Abstract
As a crucial staple crop in agricultural production, maize is extensively cultivated worldwide and plays a pivotal role in ensuring global food security. However, the significant deficiency of essential amino acids (EAA) and conditionally essential amino acids (CEAA), such as lysine (Lys), tryptophan (Trp), methionine (Met), and cysteine (Cys), leads to an imbalance of amino acids in the grain. This study investigates the regulatory mechanism of sulfur (S) application for regulating the amino acid balance of maize grains. The results demonstrate that S application has substantial effects on both the maize yield and nutritional quality. The S application resulted in an increase in maize yield by simultaneously enhancing the grain number per ear (GN) and 100-grain weight (GW), while S application elevated protein concentration through the augmentation of Cys concentration in maize grains. Furthermore, the Cys in grains optimizes the amino acid balance by regulating the ratio of other amino acids, thereby enhancing the nutritional quality of maize while ensuring a steady increase in protein concentration, simultaneously. Overall, the S application at 60–90 kg ha−1 synergistically improved both the yield and nutritional quality of maize, meeting the requirements for sustainable development in maize production. The findings offer a novel theoretical foundation and nutrient management approach for achieving high-yield and superior-quality maize production.
1. Introduction
Target 2.1 of the Sustainable Development Goals (SDG) not only challenges the world to eradicate hunger, but also emphasizes the importance of ensuring universal access to safe, nutritious, and sufficient food throughout the year [1]. Within this goal, agriculture production and food nutrition objectives partially intersect. While agriculture production aims to generate high-quality foods in large quantities, food nutrition aims to guarantee the provision of safe and healthy foods that meet the nutritional requirements of the population [2]. The integration between agricultural production and food nutrition is crucial as plant-based foods specifically serve as primary sources of calories, protein, amino acids, minerals, and vitamins for human beings; thus, playing a vital role in their development and health.
In terms of food nutrition, the essential amino acids (EAA) that cannot be synthesized de novo by humans and many ruminants include lysine (Lys), tryptophan (Trp), methionine (Met), threonine (Thr), phenylalanine (Phe), valine (Val), leucine (Leu), and isoleucine (Iso) [3]. The conditionally essential amino acids (CEAA), namely cysteine (Cys), histidine (His), and tyrosine (Tyr) are restricted to humans and certain ruminants in specific pathophysiological circumstances such as severe catabolic distress or prematurity in infants [4]. The EAA and CEAA, which are necessary for humans, requires expensive dietary supplementation [5,6]. In reality, a crucial aspect of the livestock industry development is fulfilling the human demand for EAA and CEAA. Humans can reduce their dependence on amino acids derived from animals by enriching food crops with EAA and CEAA. Consequently, food crops rich in EAA and CEAA will not only serve a nutritional function but will also play a significant economic role, given the lower production cost of plant-derived amino acids compared to animal-derived amino acids [7].
Maize (Zea mays L.), as a crucial staple crop in agricultural production, is extensively cultivated worldwide and plays a pivotal role in ensuring global food security [8]. It serves not only as a primary source of sustenance for humans but also holds significant importance in the livestock and poultry industries across the globe [9]. Maize grains consist of approximately 70% starch and around 10% protein by weight [10]. The nutritional quality of maize grains largely depends on the protein concentration and composition [11,12]. However, the pronounced deficiencies in EAA and CEAA, such as Lys, Trp, Met, and Cys, lead to an imbalance of amino acids in maize grains [13,14,15]. In particular, Lys constitutes merely 2% of the total protein concentration in maize grains, which is even less than half of the content recommended by the FAO (Food and Agricultural Organization) [16]. Consequently, how to improve the amino acid balance represents a significant challenge in agricultural production.
Sulfur (S), as an indispensable mineral nutrient for plants, is crucial for the biosynthesis of EAA and CEAA (Met and Cys) [17], vitamins (thiamine, biotin) [18], oligopeptides (glutathione, phytochelatin) [19], enzyme co-factors (Fe-S clusters), and various secondary metabolites (such as glucosinolate) [20]. Therefore, ensuring a sufficient supply of S is crucial for regulating crop growth and sustaining nutritional quality [21]. However, in recent decades, the prevalence of S deficiency in agricultural systems has been escalating [22,23,24]. This S deficiency can be attributed to several factors: (1) The implementation of environmental protection measures over the past few decades and the subsequent reduction of SO2 emissions into the atmosphere have paradoxically limited the availability of S as an input in large-scale agriculture, resulting in crop S deficiency [25,26]. (2) The application of high analysis fertilizers (N, P2O5, K2O) with little S led to the lower plant-available S supply [27]. (3) The continuous and greater exportation of S from soil to crop results in decreased S content in soil [28]. (4) Typically, the use efficiency of S in agricultural systems is relatively low (<25%) [29]. In consequence, S-containing fertilizers are currently extensively employed globally to improve crop yield and nutritional quality.
In agriculture production, S application can enhance the concentrations of EAA and CEAA in crops, particularly the S-containing amino acids such as Met and Cys [30]. The findings of previous studies have revealed a significant decrease in the concentrations of Met and Cys by 25% and 30%, respectively, in S-deficient maize grains. Concurrently, an increase of 30% in the concentrations of aspartic acid (Asp) was observed, which severely compromises the nutritional quality of maize [31]. Numerous studies indicate that increasing the levels of Met and Cys in maize can improve their nutritional quality [32,33]. It is argued that an increase in S storage might enhance the concentrations of Met and Cys in maize grains, thereby boosting the nutritional quality of maize grains [34,35]. A previous pot experiment conducted on sandy soil revealed a consistent and significant enhancement in the EAA concentration of maize grains with increasing S application [36]. However, a separate study conducted on silt loam soil demonstrated that appropriate S application can enhance maize yield, while excessive S application can result in a significant decline in maize yield [37]. It can be seen that the response of EAA concentration and yield of maize grains to S application exhibits inconsistency. Moreover, the optimal rate of S application in different soil types for synergistically enhancing maize yield and nutritional quality remains unclear.
Therefore, a field experiment was conducted to investigate the regulatory mechanism of S application in improving the amino acid balance of maize grains. This study analyzed the grain yield, protein concentration, amino acid composition, endosperm microstructure, and dietary amino acid requirements pattern for humans. The results may provide a novel theoretical foundation and perspective on enhancing the nutritional quality of maize grains by S application.
2. Materials and Methods
2.1. Experimental Region Description
Consecutive field experiments were conducted from 2017 to 2019 in Lishu County, Northeast China, specifically at the coordinates of Sankeshu (43°20′ N, 124°00′ E) and Fujiajie (43°22′ N, 124°05′ E). The present study employed two disparate soil types, specifically, a clay soil sample from Sankeshu and a sandy soil specimen from Fujiajie, based on the textural classification [38]. The initial properties of topsoil (0–20 cm) are tabulated in Table 1.

Table 1.
The physical and chemical properties of 0–20 cm topsoil at two experimental sites.
The two experimental sites, which are situated 4 km apart, exhibit identical climatic conditions. Throughout the maize growing season, the average air temperatures recorded were 20.7 °C, 20.6 °C, and 20.8 °C (2017–2019), while the total precipitation was 456 mm, 354 mm, and 533 mm, respectively. In comparison to the average of 490 mm over the past three decades, there was a significant decrease in precipitation during the maize growth period in both 2017 and 2018 but a slight increase in 2019. Detailed daily records of air temperature and precipitation throughout the maize growing seasons can be found in Figure 1.
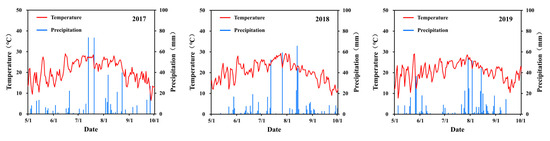
Figure 1.
Temperature and precipitation at the experimental site during maize growing seasons from 2017 to 2019.
2.2. Experimental Design
Each field experiment encompassed six S application rates with three replications, including the S0 treatment (non-S application) and five different rates of S application rates. These five S rates were: S30, S60, S90, S120, S150 (S fertilizer was applied at 30, 60, 90, 120, and 150 kg ha−1, respectively). The study employed a randomized complete block design, with each experimental plot encompassing an area of 60 m2 (6 × 10 m). In all experimental treatments, with the exception of S fertilizer, the application rates of other fertilizers remained consistent at 210 kg N, 90 kg P2O5, and 90 kg K2O per hectare. The S fertilizer used was single sulfur (S: 90%, Baker Co., Ltd., Shanghai, China). The nitrogen fertilizer consisted of a mixture of normal urea (N: 46%) and polymer-coated urea (N: 44%, the release period exceeding 2 months at water culture condition under 25 °C), in which polymer-coated urea accounted for 20%. The phosphorus fertilizer used was diammonium phosphate (DAP, N: 18%, P2O5: 46%), and the nitrogen introduced by DAP was included in the total nitrogen application. The potassium fertilizer used was potassium chloride (K2O: 60%). In each treatment, all fertilizer was utilized as a basal fertilizer and one-time applied to the soil prior to maize sowing.
The maize hybrid Liangyu 99 (Denghai Seed Industry Inc., Dandong, China) was employed in this study, and a consistent planting density of 65,000 plants per hectare. In all sites and years, the maize was sown in early May and harvested in early October. The experimental fields remained unirrigated during the maize growing period for three consecutive years, and all other management practices were carried out in accordance with the locally prevailing best management practices according to the ISSM strategy [39].
2.3. Yield and Its Components
In this experiment, consistent sampling and analytical approaches were adopted across all sites and years. At maturity stage (140 days after planting), for all S rates, maize plants were manually harvested in a designated 20 m2 area from the center of each plot to measure the grain yield. The yield was standardized to a moisture content of 14.0% to ensure consistency in the results. Additionally, the grain number per ear (GN) and 100-grain weight (GW) were measured from 15 sequential plants per plot.
2.4. Measurement of Protein Concentration in Maize Grains
The process of measuring the N concentration in maize grains involved drying the grains and grinding them into a powder. Then, the samples were digested using a concentrated sulfuric acid solution (H2SO4-H2O2), equilibrated with the deionized water, and cooled to ambient temperature. Subsequently, the N concentration in maize grains was determined using a Kjeldahl instrument (KDY-9820, KETUO, Beijing, China). The protein concentration (PC) was derived from the N concentration (NC) in maize grains, as per the following equation [40]:
PC = NC × 0.625
2.5. Analysis of Amino Acid in Maize Grains
The precisely weighed powder sample of maize grains (0.05 g) was transferred to a 20 mL hydrolysis tube, followed by the addition of 20 mL HCl solution (6 mol L−1). The sample was securely enclosed within a hydrolysis tube, which was then hermetically sealed with nitrogen and hydrolyzed at a temperature of 110 °C for a period of 24 h. Specially, for the determination of Trp, the sample hydrolyzed using 5 mol L−1 NaOH for a duration of 24 h, followed by the adjustment of the solution pH to 6 with HCl (6 mol L−1). The analysis of amino acids was conducted utilizing a high-performance liquid chromatography instrument (1260 Infinity II, Agilent, Santa Clara, CA, USA). The solution sample was injected in a split mode (15: 1, v/v) into an amino acid analysis column, which featured dimensions of Zebron ZB-AAA10 m × 0.25 mm (with a film thickness of 0.25 mm). Initially, the oven temperature was pre-established at 110 °C, and incrementally increased at a rate of 30 °C min−1 until reaching a final temperature of 320 °C. The temperature of the ion source was consistently maintained at 240 °C, and the carrier gas flow rate was steadfastly kept constant at 1.1 mL min−1 throughout the duration of the experiment [36].
2.6. Grain Endosperm Microstructure Measurements
The individual maize grain weights were computed based on the GW, and maize grains with a weight equivalent to the average single grain mass were selected through the electronic balance. The selected maize samples were meticulously sliced into two halves along the narrow side, and their endosperm microstructure was meticulously observed and photographed utilizing the optical microscope (BX51, Olympus, Tokyo, Japan). The area analysis of different types of endosperm was performed employing the image processing software (Image J 1.8, National Institutes of Health, Bethesda, MD, USA).
2.7. Data Analysis
The yield data across the different soil types, years, and S rates were analyzed using a three-way analysis of variance (ANOVA) program in Statistical Analysis System (SAS 9.2, SAS Institute Inc., Cary, NC, USA). Furthermore, amino acid concentration data across the different soil types and S rates were analyzed using a two-way ANOVA program in SAS. The mean comparisons among the S rates were conducted using Fisher’s Protected Least Significant Difference (LSD) test with a significance level of p < 0.05, when the F value was significant.
3. Results
3.1. Grain Yield and Its Components
The grain yields demonstrated a significant response to soil type and S rate but did not reflect a significant interaction between the two factors and among the three factors (Table 2). This result showed that the maize yield is closely related to soil type and S application, while inter-annual climatic differences had little effect on yield in this experiment. Overall, the grain yields exhibited a discernible trend of initial augmentation followed by subsequent attenuation with different S rates regardless of soil type. Among the different S rates, the highest yields were obtained in S90 (10,050 kg ha−1) across all three years. The yield of S150 (9259 kg ha−1) was significantly higher than that of S0 (8193 kg ha−1), but it was significantly lower than that of S90 (10,050 kg ha−1), which indicates excessive S application hinders yield improvement.

Table 2.
Effect of S application on maize yield and its components.
Similar to the yield response, the GN and GW were significantly influenced by both soil types and S rates. However, there was no significant interaction observed between the two factors and three factors. The absence of interaction showed that the response trend of GN and GW to S application was consistent in different soil types and different inter-annual. The GN and GW generally exhibited superior performance on clay soil compared to sandy soil among different soil types, with both reaching their maximum values at S90. In comparison with S0, the GN, GW, and yield of S90 increased by 3.1%, 7.3%, and 22.7%, respectively. It can be seen that the important factor in increasing yield is the synergistic improvement of GN and GW by S application.
3.2. Concentration of Grain Protein
The impact of S application on grain protein concentration is illustrated in Figure 2. The grain protein concentration significantly increased with S application, regardless of soil type. Moreover, a parabolic relationship was observed between the grain protein concentration and different S rates. Specifically, in 2017, the maximum grain protein concentration in both soils appeared at S90, reaching 10.5% (clay soil) and 10.8% (sandy soil), respectively. In 2018, the maximum grain protein concentration in clay soil appeared at S90 (10.1%), and significantly higher than that in S150, indicating that the S application of 150 kg ha−1 had begun to inhibit the accumulation of grain protein. On sandy soil, the maximum grain protein concentration was moved forward to S60 (11.3%) and was significantly higher than that of S120 and S150, indicating that the S application more than 120 kg ha−1 was no longer conducive to the accumulation of grain protein. This may be related to the fact that the yield of maize grains in sandy soils is significantly lower than that in clay soils, resulting in less S requirement for maize grains in sandy soils than in clay soils. In 2019, the maximum grain protein concentration in both soils occurred at S60, and was significantly higher than S0 and S150, but there was no significant difference compared to S30. The results showed that S application for three consecutive years increased the S accumulation in soil and increased the supply of S from soil to maize plants, and the amount of S application should be appropriately reduced. Excessive S application not only inhibits the accumulation of grain protein, but also causes waste of resources.
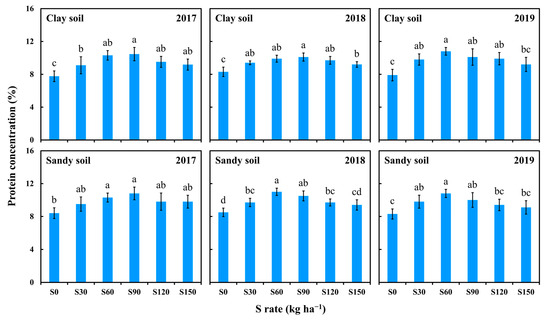
Figure 2.
Effect of S application on protein concentration in maize grains. The different letters above the bars indicate statistically significant differences (p < 0.05).
3.3. Concentrations of Amino Acids
Amino acids are the basic unit of proteins and determine the nutritional value of proteins. The amino acid composition of grain protein was further assessed by conducting a comprehensive analysis on the concentration of various amino acids in maize grains. In this study, all other amino acids were not significantly affected by soil type except for the Glu, Trp, Met, and Cys (Table 3). Glu is the most abundant amino acid in maize grains, while Trp is the least abundant amino acid in maize grains, and Met and Cys are S-containing amino acids. This result may be due to differences in the sensitivity of these amino acids to S supply of different soil types. All amino acids significantly responded to the S rate but not to the soil type × S rate interaction. Among all amino acids, the levels of Asp, Glu, Gly, His, Thr, Trp, Leu, Phe, and Lys exhibited a parabolic relationship with different S rates. Ser, Arg, Met, Tyr, Val, and Cys demonstrated a continuous increase with increasing S application. Conversely, Ala, Pro, and Iso continued to decrease with increasing S application. This may be due to the diversity in the response characteristics of different amino acids to S application.

Table 3.
Concentrations of 18 amino acids in maize grains under different S rates (mg g−1).
3.4. Amino Acids Balance
In order to quantify the amino acid balance in maize grains, amino acids were divided into EAA (including CEAA), NAA, and total amino acids (TAA), and the EAA/TAA ratio was analyzed (Table 4). The EAA, NAA, TAA, and the EAA/TAA ratio were significantly affected by soil type and S rate except for the soil type effect on the ratio of EAA to TAA, but not to the soil type × S rate interaction. When comparing soil types, the concentrations of EAA, NAA, and TAA were significantly lower in clay soil compared to sandy soil; however, no significant differences were observed in the EAA/TAA ratio between clay soil and sandy soil. Regardless of soil type, S application increased EAA concentration and there was no significant change after reaching the maximum at S60, while the concentration of NAA and TAA increased significantly with S application rate and showed a parabolic relationship with different S application rates. This result means that S application simultaneously increases the concentration of EAA and NAA, and that excessive S application reduces the concentration of TAA by inhibiting the accumulation of NAA, while the concentration of EAA is not affected. Moreover, S application elevated the proportion of EAA in TAA, and the highest EAA/TAA ratio was achieved at S60, followed by no further significant changes. This implies that an S application rate of 60 kg ha−1 is sufficient to enhance EAA accumulation in protein and promote amino acid balance of maize grain.

Table 4.
Effects of S application on the concentrations of different types of amino acids in maize grains.
Based on the performance of EAA/TAA ratio at different S rates, it was evident that the differences in the EAA/TAA ratio were more pronounced for S60 and S150 compared to S0. However, no significant differences in the EAA/TAA ratio were observed between S60 and S150. Thus, the three representative S rates, namely S0, S60, and S150, were selected to conduct a comprehensive microstructural analysis of maize grain endosperm in this study. This is to further clarify the effect of S application on the amino acid balance of maize grains.
3.5. Microstructure of Maize Grain Endosperm
Generally, the maize endosperm can be classified into two distinct regions, namely the vitreous endosperm (VE) located in the outer region and the floury endosperm (FE) situated in the central region [41]. The proteins lacking EAA were mostly concentrated in the VE region [42]. The ratio of vitreous endosperm (AVE) to floury endosperm (AFE) areas typically reflects the nutritional quality of maize grains. A higher AVE to AFE ratio indicates a greater concentration of EAA-deficient proteins, suggesting a more severe degree of amino acid imbalance. The smaller AVE to AFE ratio means a more optimal amino acid balance in maize grains.
The microstructure analysis of maize endosperm showed that the maize grain size of S0 was relatively small, but the AVE (10.87 mm2) did not decrease, while the AFE (24.11 mm2) was relatively smaller, and the ratio of AVE to AFE was 0.45. This indicates that the maize grain of S0 contains less protein containing EAA and more protein containing NAA, and the amino acid imbalance in maize grains is serious. Compared with S0, the grain size of S60 was significantly larger, but the AVE (7.73 mm2) did not increase. In contrast, the AFE (34.66 mm2) increased significantly, and the ratio of AVE to AFE decreased to 0.22, indicating that S application significantly increased the protein rich EAA in maize grains. The maize grain size, AVE (9.71 mm2) and AFE (33.50 mm2) of S150 was basically the same as that of S60, and the ratio of AVE to AFE was 0.29, which showed that increasing S application had no significant effect on the amino acid composition of maize grains (Figure 3).
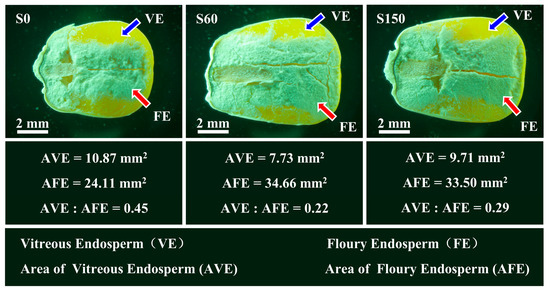
Figure 3.
Microstructure analysis of maize grain endosperm.
3.6. Dietary Amino Acid Requirement Pattern
To accurately quantify the nutritional quality of maize, we compared the proportion of EAA in grain protein with the dietary amino acid requirement pattern for humans (DAARP) established by the World Health Organization/Food and Agriculture Organization/United Nations University [43]. The results showed that the concentrations of His, Leu, Lys, Met + Cys (S-containing amino acids), Phe + Tyr (aromatic amino acids), Trp, and Val in proteins increased with the S application (Table 5). The concentration of Iso in protein decreased with the S application, while the concentration of Thr in protein first increased and then decreased with S application, but even at the highest S application rate (S150), the concentrations of Iso and Thr in protein was still higher than the DAARP. Under the conditions of non-S application (S0) and low-S application (S30), the Val concentration in protein did not meet the DAARP, while after appropriate S application (more than 60 kg ha−1), the Val concentration in protein increased significantly, reaching and exceeding the DAARP. It can be seen that S application plays an important role in promoting the amino acid balance of maize grains. Unfortunately, even under S application, the Lys concentration in protein does not meet the DAARP. The Lys concentration in protein, however, exhibited an increase with the S application, reaching its peak at 30% (clay soil) and 40% (sandy soil), as compared to S0, which provides a new possibility for improving the Lys concentration of maize grains.

Table 5.
Concentrations of EAA in protein of maize grains at different S rates (mg g−1).
3.7. Cys in Amino Acid Balance
As a typical S-containing amino acid, Cys is the basic substance that constitutes disulfide bonds in proteins and plays an important role in increasing protein concentration and promoting amino acid balance. In this study, Cys concentration continued to increase with increasing S application regardless of soil type (Figure 4a,b). The correlation analysis between protein concentration and Cys concentration showed that the protein concentration increased with increasing Cys concentration and decreased significantly after reaching its maximum at 3.96 (clay soil) and 4.06 (sandy soil), respectively (Figure 4c,d). This means that an increase in Cys concentration favors the accumulation of proteins, and as shown in the Figure 4e, the sufficient formation of the disulfide bond composed of Cys may be the main reason for the increase in protein concentration. However, as Cys concentrations continue to increase, the concentration of certain types of amino acids that constitute proteins decreases significantly, which is related to the cross-linking properties of amino acids.
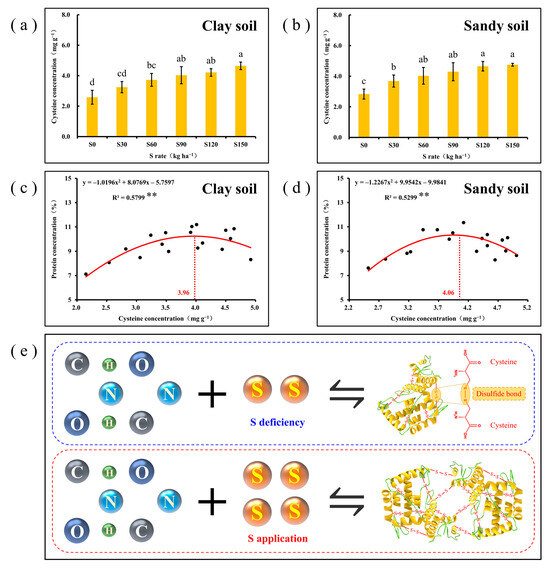
Figure 4.
(a,b) Effect of different S rates on Cys concentration in maize grains. The letters above the bars indicate the statistically significant differences among the different S rates (p < 0.05); (c,d) correlation analysis between protein concentration and Cys concentration in maize grains. The red curve indicates the correlation between the protein concentration and Cys concentration, and the black dots represents the protein concentration values at the corresponding Cys concentration. ** denotes significance at p < 0.01; (e) schematic representation of the mechanism underlying enhanced protein concentration in maize grains by S application.
When Cys concentrations are sufficient, the cross-linking of NAA and EAA with Cys exhibits selectivity. The correlation analysis showed that NAA concentration had a parabolic relationship with the increase in Cys concentration and decreased significantly after reaching the maximum at 3.55 (clay soil) and 3.77 (sandy soil), respectively (Figure 5a,b). However, the EAA concentration increased with increasing Cys concentration and remained stable after reaching its maximum at 4.54 (clay soil) and 4.55 (sandy soil), respectively (Figure 5c,d). TAA concentration showed a parabolic relationship with the increase in Cys concentration and decreased significantly after reaching the maximum at 3.98 (clay soil) and 4.13 (sandy soil), respectively (Figure 5c,d), which was caused by the decrease in NAA. This result means that the increase in Cys concentration inhibits NAA accumulation, but favors EAA accumulation, thereby promoting amino acid balance. Overall, when the concentration of Cys in maize grain falls within the range of approximately 4.0–4.5 on both soil types, it leads to the highest levels of TAA and EAA, while maintaining relatively lower NAA concentration, thereby enhancing the nutritional quality of maize (Figure 5c,d).
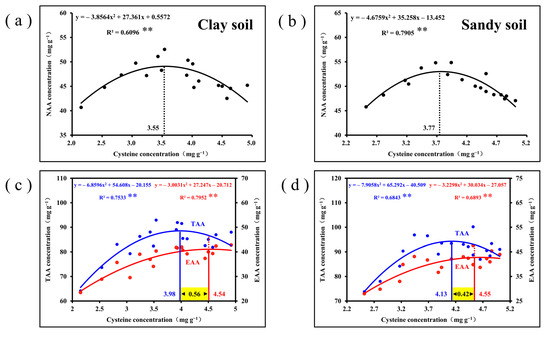
Figure 5.
(a,b) The correlation analysis between NAA and Cys concentration in maize grains; (c,d) the correlation analysis between TAA, EAA, and Cys concentration in maize grains. ** denotes significance at p < 0.01.
4. Discussion
4.1. Effects of S Application on Maize Grain Yield and Protein Concentration
As a pivotal dietary source for both humans and animals, the grain protein concentration of maize constitutes a fundamental trait in terms of nutritional quality [44]. However, in global maize production, there has been a consistent decline in grain protein concentration as grain yield increases [45,46]. Typically, the increase in maize yield leads to an average decrease in protein concentration of 0.3% per decade [47,48]. The increase in maize yield, as demonstrated by a study encompassing 45 maize varieties from the 1920s to 2001, primarily resulted from improvements in starch concentration within the grains, while simultaneously observing a decrease in protein concentration with the increase in maize yield [49]. Nutrient management emerged as a pivotal strategy to concurrently enhance maize yield and increase grain protein concentration [50]. In this study, the yield and protein concentration of maize grains simultaneously increased with S application. When the S was applied at 60–90 kg ha−1, the GN, GW, and yield of maize reached the highest level. The increase in yield can be attributed to synergistic enhancements in both GN and GW, which aligns with previous research findings [51].
The main compound responsible for S storage in maize grain is protein, which accumulates as amino acids [52]. Analysis of amino acid composition in maize grains revealed a significant increase in Cys concentration with S application (Figure 4). Cys, as a typical S-containing amino acid, serves as the foundation for protein disulfide bond formation and enhances protein stability [53]. Due to the limitations imposed by protein conformation, the elevated Cys concentration provides more potential sites and opportunities for disulfide bond formation, thereby contributing to the overall formation and enhanced the stability of proteins [54,55]. In this study, the results demonstrated a significant correlation between protein concentration and Cys concentration in maize grains, indicating that increased Cys enhances both the formation and stability of proteins. Thus, S application to increase the protein concentration of maize grain was mainly achieved by the increase in Cys concentration.
4.2. Effects of S Application on Amino Acid Balance of Maize Grains
Conventional wisdom suggests that increasing the protein concentration can improve the nutritional quality of maize grains; however, the amino acids imbalance in dietary protein may lead to serious adverse effects [56]. Therefore, achieving balanced nutrition requires not only considering protein accumulation but also taking into account the proportion of amino acids in the diet [57]. Research findings suggest that solely increasing the protein concentration in maize grains may not necessarily enhance their nutritional quality and could even potentially have an adverse impact [58]. Previous studies have demonstrated that nitrogen application can substantially elevate the protein concentration in maize grains; however, it does not uniformly increase the concentration of each amino acid within the protein. Among all amino acids, NAA concentrations such as Glu and Pro continuously rise with increasing nitrogen application rates, while EAA concentrations like Lys and Met steadily decline with higher nitrogen application rates [59]. Other studies have indicated that with increasing nitrogen application rates, zeins preferentially accumulate in maize grains while Lys and Trp concentrations progressively decrease alongside increased protein concentration [60]. Subsequent studies have demonstrated that the increase in nitrogen application rates leads to a significant rise in zeins lacking EAAs, while EAA concentration such as Lys and Thr continuously decreases, exacerbating the amino acid imbalance in grains [61,62]. Contrary to nitrogen, S can enhance EAA concentration of maize grains, particularly the concentration of S-containing amino acids like Cys and Met [30]. In this study, not only S-containing amino acids like Met and Cys increased with S application, but also EAA including His, Leu, Lys, Phe, Tyr, Thr, Trp, and Val increased with S application. This performance has been confirmed in cereal crops such as wheat and barley [63,64].
Typically, the classification of maize grain protein comprises zeins (60%), glutelins (34%), globulins (3%), and albumins (3%) [65]. Zeins, being the predominant storage protein in maize endosperm, exhibit high levels of NAA like Glu, Pro, and Ala; however, they are severely deficient in EAA such as Lys, Trp, and Met, which significantly restricts the nutritional quality of maize [66]. According to its solubility characteristics, zeins can be categorized into four subclasses, namely α-, β-, γ-, and δ-zeins [67]. Among these subclasses of zeins, γ-zeins are characterized by a high Cys, while δ-zeins exhibit a significant proportion of Met [68,69]. The β-zeins reveal high levels of two S-containing amino acids, namely Cys and Met [70]. The α-zeins constitute over 70% of the total zeins, yet they are deficient in Lys and Cys, with low levels of Met, resulting in a severe amino acid imbalance in maize grains [34]. Decreasing the concentration of α-zeins can result in a compensatory augmentation in β-, γ-, and δ-zein fractions, along with non-zein proteins [71]. In this study, the Cys concentration was increased with S application (Figure 5), and the EAA such as Lys, Trp, and Met in maize grains was also significantly increased (Table 3). The protein concentration, EAA concentration, TAA concentration, and EAA/TAA of maize grain reached the highest level when the S application was at 60–90 kg ha−1. Simultaneously, under the premise of ensuring a steady increase in protein concentration, S application resulted in a reduction of NAA concentration, including Asp, Glu, Gly, Ala, and Pro (Table 3). This effect may be attributed to the enhanced regulation of Cys by S application, leading to the inhibition of α-zein levels and compensatory increase in EAA-rich non-zein proteins. Cys residues in β- and γ-zeins undergo intermolecular cross-linking with both each other and other amino acids during the maize grain’s development period [41]. However, the absence of Cys in α-zein restricts its involvement in protein formation, thereby creating additional opportunities for EAA like Met, Lys, and Trp to be incorporated into proteins. The EAA concentrations exhibited a continuous increase with increasing Cys concentration, while the NAA concentrations initially increased and subsequently decreased with Cys concentration in this study (Figure 5). These relationships demonstrate that under sufficiently high Cys concentration, protein formation preferentially accumulates EAA while inhibiting NAA, thereby regulating the amino acid distribution in proteins and achieving a balance of amino acid in maize grains. Analysis of microstructure of the maize endosperm also confirmed this conclusion (Figure 3). Therefore, S application improved the nutritional quality of maize by regulating the amino acid balance in the grains.
5. Conclusions
The S application significantly affected the grain yield and nutritional quality of maize. S application increased the yield of maize by simultaneously increasing the GN and GW, and the protein concentration in maize grains by increasing the Cys concentration in maize grains. Simultaneously, under the premise of ensuring a steady increase in protein concentration, the Cys in grains optimized the amino acids balance by regulating the ratio of other amino acids, thereby improving the nutritional quality of maize. Overall, whether on clay soil or sandy soil, when the S application was 60–90 kg ha−1, the grain yield and nutritional quality of maize were synergistically improved, which meet the requirements for sustainable development in maize production and provide a novel theoretical foundation and nutrient management approach for achieving high-yield and superior-quality maize production.
Author Contributions
Conceptualization, H.W., S.C. and S.L.; methodology, S.C. and S.L.; software, H.W.; validation, H.W., J.F. and H.G.; formal analysis, H.W.; investigation, H.W., J.F. and H.G.; resources, S.L.; data curation, H.W.; writing—original draft preparation, H.W.; writing—review and editing, S.C. and S.L.; visualization, H.W.; supervision, S.L.; project administration, S.L.; funding acquisition, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (32202617), and the Hainan Provincial Natural Science Foundation of China (grant number 323RC417).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Correction Statement
This article has been republished with a minor correction to the Funding statement. This change does not affect the scientific content of the article.
Abbreviations
EAA: essential amino acids; CEAA: conditionally essential amino acids; TAA: total amino acids; Asp: aspartic acid; Glu: glutamic acid; Ser: serine; Gly: glycine; Arg: arginine; Ala: alanine; Pro: proline; Met: methionine; His: histidine; Thr: threonine; Tyr: tyrosine; Val: valine; Trp: tryptophan; Iso: isoleucine; Leu: leucine; Phe: phenylalanine; Lys: lysine; Cys: cysteine; S: sulfur; DAP: diammonium phosphate; GN: grain number per ear; GW: 100-grain weight; AVE: area of vitreous endosperm; AFE: area of floury endosperm; DAARP: dietary amino acid requirement pattern.
References
- SDG (Sustainable Development Goal). Sustainable Development Goals. 2019. Available online: https://sustainabledevelopment.un.org/sdg2 (accessed on 7 February 2020).
- Fischer, S.; Thomas Hilger, T.; Piepho, H.-P.; Irmgard Jordan, I.; Cadisch, G. Missing association between nutrient concentrations in leaves and edible parts of food crops—A neglected food security issue. Food Chem. 2021, 345, 128723. [Google Scholar] [CrossRef]
- Young, V.E.; El-Khoury, A.E.; Cynober, L. The notion of the nutritional essentiality of amino acids, revisited, with a note on the indispensable amino acid requirements in adults. In Amino Acid Metabolism and Therapy in Health and Nutritional Disease; Reeds, P.J., Ed.; CRC Press: Boca Raton, FL, USA, 1996; pp. 191–232. [Google Scholar]
- Fürst, P. Old and new substrates in clinical nutrition. J. Nutr. 1998, 128, 789–796. [Google Scholar] [CrossRef]
- Ali, M.; Scott, M.P.; Bakht, J. Molecular mechanism of methionine differentiation in high and low methionine maize lines. Afr. J. Biotechnol. 2011, 10, 3747–3752. [Google Scholar][Green Version]
- Li, C.S.; Xiang, X.L.; Huang, Y.C.; Zhou, Y.; An, D.; Dong, J.Q.; Zhao, C.X.; Liu, H.J.; Li, Y.B.; Wang, Q.; et al. Long-read sequencing reveals genomic structural variations that underlie creation of quality protein maize. Nat. Commun. 2020, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- EPA (Environmental Protection Agency). Predicting the Impact of Coexistence-Guided, Genetically Modified Cropping on Irish Biodiversity. STRIVE Environmental Protection Agency Program 2007–2013. 2020. Available online: http://www.epa.ie/pubs/reports/research/biodiversity/STRIVE_39_Mullins_Genetics_322web.pdf (accessed on 6 April 2020).
- Amegbor, I.; van Biljon, A.; Shargie, N.; Tarekegne, A.; Labuschagne, M. Identifying quality protein maize inbred lines for improved nutritional value of maize in Southern Africa. Foods 2022, 11, 898. [Google Scholar] [CrossRef] [PubMed]
- Tanumihardjo, S.; McCulley, L.; Roh, R.; Lopez-Ridaura, S.; Palacios-Rojas, N.; Gunaratna, N. Maize agro-food systems to ensure food and nutrition security in reference to the Sustainable Development Goals. Glob. Food Secur. 2019, 25, 100327. [Google Scholar] [CrossRef]
- Flint-Garcia, S.A.; Bodnar, A.L.; Scott, M.P. Wide variability in kernel composition, seed characteristics, and zein profiles among diverse maize inbreds, landraces, and teosinte. Theor. Appl. Genet. 2009, 119, 1129–1142. [Google Scholar] [CrossRef] [PubMed]
- Young, V.R.; Pellett, P.L. Plant proteins in relation to human protein and amino acid nutrition. Am. J. Clin. Nutr. 1994, 59, 1203S–1212S. [Google Scholar] [CrossRef]
- Mandal, S.; Mandal, R. Seed storage proteins and approaches for improvement of their nutritional quality by genetic engineering. Curr. Sci. India 2000, 79, 576–589. [Google Scholar]
- Bhan, M.K.; Bhandari, N.; Bahl, R. Management of the severely malnourished child: Perspective from developing countries. Brit. Med. J. 2003, 326, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Sethi, M.; Kumar, S.; Singh, A.; Chaudhary, D.P. Temporal profiling of essential amino acids in developing maize kernel of normal, opaque-2 and QPM germplasm. Physiol. Mol. Biol. Plants 2020, 26, 341–351. [Google Scholar] [CrossRef]
- Wu, Y.; Messing, J. Proteome balancing of the maize seed for higher nutritional value. Front. Plant Sci. 2014, 5, 240. [Google Scholar] [CrossRef]
- Maqbool, M.A.; Beshir Issa, A.; Khokhar, E.S. Quality protein maize (QPM): Importance, genetics, timeline of different events, breeding strategies and varietal adoption. Plant Breed. 2021, 140, 375–399. [Google Scholar] [CrossRef]
- Kopriva, S.; Malagoli, M.; Takahashi, H. Sulfur nutrition: Impacts on plant development, metabolism, and stress responses. J. Exp. Bot. 2019, 70, 4069–4073. [Google Scholar] [CrossRef] [PubMed]
- Gigolashvili, T.; Kopriva, S. Transporters in plant sulfur metabolism. Front. Plant Sci. 2014, 5, 442–457. [Google Scholar] [CrossRef] [PubMed]
- Zenda, T.; Liu, S.T.; Dong, A.Y.; Duan, H.J. Revisiting Sulphur-the once neglected nutrient: It’s roles in plant growth, metabolism, stress tolerance and crop production. Agriculture 2021, 11, 626. [Google Scholar] [CrossRef]
- Maruyama-Nakashita, A.; Ohkama-Ohtsu, N. Sulfur assimilation and glutathione metabolism in plants. In Glutathione in Plant Growth, Development, and Stress Tolerance; Hossain, M.A., Mostofa, M.G., Diaz-Vivancos, P., Burritt, D.J., Fujita, M., Tran, L.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 287–308. [Google Scholar]
- Koprivova, A.; Kopriva, S. Sulfur metabolism and its manipulation in crops. J. Genet. Genom. 2016, 43, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.; Jephcote, C.; Fraser, A.; Wiltshire, J.; Aston, S.; Rose, R.; Vincent, K.; Roth, B. Do UK crops and grassland require greater inputs of sulphur fertilizer in response to recent and forecast reductions in sulphur emissions and deposition? Soil Use Manag. 2016, 32, 3–16. [Google Scholar] [CrossRef]
- Aula, L.; Dhillon, J.S.; Omara, P.; Wehmeyer, G.B.; Freeman, K.W.; Raun, W.R. World sulfur use efficiency for cereal crops. Agron. J. 2019, 111, 2485–2492. [Google Scholar] [CrossRef]
- Cai, J.; Zang, F.; Xin, L.; Zhou, Q.; Wang, X.; Zhong, Y.; Huang, M.; Dai, T.; Jiang, D. Effects of cysteine and inorganic sulfur applications at different growth stages on grain protein and end-use quality in wheat. Foods 2022, 11, 3252. [Google Scholar] [CrossRef]
- Scherer, H.W. Sulfur in soils. J. Plant Nutr. Soil Sci. 2009, 172, 326–335. [Google Scholar] [CrossRef]
- Hinckley, E.L.S.; Crawford, J.T.; Fakhraei, H.; Driscoll, C.T. A shift in sulfur-cycle manipulation from atmospheric emissions to agricultural additions. Nat. Geosci. 2020, 13, 597–604. [Google Scholar] [CrossRef]
- Chien, S.H.; Gearhart, M.M.; Villagarcia, S. Comparison of ammonium sulfate with other nitrogen and sulfur fertilizers in increasing crop production and minimizing environmental impact: A review. Soil Sci. 2011, 176, 327–335. [Google Scholar] [CrossRef]
- Kovar, J.L. Maize response to sulfur fertilizer in three Iowa Soils. Commun. Soil Sci. Plant Anal. 2021, 52, 905–915. [Google Scholar] [CrossRef]
- Eriksen, J. Soil sulfur cycling in temperate agricultural systems. Adv. Agron. 2009, 102, 55–89. [Google Scholar]
- Liu, S.; Cui, S.; Zhang, X.; Wang, Y.; Mi, G.; Gao, Q. Synergistic regulation of nitrogen and sulfur on redox balance of maize leaves and amino acids balance of grains. Front. Plant Sci. 2020, 11, 576718. [Google Scholar] [CrossRef] [PubMed]
- Baudet, J.; Huet, J.C.; Jolivet, E.; Lesaint, C.; Mossé, J.; Pernollet, J.C. Chaises in accumulation of seed nitrogen compounds in maize under conditions of sulphur deficiency. Physiol. Plant. 1986, 68, 608–614. [Google Scholar] [CrossRef]
- Galili, G.; Amir, R. Fortifying plants with the essential amino acids lysine and methionine to improve nutritional quality. Plant Biotechnol. J. 2013, 11, 211–222. [Google Scholar] [CrossRef]
- Hintch, T.D.; Lauter, A.M.; Kinney, S.M.; Lubberstedt, T.; Frei, U.; Duangpapeng, P.; Edwards, J.W.; Scott, M.P. Development of maize inbred lines with elevated grain methionine concentration from a high methionine population. Crop Sci. 2023, 63, 2417–2425. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, W.; Messing, J. Balancing of sulfur storage in maize seed. BMC Plant Biol. 2012, 12, 77–85. [Google Scholar] [CrossRef]
- Planta, J.; Xiang, X.L.; Leustek, T.; Messing, J. Engineering sulfur storage in maize seed proteins without apparent yield loss. Proc. Natl. Acad. Sci. USA 2017, 114, 11386–11391. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cui, S.; Ying, F.; Nasar, J.; Wang, Y.; Gao, Q. Simultaneous improvement of protein concentration and amino acid balance in maize grains by coordination application of nitrogen and sulfur. J. Cereal Sci. 2021, 99, 103189. [Google Scholar] [CrossRef]
- Li, N.; Yang, Y.; Wang, L.Q.; Zhou, C.J.; Jing, J.Y.; Sun, X.; Tian, X.X. Combined effects of nitrogen and sulfur fertilization on maize growth, physiological traits, N and S uptake, and their diagnosis. Field Crop Res. 2019, 242, 107593. [Google Scholar] [CrossRef]
- Gerakis, A.; Baer, B. A computer program for soil textural classification. Soil Sci. Soc. Am. J. 1999, 63, 807–808. [Google Scholar] [CrossRef]
- Chen, X.; Cui, Z.; Fan, M.; Vitousek, P.; Zhao, M.; Ma, W.; Wang, Z.; Zhang, W.; Yan, X.; Yang, J.; et al. Producing more grain with lower environmental costs. Nature 2014, 514, 486–489. [Google Scholar] [CrossRef] [PubMed]
- ISO 5983-1; Animal Feeding Stuffs-Determination of Nitrogen Content and Calculation of Crude Protein Content-Part 1: Kjeldahl Method. International Organization for Standardization: Geneva, Switzerland, 2005; pp. 1–10.
- Gibbon, B.C.; Larkins, B.A. Molecular genetic approaches to developing quality protein maize. Trends Genet. 2005, 21, 227–233. [Google Scholar] [CrossRef]
- Holding, D.R. Recent advances in the study of prolamin storage protein organization and function. Front. Plant Sci. 2014, 5, 276–284. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Joint WHO/FAO/UNU Expert Consultation: Protein and Amino Acid Requirements in Human Nutrition; Technical Report 935; World Health Organization: Rome, Italy, 2007; pp. 1–265. [Google Scholar]
- Yu, B.G.; Chen, X.X.; Zhou, C.X.; Ding, T.B.; Wang, Z.H.; Zou, C.Q. Nutritional composition of maize grain associated with phosphorus and zinc fertilization. J. Food Compos. Anal. 2022, 114, 104775. [Google Scholar] [CrossRef]
- Duvick, D.N.; Cassman, K.G. Post-green revolution trends in yield potential of temperate maize in the North-Central United States. Crop Sci. 1999, 39, 1622–1630. [Google Scholar] [CrossRef]
- Ciampitti, I.A.; Vyn, T.J. Physiological perspectives of changes over time in maize yield dependency on nitrogen uptake and associated nitrogen efficiencies: A review. Field Crop Res. 2012, 133, 48–67. [Google Scholar] [CrossRef]
- Duvick, D.N. The contribution of breeding to yield advances in maize (Zea mays L.). Adv. Agron. 2005, 86, 83–145. [Google Scholar]
- Chen, Y.L.; Xiao, C.X.; Wu, D.L.; Xia, T.T.; Chen, Q.W.; Chen, F.J.; Yuan, L.X.; Mi, G.H. Effects of nitrogen application rate on grain yield and grain nitrogen concentration in two maize hybrids with contrasting nitrogen remobilization efficiency. Eur. J. Agron. 2015, 62, 79–89. [Google Scholar] [CrossRef]
- Scott, M.P.; Edwards, J.W.; Bell, C.P.; Schussler, J.R.; Smith, J.S. Grain composition and amino acid content in maize cultivars representing 80 years of commercial maize varieties. Maydica 2006, 51, 417–423. [Google Scholar]
- Zhang, L.; Liang, Z.; He, X.; Meng, Q.; Hu, Y.; Schmidhalter, U.; Zhang, W.; Zou, C.; Chen, X. Improving grain yield and protein concentration of maize (Zea mays L.) simultaneously by appropriate hybrid selection and nitrogen management. Field Crop Res. 2020, 249, 107754. [Google Scholar] [CrossRef]
- Ma, Y.Z.; Zhang, H.; Xue, Y.F.; Gao, Y.B.; Qian, X.; Dai, H.C.; Liu, K.C.; Li, Q.Q.; Li, Z.X. Effect of sulfur fertilizer on summer maize grain yield and soil water utilization under different irrigation patterns from anthesis to maturity. Agric. Water Manag. 2021, 250, 106828. [Google Scholar] [CrossRef]
- Sriperm, N.; Pesti, G.M.; Tillman, P.B. The distribution of crude protein and amino acid content in maize grain and soybean meal. Anim. Feed Sci. Technol. 2010, 159, 131–137. [Google Scholar] [CrossRef]
- Depuydt, M.; Messens, J.; Collet, J.F. How proteins form disulfide bonds. Antioxid. Redox Signal. 2011, 15, 49–66. [Google Scholar] [CrossRef]
- Hogg, P.J. Disulfide bonds as switches for protein function. Trends Biochem. Sci. 2003, 28, 210–214. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Y.; Luo, X.; Li, J.; Reed, S.A.; Xiao, H.; Young, T.S.; Schultz, P.G. Enhancing protein stability with extended disulfide bonds. Proc. Natl. Acad. Sci. USA 2016, 113, 5910–5915. [Google Scholar] [CrossRef]
- Maurin, A.-C.; Benani, A.; Lorsignol, A.; Brenachot, X.; Parry, L.; Carraro, V.; Guissard, C.; Averous, J.; Jousse, C.; Bruhat, A. Hypothalamic eIF2α Signaling Regulates Food Intake. Cell Rep. 2014, 6, 438–444. [Google Scholar] [CrossRef]
- Kim, S.W.; Chen, H.; Parnsen, W. Regulatory role of amino acids in pigs fed on protein-restricted diets. Curr. Protein Pept. Sci. 2019, 20, 132–138. [Google Scholar] [CrossRef]
- Wu, Y.R.; Messing, J. RNA interference can rebalance the nitrogen sink of maize seeds without losing hard endosperm. PLoS ONE 2012, 7, e32850. [Google Scholar]
- MacGregor, J.M.; Taskovitch, L.T.; Martin, W.P. Effect of nitrogen fertilizer and soil type on the amino acid content of corn grain. Agron. J. 1961, 53, 211–214. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Warren, H.L.; Huber, D.M.; Bressan, R.A. Interactions between the kernel N sink, grain yield and protein nutritional quality of maize. J. Sci. Food Agric. 1983, 34, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.Y.; Dweikat, I.; Huber, D.M.; Warren, H.L. Interrelationship of nitrogen nutrition with maize (Zea mays) grain yield, nitrogen use efficiency and grain quality. J. Sci. Food Agric. 1992, 58, 1–8. [Google Scholar] [CrossRef]
- Lošák, T.; Hlušek, J.; Filipčík, R.; Pospíšilová, L.; Maňásek, J.; Prokeš, K.; Bunka, F.; Krácmar, S.; Martensson, A.; Orosz, F. Effect of nitrogen fertilization on metabolisms of essential and non-essential amino acids in field-grown grain maize (Zea mays L.). Plant Soil Environ. 2010, 56, 574–579. [Google Scholar] [CrossRef]
- Habtegebrial, K.; Singh, B.R. Response of wheat cultivars to nitrogen and sulfur for crop yield, nitrogen use efficiency, and protein quality in the semiarid region. J. Plant Nutr. 2009, 32, 1768–1787. [Google Scholar] [CrossRef]
- Holopainen, U.R.M.; Rajala, A.; Jauhiainen, L.; Wilhelmson, A.; Home, S.; Kauppila, R.; Peltonen-Sainio, P. Influence of sulphur application on hordein composition and malting quality of barley (hordeum vulgare L.) in northern European growing conditions. J. Cereal Sci. 2015, 62, 151–158. [Google Scholar] [CrossRef]
- Salamini, S.A.; Soavc, C. Zein: Genetics and biochemistry. In Maize for Biological Research; Sheridan, W.F., Ed.; University of North Dakota Press: Grand Forks, ND, USA, 1982; pp. 155–160. [Google Scholar]
- Coleman, C.E.; Larkins, B.A. The prolamins of maize. In Seed Proteins; Shewry, P.R., Casey, R., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999; pp. 109–139. [Google Scholar]
- Esen, A. Proposed nomenclature for the alcohol-soluble proteins (zeins) of maize (Zea mays L.). J. Cereal Sci. 1987, 5, 117–128. [Google Scholar] [CrossRef]
- Kirihara, J.A.; Petri, J.B.; Messing, J. Isolation and sequence of a gene encoding a methionine-rich 10-kDa zein protein from maize. Gene 1988, 71, 359–370. [Google Scholar] [CrossRef]
- Swarup, S.; Timmermans, M.C.; Chaudhuri, S.; Messing, J. Determinants of the high-methionine trait in wild and exotic germplasm may have escaped selection during early cultivation of maize. Plant J. 1995, 8, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Randall, J.; Sutton, D.; Ghoshroy, S.; Bagga, S.; Kemp, J.D. Co-ordinate expression of β- and δ-zeins in transgenic tobacco. Plant Sci. 2004, 167, 367–372. [Google Scholar] [CrossRef]
- Landry, J. A linear model for quantitating the accumulation of zeins and their fractions (α+δ, β&γ) in developing endosperm of wild-type and mutant maizes. Plant Sci. 2002, 163, 111–115. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).