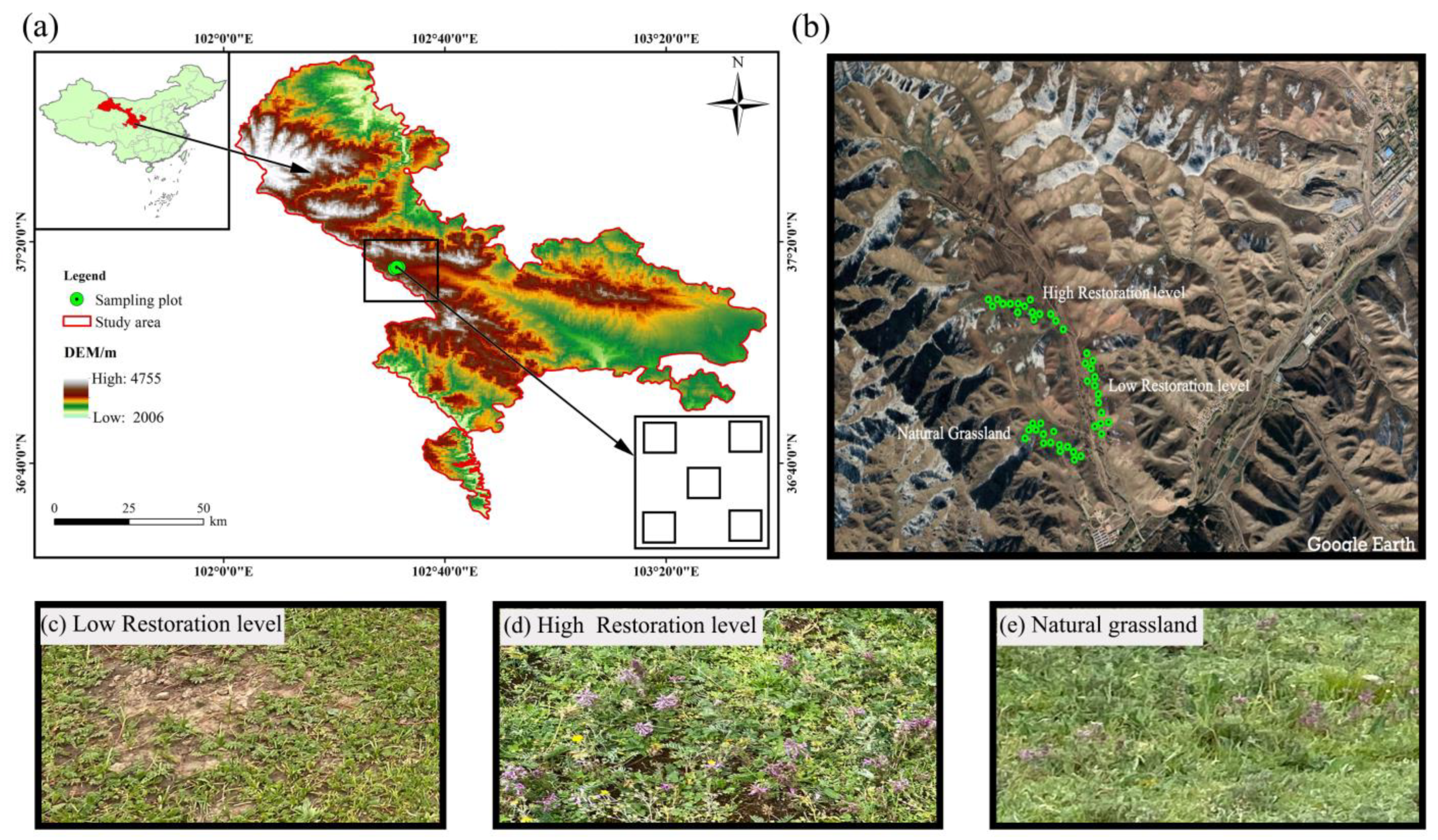


| Recovered Degree | Geography Coordinate | Altitude/m | Above-Ground Biomass (g m−2) | Vegetation Cover |
|---|---|---|---|---|
| Low restoration level (LR) | 37°25′67″ N 102°52′24″ E | 3294 | 130.73 ± 5.64 c | 0.25 ± 0.15 b |
| High restoration level (HR) | 37°25′61″ N 102°52′82″ E | 3292 | 360.85 ± 2.74 b | 0.77 ± 0.03 a |
| Natural grassland (NG) | 37°25′62″ N 102°52′18″ E | 3295 | 426.36 ± 9.05 a | 0.88 ± 0.05 a |
| Parameters | LR | HR | NG | p |
|---|---|---|---|---|
| Density (n m−2) | 269.10 ± 45.49 c | 377.33 ± 11.55 b | 385.16 ± 17.63 a | 0.354 |
| Height (cm) | 7.79 ± 0.49 b | 12.96 ± 4.04 a | 15.24 ± 0.17 a | <0.001 |
| Shannon–Wiener index | 1.69 ± 0.33 b | 2.20 ± 0.23 a | 2.32 ± 0.29 a | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Feng, Q.; Zhu, M.; Yang, L.; Zhang, C.; Zhang, J.; Wang, Z.; Feng, Y. Changes in Nutrient-Regulated Soil Microbial Communities in Soils Concomitant with Grassland Restoration in the Alpine Mining Region of the Qilian Mountains. Agronomy 2023, 13, 3052. https://doi.org/10.3390/agronomy13123052
Yang X, Feng Q, Zhu M, Yang L, Zhang C, Zhang J, Wang Z, Feng Y. Changes in Nutrient-Regulated Soil Microbial Communities in Soils Concomitant with Grassland Restoration in the Alpine Mining Region of the Qilian Mountains. Agronomy. 2023; 13(12):3052. https://doi.org/10.3390/agronomy13123052
Chicago/Turabian StyleYang, Xiaomei, Qi Feng, Meng Zhu, Linshan Yang, Chengqi Zhang, Jutao Zhang, Zhiyang Wang, and Yonglin Feng. 2023. "Changes in Nutrient-Regulated Soil Microbial Communities in Soils Concomitant with Grassland Restoration in the Alpine Mining Region of the Qilian Mountains" Agronomy 13, no. 12: 3052. https://doi.org/10.3390/agronomy13123052
APA StyleYang, X., Feng, Q., Zhu, M., Yang, L., Zhang, C., Zhang, J., Wang, Z., & Feng, Y. (2023). Changes in Nutrient-Regulated Soil Microbial Communities in Soils Concomitant with Grassland Restoration in the Alpine Mining Region of the Qilian Mountains. Agronomy, 13(12), 3052. https://doi.org/10.3390/agronomy13123052











