Changes in Soil Chemical Properties and Rhizosphere Bacterial Community Induced by Soil Amendments Associated with Reduction in Cadmium Accumulation by Rice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Description and Experimental Design
2.2. Sample Collection
2.3. Chemical Analysis
2.4. DNA Extraction, PCR Amplification, and High-Throughput Sequencing
2.5. Statistical Analysis
3. Results and Discussion
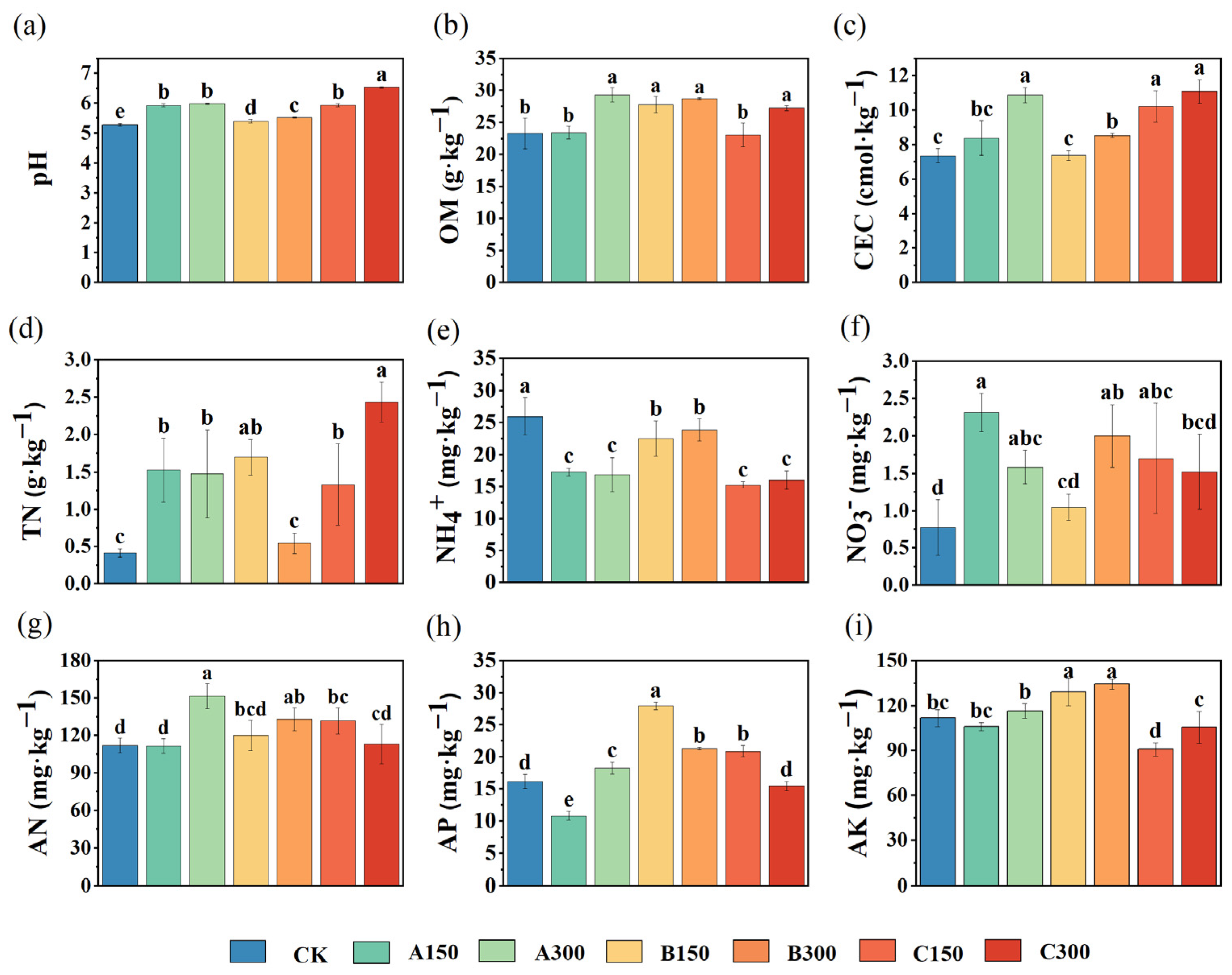
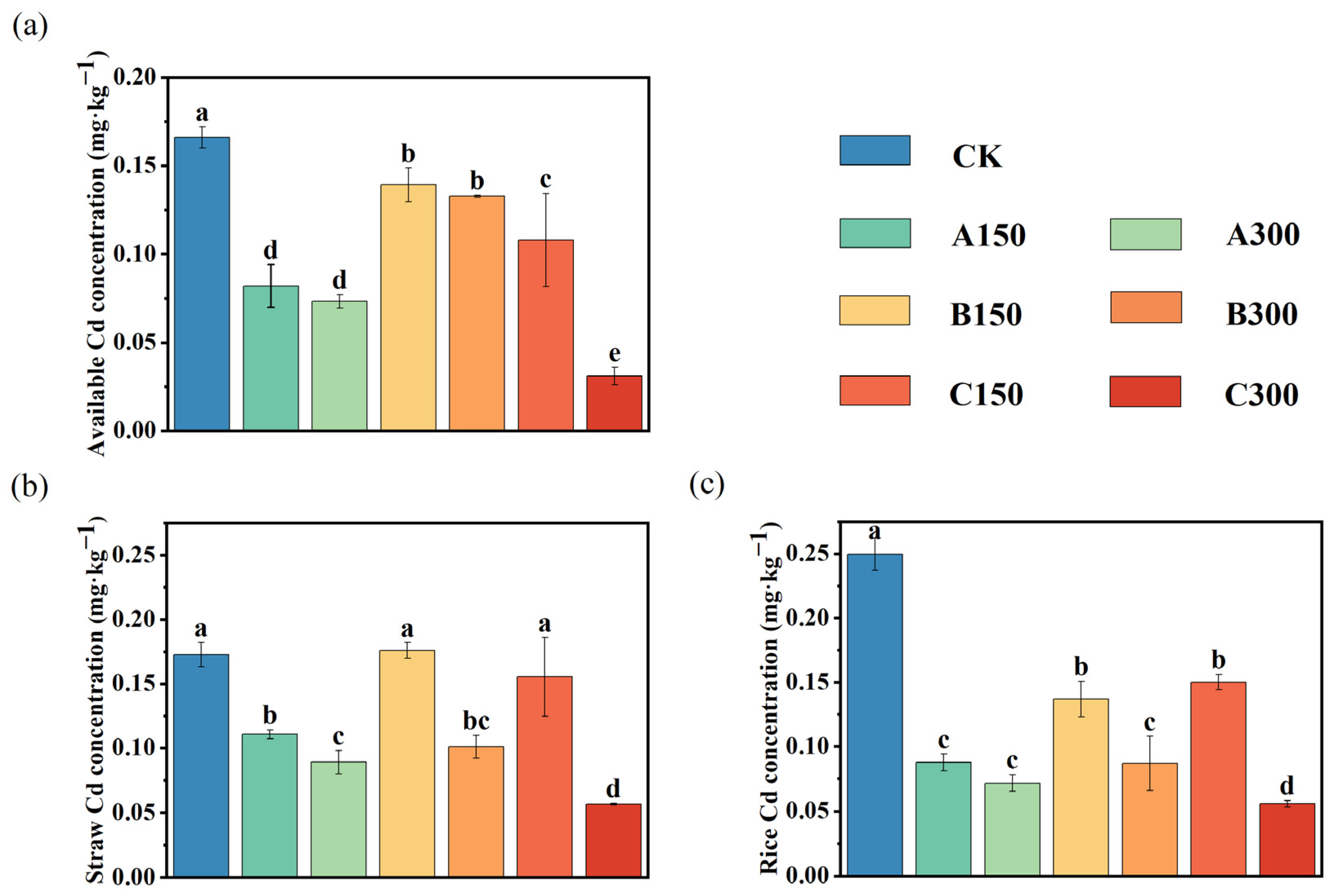
3.1. Effects of Soil Amendments on Soil Chemical Properties and Total Cd Accumulation in Rice
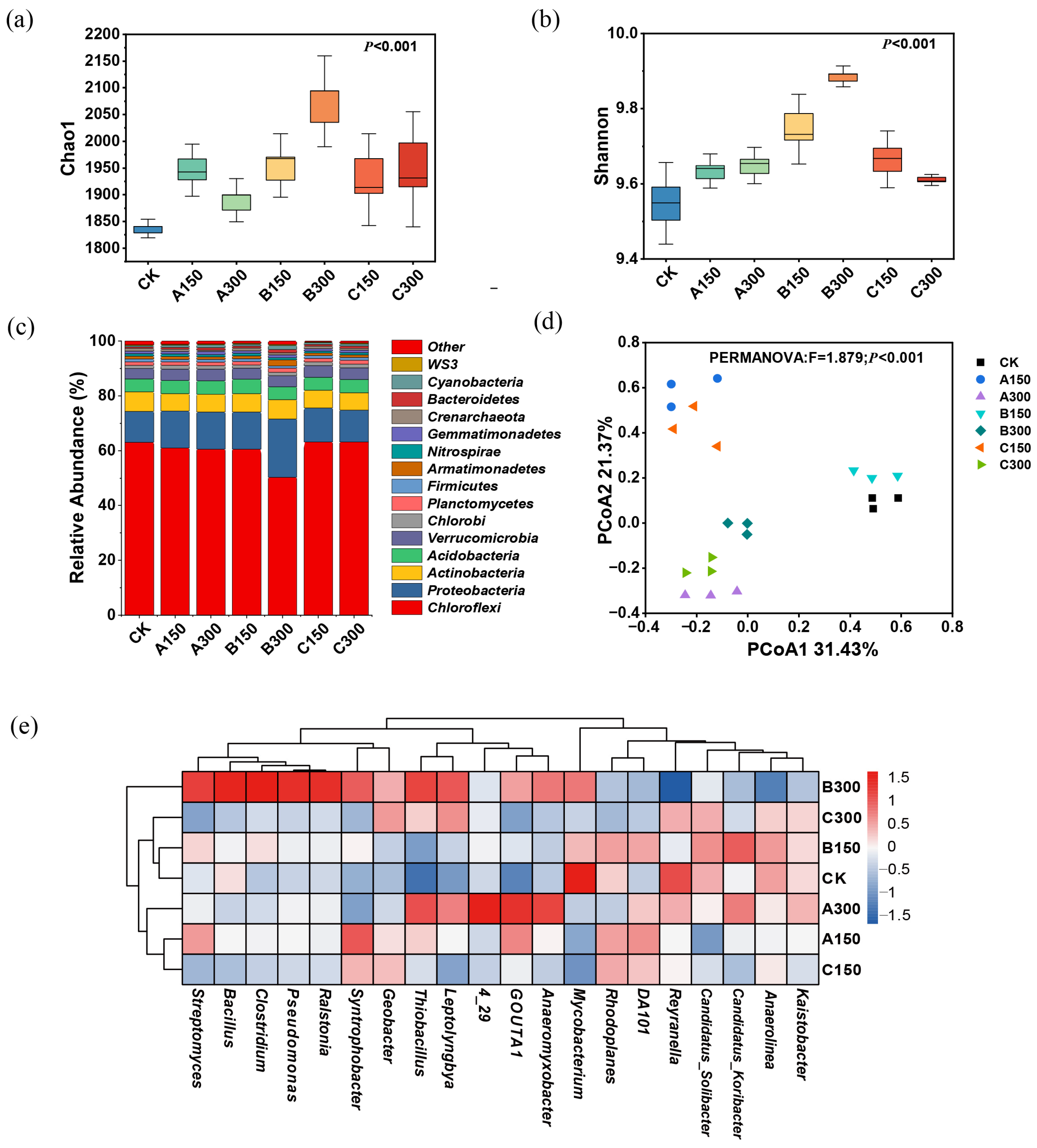
3.2. Effects of Soil Amendments on Rhizosphere Bacterial Community Diversity and Composition
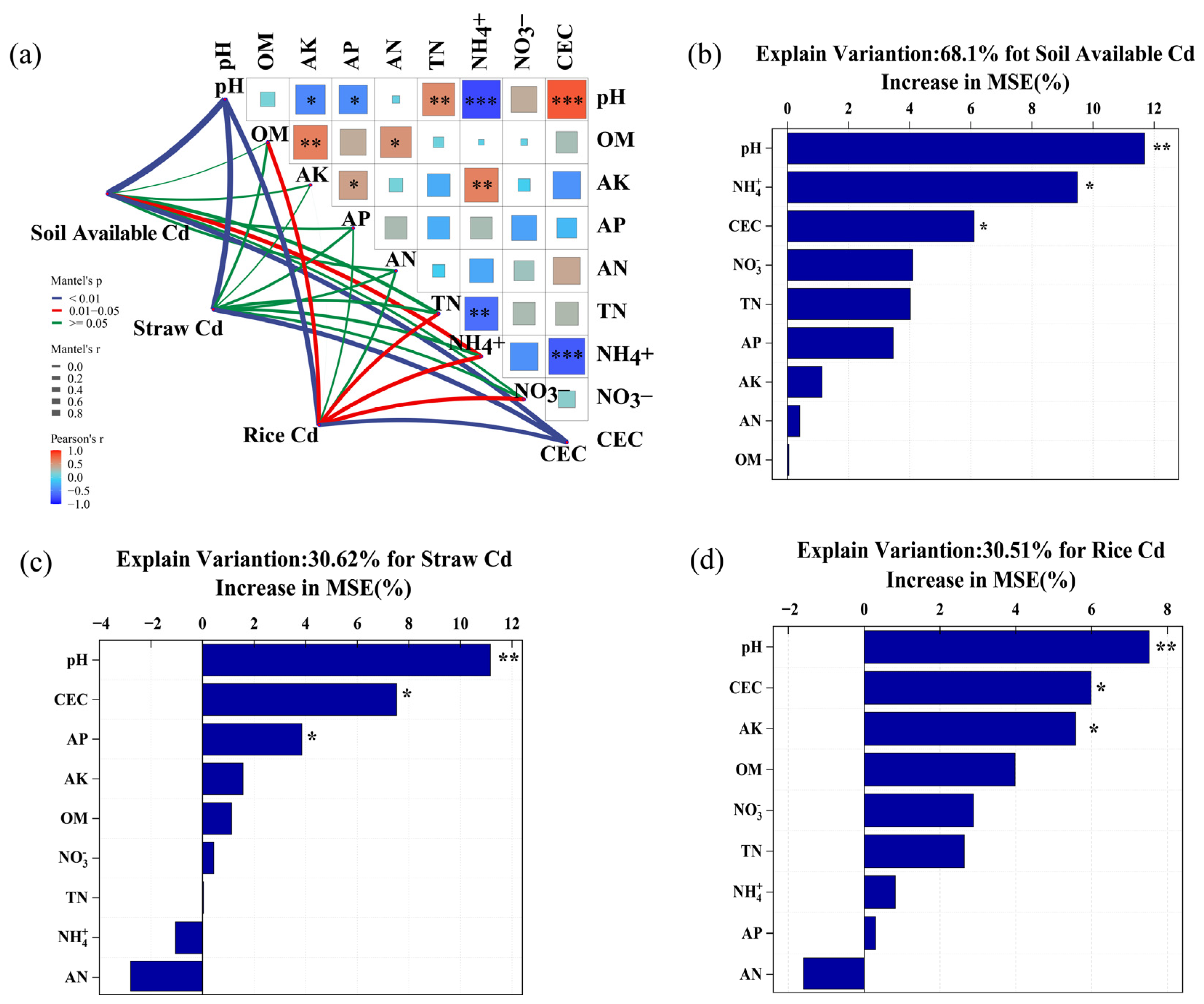
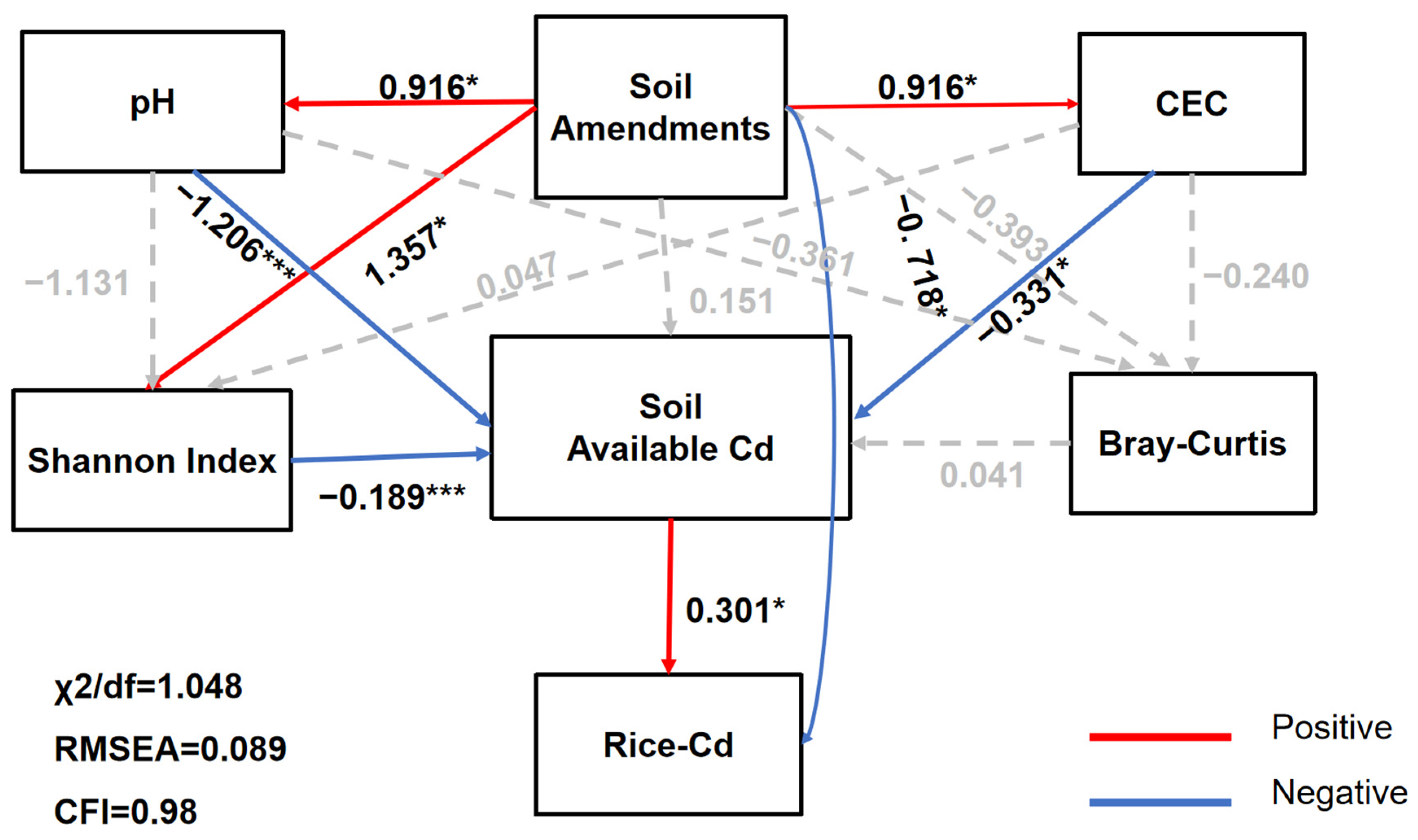
3.3. Correlations between Soil Variables and Bacterial Community Diversity and Composition
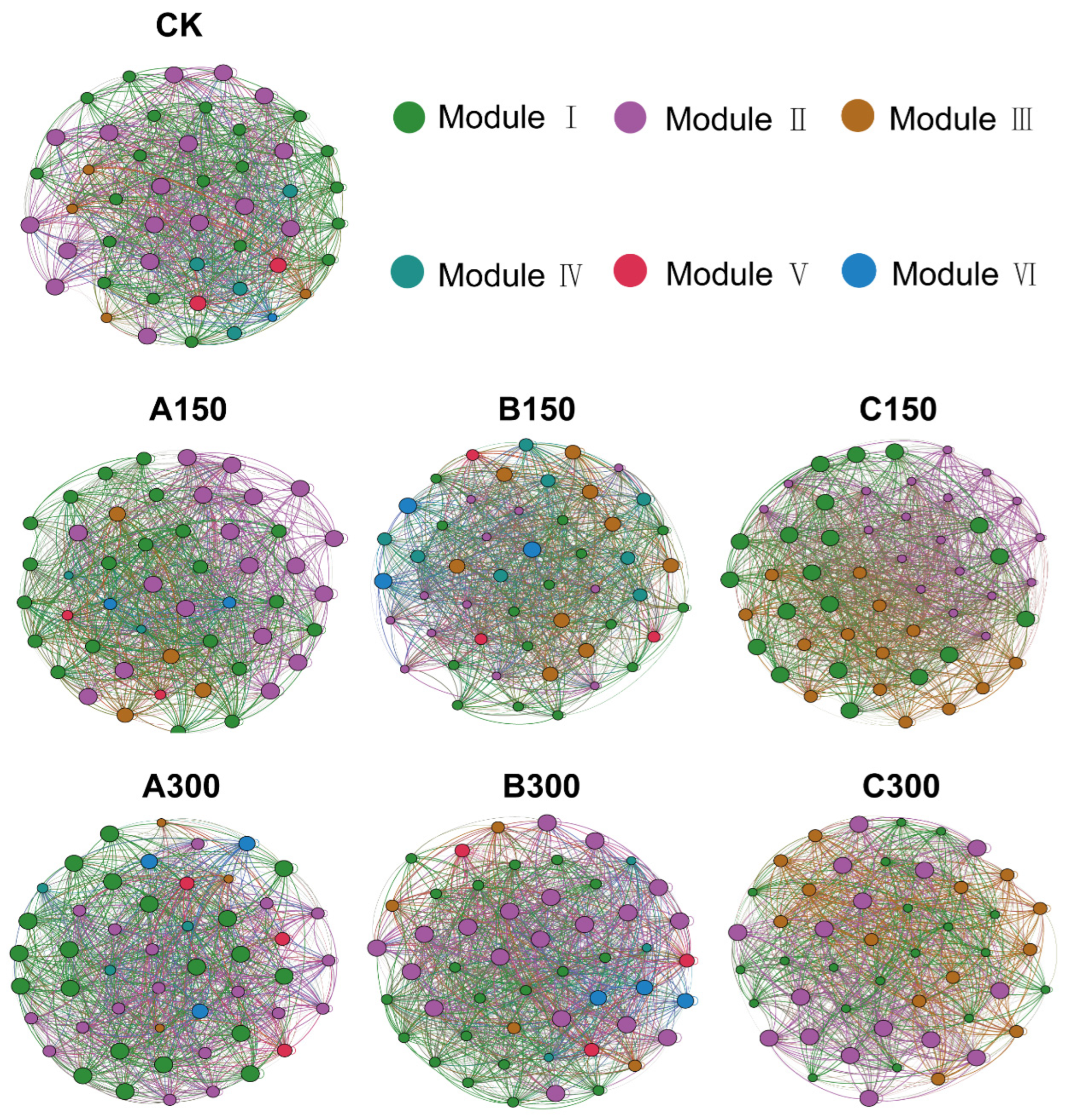
3.4. Microbial Co-Occurrence Networks
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ye, X.; Ma, Y.; Sun, B. Influence of soil type and genotype on Cd bioavailability and uptake by rice and implications for food safety. J. Environ. Sci. 2012, 24, 1647–1654. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Statistics Division of Food and Agriculture; Organization of the United Nations: Rome, Italy, 2019; Available online: http://www.fao.org/faostat/en/#data (accessed on 28 October 2023).
- Li, G.Q.; Gao, P.K.; Wu, Y.Q.; Tian, H.M.; Dai, X.C.; Wang, Y.S.; Cui, Q.F.; Zhang, H.Z.; Dong, H.P.; Ma, T. Microbial abundance and community composition influence production performance in a lowetemperature petroleum reservoir. Environ. Sci. Technol. 2014, 48, 5336–5344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; He, Y.; Lin, D.; Yao, Y.P.; Song, N.N.; Wang, F.L. Co-application of biochar and nitrogen fertilizer promotes rice performance, decreases cadmium availability, and shapes rhizosphere bacterial community in paddy soil. Environ. Pollut. 2022, 308, 119624. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; Yin, L.; Deng, X.P.; Zhang, Z.Y. Combined application of silicon and nitric oxide jointly alleviated cadmium accumulation and toxicity in maize. J. Hazard. Mater. 2020, 395, 122679. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Duan, Z.Q.; Zhang, L.X.; Sun, D.; Li, X. The Status and Research Progress of Cadmium Pollution in Rice-(Oryza sativa L.) and Wheat-(Triticum aestivum L.) Cropping Systems in China: A Critical Review. Toxics 2022, 10, 794. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Yin, D.X.; Peng, B.; Tan, C.Y.; Liu, Y.G.; Tan, X.F.; Wu, S.X. Efficiency and mechanisms of Cd removal from aqueous solution by biochar derived from water hyacinth (Eichornia crassipes). J. Environ. Manag. 2015, 153, 68–73. [Google Scholar] [CrossRef]
- Cui, L.Q.; Yan, J.L.; Yang, Y.G.; Li, L.Q.; Quan, G.X.; Ding, C.; Chen, T.M.; Fu, Q.; Chang, A. Influence of biochar on microbial activities of heavy metals contaminated paddy fields. BioResources 2013, 8, 5536–5548. [Google Scholar] [CrossRef]
- Jiang, Y.X.; Hu, T.; Peng, O.; Chen, A.W.; Tie, B.Q.; Shao, J.H. Impact of heavy metal passivators on the nitrogenase activity and diazotrophic community in a cadmium-contaminated paddy field. Int. Biodeterior. Biodegrad. 2022, 175, 105506. [Google Scholar] [CrossRef]
- Sun, Y.B.; Sun, G.H.; Xu, Y.M.; Liu, W.T.; Liang, X.F.; Wang, L. Evaluation of the effectiveness of sepiolite, bentonite, and phosphate amendments on the stabilization remediation of cadmium-contaminated soils. J. Environ. Manag. 2016, 166, 204–210. [Google Scholar] [CrossRef]
- Hong, Y.; Li, D.; Xie, C.; Zheng, X.X.; Yin, J.; Li, Z.D.; Zhu, Z.Q. Combined apatite, biochar, and organic fertilizer application for heavy metal co-contaminated soil remediation reduces heavy metal transport and alters soil microbial community structure. Sci. Total Environ. 2022, 851, 158033. [Google Scholar] [CrossRef]
- Guo, J.H.; Yan, C.Z.; Luo, Z.X.; Fang, H.D.; Hu, S.G.; Cao, Y.L. Synthesis of a novel ternary HA/Fe-Mn oxides-loaded biochar composite and its application in cadmium(II) and arsenic(V) adsorption. J. Environ. Sci. 2019, 85, 168–176. [Google Scholar] [CrossRef]
- Cheng, Z.Y.; Shi, J.C.; He, Y.; Wu, L.S.; Xu, J.M. Assembly of root-associated bacterial community in cadmium contaminated soil following five-year consecutive application of soil amendments: Evidences for improved soil health. J. Hazard. Mater. 2022, 426, 128095. [Google Scholar] [CrossRef]
- Meng, L.; Huang, T.H.; Shi, J.C.; Chen, J.; Zhong, F.L.; Wu, L.S.; Xu, J.M. Decreasing cadmium uptake of rice (Oryza sativa L.) in the cadmium-contaminated paddy field through different cultivars coupling with appropriate soil amendments. J. Soils Sediments 2019, 19, 1788–1798. [Google Scholar] [CrossRef]
- Luo, X.L.; Zhao, B.C.; Peng, M.G.; Shen, R.Y.; Mao, L.Q.; Zhang, W.Y. Effects of Inorganic Passivators on Gas Production and Heavy Metal Passivation Performance during Anaerobic Digestion of Pig Manure and Corn Straw. Int. J. Environ. Res. Public Health 2022, 19, 14094. [Google Scholar] [CrossRef]
- Yang, Y.J.; Xiong, J.; Tao, L.X.; Cao, Z.Z.; Tang, W.; Zhang, J.P.; Yu, X.Y.; Fu, G.F.; Zhang, X.F.; Lu, Y.L. Regulatory mechanisms of nitrogen (N) on cadmium (Cd) uptake and accumulation in plants: A review. Sci. Total Environ. 2020, 708, 135186. [Google Scholar] [CrossRef]
- Liu, M.S.; Almatrafi, E.; Zhang, Y.; Xu, P.; Song, B.; Zhou, C.Y.; Zhu, Y. A critical review of biochar-based materials for the remediation of heavy metal contaminated environment: Applications and practical evaluations. Sci. Total Environ. 2022, 806 Pt 1, 150531. [Google Scholar] [CrossRef]
- Li, Q.; Yin, J.; Wu, L.L.; Li, S.L.; Chen, L. Effects of biochar and zero valent iron on the bioavailability and potential toxicity of heavy metals in contaminated soil at the field scale. Sci. Total Environ. 2023, 897, 165386. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.T.; Zhou, H.; Gu, J.F.; Liao, B.H.; Peng, P.Q.; Zeng, Q.R. Effects of a combined amendment on Pb, Cd, and as availability andaccumulation in rice planted in contaminated paddy soil. Soil Sediment Contam. Int. J. 2017, 26, 70–83. [Google Scholar] [CrossRef]
- Chen, X.M.; Zhao, Y.; Zeng, C.C.; Li, Y.L.; Zhu, L.J.; Wu, J.Q.; Chen, J.; Wei, Z.M. Assessment contributions of physicochemical properties and bacterial community to mitigate the bioavailability of heavy metals during composting based on structural equation models. Bioresour. Technol. 2019, 289, 121657. [Google Scholar] [CrossRef] [PubMed]
- He, L.Z.; Zhong, H.; Liu, G.X.; Dai, Z.; Xu, J.M. Remediation of heavy metal contaminated soils by biochar: Mechanisms, potential risks and applications in China. Environ. Pollut. 2019, 252, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Zhang, J.; Li, X.S.; Li, R.D. Effects of typical modified passivators on speciation of heavy metals in protein extracted from sewage sludge. Environ. Sci. Pollut. Res. Int. 2019, 26, 10875–10886. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, P.B.; Zhan, Q.; Bocharnikova, E.; Matichenkov, V. Regulation of As and Cd accumulation in rice by simultaneous application of lime or gypsum with Si-rich materials. Environ. Sci. Pollut. Res. Int. 2021, 28, 7271–7280. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Wu, X.S.; Teng, Z.N.; Lou, X.Y.; Han, X.B.; Li, Z.X.; Li, G. Effects of adding biochar on the preservation of nitrogen and passivation of heavy metal during hyperthermophilic composting of sewage sludge. J. Air Waste Manag. Assoc. 2023, 73, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Oustriere, N.; Marchand, L.; Galland, W.; Gabbon, L.; Lottier, N.; Motelica, M.; Mench, M. Influence of biochars, compost and iron grit, alone and in combination, on copper solubility and phytotoxicity in a Cu-contaminated soil from a wood preservation site. Sci. Total Environ. 2016, 566, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Hamid, Y.; Tang, L.; Hussain, B.; Usman, M.; Gurajala, H.K.; Rashid, M.S.; He, Z.L.; Yang, X. Efficiency of lime, biochar, Fe containing biochar and composite amendments for Cd and Pb immobilization in a co-contaminated alluvial soil. Environ. Pollut. 2020, 257, 113609. [Google Scholar] [CrossRef] [PubMed]
- Li, P.F.; Liu, M.; Ma, X.Y.; Wu, M.; Jiang, C.Y.; Liu, K.; Liu, J.; Li, Z.P. Responses of microbial communities to a gradient of pig manure amendment in red paddy soils. Sci. Total. Environ. 2020, 705, 135884. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Q.; Xu, R.; Sun, X.X.; Han, F.; Xiao, E.Z.; Chen, L.; Qiu, L.; Sun, W.M. Microbiome–environment interactions in antimony-contaminated rice paddies and the correlation of core microbiome with arsenic and antimony contamination. Chemosphere 2021, 263, 128227. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.M.; Chen, B.L.; Zhu, L.Z.; Xing, B.S. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.L.; Dai, W.J.; Zhao, Z.L.; Zheng, J.T.; Huang, F.; Mei, C.; Huang, S.T.; Liu, C.F.; Wang, P.; Xiao, R.B. Effect of rice straw biochar on three different levels of Cd-contaminated soils: Cd availability, soil properties, and microbial communities. Chemosphere 2022, 301, 134551. [Google Scholar] [CrossRef]
- Li, X.Q.; Meng, D.L.; Li, J.; Yan, M.L. Response of soil microbial communities and microbial interactions to long-term heavy metal contamination. Environ. Pollut. 2017, 231, 908–917. [Google Scholar] [CrossRef]
- Cui, H.B.; Fan, Y.C.; Yang, J.; Xu, L.; Zhu, Z.Q. In situ phytoextraction of copper and cadmium and its biological impacts in acidic soil. Chemosphere 2016, 161, 233–241. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Adrees, M.; Rizvi, H.; Rehman, M.Z.; Hannan, F.; Qayyum, M.F.; Hafeez, F.; OK, Y.S. Cadmium stress in rice: Toxic effects, tolerance mechanisms and management: A critical review. Environ. Sci. Pollut. Res. 2016, 23, 17859–17879. [Google Scholar] [CrossRef] [PubMed]
- Woldetsadik, D.; Drechsel, P.; Keraita, B.; Marschner, B.; Itanna, F.; Gebrekidan, H. Effects of biochar and alkaline amendments on cadmium immobilization, selected nutrient and cadmium concentrations of lettuce (Lactuca sativa) in two contrasting soils. SpringerPlus 2016, 5, 397. [Google Scholar] [CrossRef]
- GB 15618-2018; Soil Environmental Quality Agricultural Land Soil Pollution Risk Control Standards. China Environmental Science Press: Beijing, China, 2018.
- Edwards, J.; Santos-Medellín, C.; Sundaresan, V. Extraction and 16S rRNA sequence analysis of microbiomes associated with rice Roots. Bio-Protocol 2018, 8, e2884. [Google Scholar] [CrossRef]
- Chen, L.; Guo, L.; Zhou, Q.; Liu, M.; Zhan, S.; Pan, X.; Zeng, Y. Response of soil fertility and Cu and Cd availability to biochar application on paddy soils with diferent acidifcation levels. Biomass Convers. Biorefinery 2020, 12, 1493–1502. [Google Scholar] [CrossRef]
- Chen, L.; Guo, L.; Liao, P.; Xiong, Q.; Deng, X.; Gao, H.; Wei, H.; Dai, Q.; Pan, X.; Zeng, Y.; et al. Rice straw biochar reduces Cd accumulation and promotes Cu accumulation in rice: Irrigation regime is the driving factor. J. Soil Sediments 2022, 23, 193–205. [Google Scholar] [CrossRef]
- Shidan, B. Soil Agrochemical Analysis, 3rd ed.; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Luo, W.X.; Yang, S.N.; Khan, M.A.; Liu, D. Mitigation of Cd accumulation in rice with water management and calcium-magnesium phosphate fertilizer in field environment. Environ. Geochem. Health 2020, 42, 3877–3886. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.G.; Ying, Y.Q.; Lu, S.G. Si-Ca-K-Mg amendment reduces the phytoavailability and transfer of Cd from acidic soil to rice grain. Environ. Sci. Pollut. Res. Int. 2020, 27, 33248–33258. [Google Scholar] [CrossRef]
- Yang, W.H.; Li, C.J.; Wang, S.S.; Zhou, B.Q.; Mao, Y.L. Influence of biochar and biochar-based fertilizer on yield, quality of tea and microbial community in an acid tea orchard soil. Appl. Soil Ecol. 2021, 166, 104005. [Google Scholar] [CrossRef]
- Wang, Z.W.; Liu, S.R.; Ruan, Y.Z.; Wang, Q.; Zhang, Z.J. Comparison of Biochar- and Lime-Adjusted pH Changes in N2O Emissions and Associated Microbial Communities in a Tropical Tea Plantation Soil. Agronomy 2023, 13, 1144. [Google Scholar] [CrossRef]
- Xu, D.M.; Fu, R.B.; Wang, J.X.; Shi, Y.X.; Guo, X.P. Chemical stabilization remediation for heavy metals in contaminated soils on the latest decade: Available stabilizing materials and associated evaluation methods-a critical review. J. Clean Prod. 2021, 321, 128730. [Google Scholar] [CrossRef]
- Garcia, M.A.; Chimenos, J.M.; Fernández, A.I.; Miralles, L.; Segarra, M. Low-grade MgO used to stabilize heavy metals in highly contaminated soils. Chemosphere 2004, 56, 481–491. [Google Scholar] [CrossRef]
- Liu, J.; Ma, J.; He, C.W.; Li, X.L.; Zhang, W.J.; Xu, F.S.; Lin, Y.J.; Wang, L.J. Inhibition of cadmium ion uptake in rice (Oryza sativa) cells by a wall-bound form of silicon. New Phytol. 2013, 200, 691–699. [Google Scholar] [CrossRef]
- Neumann, D.; zur Nieden, U. Silicon and heavy metal tolerance of higher plants. Phytochemistry 2001, 56, 685–692. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Zhang, H.; Shao, J.A.; Zhang, S.H.; Zhang, X.; Chen, H.P. Effect of phosphorus-modified biochars on immobilization of Cu (II), Cd (II), and As (V) in paddy soil. J. Hazard. Mater. 2019, 390, 121349. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.N.; Xu, C.Y.; Tahmasbian, I.; Che, R.X.; Xu, Z.H.; Zhou, X.H.; Wallace, H.M.; Bai, S.H. Effects of biochar on soil available inorganic nitrogen: A review and meta-analysis. Geoderma 2017, 288, 79–96. [Google Scholar] [CrossRef]
- Yi, X.Y.; Ji, L.F.; Hu, Z.M.; Yang, X.D.; Li, H.T.; Jiang, Y.Y.; Ruan, J.Y. Organic amendments improved soil quality and reduced ecological risks of heavy metals in a long-term tea plantation field trial on an Alfisol. Sci. Total Environ. 2022, 828, 156017. [Google Scholar] [CrossRef]
- Jezequel, K.; Perrin, J.; Lebeau, T. Bioaugmentation with a Bacillus sp. to reduce the phytoavailable Cd of an agricultural soil: Comparison of free and immobilized microbial inocula. Chemosphere 2005, 59, 1323–1331. [Google Scholar] [CrossRef]
- Yang, Y.L.; Hu, X.J.; Wang, H.F.; Zhong, X.L.; Chen, K.S.; Huang, B.; Qian, C.X. Corncob biochar combined with Bacillus subtilis to reduce Cd availability in low Cd-contaminated soil. RSC Adv. 2022, 12, 47. [Google Scholar] [CrossRef]
- Bian, F.F.; Zhong, Z.K.; Zhang, X.P.; Li, Q.L.; Huang, Z.Y. Bamboo-based agroforestry changes phytoremediation efficiency by affecting soil properties in rhizosphere and non-rhizosphere in heavy metal-polluted soil (Cd/Zn/Cu). J. Soils Sediments 2022, 23, 368–378. [Google Scholar] [CrossRef]
- Ali, A.; Guo, D.; Li, Y.; Shaheen, S.M.; Wahid, F.; Antoniadis, V.; Abdelrahman, H.; Al-Solaimani, S.G.; Li, R.; Tsang, D.C.W.; et al. Streptomyces pactum addition to contaminated mining soils improved soil quality and enhanced metals phytoextraction by wheat in a green remediation trial. Chemosphere 2021, 273, 129692. [Google Scholar] [CrossRef] [PubMed]
- Song, A.; Li, Z.M.; Wang, E.Z.; Xu, D.Y.; Wang, S.; Bi, J.J.; Fan, F.L. Supplying silicon alters microbial community and reduces soil cadmium bioavailability to promote health wheat growth and yield. Sci. Total Environ. 2021, 796, 148797. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Han, L.F.; Yang, Y.; Xia, X.H.; Yang, Z.F.; Wu, F.C.; Li, F.B.; Feng, Y.F.; Xing, B.S. Application of hydrochar altered soil microbial community composition and the molecular structure of native soil organic carbon in a paddy soil. Environ. Sci. Technol. 2020, 54, 2715–2725. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Shao, L.M.; Zhang, H.; He, P.J.; Lü, F. Quantifying the contributions of surface area and redox-active moieties to electron exchange capacities of biochar. J. Hazard. Mater. 2020, 394, 122541. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.J.; Wang, Y.; Wang, F.; Xia, S.Q.; Zhao, J.F. Struvite-supported biochar composite effectively lowers cu bio-availability and the abundance of antibioticresistance genes in soil. Sci. Total Environ. 2020, 724, 13829. [Google Scholar] [CrossRef]
- Jiang, Z.W.; Yang, S.H.; Pang, Q.Q.; Xu, Y.; Chen, X.; Sun, X.; Qi, S.T.; Yu, W.Q. Biochar improved soil health and mitigated greenhouse gas emission from controlled irrigation paddy field: Insights into microbial diversity. J. Clean. Prod. 2021, 318, 128595. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, Y.; Liu, M.; Wei, L.; Gao, Y.; Ruan, Y.; Wang, Q.; Zhang, Z. Changes in Soil Chemical Properties and Rhizosphere Bacterial Community Induced by Soil Amendments Associated with Reduction in Cadmium Accumulation by Rice. Agronomy 2023, 13, 3051. https://doi.org/10.3390/agronomy13123051
Xin Y, Liu M, Wei L, Gao Y, Ruan Y, Wang Q, Zhang Z. Changes in Soil Chemical Properties and Rhizosphere Bacterial Community Induced by Soil Amendments Associated with Reduction in Cadmium Accumulation by Rice. Agronomy. 2023; 13(12):3051. https://doi.org/10.3390/agronomy13123051
Chicago/Turabian StyleXin, Yu, Min Liu, Lanchun Wei, Yu Gao, Yunze Ruan, Qing Wang, and Zhijun Zhang. 2023. "Changes in Soil Chemical Properties and Rhizosphere Bacterial Community Induced by Soil Amendments Associated with Reduction in Cadmium Accumulation by Rice" Agronomy 13, no. 12: 3051. https://doi.org/10.3390/agronomy13123051
APA StyleXin, Y., Liu, M., Wei, L., Gao, Y., Ruan, Y., Wang, Q., & Zhang, Z. (2023). Changes in Soil Chemical Properties and Rhizosphere Bacterial Community Induced by Soil Amendments Associated with Reduction in Cadmium Accumulation by Rice. Agronomy, 13(12), 3051. https://doi.org/10.3390/agronomy13123051




