Comparative Study of Traditional and Environmentally Friendly Zinc Sources Applied in Alkaline Fluvisol Soil: Lettuce Biofortification and Soil Zinc Status
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pot Experiment
2.2. Plant Analysis
2.3. Soil Analysis
2.4. Statistical Analysis
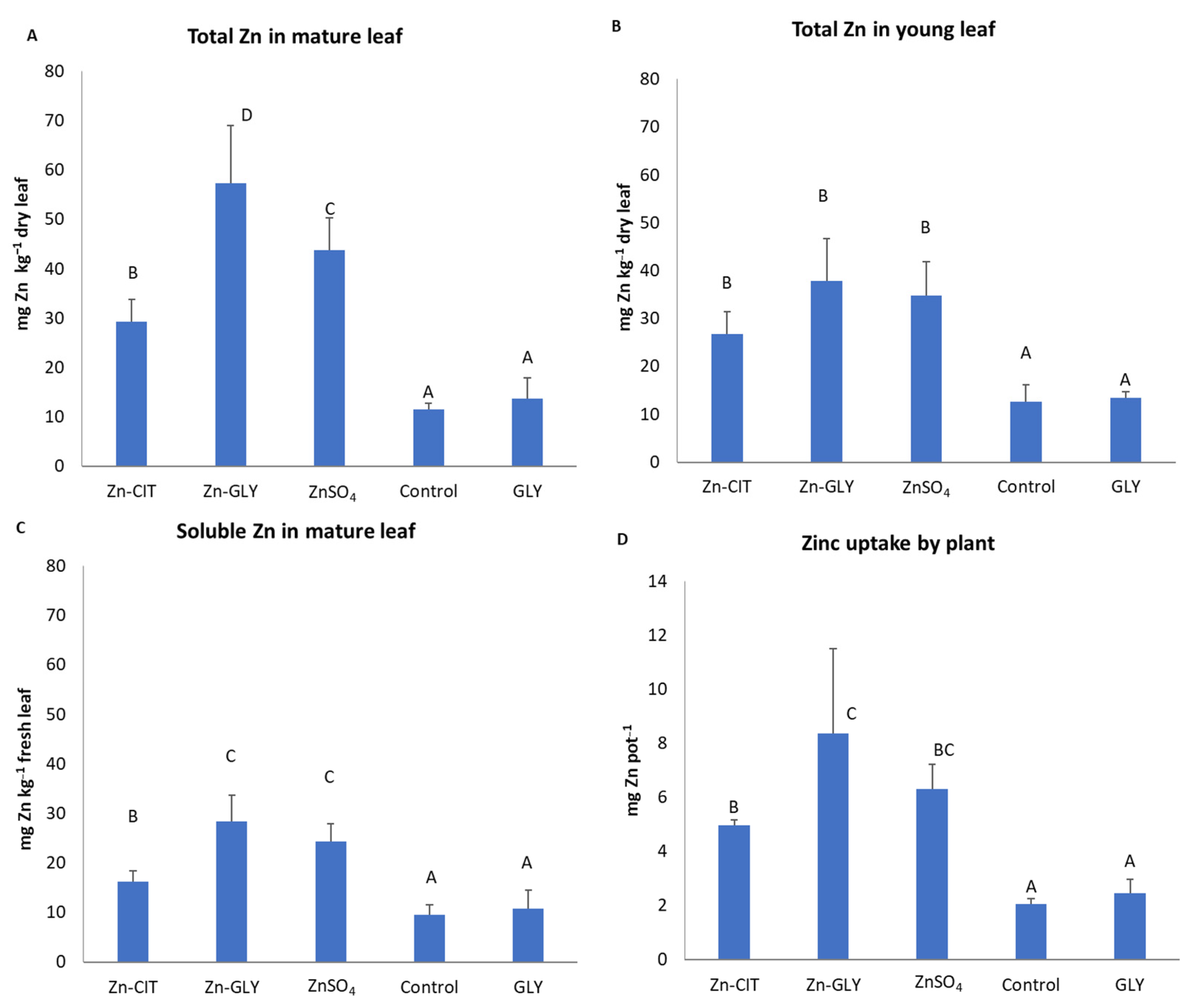
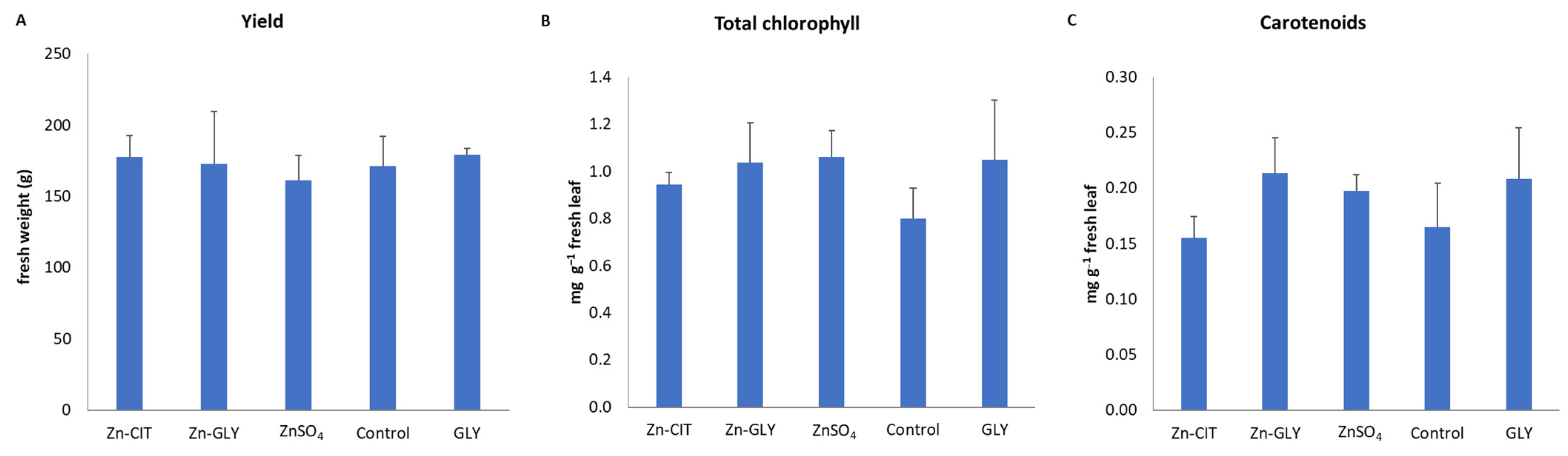
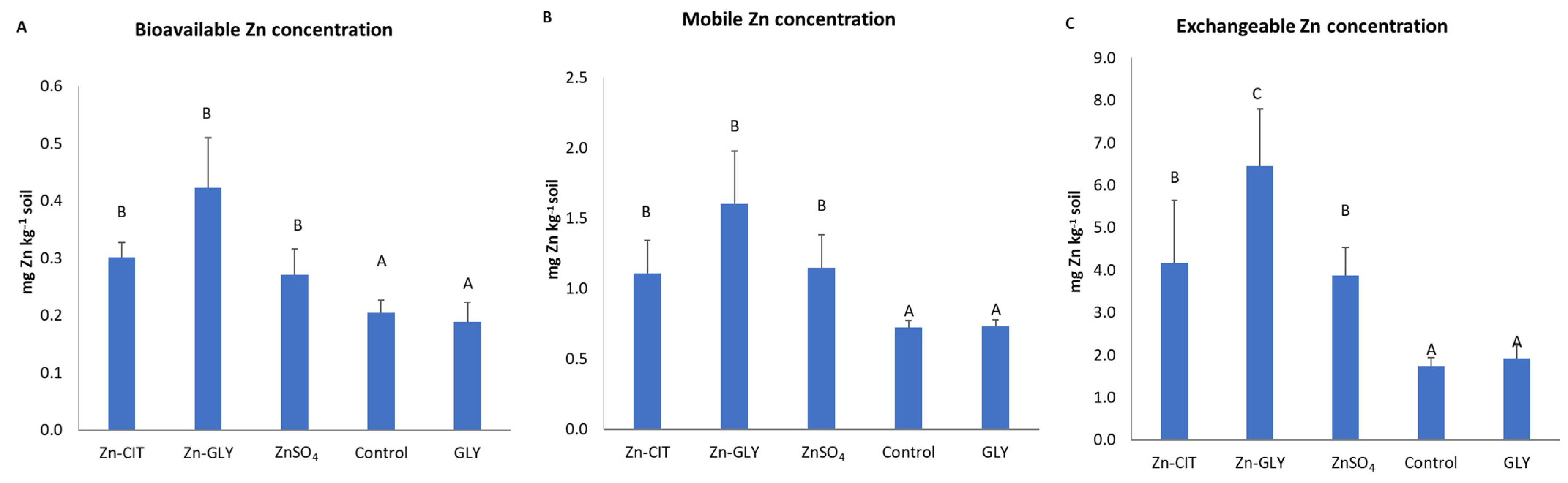
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khan, S.T.; Malik, A.; Alwarthan, A.; Shaik, M.R. The Enormity of the Zinc Deficiency Problem and Available Solutions; an Overview. Arab. J. Chem. 2022, 15, 103668. [Google Scholar] [CrossRef]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in Plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef]
- Hunt, J.R. Bioavailability of Iron, Zinc, and Other Trace Minerals from Vegetarian Diets. Am. J. Clin. Nutr. 2003, 78, 633S–639S. [Google Scholar] [CrossRef]
- Kaur, K.; Gupta, R.; Saraf, S.A.; Saraf, S.K. Zinc: The Metal of Life. Compr. Rev. Food Sci. Food Saf. 2014, 13, 358–376. [Google Scholar] [CrossRef]
- Liu, M.; Xu, M.; Yu, H.; Fu, H.; Tang, S.; Ma, Q.; Li, Y.; Wu, L. Spraying ZnEDTA at High Concentrations: An Ignored Potential for Producing Zinc-Fortified Pear (Pyrus spp.) Fruits without Causing Leaf and Fruitlet Burns. Sci. Hortic. 2023, 322, 112380. [Google Scholar] [CrossRef]
- de Moraes, C.C.; Silveira, N.M.; Mattar, G.S.; Sala, F.C.; Mellis, E.V.; Purquerio, L.F.V. Agronomic Biofortification of Lettuce with Zinc under Tropical Conditions: Zinc Content, Biomass Production and Oxidative Stress. Sci. Hortic. 2022, 303, 111218. [Google Scholar] [CrossRef]
- de Almeida, H.; Carmona, V.M.V.; Inocêncio, M.F.; Furtini Neto, A.E.; Cecílio Filho, A.B.; Mauad, M. Soil Type and Zinc Doses in Agronomic Biofortification of Lettuce Genotypes. Agronomy 2020, 10, 2–10. [Google Scholar] [CrossRef]
- Meneghelli, C.M.; Fontes, P.C.R.; do Milagres, C.C.; da Silva, J.M.; Junior, E.G. Zinc-Biofortified Lettuce in Aeroponic System. J. Plant Nutr. 2021, 44, 2146–2156. [Google Scholar] [CrossRef]
- FAOSTAT The Food and Agriculture Organization (FAO). Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 15 November 2023).
- Cervera-Mata, A.; Fernández-Arteaga, A.; Navarro-Alarcón, M.; Hinojosa, D.; Pastoriza, S.; Delgado, G.; Rufián-Henares, J.Á. Spent Coffee Grounds as a Source of Smart Biochelates to Increase Fe and Zn Levels in Lettuces. J. Clean. Prod. 2021, 328, 129548. [Google Scholar] [CrossRef]
- Alloway, B.J. Zinc in Soils and Crop Nutrition, 2nd ed.; International Zinc Association: Brussels, Belgium; Paris, France, 2008. [Google Scholar]
- Ganoe, K.; Ketterings, Q.; Herendeen, N. Zinc. Agronomy Fact Sheet Series. Ithaca, NY, USA. 2007. Available online: http://nmsp.cals.cornell.edu/publications/factsheets/factsheet32.pdf (accessed on 15 November 2023).
- Longnecker, N.E.; Robson, A.D. Distribution and Transport of Zinc in Plants. Zinc Soils Plants 1993, 55, 79–91. [Google Scholar] [CrossRef]
- Xu, M.; Liu, M.; Liu, F.; Zheng, N.; Tang, S.; Zhou, J.; Ma, Q.; Wu, L. A Safe, High Fertilizer-Efficiency and Economical Approach Based on a Low-Volume Spraying UAV Loaded with Chelated-Zinc Fertilizer to Produce Zinc-Biofortified Rice Grains. J. Clean. Prod. 2021, 323, 129188. [Google Scholar] [CrossRef]
- Alvarez, J.M.; Almendros, P.; Gonzalez, D. Residual Effects of Natural Zn Chelates on Navy Bean Response, Zn Leaching and Soil Zn Status. Plant Soil 2009, 317, 277–291. [Google Scholar] [CrossRef]
- Almendros, P.; Obrador, A.; Gonzalez, D.; Alvarez, J.M. Biofortification of Zinc in Onions (Allium cepa L.) and Soil Zn Status by the Application of Different Organic Zn Complexes. Sci. Hortic. 2015, 186, 254–265. [Google Scholar] [CrossRef]
- Gangloff, W.J.; Westfall, D.G.; Peterson, G.A.; Mortvedt, J.J. Relative Availability Coefficients of Organic and Inorganic Zn Fertilizers. J. Plant Nutr. 2002, 25, 259–273. [Google Scholar] [CrossRef]
- De Liñán, C. Vademécum de Productos Fitoranitarios y Nutricionales 2022; Ediciones Agrotécnicas SL: Madrid, Spain, 2022; ISBN 9788417596064. [Google Scholar]
- Mcbeath, T.M.; Mclaughlin, M.J. Efficacy of Zinc Oxides as Fertilisers. Plant Soil 2014, 374, 843–855. [Google Scholar] [CrossRef]
- Chandrika, K.S.V.P.; Patra, D.; Yadav, P.; Qureshi, A.A.; Gopalan, B. Metal Citrate Nanoparticles: A Robust Water-Soluble Plant Micronutrient Source. RSC Adv. 2021, 11, 20370–20379. [Google Scholar] [CrossRef]
- Mosa, W.F.A.; Ali, H.M.; Abdelsalam, N.R. The Utilization of Tryptophan and Glycine Amino Acids as Safe Alternatives to Chemical Fertilizers in Apple Orchards. Environ. Sci. Pollut. Res. 2021, 28, 1983–1991. [Google Scholar] [CrossRef]
- Zargar Shooshtari, F.; Souri, M.K.; Hasandokht, M.R.; Jari, S.K. Glycine Mitigates Fertilizer Requirements of Agricultural Crops: Case Study with Cucumber as a High Fertilizer Demanding Crop. Chem. Biol. Technol. Agric. 2020, 7, 19. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Mirbolook, A.; Lakzian, A.; Sadaghiani, M.R. Fortification of Bread Wheat Using Synthesized Zn- Glycine and Zn-Alanine Chelates in Comparison with ZnSO4 in a Calcareous Soil. Commun. Soil Sci. Plant Anal. 2020, 51, 1048–1064. [Google Scholar] [CrossRef]
- Xu, M.; Du, L.; Liu, M.; Zhou, J.; Pan, W.; Fu, H.; Zhang, X.; Ma, Q.; Wu, L. Glycine-Chelated Zinc Rather than Glycine-Mixed Zinc Has Lower Foliar Phytotoxicity than Zinc Sulfate and Enhances Zinc Biofortification in Waxy Corn. Food Chem. 2022, 370, 131031. [Google Scholar] [CrossRef] [PubMed]
- Ström, L.; Owen, A.G.; Godbold, D.L.; Jones, D.L. Organic Acid Behaviour in a Calcareous Soil Implications for Rhizosphere Nutrient Cycling. Soil Biol. Biochem. 2005, 37, 2046–2054. [Google Scholar] [CrossRef]
- Palomo, L.; Claassen, N.; Jones, D.L. Differential Mobilization of P in the Maize Rhizosphere by Citric Acid and Potassium Citrate. Soil Biol. Biochem. 2006, 38, 683–692. [Google Scholar] [CrossRef]
- Gramlich, A.; Tandy, S.; Frossard, E.; Eikenberg, J.; Schulin, R. Availability of Zinc and the Ligands Citrate and Histidine to Wheat: Does Uptake of Entire Complexes Play a Role? J. Agric. Food Chem. 2013, 61, 10409–10417. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA Soil Test for Zinc, Iron, Manganese, and Copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Ministerio de la Presidencia, Relaciones con las Cortes y Memoria Democrática. RD 1051 Real Decreto 1051/2022, de 27 de Diciembre, Por El Que Se Establecen Normas Para La Nutrición Sostenible En Los Suelos Agrarios. Boletín Of. Del Estado 2022, 312, 188873–188916. [Google Scholar]
- Ramos, C.; Pomares, F. Abonado de Los Cultivos Hortícolas. Guía Práctica La Fertilizacion Racional de los Cultivos en España. Parte II 2010, 181–192. Available online: https://www.mapa.gob.es/es/agricultura/publicaciones/02_FERTILIZACI%C3%93N(BAJA)_tcm30-57891.pdf#page=61 (accessed on 15 November 2023).
- Almendros, P.; González, D.; Ibañez, M.A.; Fernández, M.D.; García-Gomez, C.; Smolders, E.; Obrador, A. Can Diffusive Gradients in Thin Films (DGT) Technique and Chemical Extraction Methods Successfully Predict Both Zn Bioaccumulation Patterns in Plant and Leaching to Groundwater in Soils Amended with Engineered ZnO Nanoparticles? J. Soil Sci. Plant Nutr. 2020, 20, 1714–1731. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Lichtenthaler, H. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzym. 1987, 148, 350–382. [Google Scholar]
- Almendros, P.; Gonzalez, D.; Alvarez, J.M. Residual Effects of Organic Zn Fertilizers Applied before the Previous Crop on Zn Availability and Zn Uptake by Flax (Linum usitatissium). J. Plant Nutr. Soil Sci. 2013, 176, 603–615. [Google Scholar] [CrossRef]
- Feng, M.H.; Shan, X.Q.; Zhang, S.Z.; Wen, B. A Comparison of the Rhizosphere-Based Method with DTPA, EDTA, CaCl2, and NaNO3 Extraction Methods for Prediction of Bioavailability of Metals in Soil to Barley. Environ. Pollut. 2005, 137, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Kraus, U.; Wiegand, J. Long-Term Effects of the Aznalcóllar Mine Spill—Heavy Metal Content and Mobility in Soils and Sediments of the Guadiamar River Valley (SW Spain). Sci. Total Environ. 2006, 367, 855–871. [Google Scholar] [CrossRef] [PubMed]
- Simard, R. Ammonium Acetate-Extractable Elements. In Soil Sampling and Methods of Analysis; Carter, M., Gregorich, E., Eds.; Canadian Society of Soil Science: Ottawa, ON, Canada, 2008. [Google Scholar]
- Hall, L.; Chang-Yen, I. An Evaluation of the Extraction Efficiencies of Some Common Extractants for Fe, Cr, Mn, Ni, Pb and Cu on Five Grain-Size Fractionated, Tropical Marine Sediments. Environ. Pollut. 1989, 56, 51–63. [Google Scholar] [CrossRef]
- Maynard, D.N.; Hochmuth, G.J. Knott’s Handbook for Vegetable Growers, 6th ed.; Hochmuth, G., Sideman, R., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2022; ISBN 978-1-119-81107-7. [Google Scholar]
- EFSA. Panel on Dietetic Products Nutrition and Alergies Scientific Opinion on Dietary Reference Values for Zinc. EFSA J. 2014, 12, 3844. [Google Scholar] [CrossRef]
- Rengel, Z. Exchange Complex. In Handbook of Soil Acidity; CRC Press: Boca Raton, FL, USA, 2003; pp. 629–643. ISBN 9780429223099. [Google Scholar] [CrossRef]
- Martell, A.; Smith, R. Critical Stability Constants; Springer: New York, NY, USA, 1982; ISBN 9781461567660. [Google Scholar]
- Sachdev, P.; Lindsay, W.L.; Deb, D.L. Activity Measurements of Zinc in Soils of Different PH Using EDTA. Geoderma 1992, 55, 247–257. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Increase in Membrane Permeability and Exudation in Roots of Zinc Deficient Plants. J. Plant Physiol. 1988, 132, 356–361. [Google Scholar] [CrossRef]
- Brown, P.H.; Cakmak, I.; Zhang, Q. Form and Function of Zinc Plants. In Zinc in Soils and Plants; Robson, A.D., Ed.; Springer: Dordrecht, The Netherlands, 1993; pp. 93–106. ISBN 978-94-011-0878-2. [Google Scholar]
- Zhou, J.R.; Erdman, J.W. Phytic Acid in Health and Disease. Crit. Rev. Food Sci. Nutr. 1995, 35, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Chakraborty, M.; Padhan, D.; Saha, B.; Murmu, S.; Batabyal, K.; Seth, A.; Hazra, G.C.; Mandal, B.; Bell, R.W. Agronomic Biofortification of Zinc in Rice: Influence of Cultivars and Zinc Application Methods on Grain Yield and Zinc Bioavailability. F. Crop. Res. 2017, 210, 52–60. [Google Scholar] [CrossRef]
- Mou, B. Genetic Variation of Beta-Carotene and Lutein Contents in Lettuce. J. Am. Soc. Hortic. Sci. 2005, 130, 870–876. [Google Scholar] [CrossRef]
- Kobayashi, K.; Tsurumizu, A.; Toyoda, M.; Y, S. Contents of Chlorophylls, B-Carotene and Pesticide Residues in Butter Head Lettuce Produced by Various Cultivation Methods. Nippon. Shokuhin Kogyo Gakkaishi 1989, 36, 676–681. [Google Scholar] [CrossRef]
| Treatment | LMWOAs | Water-Soluble | NH4Ac 1 M |
|---|---|---|---|
| Zn-CIT | 0.56 ± 0.05 | 2.05 ± 0.44 | 7.72 ± 2.75 |
| Zn-GLY | 0.79 ± 0.16 | 2.97 ± 0.70 | 11.95 ± 2.50 |
| ZnSO4 | 0.50 ± 0.08 | 2.13 ± 0.43 | 7.17 ± 1.24 |
| Control | 0.49 ± 0.04 | 1.74 ± 0.19 | 4.18 ± 0.66 |
| GLY | 0.44 ± 0.08 | 1.62 ± 0.22 | 4.03 ± 0.20 |
| Plant and Soil Factors | Bioavailable Zn Concentration (LMWOAs) | Mobile Zn Concentration (Water Soluble) | Exchangeable Zn Concentration (NH4Ac 1 M) | Soil pH | Electrical Conductivity |
|---|---|---|---|---|---|
| Total Zn concentration in mature leaves | 0.839 ** | 0.890 *** | 0.827 ** | −0.591 * | NS |
| Total Zn concentration in young leaves | 0.741 * | 0.857 *** | 0.769 ** | −0.487 * | NS |
| Soluble Zn concentration in mature leaves | NS | 0.616 * | 0.727 * | NS | NS |
| Soil pH | −0.573 * | −0.512 * | −0.523 * | -- | −0.827 ** |
| Level | Reference Value | Reference |
|---|---|---|
| Zn concentration in plant: | ||
| Appropriate for the plant | 15–20 mg Zn kg−1 of leaves | Broadley et al. [2] |
| Optimal for the plant | 20–60 mg Zn kg−1 of leaves | Maynard and Hochmuth [40] |
| Daily dietary Zn requirement: | ||
| Women Men Children over 7 years old | 10.1 mg 12.85 mg 8.55 mg | European Commission [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz, R.; Gascó, G.; Méndez, A.; Sanchez-Martín, L.; Obrador, A.; Almendros, P. Comparative Study of Traditional and Environmentally Friendly Zinc Sources Applied in Alkaline Fluvisol Soil: Lettuce Biofortification and Soil Zinc Status. Agronomy 2023, 13, 3014. https://doi.org/10.3390/agronomy13123014
Ortiz R, Gascó G, Méndez A, Sanchez-Martín L, Obrador A, Almendros P. Comparative Study of Traditional and Environmentally Friendly Zinc Sources Applied in Alkaline Fluvisol Soil: Lettuce Biofortification and Soil Zinc Status. Agronomy. 2023; 13(12):3014. https://doi.org/10.3390/agronomy13123014
Chicago/Turabian StyleOrtiz, Raquel, Gabriel Gascó, Ana Méndez, Laura Sanchez-Martín, Ana Obrador, and Patricia Almendros. 2023. "Comparative Study of Traditional and Environmentally Friendly Zinc Sources Applied in Alkaline Fluvisol Soil: Lettuce Biofortification and Soil Zinc Status" Agronomy 13, no. 12: 3014. https://doi.org/10.3390/agronomy13123014
APA StyleOrtiz, R., Gascó, G., Méndez, A., Sanchez-Martín, L., Obrador, A., & Almendros, P. (2023). Comparative Study of Traditional and Environmentally Friendly Zinc Sources Applied in Alkaline Fluvisol Soil: Lettuce Biofortification and Soil Zinc Status. Agronomy, 13(12), 3014. https://doi.org/10.3390/agronomy13123014








