Plant and Microorganism Combined Degradation of Bensulfuron Herbicide in Eight Different Agricultural Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Microorganisms, and Culture Medium
2.2. Agricultural Soil Samples
2.3. Inoculum Formulation
2.4. Biodegradation of Pesticides in Liquid Medium
2.5. Biodegradation of BN in Different Types of Soils
2.6. Biodegradation of BN in Sterile and Non-Sterile Soil
2.7. Combined Phytoremediation of Residual BN in Agricultural Soil
2.8. Analytical Method
2.9. Degradation Kinetics
2.10. Statistical Analyses
3. Results and Discussion
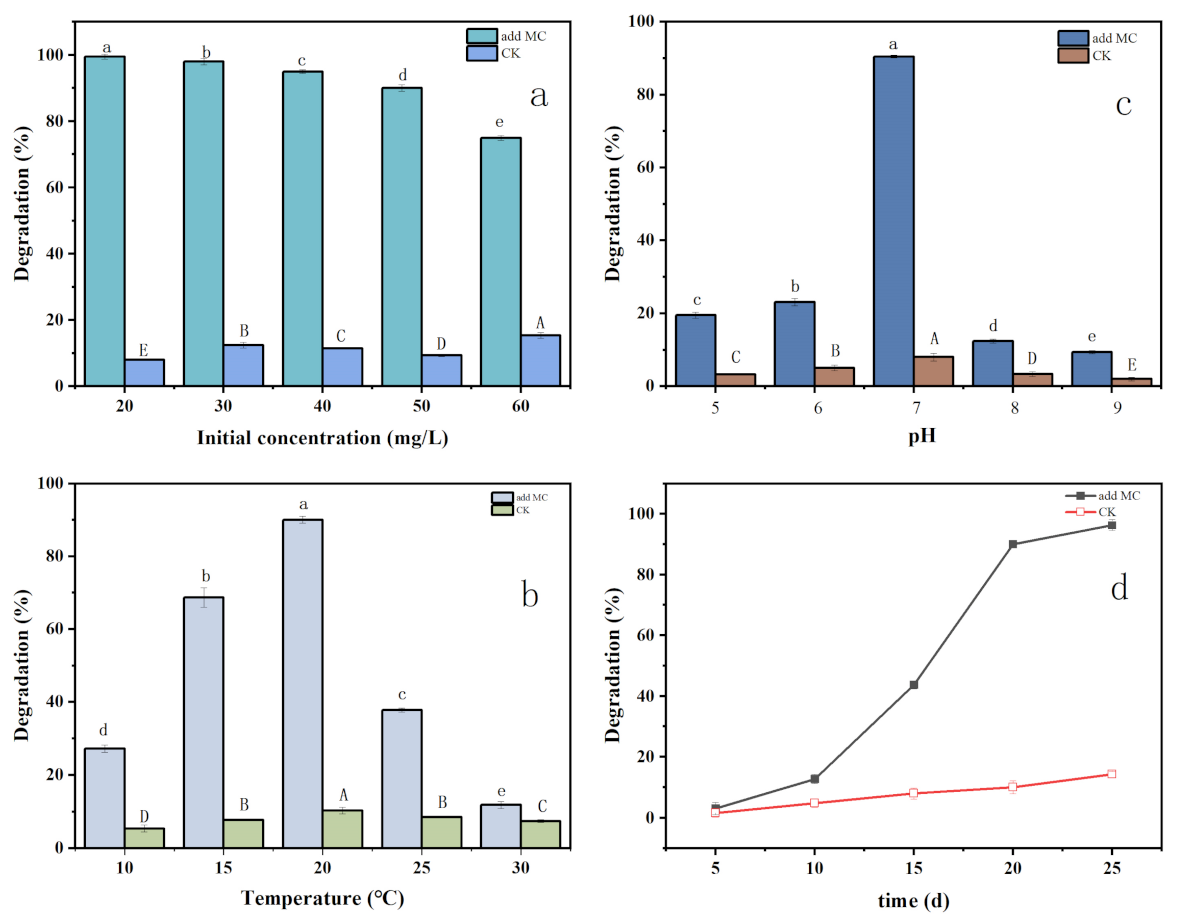
3.1. Analysis and Explanation of Biodegradation in Liquid Medium
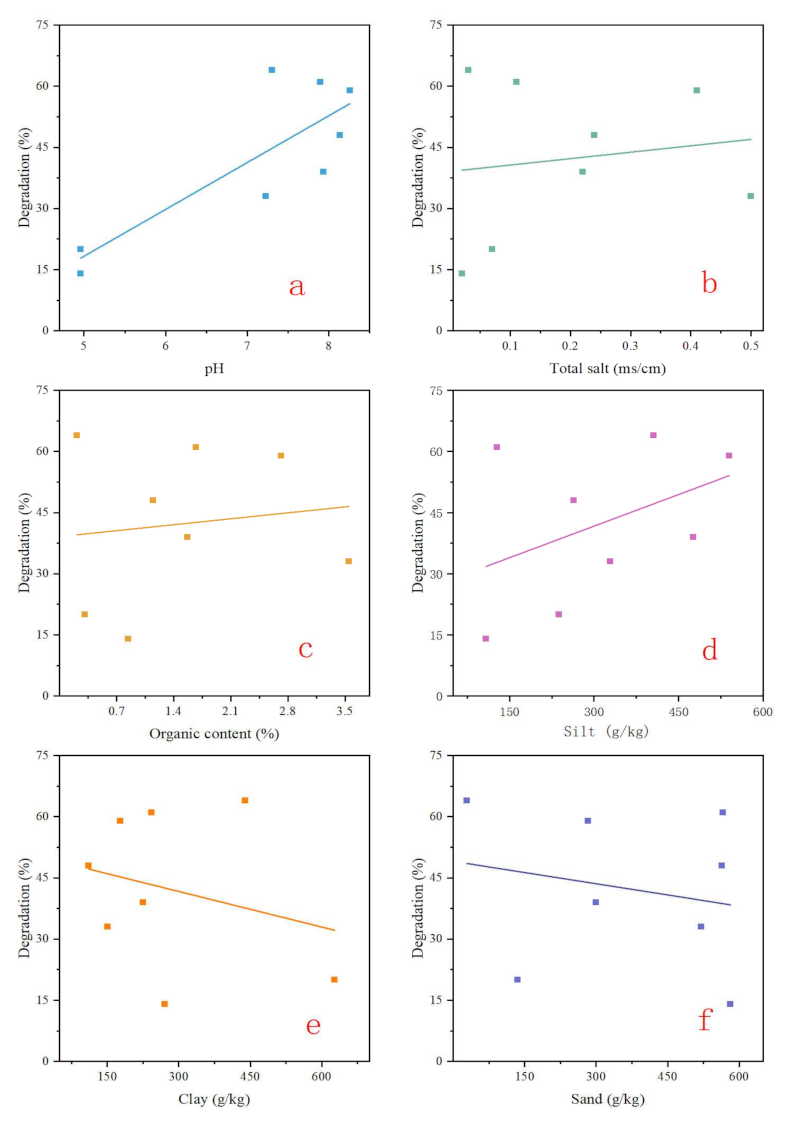
3.2. Soil Properties
3.3. Combined Phytoremediation of Residual BN in Agricultural Soil
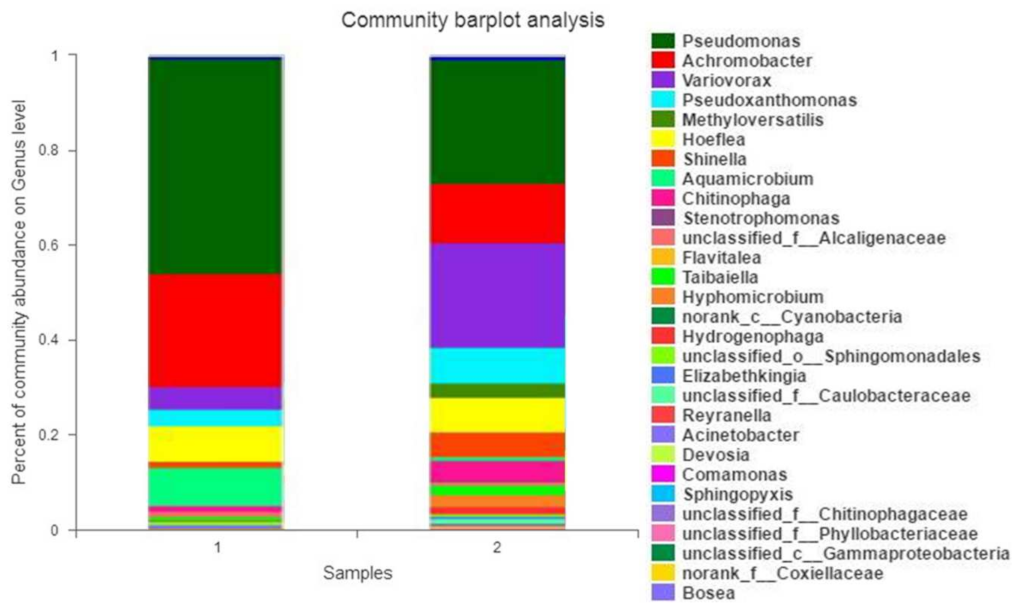
3.4. Composition Changes in MC before and after Degradation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Devendar, P.; Yang, G. Sulfur-Containing Agrochemicals. Top. Curr. Chem. 2017, 375, 82. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.M. Mode of Action, Crop Selectivity, and Soil Relations of The Sulfonylurea Herbicides. Pestic. Sci. 1990, 29, 263–281. [Google Scholar] [CrossRef]
- Sarmah, A.K.; Sabadie, J. Hydrolysis of Sulfonylurea Herbicides in Soils and Aqueous Solutions: A Review. J. Agric. Food Chem. 2002, 50, 6253–6265. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.S.; Hu, J. Research Progress of Sulfonylurea Herbicides and Weeds’ Resistance. Weed Sci. 2006, 4, 145. [Google Scholar] [CrossRef]
- Guo, M.; Shan, Z.J.; Shi, L.L.; Song, N.H.; Xu, J. Degradation and Adsorption Characteristics of Three Sulfonylurea Herbicides in Soil. J. Environ. Sci. 2012, 32, 1459–1464. [Google Scholar] [CrossRef]
- Sarmah, A.K.; Kookana, R.S.; Alston, A.M. Fate and behavior of triasulfuron, metsulfuron-methyl, and chlorsulfuron in the Australian soil environment: A review. Aust. J. Agric. Res. 1998, 49, 775–790. [Google Scholar] [CrossRef]
- Schneiders, G.E.; Koeppe, M.K.; Naidu, M.V.; Horne, P.; Brown, A.M.; Mucha, C.F. The fate of Rimsulfuron in the Environment. J. Agric. FoodChem. 1993, 41, 2404–2410. [Google Scholar] [CrossRef]
- Durner, J.; Gailus, V.; Boger, P. New Aspects on Inhibition of Plant Acetolactate Synthase by Chlorsulfuron and Imazaquin. Plant Physiol. 1991, 95, 1144–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.Y.; Liu, W.P.; Zhang, Y.S.; Liu, K.K. Action Mechanisms of Acetolactate Synthase-inhibiting Herbicides. Pestic. Biochem. Physiol. 2007, 89, 89–96. [Google Scholar] [CrossRef]
- Geng, Q.Q.; Li, T.L.; Wang, X.; Chu, W.J.; Cai, M.L.; Xie, J.C.; Ni, H.W. The mechanism of bensulfuron-methyl complexation with β-cyclodextrin and 2-hydroxypropyl-β-cyclodextrin and effect on soil adsorption and bio-activity. Sci. Rep. 2019, 9, 1882. [Google Scholar] [CrossRef]
- Boldt, T.S.; Jacobsen, C.S. Different Toxic Effects of The Sulfonylurea Herbicides Metsulfuron Methyl, Chlorsulfuron, and Thifensulfuron Methyl on Fluorescent Pseudomonads Isolated from an Agricultural Soil. Fems Microbiol. Lett. 1998, 161, 29–35. [Google Scholar] [CrossRef]
- Zheng, Y.Q. Research Progress and Prospect of Pesticide Residues. Plant Prot. 2013, 39, 90–98. [Google Scholar]
- Tomco, P.L.; Seefeldt, S.S.; Rodriguez-Baisi, K.; Hatton, J.J.; Duddleston, K.N. Sub-Arctic Field Degradation of Metsulfuron-Methyl in Two Alaskan Soils and Microbial Community Composition Effects. Water Air Soil Pollut. 2020, 231, 157. [Google Scholar] [CrossRef]
- Wang, M.X. Control Effect of Sulfosulfuron on Eupatorium Adenophorum and Its Effect on Detoxifying Enzymes in Eupatorium Hendel; Fujian Agriculture and Forestry University: Fuzhou, China, 2009. [Google Scholar]
- Wang, Y.J.; Hong, X.J.; Song, W.Y.; Wang, D.M. Degradation Effect of Chlorimuron-ethyl Degrading Bacteria. Heilongjiang Agric. Sci. 2012, 6, 73–76. [Google Scholar]
- Zhang, X.J. Sulfonylurea Herbicide-rimsulfuron. Mod. Agrochem. 2010, 9, 44–50. [Google Scholar]
- Ou, X.M.; Bu, H.Y. Research Progress on Hydrochemical Degradation Mechanism of Sulfonylurea Herbicides. J. Agric. Environ. Sci. 2007, 5, 1607–1614. [Google Scholar]
- Guan, J.Y. Study on Sample Pretreatment Technology for Residue Analysis of Sulfonylurea Herbicides in Complex Samples; Central China Normal University: Wuhan, China, 2012. [Google Scholar]
- Liang, Y.; Wang, W.; Shen, Y.; Liu, Y.; Liu, X.J. Effects of home preparation on organophosphorus pesticide residues in raw cucumber. Food Chem. 2012, 133, 636–640. [Google Scholar] [CrossRef]
- Fahl, G.M.; Kreft, L.; Altenburger, R.; Faust, M.; Boedeker, W.; Grimme, L.H. pH-Dependent Sorption, Bioconcentration, and Algal Toxicity of Sulfonylurea Herbicides. Aquat. Toxicol. 1995, 31, 175–187. [Google Scholar] [CrossRef]
- Al-Nasir, F.M.; Jiries, A.G.; Al-Rabadi, G.J.; Alu’datt, M.H.; Tranchant, C.C.; Al-Dalain, S.A.; Alrabadi, N.; Madanat, O.Y.; Al-Dmour, R.S. Determination of pesticide residues in selected citrus fruits and vegetables cultivated in the Jordan Valley. Lwt-Food Sci. Technol. 2020, 123, 109005. [Google Scholar] [CrossRef]
- Silva, F.B.; Costa, A.C.; Müller, C.; Nascimento, K.T.; Batista, P.F.; Vital, R.G.; Megguer, C.A.; Jakelaitis, A.; Domingos, M. Dipteryx alata, a tree native to the Brazilian Cerrado, is sensitive to the herbicide nicosulfuron. Ecotoxicology 2020, 29, 217–225. [Google Scholar] [CrossRef]
- Boutin, C.; Strandberg, B.; Carpenter, D.; Mathiassen, S.K.; Thomas, P.J. Herbicide impact on non-target plant reproduction: What are the toxicological and ecological implications? Environ. Pollut. 2014, 185, 295–306. [Google Scholar] [CrossRef] [Green Version]
- Ji, B.; Du, J.G.; Qi, H.S.; Zheng, Y.; Chen, J. Application of Microbial Agents in Soil Remediation. Jilin Agric. 2016, 6, 93–97. [Google Scholar] [CrossRef]
- Su, W.C.; Hao, H.D.; Wu, R.H.; Xu, H.L.; Xue, F.; Lu, C.T. Degradation of Mesotrione Affected by Environmental Conditions. Bull. Environ. Contam. Toxicol. 2017, 98, 212–217. [Google Scholar] [CrossRef]
- Berger, B.M.; Wolfe, N.L. Hydrolysis and Biodegradation of Sulfonylurea Herbicides in Aqueous Buffers and Anaerobic Water-sediment Systems: Assessing Fate Pathways Using Molecular Descriptors. Environ. Toxicol. Chem. 1996, 15, 1500–1507. [Google Scholar] [CrossRef]
- Boschin, G.; D’Agostina, A.; Arnoldi, A.; Marotta, E.; Zanardini, E.; Negri, M.; Valle, A.; Sorlini, C. Biodegradation of chlorsulfuron and metsulfuron-methyl by Aspergillus niger in laboratory conditions. J. Environ. Sci. Health Part B-Pestic. Food Contam. Agric. Wastes 2003, 38, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Zhao, Y.H.; Ding, H.T.; Lin, X.Y.; Zheng, H.B. Co-Metabolic Degradation of Bensulfuron-Methyl In Laboratory Conditions. J. Hazard. Mater. 2008, 158, 208–214. [Google Scholar] [CrossRef]
- Song, J.L.; Gu, J.G.; Zhai, Y.; Wu, W.; Wang, H.S.; Ruan, Z.Y.; Shi, Y.H.; Yan, Y.C. Biodegradation of nicosulfuron by a Talaromyces flavus LZM1. Bioresour. Technol. 2013, 140, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, D.; Si, H.; Wang, J.; Parales, R.E.; Zhang, J. Biotransformation of the herbicide nicosulfuron residues in soil and seven sulfonylurea herbicides by Bacillus subtilis YB1: A climate chamber study. Environ. Pollut. 2020, 263, 114492. [Google Scholar] [CrossRef]
- Feng, W.M.; Wei, Z.; Song, J.L.; Qin, Q.; Yu, K.M.; Li, G.C.; Zhang, J.Y.; Wu, W.; Yan, C. Hydrolysis of nicosulfuron under acidic environment caused by oxalate secretion of a novel Penicillium oxalicum strain YC-WM1. Sci. Rep. 2017, 7, 647. [Google Scholar] [CrossRef] [Green Version]
- Valle, A.; Boschin, G.; Negri, M.; Abbruscato, P.; Sorlini, C.; D’Agostina, A.; Zanardini, E. The Microbial Degradation of Azimsulfuron and Its Effect on The Soil Bacterial Community. J. Appl. Microbiol. 2006, 101, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Mikeskova, H.; Nocotny, C.; Svobodova, K. Interspecific Interactions in Mixed Microbial Cultures in a Biodegradation Perspective. Appl. Microbiol. Biotechnol. 2012, 95, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.F.; Liu, J.W.; Hu, C.C.; Yang, L.; Du, H.F.; Hu, K.; Huang, Y.J.; Yang, X.Q.; Jiang, Q.; Zhang, S.S. Liquid Chromatographic Behavior of Two Alanine-Substituted Calix[4]Arene-Bonded Silica Gel Stationary Phases. J. Sep. Sci. 2014, 37, 3268–3275. [Google Scholar] [CrossRef]
- Wu, C.X.; Nie, G.; Gao, L.M.; Wang, G.C.; Chen, B.K.; Xu, Y.Q.; Li, T. Research Progress on Microbial Degradation of Sulfonylurea Herbicides. Pestic. Sci. Manag. 2014, 35, 11–18. [Google Scholar]
- Morgante, V.; Flores, C.; Fadic, X.; Gonzalez, M.; Hernandez, M.; Cereceda-Balic, F.; Seeger, M. Influence of microorganisms and leaching on simazine attenuation in agricultural soil. J. Environ. Manag. 2012, 95, S300–S305. [Google Scholar] [CrossRef]
- Wang, M.Y.; Ma, Y.; Wang, H.Y.; Cao, G.; Li, Z.M. Kinetics of the Chemical Hydrolysis and 3D-QSAR Study of 5-Substituted Benzenesulfonylurea Compounds. Chem. J. Chin. Univ. Chin. 2016, 37, 1636–1642. [Google Scholar] [CrossRef]
- Boschin, G.; Valle, A.; D'Aqostina, A.; Negri, M.; Antonini, C.; Zanardini, E.; Sorlini, C.; Arnoldi, A. Chemical and microbial degradation of triflusulfuron-methyl and azimsulfuron under laboratory conditions. Pestic. Air Plant Soil Water Syst. 2003, 217–222. [Google Scholar]
- Ma, J.P.; Jiang, L.H.; Wu, G.G.; Xia, Y.; Lu, W.H.; Li, J.H.; Chen, L.X. Determination of six sulfonylurea herbicides in environmental water samples by magnetic solid-phase extraction using multi-walled carbon nanotubes as adsorbents coupled with high-performance liquid chromatography. J. Chromatogr. A 2016, 1466, 12–20. [Google Scholar] [CrossRef]
- Lang, Y.H.; Jiang, X.; Zhao, Q.G.; He, W.X. Advances in researches of environmental behavior of sulfonylurea herbicides in soil. Chin. J. Appl. Ecol. 2002, 9, 1187–1190. [Google Scholar] [CrossRef]
- Li, D.B. Degradation of Chlorimuron-Ethyl and Its Effect on Soil Microecology; Northeast Forestry University: Harbin, China, 2012. [Google Scholar]
- Guo, J. Degradation Characteristics of Chlorimuron-ethyl Degrading Strain N80 and Remediation of Contaminated Soil; Jilin Agricultural University: Changchun, China, 2012. [Google Scholar]
- Wang, Y.; Wang, Z.Q. Effect of planting years on microbial community and functional diversity of greenhouse vegetable soils. Acta Agric. Zhejiangensis 2013, 25, 567–576. [Google Scholar]
- Liu, B.R.; Niu, S.F.; Zhang, W.W. Effects of soil particle size on enzyme activities and the amountof soilmicroorganism in rhizosphere of Caragana korshinskii in desert steppe. Acta Ecol. Sin. 2019, 39, 9171–9178. [Google Scholar] [CrossRef]
- Li, A.H. Study on Interaction between Sulfonylurea Herbicides and Catalase; Huazhong Agricultural University: Wuhan, China, 2008. [Google Scholar]
- Li, D.B. Study on the Degradation of Chlorosulfuron and Its Impact on Soil Microecology; Northeast Forestry University: Harbin, China, 2012. [Google Scholar]
- Ai, S.Q.; Zhao, Y.Q.; Sun, Z.Y. Changes in Microbial Community Structure during Cellulose Degradation by Compound Bacteria. J. Bioeng. 2018, 34, 1794–1808. [Google Scholar] [CrossRef]

| Number | Source | Type |
|---|---|---|
| 1 | Hengxian County, Nanning, Guangxi Province | Brick red soil, ball clay |
| 2 | Lichuan County, Fuzhou, Jiangxi Province | Red soil, sandy clay loam |
| 3 | Qingpu District, Huaian City, Jiangsu Province | Rice soil, sandy loam |
| 4 | Meishan City, Sichuan Province | Purple soil, silty clay |
| 5 | Qinxian County, Changzhi City, Shanxi Province | Loess, silty loam |
| 6 | Tengzhou, Shandong Province | Cinnamon soil, loam |
| 7 | Shenyang, Liaoning Province | Brown loam, sandy loam |
| 8 | Fusong County, Baishan City, Jilin Province | Black soil, sandy loam |
| Number | Experimental Arrangement |
|---|---|
| 1 | BN (sterile) |
| 2 | BN (non-sterile) |
| 3 | BN + DL (non-sterile) |
| 4 | BN + MC (non-sterile) |
| 5 | BN + DL + MC (non-sterile) |
| Kinetic Equation | Half-Life (d) | K (×10−2)(d−1) | |
|---|---|---|---|
| MC added | Ct = 50·e−0.0383t | 18.10 | 3.83 |
| CK | Ct = 50·e−0.0032t | 214.87 | 0.32 |
| Number | pH | Total Salt (mSiemens/cm) | Organic Content (%) | Clay (g/kg) | Silt (g/kg) | Sand (g/kg) | Microbial Degradation (%) |
|---|---|---|---|---|---|---|---|
| 1 | 4.96 | 0.07 | 0.31 | 627.41 | 237.69 | 134.90 | 20.00 |
| 2 | 4.96 | 0.02 | 0.84 | 270.71 | 107.70 | 581.82 | 14.00 |
| 3 | 8.14 | 0.24 | 1.15 | 110.34 | 263.70 | 563.89 | 48.00 |
| 4 | 7.31 | 0.03 | 0.21 | 439.54 | 405.77 | 28.63 | 64.00 |
| 5 | 8.26 | 0.41 | 2.72 | 177.42 | 540.04 | 282.54 | 59.00 |
| 6 | 7.94 | 0.22 | 1.57 | 224.62 | 476.09 | 299.29 | 39.00 |
| 7 | 7.90 | 0.11 | 1.67 | 243.18 | 127.63 | 566.29 | 61.00 |
| 8 | 7.23 | 0.50 | 3.55 | 150.80 | 328.88 | 520.32 | 33.00 |
| Treatments | Stem Length (cm) | Stem Dry Weight (×10−2) (g) | Root Dry Weight (×10−3) (g) |
|---|---|---|---|
| DL only | 32.14 | 57.30 | 85.38 |
| DL + BN | 35.35 | 47.60 | 51.23 |
| DL + BN + 3% MC | 35.49 | 54.96 | 64.20 |
| DL + BN + 5% MC | 32.85 | 49.49 | 51.37 |
| DL + BN + 10% MC | 32.88 | 45.97 | 48.90 |
| Treatment | Degradation (%) | S-UR (ug/g) | S-CAT (mmol/g) |
|---|---|---|---|
| Initial | 30.00 | 1388.00 | 87.60 |
| Add 3% MC | 61.00 | 1510.00 | 93.20 |
| Add DL | 57.30 | 1288.00 | 82.12 |
| Add DL and 3% MC | 79.00 | 1837.00 | 99.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, X.; Liu, W.; Ge, L. Plant and Microorganism Combined Degradation of Bensulfuron Herbicide in Eight Different Agricultural Soils. Agronomy 2022, 12, 2989. https://doi.org/10.3390/agronomy12122989
Zhang Y, Wang X, Liu W, Ge L. Plant and Microorganism Combined Degradation of Bensulfuron Herbicide in Eight Different Agricultural Soils. Agronomy. 2022; 12(12):2989. https://doi.org/10.3390/agronomy12122989
Chicago/Turabian StyleZhang, Yanan, Xin Wang, Wenrui Liu, and Ling Ge. 2022. "Plant and Microorganism Combined Degradation of Bensulfuron Herbicide in Eight Different Agricultural Soils" Agronomy 12, no. 12: 2989. https://doi.org/10.3390/agronomy12122989
APA StyleZhang, Y., Wang, X., Liu, W., & Ge, L. (2022). Plant and Microorganism Combined Degradation of Bensulfuron Herbicide in Eight Different Agricultural Soils. Agronomy, 12(12), 2989. https://doi.org/10.3390/agronomy12122989







