Abstract
Legumes are indispensable crops in sustainable agricultural systems because of their capability for biological nitrogen fixation owing to symbiosis with rhizobia and soil fertility restoration. Fungal pathogens from the genera Fusarium cause rotting and wilting and produce mycotoxins in plant tissues. The use of fungicides in sustainable agricultural systems is limited; therefore, the application of biological agents with antifungal activity against Fusarium spp. is desirable. Lactic acid bacteria (LAB) are promising control agents that produce a wide spectrum of functional metabolites. Lactiplantibacillus plantarum and other lactobacilli are the most intensively studied genera of LAB in relation to antifungal activity against Fusarium spp. However, LAB strains belonging to the lactobacilli and lactococci genera have not yet been isolated and characterised from legumes. Therefore, we aimed to obtain wild strains of LAB from legumes, screen them for functional characteristics with respect to their antifungal activity, and compare their antifungal activity against isolates of Fusarium spp. from legumes. Consequently, 31 LAB isolates belonging to 10 species were obtained and identified from legumes. Their functional properties, including genetics and proteomics, short-chain organic acid production, and antifungal activity against five Fusarium spp., of Lactiplantibacillus plantarum, Lacticaseibacillus paracasei, and Lactiplantibacillus pentosus isolates, were studied. Cell-free supernatants of L. plantarum and L. pentosus showed significant suppression of mycelial growth and conidial germination.
1. Introduction
Legumes are used as food and feed because of their highly nutritious seeds and have many uses in agricultural systems. Legume plants are grown as green fodder and fertiliser. They are ploughed into the soil to improve and maintain soil fertility because of the nitrogen fixation by rhizobia in the plant root nodules. Legumes are critical for soil restoration in sustainable agricultural systems with limited or restricted agrochemical use. Legumes are vulnerable to a wide spectrum of fungal pathogens during the growing season. Seed crops are attacked by soil-borne pathogens, including ascomycetous and oomycetous fungi [1]. The genus Fusarium represents ascomycetous micromycetes that cause wild rot and vascular wilt and produce a spectrum of mycotoxins in plant tissues. It includes more than 120 formae specialis based on the host species specificity. Owing to the magnitude of economic losses, mycotoxin production, and complex control, Fusarium spp. are among the top ten plant pathogens worldwide [2,3]. In sustainable agriculture, the use of beneficial microorganisms, micromycetes, and their products has been cited as an effective biological control strategy against Fusarium spp. [4].
Lactic acid bacteria (LAB) represent a heterogeneous phylogenetic group whose members occur in many ecological niches, including plants, soil, water, food and feed, insects, animals, and humans. Their interactions with other organisms are based on mutualism, commensalism, and symbiosis [5]. The current taxonomic classification of LAB is based on a polyphasic approach [6,7]. It includes genetic and phenotypic descriptions of 261 Lactobacillus species in 26 lineages, with 23 new genera. The role of LAB as fermentative and probiotic microorganisms is well known, as is their use in food (dairy, bakery, and wine) and feed industries and human and veterinary medicines. Current knowledge on plant and soil microbiomes allows the use of LAB or their products as protective agents, having antimicrobial, antifungal, and biostimulant activities [8,9,10]. LAB colonise all plant parts at varying concentrations depending on the geographic and environmental conditions, host species, phenology, and many other factors. LAB have never represented the dominant population in plants and soils, and their concentration ranking is 102–104 CFU·g−1 [11,12]. Studies on plant-associated LAB have confirmed that LAB strains isolated from the same plants show genetic relatedness, similar carbohydrate utilisation, and stress adaptability [13,14]. LAB strains from competing sources (silage and sourdough) showed a stronger antifungal effect than those from a uniform matrix [15,16].
The antifungal activity of LAB results from metabolites regulated by gene expression and is considered an intraspecific trait [17]. Short-chain organic acids, bacteriocins, chitinolytic enzymes, and volatile compounds are believed to be the metabolites that inhibit fungal growth and spore germination [15,18]. Lactiplantibacillus plantarum and other lactobacilli are the most intensively studied genera of LAB in relation to their antifungal activity against Fusarium spp. [19,20]. However, LAB strains belonging to the lactobacilli and lactococci genera have not yet been isolated and characterised from legumes. Therefore, the aim of this study was to obtain wild strains of LAB from legumes, screen them for functional characteristics with respect to their antifungal activity, and compare their antifungal activity against isolates of Fusarium spp. from legumes.
2. Materials and Methods
2.1. Isolation and Identification of LAB
The leaves, stems, flowers, and roots of legumes (peas, soya, and lupins) were separated in the laboratory. The plant material was collected from experimental fields of the Faculty of Agriculture and Technology, the University of South Bohemia, the Czech Republic (48.9805606 N, 14.6182772 E); the Faculty of Agrobiology, Food and Natural Sciences, the Czech University of Life Science, Prague, the Czech Republic (50.054247 N, 14.594223 E); and in the fields of the farm Soběkury, the Czech Republic (49.5760789 N, 13.2389956 E). The plant tissues were homogenised in sterile mortars. Then, 1 g of the homogenised material was diluted in sterile Ringer’s solution (Sigma-Aldrich, St. Louis, MO, USA) and standardised using serial dilution [21]. Within five days, cultivations of each dilution were performed anaerobically at 30 °C on de Man, Rogosa, and Sharpe (MRS) agar (Merck KGaA, Darmstadt, Germany) with vancomycin (0.05%; Glenham Life Sciences, Corsham, UK) [22]. Bacterial counts (CFU·g−1) of the LAB were taken for serial dilution in triplicate. All bacterial isolates were examined under a microscope (Olympus BX43, Sindzuku, Japan) using a staining assay (G+ staining, catalase-negative) to determine the cell morphology. Catalase-negative G+ cocci and lactobacilli were identified using 16S rDNA analysis and tested for class IIa bacteriocins, chitinases, and their extracellular products. The bacterial DNA was extracted using the DNeasy® UltraClean® Microbial Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. The DNA was quantified using the Qubit® Fluorometer 3.0, (Life Technologies, Waltham, MA, USA), and the dsDNA was quantified using the Qubit® Fluorometer 3.0 and dsDNA HS Assay Kit (Life Technologies, Waltham, MA, USA). Lastly, the DNA was stored at −40 °C for further PCR amplification.
2.2. Isolation and Identification of Fusaria
The Fusarium spp. isolates were obtained from the seeds and seedlings of legumes and soil samples from legume fields. Dichloran-glycerol (DG18) (VWR, Leuven, Belgium) with chloramphenicol (AppliChem GmbH, Darmstadt, Germany) and dichloran rose-bengal chloramphenicol media (DRBC) (OXOID Ltd., Hampshire, UK) were used for primary isolation. The isolates were cultured in potato dextrose agar (PDA) (OXOID Ltd., Hampshire, UK) at 25 °C [23]. The isolates were examined microscopically (Olympus BX 43, Sindzuku, Japan) and identified using DNA sequencing of the ITS region [24] and barcoding [25]. The identified isolates were stored on agar plates and as freezing cultures in PDA mixed with glycerol (2%) at −40 °C. DNA isolation from the mycelia was performed in microtubes containing glass beads (G1145, Sigma-Aldrich, Darmstadt, Germany) and 20 µL 0.5 M NaOH [26]. The vials were vortexed using a vortex adapter Omni 24 (Omni International, Kenesaw, GA, USA), homogenised at maximum power for 5 min, and centrifuged at 13,000× g for 2 min. The supernatant was transferred into sterile microtubes and diluted 10-fold in 10 mM Tris-HCl (pH 8.5) (Life Technologies, Waltham, MA, USA). The DNA was quantified using the Qubit® Fluorometer 3.0, (Life Technologies, Waltham, MA, USA), and the dsDNA was quantified using the Qubit® Fluorometer 3.0 and dsDNA HS Assay Kit (Life Technologies, Waltham, MA, USA). Lastly, the DNA was stored at −40 °C for further PCR amplification.
2.3. Functional Characteristics of Lactobacilli
The genes encoding class IIa bacteriocins and chitinases were identified in the bacterial cultures. The lactobacilli were cultivated in MRS broth (Merck KGaA, Darmstadt, Germany) and enriched with fructose (Lachner, Neratovice, the Czech Republic), glucose (Lachner, Neratovice, the Czech Republic), sodium gluconate (Sigma-Aldrich, Steinheim, Germany), and maltose (Glentham, Corsham, UK) [MRS-FGGM] in 50 mL falcons at 30 °C for 24 h [27].
To determine the extracellular metabolites of the lactobacilli, cell-free supernatants (CFS) were obtained using the following procedures. The lactobacilli were cultivated in MRS-FGGM broth in 50 mL falcons at 30 °C for 24 h as described above. The falcons with the lactobacilli were then centrifuged for 15 min (16,000× g). The supernatants were filtered through a sterile microbial filter (Chromafil CA/S; Macherey-Nagel, Germany) to obtain the CFS.
2.3.1. PCR Amplification of Class IIa Bacteriocin Genes
The class IIa genes were amplified using diverse primer pairs (Table S1) [28]. The PCR assay mixture (25 µL) consisted of 0.5 µL of each primer (10 µM), 1 µL of DNA template, 12.5 µL of 2× PPP Taq MasterMix (TopBio, Prague, the Czech Republic), and 10.5 µL of ddH2O. The PCR amplification was performed using a Biometra thermal cycler (Analytic Jena GmbH+Co, Jena, Germany) as follows: pre-heating at 95 °C for 2 min, 35 cycles each of denaturation at 95 °C for 30 s, annealing at 44 °C for 30 s, and extension at 72 °C for 60 s, followed by a final extension at 72 °C for 8 min. The PCR products were visualised on a GelRed®-stained (Biotium, Fremont, QC, Canada) 2% SeaKem® LE agarose gel (SeaKem® LE; Lonza, Rockland, ME, USA) at 60 V for 120 min and viewed using the GeneGenius Bio Imaging System (Syngene, Frederick, MD, USA).
2.3.2. PCR Amplification of the Chitinase Gene
The chitinase (chiA) gene was detected by using two different primer pairs, namely chiAF (5′-ACCCTTCCCACTTTCAAGCC-3′) and chiAR (5′-ATATGAGCGTCAGCTCCTCC-3′) and chiFEMSF (5′-GATATCGACTGGGAGTTCCC-3′), and chiFEMSR (5′-CATAGAAGTCGTAGGTCATC-3′), according to Liu et al. (2015) [29]. The PCR reaction (25 µL) included 0.5 µL of each primer (10 µM), 1 µL of DNA template, 12.5 µL of 2× PPP Taq MasterMix (TopBio, Prague, the Czech Republic), and 10.5 µL of ddH2O. The PCR products were amplified using Biometra thermocycler (Analytic Jena GmbH+Co, Jena, Germany) as follows: pre-heating at 95 °C for 2 min, 35 cycles each of denaturation at 95 °C for 30 s, annealing at 52/46 °C for 30 s, and extension at 72 °C for 60 s, followed by a final extension at 72 °C for 8 min. The PCR products were separated on a GelRed®-stained (Biotium Inc., Fremont, QC, Canada) 2% SeaKem® LE agarose gel (Lonza, Basil, Switzerland) at 60 V for 120 min and viewed using the GeneGenius Bio Imaging System (Syngene, Frederick, MD, USA).
2.3.3. Chitinase Production
The extracellular proteins and peptides were isolated and visualised as described by [15], with minor changes. The Amicon ProAffinity Concentrator 30.000 NMWL (Merck Millipore Ltd., Tullagreen, Ireland) was used according to the instruction manual to concentrate the extracellular proteins and peptides in the CFS. The proteins and peptides were isolated using a ProteoExtract protein precipitation kit (Merck KGaA, Darmstadt, Germany) according to the manufacturer’s instructions. The obtained pellets were dried and resuspended in 20 µL of tricine sample buffer (BIO-RAD, Hercules, CA, USA) supplemented with 2-mercaptoethanol (2% v/v; BIO-RAD, Hercules, CA, USA) and heated at 70 °C for 10 min. The resolving (12%) and stacking (4%) gels and buffers were prepared according to Haider et al. (2012) [30]. Precision Plus Protein™ Dual Xtra Standards (BIO-RAD, Hercules, CA, USA) and polypeptide SDS-PAGE molecular weight standards (BIO-RAD, Hercules, CA, USA) were loaded in the wells together with the samples (8 µL) under a constant voltage of 125 V until the dye front touched the bottom. A Mini-Protean Tetra Cell apparatus for 1D vertical electrophoresis (Bio-Rad, Hercules, CA, USA) was used. The gels were washed and fixed in a fixing solution (50:10:40/methanol: acetic acid: H2O) for 25–30 min and repeatedly washed in deionised water. The products were stained using GelCode Blue Safe Protein (Thermo Scientific, Rockford, IL, USA) in a rocking bath for 1 h. The gels were destained in 10% acetic acid solution. The peptides and proteins were determined directly from the concentrates using 30.000 NMWL on a timsTOF Pro (Bruker Daltonics GmbH, Leipzig, Germany) using the UltiMate 3000 nano ultra-high-performance liquid chromatography system (Bruker Daltonics GmbH, Leipzig, Germany). The MaxQuant software (Max Plant Institute of Biochemistry, Munich, Germany) and the UniProt database were used for data analysis.
2.3.4. Production of Organic Acids
The production of short-chain organic acids (lactic and acetic acids) by the lactobacilli was determined in the CFS using an isotachophoretic analyser EA 02 (VILLA Labeco, Nitra, Slovakia). The results were presented in mg·100 mL−1. The pH values of the cultivation media were measured in triplicate using an inoLab pH 730 pH meter (WTW, Weilheim in Oberbayern, Germany).
2.4. Antifungal Assays
2.4.1. Agar Plug Diffusion Method
The lactobacilli were cultivated in MRS-FGGM in 50 mL falcons at 30 °C for 24 h. The conidial suspension of the Fusarium isolates was estimated to be 105 in 1 mL by using a Bürker counting chamber and serial dilution [23]. A drop of Fusarium conidial suspension (100 μL) was placed in a Petri dish and mixed with PDA medium cooled to 45 °C. (OXOID Ltd., Hampshire, UK). After the solidification of the PDA, five plugs (avr. 6 mm) of each Lactobacillus isolate were placed on Petri dishes inoculated with Fusarium under sterile conditions. The growth of mycelia was observed daily for five days, and the mycelial growth was evaluated using the following index scale: 1—clear Petri dish; 2—a clear area around and among the Lactobacillus plugs, and the mycelia occur on the outer side of plugs; 3—a few fungal colonies among the plugs, and the mycelia occur on the outer side of plugs; 4—Petri dish completely covered by mycelia. Three Petri dishes for each combination of the Fusarium strain and Lactobacillus isolates were used in the triplicate experiment.
2.4.2. Antifungal Activity of Extracellular Products
The effect of the cell-free supernatants of lactobacilli (Table 1) on the radial growth of mycelia from the plugs was observed to determine the antifungal activity [31]. Five Fusarium spp. were cultivated on PDA medium (OXOID Ltd., Hampshire, UK) at 25 °C for five days. For this purpose, a drop (1 mL) with an estimated concentration of 1 × 10−5 conidia was placed in the bottom of the Petri dishes and mixed with tempered PDA medium. The lactobacilli were cultivated in MRS-FGGM in 50 mL falcons at 30 °C for 24 h. The falcons with lactobacilli were centrifuged (22,740× g) for 10 min. Without disturbing the pellets, the supernatant was filtrated via sterile microbial filters (0.22 µL; Filtratech, France) to obtain the cell-free supernatants (CFS). Thereafter, an ultrafiltration device, the Amicon 30 MWCO (Merck Millipore Ltd., Tullagreen, Ireland), was used to divide the CFS into two fractions: a concentrate with proteins ≥ 30 kDa (CFS F1) and filtrate containing proteins ≤ 30 kDa (CFS F2). The CFS F1 or CFS F2 was separately mixed with tempered PDA (20%) and poured into a Petri dish to compare their inhibitory effects. Mycelial plugs (diameter 6 mm) were cut from the Fusarium plates and placed on solidified PDA with the CFS F1 and CFS F2 (20%). The mycelial growth from the plugs on PDA with 20% sterile water and PDA alone were used as controls. The radial growth of the mycelia was measured after 24 h for five days from the edge of the discs in the four cross positions. The experiment and all the measurements were repeated three times.

Table 1.
The isolates of lactobacilli from legumes. The isolates with acronyms were screened for class IIa bacteriocins, the chiA gene, extracellular chitinase production, short-chain organic acid production, and antifungal assays.
The confluent layer of fungal mass was observed in Petri dishes filled with buffered peptone water (1.5 mL) (BPW) [Granucult®, Merck KGaA, Darmstadt, Germany], inoculated with 100 µL conidial suspension, for 24 h to determine the influence of 20% (w/v) CFS F2 (2–30 kDa) on the conidia germination and mycelial growth. The confluent layer was measured using a CytoSMART Lux2 (Axion BioSystems, Atlanta, GA, USA) at 30 min intervals and expressed as a percentage of the observed area. At the beginning and the end of each experiment, the PDA plates were inoculated with 100 µL of complex suspension to establish the concentration and viability of the Fusarium spp. strains.
2.5. Data Processing
The number of LAB on the legumes (CFU·mL−1) was log-transformed using a common logarithm (log 10×) and analysed using non-parametric Kruskal–Wallis (H) tests (one-way non-parametric ANOVA for independent samples with different sample sizes) in Statistica software v 12.1. (StatSoft Europe, Hamburg, Germany) due to the unequal number of samples. The data for the physicochemical parameters (pH, short-chain organic acid production) of the CFS and PDA with CFS (20%) were subjected to statistical analysis using ANOVA with factorial design in Statistica software v 12.1 (StatSoft Europe, Hamburg, Germany). The data obtained from the antifungal assays were subjected to ANOVA with a factorial design [32]. The data based on the index scale were first log-transformed using the natural logarithm (log ×) due to the linear data distribution. Tukey’s HSD test at p ≤ 0.05 was used to determine significant differences between the tested isolates and interactions, and the data were presented as the mean and standard error of the mean due to the discrepancy between the sample means. Linear regression models (Statistica software v 12.1, StatSoft Europe, Hamburg, Germany) were used to determine the correlation between the confluence and the final concentration of viable conidia in the BWP (CFU·mL−1) based on the coefficient ß [32].
3. Results and Discussion
3.1. Isolation and Identification of LAB
In total, 31 isolates of LAB belonging to 10 species were obtained and identified from legume plants (Table 1) during the vegetative season. Six isolates of L. plantarum, one of L. paracasei, and two of L. pentosus were characterised based on the presence of genes, protein products, organic acid production, and antifungal activity against five Fusarium spp., together with three strains of L. plantarum from the CCDM collection (Table 1). Lactiplantibacillus plantarum, Leuconostoc meseteroides, and Leuconostoc lactis occurred most frequently in the legumes. Other lactobacilli such as Lacticaseibacillus paracasei, Lactiplantibacillus pentosus, Loigolactibacillus coryniformis, and Sporolactibacillus nakymae occurred less frequently. The species pool of representative LAB in legumes generally corresponds with the data obtained from plants [13]. However, the dataset was originally for legumes (pea, soya, and lupine) cultured in Central Europe. LAB strains belonging to the lactobacilli and lactococci genera have not yet been isolated and characterised from legumes.
The colonisation of legume plants (lupine, pea, and soya) by LAB varied from an average of 102 to a maximum of 104 CFU·g−1 during legume flowering. Although the LAB colonisation of the aerial parts of the legumes appeared higher than the roots, there were no significant differences in CFU·g−1 between the aboveground parts and roots (Kruskal–Wallis test, H(1.76) = 2.49; p ≤ 0.115) (Figure S1). Similarly, the legume species (pea, soya, and lupine) did not influence the CFU·g−1 of the LAB (Kruskal–Wallis test, H(2.76) = 1.57; p ≤ 0.46) (Figure S2). LAB are present in legumes in minor amounts compared to other microorganisms, especially in the root system, and their natural role is unclear. The LAB isolates from the legumes in this study belong to well-known plant-associated genera [5]. L. plantarum isolates were the most abundant. This nomadic species possesses a spectrum of genes and metabolic products with protective effects against fungal and bacterial pathogens [13,33].
3.2. Isolation and Identification of Fusarium spp.
The Fusarium isolates from legumes belonged to the following five species: F. proliferatum, F. oxysporum, F. avenaceum, F. culmorum, and F. sporotrichoides. Isolates with consistent barcoding results were used for the antifungal assays (Table 2). The presence of Fusarium spp. in legumes has been reported in many studies [3], including races and pathotypes specialised in legume species (such as lentils and peas) [34]. The isolates obtained from the seeds and seedlings of the legumes (pea, soybean, and cowpea) were identified using DNA barcoding as F. oxysporum, F. avenaceum, F. proliferatum, F. culmorum, and F. sporotrichoides. This spectrum corresponds to the data reported by Pflughöft et al. (2012) [35]. The strains were deposited onto agar slants and as frozen cultures (−70 °C) in CCDBC (Culture Collection of Dairy and Bakery Contaminants, MILCOM Ltd., Prague, the Czech Republic).

Table 2.
The list of Fusarium spp. used in antifungal assays, including CCDBC (Culture Collection of Dairy and Bakery Contaminants, Milcom Ltd., Prague, the Czech Republic) acronyms and accession numbers (the * Gene Bank and NCBI databases).
Representatives of these species were tested against L. plantarum, L. paracasei, L. pentosus, and their metabolites. Representatives of Fusarium spp. show high ecological plasticity and adaptability and survive endophytically in legume grains.
3.3. Functional Characteristics of Lactobacilli
3.3.1. Bacteriocin Gene Class IIa
The L. plantarum isolates and strains differed in the spectra of PCR products generated when amplified using primers complementary to four clades (six primer pairs) of class IIa bacteriocins (Table 3, Figures S3–S8). Variability in the genes encoding the class IIa bacteriocins in the five clades was found in the L. plantarum isolates. Compared to silage strains (CCDM 191 and 196) and ATCC14917, the legume isolates (A, B, C, D, E, J, and CCDM 1110) did not yield any product when using clade 2 amplification primers. Variability was also noted in the products obtained using the second primer pair for clade 5. Unlike ATCC14917, none of the L. plantarum isolates or strains generated PCR products using the second primer pair for clade 4. PCR products were not obtained from L. paracasei or L. pentosus isolates using any of the six primer pairs.

Table 3.
The gene profile coding class IIa bacteriocins of isolates L. plantarum A, B, C, D, E, I, and J from legumes; the collection strains CDDM 191, 196, and 1110; and the reference strain ATCC14917. The sign + represents the amplified PCR product in Clades 1–5, and the sign − no amplified product.
Although class IIa bacteriocin production was originally associated with antimicrobial activity [36], antifungal activity of L. plantarum strains with bacteriocin production against yeast and filamentous fungi has also been reported [37]. Nevertheless, screening of peptide and protein products using mass spectrophotometry did not confirm the extracellular production of class IIa bacteriocins. The conditions for gene expression that define peptide products remain unknown. The antifungal activity of strains with known bacteriocin production [38,39] has been tested against a limited spectrum of fungi using in vitro assays; however, the screened genes in clades 1 to 5 have never been tested simultaneously. Therefore, it remains unclear whether the strain variability in clades can influence the production of antifungal metabolites with target activity, especially when isolates are from similar competitive environments. Our recent study [15] confirmed that strains from microbiologically rich environments, such as silages, have considerable inhibitory effects on a spectrum of yeasts.
3.3.2. Chitinase Genes and Extracellular Chitinase Production
The gene chiA, which encodes chitinase (Figure S9), and chitinase precursors were successfully amplified in all isolates and strains except for isolate F (L. paracasei) (Table 4).

Table 4.
The presence of the chitinase gene chiA in DNA of lactobacilli isolated from legumes (A, B, C, D, E, J—L. plantarum, F—L. paracasei, G, H—L. pentosus) and strains from silage (CCDM 191, 196, 1110—L. plantarum) and its extracellular production as LACO chitin-binding protein (22,199 kDa) into culture media (MRS-FGGM). The sign + represents the amplified PCR product in Clades 1–5, and the sign − no amplified product.
The protein products matching the LACO chitin-binding protein (CBP) were produced extracellularly in the medium by most isolates, except for C (L. plantarum), F (L. paracasei), and G (L. pentosus) (Figure 1 and Figure 2). In addition, the CCDM strains produced CBP extracellularly, although the pattern on the tricine gel differed from those of the products of lactobacilli isolated from the legumes (Figure 3).
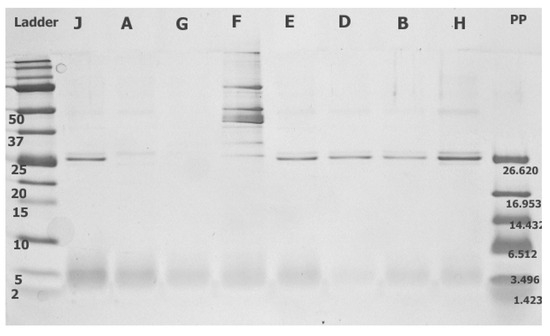
Figure 1.
The spectrum of extracellular proteins and peptides produced by lactobacilli isolated from legumes (L. plantarum) [J, A, E, D, B], (L. paracasei) [F], (L. pentosus) [G, H] contained in filtrate <30 kDa visualised with 15% Tricine-PAGE. The bands in the 25 kDa spectrum were determined as LACO chitin-binding protein (22.19 kDa).
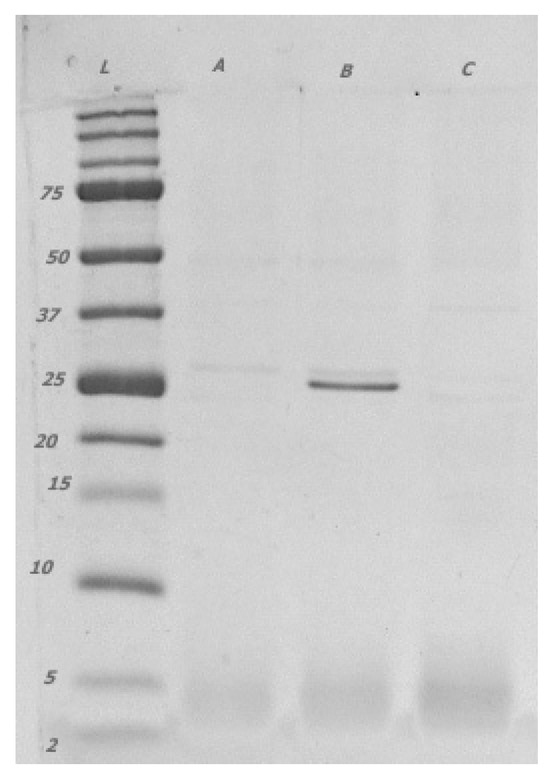
Figure 2.
The spectrum of extracellular proteins and peptides produced by lactobacilli isolated from legumes (L. plantarum) [A, B, C] contained in filtrate <30 kDa visualised with 15% Tricine-PAGE. [L] protein ladder. The bands in the 25 kDa spectrum were determined as LACO chitin-binding protein (22.19 kDa).
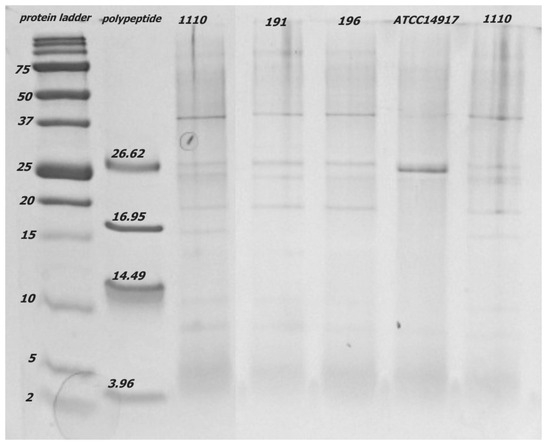
Figure 3.
The spectrum of extracellular proteins and peptides in filtrate <30 kDa produced by L. plantarum from CCDM 1110, 191, 196, and ATCC14917 visualised with 15% Tricine-PAGE. The bands in the 25 kDa spectrum were determined as LACO chitin-binding protein (22.19 kDa).
Notably, similar products were amplified in both isolates of L. pentosus, probably because of their phylogenetic similarity to L. plantarum [40]. Extracellular expression of the LACO CBP was detected by using Tricine-PAGE, except in three isolates (C, E, and F), and was confirmed using mass chromatography. The Tricine gel signal reflects the extracellularly produced protein and peptide concentrations, which depend on the density of bacterial cells in the nutritional medium. Although the initial inoculum was equal for all the isolates and strains of lactobacilli, the growth of particular isolates in time and the amount of extracellular proteins might differ. The size of the protein fraction in the CFS played an with CFS essential role in the antifungal effect because the suppression of mycelial growth on the PDA F2 (≤30 kDa) was evident. Only the protein LACO CBP (22.19 kDa) was identified as a protein with possible antifungal activity in the fraction CFS F2 (≤30 kDa) and Tricine-PAGE products [41,42]. Subsequently, several other proteins in the range of approximately 25 kDa were identified, and their functions have been previously described as 9LACO WxL domain-containing proteins [43] and 9LACO LysM peptidoglycan-binding domain-containing protein OS [44]. Although the direct antifungal effects of these proteins have not been tested, they may be involved in the interactions between eukaryotic and prokaryotic cells.
3.3.3. Production of Organic Acids
All isolates and strains of lactobacilli significantly acidified the medium compared to the pH values of MRS-FGGM. The culture medium (MRS-FGGM) was most intensively acidified by the strain CCDM 1110, but the pH values of the strains and isolates of lactobacilli were not significantly different (Table 5). Similarly, the addition of CFS (<30 kDa) significantly decreased the pH of the PDA. The pH did not vary significantly among the strains and isolates of lactobacilli, although the lowest pH value was measured in the PDA supplemented with CCDM 1110. Isolate J (L. plantarum) from the legumes decreased the pH of MRS-FGGM and PDA to a level similar to that of the CCDM strains from silages. The production of short-chain organic acids varied significantly within the strains of lactobacilli. The CCDM 1110, 191, and 196 strains produced significantly higher amounts of lactic and acetic acids than the legume isolates. Isolates G (L. pentosus) and J (L. plantarum) produced similar amounts of acetic acid as the CCDM strains. Considering that pH and short-chain organic acids play crucial roles in antifungal [45] and anti-aflatoxigenic activities [46], strain CCDM 1110 significantly exceeded the production of lactic and acetic acids. The amounts of lactic and acetic acid were the same in both media, except for isolate E (L. paracasei). Strains CCDM 191 and 196, whose lactic and acetic acid production was recently reported [15], were used as positive controls. Phenylated forms of lactic acid and other organic acids are most effective in terms of the antifungal activity of lactobacilli [47,48].

Table 5.
The acidity of cell-free supernatants (CFS; MRS-FGGM) after 24 h cultivation of lactobacilli, PDA with CFS2 (20% v/v), and the content of lactic and acetic acids (mg·100 mL−1) in CFS (MRS-FGGM). The values are represented by the averages and standard error of the mean (s.e.m.). The index letters denote the significant variability in the production of short-chain organic acids within the lactobacilli.
3.4. Antifungal Assays
3.4.1. Agar Plug Assay
The growth of five Fusarium spp. (Table 6) was observed on the PDA with five agar plugs (MRS-FGGM) containing cells and metabolites of lactobacilli (Table 1). The evaluation of the experiment was assessed based on an index scale of 1 to 5. The significant difference in the inhibitory effect of lactobacilli on Fusarium spp. showed (Table 6, Figure 4) that the strains L. plantarum B, J, CCDM 1110, and L. pentosus G and H limited mycelial growth, such that mycelia grew only on the outer side of the plugs and the edges of the Petri dishes (Table S2, Figures S10 and S11). Five isolates of Fusarium spp. were found to be differentially sensitive to the tested Lactobacillus strains (Table 6) and their metabolites. F. avenaceum was significantly more sensitive than the other isolates; thus, its mycelia barely grew. The isolates F. oxysporum and F. culmorum [34], which were the most abundant and aggressive species, were generally less sensitive. Despite this, the zones of inhibition around the plugs were evident. Regarding the population ecology of L. plantarum and L. pentosus in the legumes, the cell numbers and, consequently, the concentration of metabolites could not reach the concentration obtained using agar plugs containing lactobacilli. The use of by-products of lactobacilli isolates with proven antifungal activity is a promising step to attain protective and growth-promoting effects.

Table 6.
The variability in the inhibitory effect of MRS-FGGM agar plugs with lactobacilli on the growth of five Fusarium spp. The significance of factors based on log-transformed data from the index scale (Factorial ANOVA, Statistica soft. v.12.1., p ≤ α ≤ 0.05). The index asterisk denotes the significant variability.
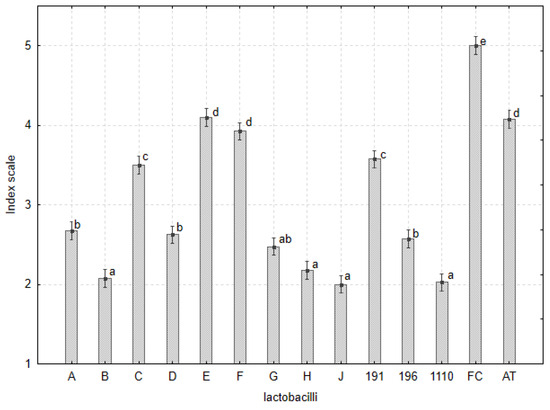
Figure 4.
The inhibitory effect of lactobacilli on the growth of five Fusarium spp. in agar plug assay based on the index scale represented by mean and s.e.m. The index letters denote the significant variability within the lactobacilli strains. Isolates A, B, C, D, E, and J of L. plantarum; F of L. paracasei; G and H of L. pentosus from legumes; CCDM 191, 196, and 1110 of L. plantarum from silage; FC-Fusarium spp. Control; and AT-ATTC14917.
3.4.2. Mycelia Growth
The CFS fractions in the MRS-FGGM, the Lactobacillus isolates, and the intraspecific sensitivity of Fusarium sp. influenced the mycelial growth of the five Fusarium spp. (Table 7, Figure 5).

Table 7.
The significance of the factors influencing the inhibitory effect of CFS of lactobacilli, CFS1 ≥ 30 kDa, and CFS2 ≤ 30 kDa, against five isolates of Fusarium spp. The index asterisk denotes the significant variability.
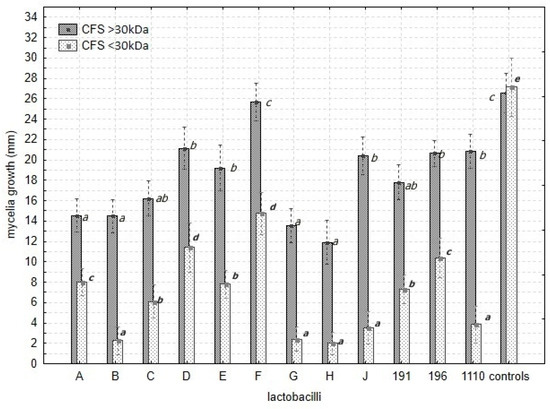
Figure 5.
The effect of cell-free supernatants, CFS F1 >30 kDa (dark bars) and CFS F2 < 30 kDa (dotted bars), mixed with PDA (20%) on radial growth of Fusarium spp. The significant effect of CFS F1 and CFS F2 fractions is highlighted by index values. The index letters denote the significant variability within the lactobacilli.
The extracellular products in CFS F1 and CFS F2 significantly inhibited the growth of Fusarium spp. compared with the controls (Figure 4, Table 7 and Table S3). Significant variability in the inhibitory effect of the CFS was also noted among the Lactobacillus isolates (Table 7 and Table S3, Figure 5). The supplement of CFS F2 (≤30 kDa) relating to the B, G, H, and J isolates and the strain CCDM 1110 medium significantly suppressed the growth of mycelia in contrast to the control and other CFS F2 (≤30 kDa) (Figure 6, Figure 7 and Figure 8).
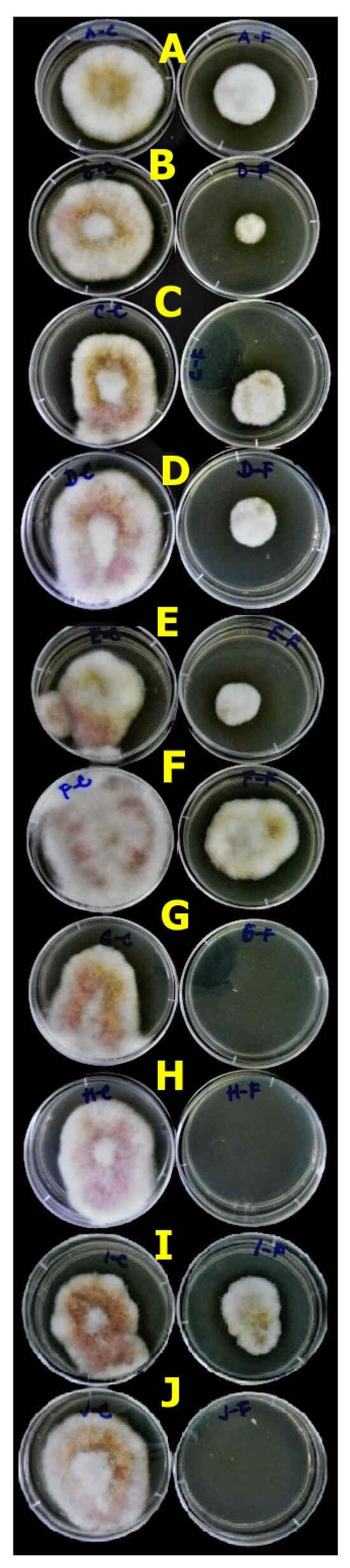
Figure 6.
The variability in the antifungal effect of isolates from legumes on Fusarium culmorum after 5 days of cultivation at 25 °C. The left row represents the variant PDA supplemented with CFS F1 (≥30 kDa) and the right row with CFS F2 (≤30 kDa).
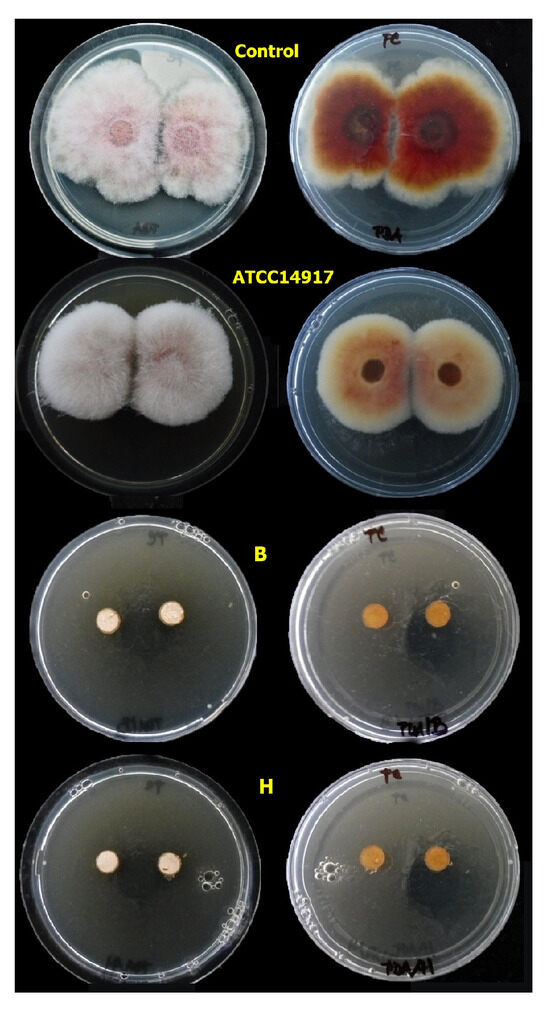
Figure 7.
The mycelial growth of Fusarium culmorum on PDA with 20% of CFS F2 from lactobacilli (L. plantarum) [control, ATCC14917, B], (L. pentosus) [H] with antifungal activity after 5 days of cultivation at 25 °C.
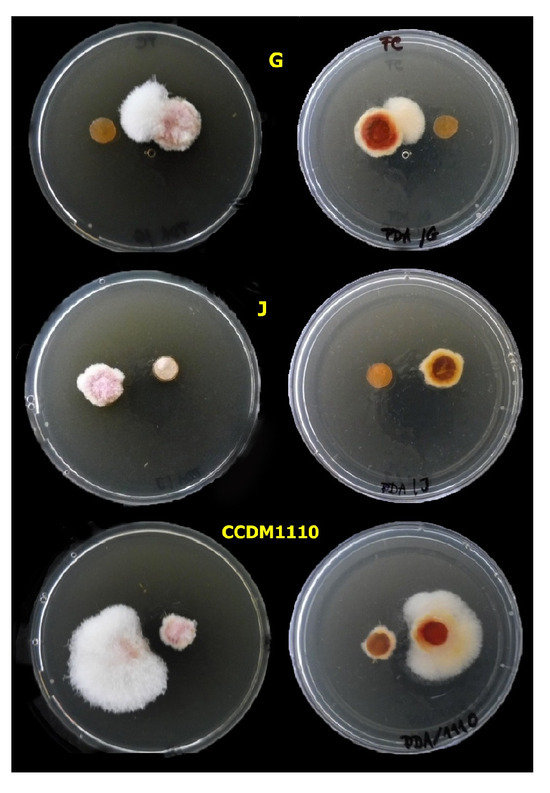
Figure 8.
The mycelial growth of Fusarium culmorum on PDA with 20% of CFS F2 from lactobacilli (L. pentosus) [G] and (L. plantarum) [J, CCDM1110] with antifungal activity after 5 days of cultivation at 25 °C.
The antifungal effects also included sensitivity against all five Fusarium spp. The most sensitive Fusarium sp. to CFS F1 and CFS F2 was F. avenaceum (H1), and the most resistant was F. oxysporum (D1) (Figure S12). The radial growth of the mycelia reached almost the same level in all Fusarium spp. cultured on the PDA (control II) and PDA supplemented with MRS-FGGM (20%; control I). The values from the controls significantly exceeded those from the experimental variants with CFSs.
The results of both antifungal assays supported each other. Plugs with living cells of the isolates B, G, H, and J and strain CCDM 1110 significantly inhibited mycelial growth, and the CFS from identical isolates suppressed the growth of Fusarium spp. Using a confluence test, these isolates and the strain CCDM 1110 (Figures S13 and S14) were tested for their ability to inhibit mycelial growth and conidial germination within 24 h in the BPW and BPW with 20% CFS. The addition (20% v/v) of the CFS to the BPW significantly decreased the mycelial growth (Figure 9, Table 8 and Table S4) and spore germination (Figure 10, Table 9 and Table S5).
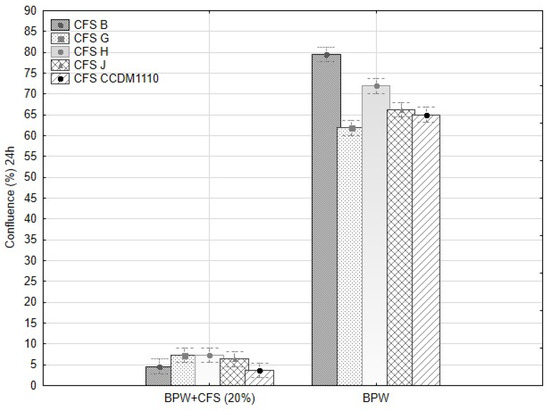
Figure 9.
The confluence of mycelia of five tested Fusarium sp. after 24 h of cultivation (25 °C). The comparison of mycelia growth in BPW medium with 20% of CFS from lactobacilli and BPW alone.

Table 8.
The significance of the factors influencing the inhibitory effect of CFS of lactobacilli, CFS F1 ≥ 30 kDa and CFS F2 ≤ 30 kDa, against five isolates of Fusarium spp. (Factorial ANOVA, Statistica soft. v.12.1., p ≤ α ≤ 0.05). The index asterisk denotes the significant variability.
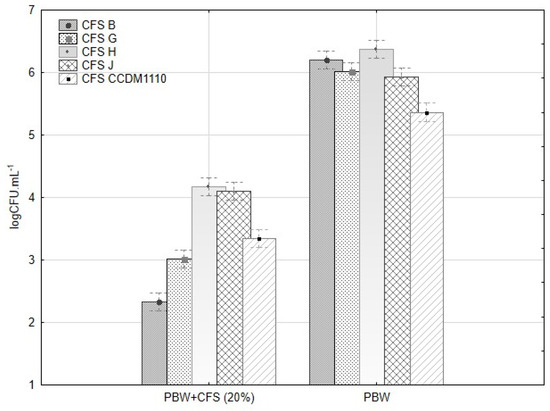
Figure 10.
The influence of metabolites from lactobacilli on the germinability of conidia of five tested Fusarium sp. within 24 h. The CFU·mL−1 (log10) of Fusarium spp. after 24 h cultivation in BPW with 20% of CFS and BPW alone. The start inoculum was estimated to be 1.105 CFU·mL−1.

Table 9.
The factors influencing the germinability of conidia incubated 24 h in BPW with CFS from lactobacilli and PWB alone. The isolates that significantly inhibited mycelia growth were used (B, J, G, H, and CCDM1110) [Factorial ANOVA, Statistica soft. v. 12.1. p ≤ 0.05]. The index asterisk denotes the significant variability.
The CFU·mL−1 of the remaining Fusarium strains decreased significantly (Table S4). The confluence (%) of mycelia in the BWP with CFS was positively correlated with the final CFU·mL−1 after 24 h (R = 0.952, F(3,146) = 474.5, p ≤ 0.001, β = 0.93). The CFS from B and G completely inhibited the spore germination of F. avenaceum (H1) (Table S5). The sensitivity of the hyphal tips and young hyphae to environmental stress was higher than that of the conidia, although the sensitivity depends on the environmental and physiological factors of the fungal strain/isolate. The suppression of mycelial growth and decrease in germination of the Fusarium conidia within 24 h are important findings that may help design the application of CFS for legume cultivation and fungal disease control. Thus, isolate B (L. plantarum) appears to be a promising candidate for biological control. The other effective isolates (G, H, J, and CCDM 1110) partially suppressed conidial germination, but the inhibition of the mycelia was comparable to B. Strains of L. plantarum could suppress the conidial germination of post-harvest pathogens by 80–90% [49]. The isolate MYS6 (L. plantarum) inhibited the hyphal growth and germination of the conidia of F. proliferatum and eliminated mycotoxins from fermented legume foods and feeds [36,37]. The characterisation of the effective isolates and the strain CCDM 1110 differed only in the PCR products of clades 4-1 and 5-2 (isolates B, CCDM 1110, and J), and CCDM 1110 produced significantly high amounts of lactic and acetic acids. The characterisation of the isolates based on the available molecular and proteomic analyses supported the results of antifungal bioassays using a spectrum of Fusarium spp. from the legumes.
4. Conclusions
Lactobacillus isolates, including L. plantarum and L. pentosus, from legumes were effective in suppressing the mycelial growth and conidial germination of five Fusarium species. Their genetic backgrounds showed a predisposition to producing class IIa bacteriocins and chitinases. High levels of lactic and acetic acids and LACO CBP were detected in their extracellular matrix. However, the antifungal activity may be due to many other molecules and factors that were not detected or described in the present study. The results showed that the strains of L. plantarum (B and J) and L. pentosus (G and H) from legumes inhibited the mycelial growth and conidial germination of Fusarium spp. more effectively than the strains from silages (CCDM 191, 196, and 1110). The application of concentrated cell-free ferments from L. plantarum and L. pentosus with antifungal activity for seed coating or spraying the green parts of legumes against Fusarium spp. will prove to be an effective method for the biological control of legumes. The next necessary step is to parameterise the optimal growth curve of the effective strains to produce the desired by-products with protective properties. Given the promising preliminary results from the seed coating trials, the composition of the medium to produce functional products from lactobacilli with plant-promoting and antifungal activity is a crucial step to obtain an effective biocontrol product with stability and persistence in the field environment.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy13122911/s1. Table S1: Table of primers used for characterisation of lactobacilli. Table S2: The interactions among lactobacilli and five Fusarium species based on the index scale value. The data represent the mean and s.e.m. of index values and log-transformed index values. The significant differences are denoted with index letters. (ANOVA, post hoc Tukey’s HSD test, Statistica soft. v.12.1., p ≤ α ≤ 0.05). Table S3: The radial growth of mycelia Fusarium spp. on PDA supplemented with CFS F1 and CFS F2 (20% v.v) from lactobacilli, PDA itself (control), and PDA with MRS-FGGM (20% v.v). The values are represented by the averages and standard error of the mean (s.e.m.) [Factorial ANOVA, post hoc Tukey’s HSD test, Statistica soft. v.12.1., p ≤ α ≤ 0.05]. The index letters indicate the significant differences. Table S4: The confluence of mycelia of five tested Fusarium sp. after 24 h of cultivation (25 °C). The comparison of mycelia growth in PBW medium with 20% of CFS from lactobacilli and PBW alone. The start inoculum was estimated to be 1.105 CFU·mL−1. Data represent mean ± s.e.m. including three replicates of experiment. (ANOVA, post hoc Tukey’s HSD test, Statistica soft. v.12.1., p ≤ α ≤ 0.05). Table S5: The influence of metabolites from lactobacilli on the germinability of conidia of five tested Fusarium spp. within 24 h. The CFU·mL−1 (log10) of Fusarium spp. after 24 h cultivation in PBW with 20% of CFS and PBW alone. The start inoculum was estimated to be 1.105 CFU·mL−1. Data represent mean ± s.e.m. including three replicates of experiment. (ANOVA, post hoc Tukey’s HSD test, Statistica soft. v.12.1., p ≤ α ≤ 0.05). Figure S1: The variability in the concentration of lactic acid bacteria (CFU.g−1) on aboveground and root parts of legumes represented as average, s.e.m., and std (ANOVA, Statistica soft. v.12.1., p ≤ α ≤ 0.05). Figure S2: The variability in the concentration of lactic acid bacteria (CFU.g−1) on legumes (pea, soya, lupine) represented as average, s.e.m., and std [ANOVA, Statistica soft. v.12.1., p ≤ α ≤ 0.05]. Figure S3: Clade 1 class 2a bacteriocin gene amplification. O’RangeRulerTM 50 bp DNA Ladder and GeneRulerTM DNA Ladder Mix were used as molecular markers. PC—positive control represented by strain ATTCC 1491. Figure S4: Clade 2 class 2a bacteriocin gene amplification. O’RangeRulerTM 50 bp DNA Ladder and GeneRulerTM DNA Ladder Mix were used as molecular markers. PC—positive control represented by strain ATTCC 1491. Figure S5: Clade 4 class 2a bacteriocin gene amplified using first primer pair. O’RangeRulerTM 50 bp DNA Ladder and GeneRulerTM DNA Ladder Mix were used as molecular markers. PC—positive control represented by strain ATTCC 1491. Figure S6. Clade 4 class 2a bacteriocin gene amplified using second primer pair. O’RangeRulerTM 50 bp DNA Ladder and GeneRulerTM DNA Ladder Mix were used as molecular markers. PC—positive control represented by strain ATTCC 1491. Figure S7: Clade 5 class 2a bacteriocin gene amplified using first primer pair. O’RangeRulerTM 50 bp DNA Ladder and GeneRulerTM DNA Ladder Mix were used as molecular markers. PC—positive control represented by strain ATTCC 1491. Figure S8: Clade 5 Class 2a bacteriocin gene amplified using a second primer pair. O’RangeRulerTM 50 bp DNA Ladder and GeneRulerTM DNA Ladder Mix were used as molecular markers. PC—positive control represented by strain ATTCC 1491. Figure S9: Chitinase gene amplification using ChiFMSF and ChiFEMSR primers. GeneRulerTM DNA Ladder Mix was used as molecular marker. PC—positive control represented by strain ATTCC 1491. Figure S10: The inhibitory effect of lactobacilli isolates belonging to L. plantarum (A, B, C, D, E) against Fusarium culmorum (FC) in agar plug assay after five days of the experiment. Figure S11. The inhibitory effect of isolates belonging to L. plantarum (CCDM1110, CCDM 196, J), L. paracasei (F), and L. pentosus (G, H) against Fusarium culmorum (FC) in agar plugs assay after five days of the experiment. Figure S12: The differences in mycelial growth of Fusarium spp. on PDA with CFS (20%). [Factorial ANOVA, post hoc Tukey’s HSD test, Statistica soft. v.12.1., p ≤ α ≤ 0.05]. Figure S13: The confluence of Fusarium sporotrichoides (L3) in BPW with CFS (20%) from CCDM 1110 (L. plantarum) within 20 h at 25 °C. Figure S14: The confluence of Fusarium oxysporum (D1) in BPW with CFS (20%) from CCDM 1110 (L. plantarum) within 20 h at 25 °C.
Author Contributions
Conceptualization, M.K., J.C., O.B. and A.B.; methodology, M.K., J.C., O.B. and A.B.; software, J.C., O.B. and M.K.; validation, J.C., M.K. and O.B.; formal analysis, M.K., O.B., J.C. and J.L.; investigation, M.K., J.C. and A.B.; resources, J.L. and A.B.; data curation, J.C. and M.K.; writing—original draft preparation, M.K. and J.C.; writing, M.K. and J.C.; visualization, M.K. and J.C.; supervision, M.K., A.B. and P.K.; project administration, M.K. and P.K.; funding acquisition, M.K. and P.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Ministry of Agriculture of the Czech Republic, grant numbers QK22010255, MZe-RO1423.
Data Availability Statement
The data that support the findings of this study are present in the supplementary material.
Acknowledgments
We thank farmer Hodan and the Czech University of Life Sciences, Prague, for providing the legume plant samples. We acknowledge the technicians and laboratory staff from the Dairy Research Institute and the Faculty of Agriculture and Technology in České Budějovice for managing the samples and materials. We are grateful to Peter Konik from the Laboratory of Proteomics, the Faculty of Sciences, the University of South Bohemia, for the precise analysis of the Lactobacilli extracellular products.
Conflicts of Interest
Authors Miloslava Kavková, Olga Bazalová, and Jaromír Cihlář were employed by the company Dairy Research Institute Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Wille, L.; Messmer, M.M.; Studer, B.; Hohmann, P. Insights to plant–microbe interactions provide opportunities to improve resistance breeding against root diseases in grain legumes. Plant Cell Environ. 2019, 42, 20–40. [Google Scholar] [CrossRef]
- Arie, T. Fusarium diseases of cultivated plants, control, diagnosis, and molecular and genetic studies. J. Pestic. Sci. 2019, 44, 275–281. [Google Scholar] [CrossRef]
- Sampaio, A.M.; De Sousa Araújo, S.; Rubiales, D.; Patto, M.C.V. Fusarium wilt management in legume crops. Agronomy 2020, 10, 1073. [Google Scholar] [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological Control of Plant Pathogens: A Global Perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef]
- Duar, R.M.; Lin, X.B.; Zheng, J.; Martino, M.E.; Grenier, T.; Pérez-Muñoz, M.E.; Leulier, F.; Gänzle, M.; Walter, J. Lifestyles in transition: Evolution and natural history of the genus Lactobacillus. FEMS Microbiol. Rev. 2017, 41, 27–48. [Google Scholar] [CrossRef]
- Oberg, T.S.; McMahon, D.J.; Culumber, M.D.; McAuliffe, O.; Oberg, C.J. Invited review: Review of taxonomic changes in dairy-related lactobacilli. J. Dairy Sci. 2022, 105, 2750–2770. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Enebe, M.C.; Babalola, O.O. The impact of microbes in the orchestration of plants’ resistance to biotic stress: A disease management approach. Appl. Microbiol. Biotechnol. 2019, 103, 9–25. [Google Scholar] [CrossRef]
- Lamont, J.R.; Wilkins, O.; Bywater-Ekegärd, M.; Smith, D.L. From yoghurt to yield: Potential applications of lactic acid bacteria in plant production. Soil Biol. Biochem. 2017, 111, 1–9. [Google Scholar] [CrossRef]
- Raman, J.; Kim, J.S.; Choi, K.R.; Eun, H.; Yang, D.; Ko, Y.J.; Kim, S.J. Application of Lactic Acid Bacteria (LAB) in Sustainable Agriculture: Advantages and Limitations. Int. J. Mol. Sci. 2022, 23, 7724. [Google Scholar] [CrossRef]
- Di Cagno, R.; De Angelis, M.; Calasso, M.; Vincentini, O.; Vernocchi, P.; Ndagijimana, M.; De Vincenzi, M.; Dessi, M.R.; Guerzoni, M.E.; Gobbetti, M. Quorum sensing in sourdough Lactobacillus plantarum DC400: Induction of plantaricin a (PlnA) under co-cultivation with other lactic acid bacteria and effect of PlnA on bacterial and Caco-2 cells. Proteomics 2010, 10, 2175–2190. [Google Scholar] [CrossRef]
- Ruiz Rodríguez, L.G.; Mohamed, F.; Bleckwedel, J.; Medina, R.; De Vuyst, L.; Hebert, E.M.; Mozzi, F. Diversity and functional properties of lactic acid bacteria isolated from wild fruits and flowers present in northern Argentina. Front. Microbiol. 2019, 10, 1091. [Google Scholar] [CrossRef]
- Yu, A.O.; Leveau, J.H.J.; Marco, M.L. Abundance, diversity and plant-specific adaptations of plant-associated lactic acid bacteria. Environ. Microbiol. Rep. 2020, 12, 16–29. [Google Scholar] [CrossRef]
- Yu, A.O.; Goldman, E.A.; Brooks, J.T.; Golomb, B.L.; Yim, I.S.; Gotcheva, V.; Angelov, A.; Kim, E.B.; Marco, M.L. Strain diversity of plant-associated Lactiplantibacillus plantarum. Microb. Biotechnol. 2021, 14, 1990–2008. [Google Scholar] [CrossRef]
- Kavková, M.; Cihlář, J.; Dráb, V.; Bazalová, O.; Dlouhá, Z. The Interactions among Isolates of Lactiplantibacillus plantarum and Dairy Yeast Contaminants: Towards Biocontrol Applications. Fermentation 2022, 8, 14. [Google Scholar] [CrossRef]
- Palla, M.; Cristani, C.; Giovannetti, M.; Agnolucci, M. Large genetic intraspecific diversity of autochthonous lactic acid bacteria and yeasts isolated from PDO Tuscan bread sourdough. Appl. Sci. 2020, 10, 1043. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C.; Zdolec, N. Lactobacillus plantarum with Functional Properties: An Approach to Increase Safety and Shelf-Life of Fermented Foods. BioMed Res. Int. 2018, 2018, 9361614. [Google Scholar] [CrossRef]
- Bergsma, S.; Euverink, G.J.W.; Charalampogiannis, N.; Poulios, E.; Janssens, T.K.S.; Achinas, S. Biotechnological and Medical Aspects of Lactic Acid Bacteria Used for Plant Protection: A Comprehensive Review. BioTech 2022, 11, 40. [Google Scholar] [CrossRef]
- Ristić, D.; Vucurović, I.; Aleksić, G.; Nikolić, B.; Djurović, S.; Starović, M. Application of different combinations of lactic acid, phototrophic bacteria and yeast mixtures in control of seed and seedlings pathogens of tomato and pepper. Pestic. i Fitomedicina 2021, 36, 73–82. [Google Scholar] [CrossRef]
- Steglińska, A.; Kołtuniak, A.; Motyl, I.; Berłowska, J.; Czyżowska, A.; Cieciura-Włoch, W.; Okrasa, M.; Kręgiel, D.; Gutarowska, B. Lactic Acid Bacteria as Biocontrol Agents against Potato (Solanum tuberosum L.) Pathogens. Appl. Sci. 2022, 12, 7763. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Hamilton-Miller, J.M.T.; Shah, S. Comparative in-vitro activity of ketolide HMR 3647 and four macrolides against Gram-positive cocci of known erythromycin susceptibility status. J. Antimicrob. Chemother. 1998, 61, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.A.; Houbraken, J.; Thrane, U.; Frisvad, J.C.; Andersen, B. Food and Indoor Fungi, 1st ed.; Crous, P.W., Samson, R.A., Eds.; CBS-KNAW Fungal Biodiversity Centre Utrecht: Utrecht, The Netherlands, 2010; p. 390. [Google Scholar]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Bolchacova, E.; Voigt, K.; Crous, P.W.; et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [PubMed]
- Boutigny, A.L.; Gautier, A.; Basler, R.; Dauthieux, F.; Leite, S.; Valade, R.; Aguayo, J.; Ioos, R.; Laval, V. Metabarcoding targeting the EF1 alpha region to assess fusarium diversity on cereals. PLoS ONE 2019, 14, e0207988. [Google Scholar] [CrossRef] [PubMed]
- Kavková, M.; Cihlář, J.; Dráb, V.; Bár, L. Differentiation of Penicillium roqueforti from closely related species contaminating cheeses and dairy environment. Fermentation 2021, 7, 222. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef]
- Wieckowicz, M.; Schmidt, M.; Sip, A.; Grajek, W. Development of a PCR-based assay for rapid detection of class IIa bacteriocin genes. Lett. Appl. Microbiol. 2011, 52, 281–289. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, D.; Miao, L. Characterization of thermotolerant chitinases encoded by a Brevibacillus laterosporus strain isolated from a suburban wetland. Genes 2015, 6, 1268–1282. [Google Scholar] [CrossRef]
- Haider, S.R.; Reid, J.H.; Sharp, B.L. Tricine-SDS-PAGE. In Protein Electrophoresis: Methods in Molecular Biology; Kurien, B.T., Hal Scofield, R., Eds.; Humana Press: Totowa, NJ, USA, 2012; Volume 869, pp. 81–91. [Google Scholar] [CrossRef]
- Demirbaş, F.; İspirli, H.; Kurnaz, A.A.; Yilmaz, M.T.; Dertli, E. Antimicrobial and functional properties of lactic acid bacteria isolated from sourdoughs. LWT Food Sci Technol. 2017, 79, 361–366. [Google Scholar] [CrossRef]
- Matchado, M.S.; Lauber, M.; Reitmeier, S.; Kacprowski, T.; Baumbach, J.; Haller, D.; List, M. Network analysis methods for studying microbial communities: A mini-review. Comp. Struct. Biotech. J. 2021, 19, 2687–2698. [Google Scholar] [CrossRef]
- Martino, M.E.; Bayjanov, J.R.; Caffrey, B.E.; Wels, M.; Joncour, P.; Hughes, S.; Gillet, B.; Kleerebezem, M.; van Hijum, S.A.F.T.; Leulier, F. Nomadic lifestyle of Lactobacillus plantarum revealed by comparative genomics of 54 strains isolated from different habitats. Environ. Microbiol. 2016, 18, 4974–4989. [Google Scholar] [CrossRef]
- Šišić, A.; Baćanović-Šišić, J.; Karlovsky, P.; Wittwer, R.; Walder, F.; Campiglia, E.; Radicetti, E.; Friberg, H.; Baresel, J.P.; Finckh, M.R. Roots of symptom-free leguminous cover crop and living mulch species harbor diverse Fusarium communities that show highly variable aggressiveness on pea (Pisum sativum). PLoS ONE 2018, 13, e0191969. [Google Scholar] [CrossRef]
- Pflughöft, O.; Merker, C.; von Tiedemann, A.; Schäfer, B.C. Zur Verbreitung und Bedeutung von Pilzkrankheiten in Körnerfuttererbsen (Pisum sativum L.) in Deutschland. Gesunde Pflanz 2012, 64, 39–48. [Google Scholar] [CrossRef]
- Eijsink, V.G.H.; Axelsson, L.; Diep, D.B.; Håvarstein, L.S.; Holo, H.; Nes, I.F. Production of class II bacteriocins by lactic acid bacteria; an example of biological warfare and communication. Antonie Van Leeuwenhoek 2002, 81, 639–654. [Google Scholar] [CrossRef]
- Zhao, S.; Hao, X.; Yang, F.; Wang, Y.; Fan, X.; Wang, Y. Antifungal Activity of Lactobacillus plantarum ZZUA493 and Its Application to Extend the Shelf Life of Chinese Steamed Buns. Foods 2022, 11, 195. [Google Scholar] [CrossRef]
- Deepthi, B.V.; Rao, K.P.; Chennapa, G.; Naik, M.K.; Chandrashekara, K.T.; Sreenivasa, M.Y. Antifungal attributes of Lactobacillus plantarum MYS6 against fumonisin producing Fusarium proliferatum associated with poultry feeds. PLoS ONE 2016, 11, e0155122. [Google Scholar] [CrossRef]
- Mani-López, E.; Arrioja-Bretón, D.; López-Malo, A. The impacts of antimicrobial and antifungal activity of cell-free supernatants from lactic acid bacteria in vitro and foods. Compr. Rev. Food Sci. Food Saf. 2022, 21, 604–641. [Google Scholar] [CrossRef]
- Torriani, S.; Felis, G.E.; Dellaglio, F. Differentiation of Lactobacillus plantarum, L. pentosus, and L. paraplantarum by recA Gene Sequence Analysis and Multiplex PCR Assay with recA Gene-Derived Primers. Appl. Environ. Microbiol. 2001, 67, 3450–3454. [Google Scholar] [CrossRef]
- Horváth-Szanics, E.; Perjessy, J.; Klupacs, A.; Takacs, K.; Nagy, A.; Koppany-Szabo, E.; Hegyi, F.; Nemeth-Szerdahelyi, E.; Du, M.Y.; Wang, Z.R.; et al. Study of chitinase and chitinolytic activity of Lactobacillus strains. Acta Aliment. 2020, 49, 214–224. [Google Scholar] [CrossRef]
- Sánchez, B.; Ruiz, L.; Gueimonde, M.; Margolles, A. Omics for the study of probiotic microorganisms. Food Res. Int. 2013, 54, 1061–1071. [Google Scholar] [CrossRef]
- Galloway-Peña, J.R.; Liang, X.; Singh, K.V.; Yadav, P.; Chang, C.; La Rosa, S.L.; Shelburne, S.; Ton-That, H.; Höök, M.; Murray, B.E. The identification and functional characterization of WxL proteins from Enterococcus faecium reveal surface proteins involved in extracellular matrix interactions. J. Bacteriol. 2015, 197, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Visweswaran, G.R.R.; Leenhouts, K.; Van Roosmalen, M.; Kok, J.; Buist, G. Exploiting the peptidoglycan-binding motif, LysM, for medical and industrial applications. Appl. Microbiol. Biotechnol. 2014, 98, 4331–4345. [Google Scholar] [CrossRef] [PubMed]
- Hirozawa, M.T.; Ono, M.A.; Suguiura, I.M.D.S.; Bordini, J.G.; Ono, E.Y.S. Lactic acid bacteria and Bacillus spp. as fungal biological control agents. J. Appl. Microbiol. 2023, 134, lxac083. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.; Venancio, A.; Abrunhosa, L. Antifungal effect of organic acids from lactic acid bacteria on Penicillium nordicum. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2018, 35, 1803–1818. [Google Scholar] [CrossRef]
- Russo, P.; Arena, M.P.; Fiocco, D.; Capozzi, V.; Drider, D.; Spano, G. Lactobacillus plantarum with broad antifungal activity: A promising approach to increase safety and shelf-life of cereal-based products. Int. J. Food Microbiol. 2017, 247, 48–54. [Google Scholar] [CrossRef]
- Ström, K.; Sjögren, J.; Broberg, A.; Schnürer, J. Lactobacillus plantarum MiLAB 393 produces the antifungal cyclic dipeptides cyclo(L-Phe-L-Pro) and cyclo(L-Phe-trans-4-OH-L-Pro) and 3-phenyllactic acid. Appl. Environ. Microbiol. 2002, 68, 4322–4327. [Google Scholar] [CrossRef]
- Živcović, S.T.; Stošić, S.T.; Ristič, D.T.; Vačurović, I.B.; Stevanović, M.L. The antagonistic potential of Lactobacillus plantarum against some postharvest pathogenic fungi. Zb. Matice Srp. Za Prir. Nauk. 2019, 136, 79–88. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).