The Relationship between Endogenous Hormone Content and Related Gene Expression and Tillering in Wild Kentucky Bluegrass
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Condition
2.2. Measurement of IAA, ZT and SL Contents
2.3. Determination of Transcription and Expression of Hormone Related Genes
2.4. Data Statistics
3. Results
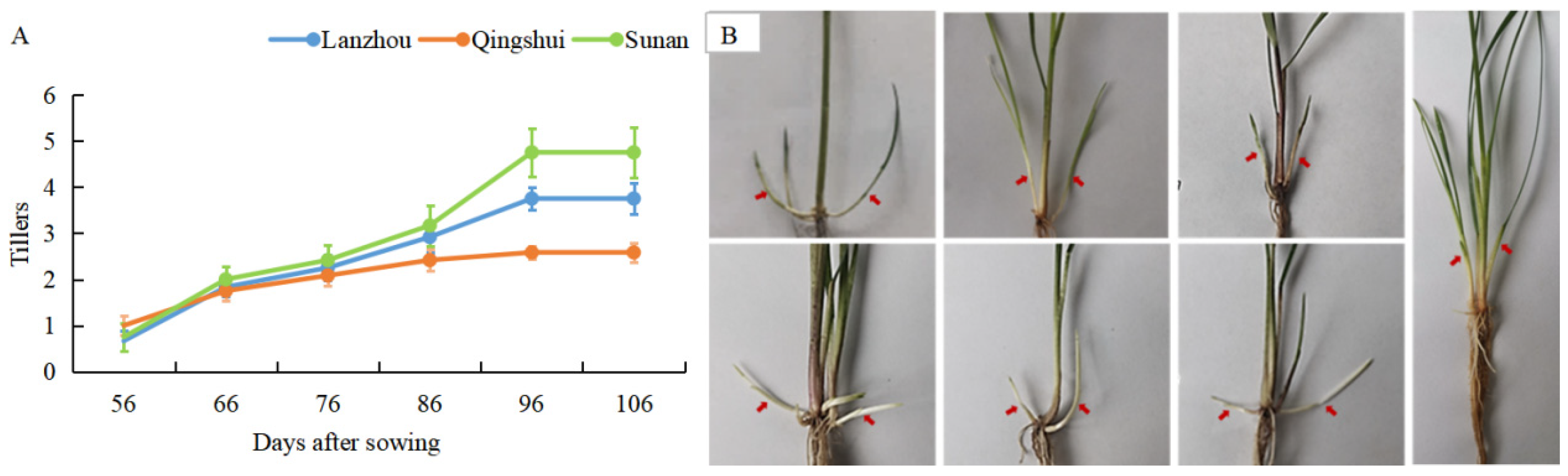
3.1. Study on Tillering Ability of Kentucky Bluegrass
3.2. Comparison of Endogenous Hormone Levels in Stems and Roots of Sunan and Qingshui
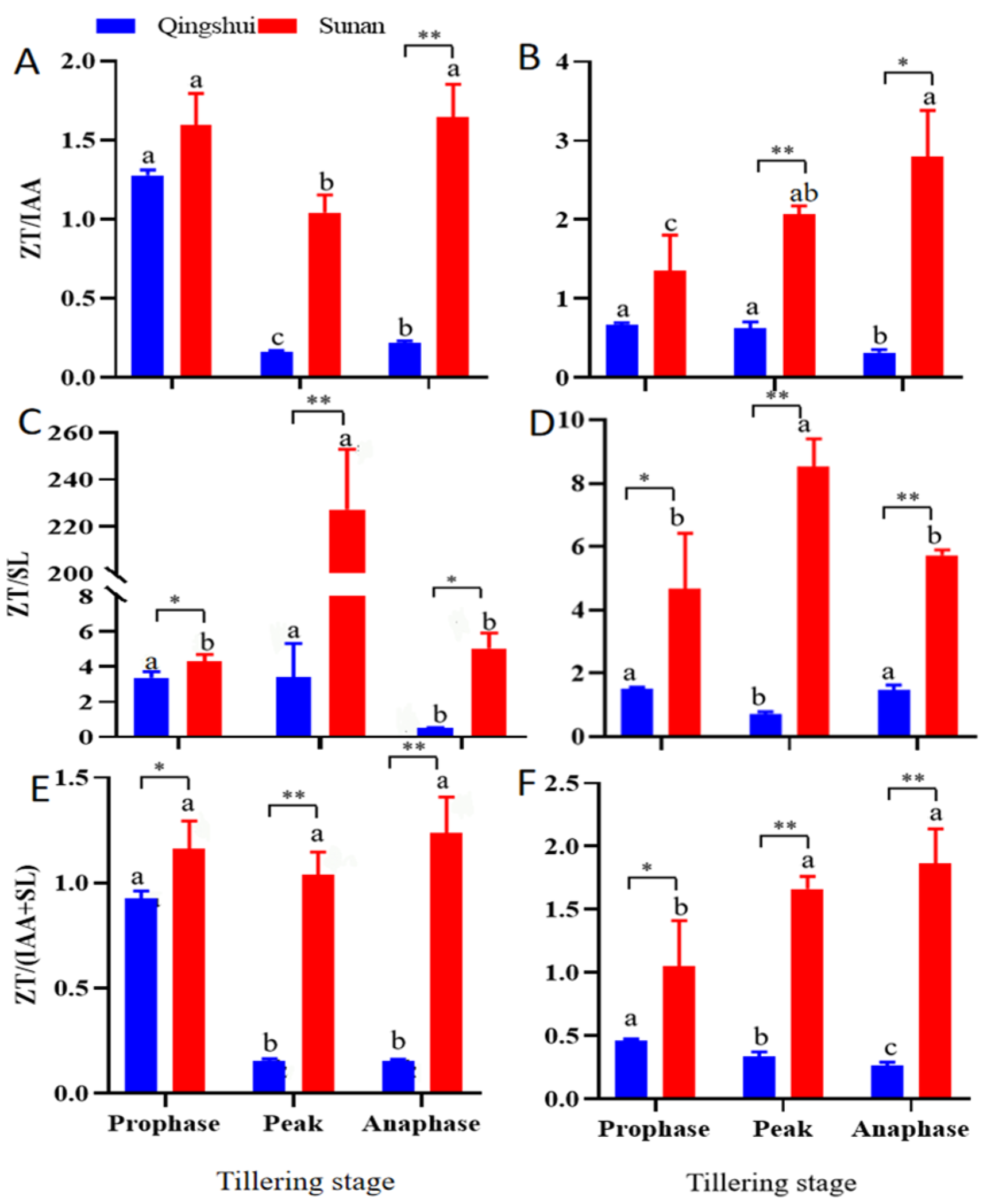
3.3. Comparison and Ratio Analysis of Contents of Three Kinds of Endogenous Hormones
3.4. Correlation between Endogenous Hormone Levels and Tillering Strength
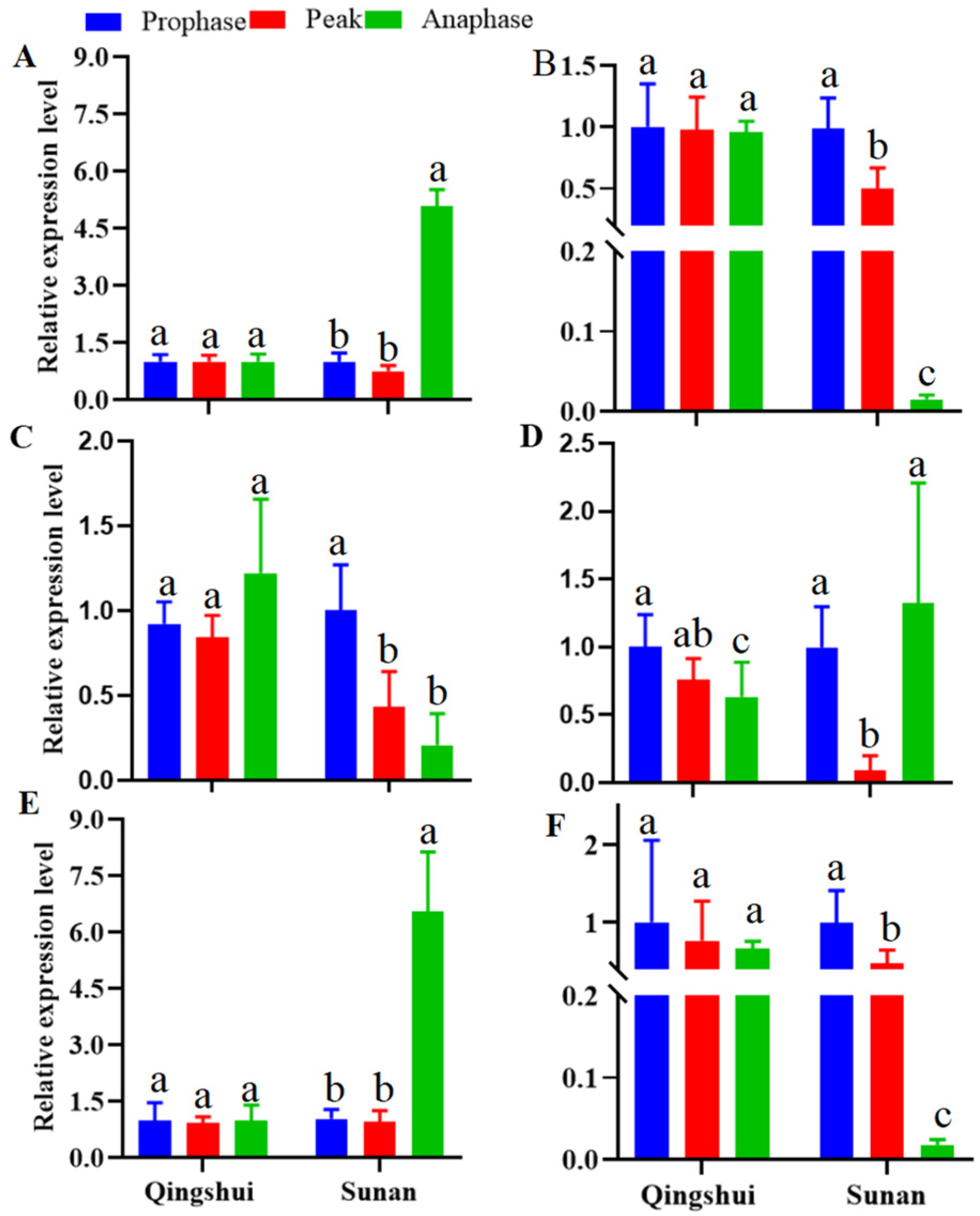
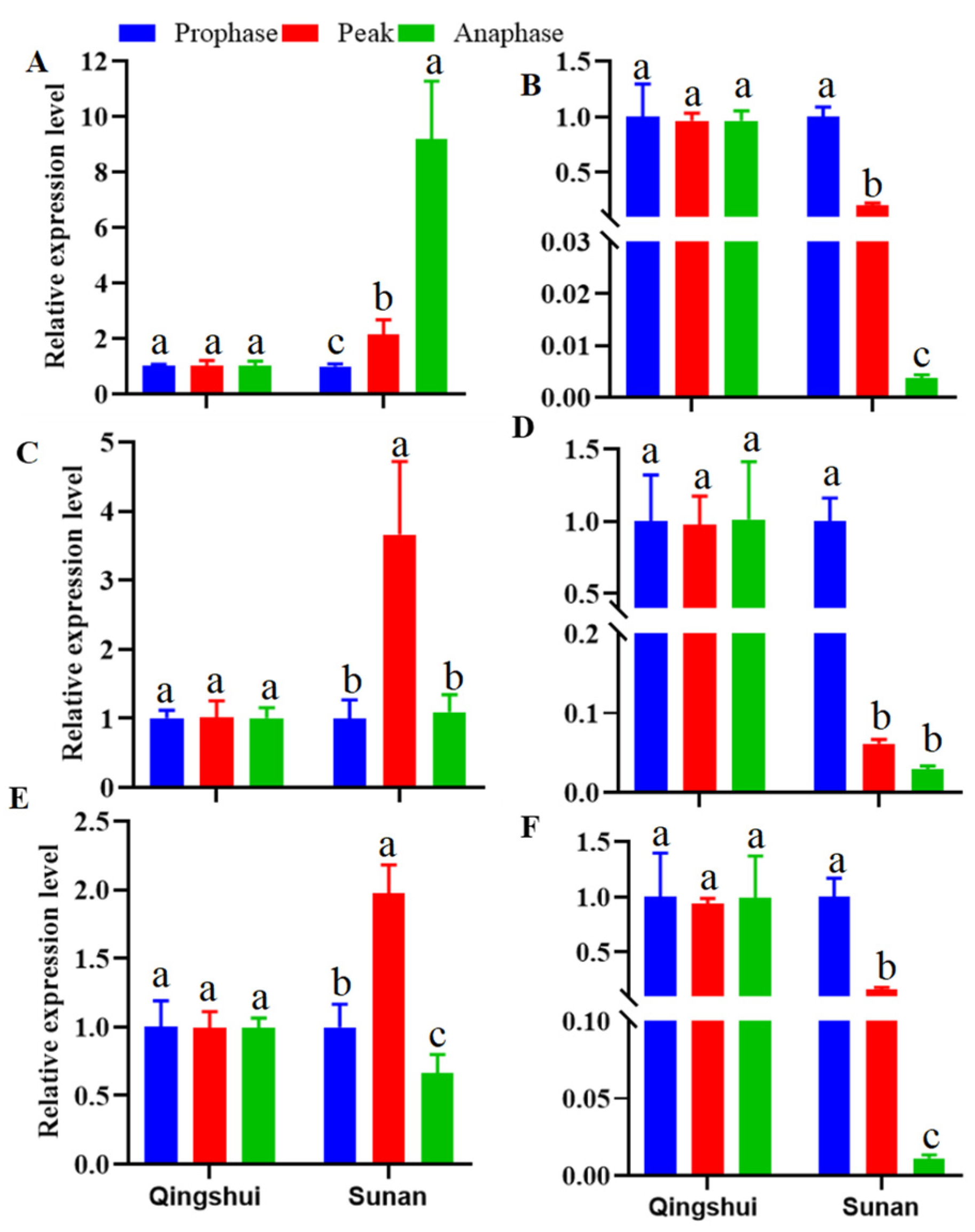
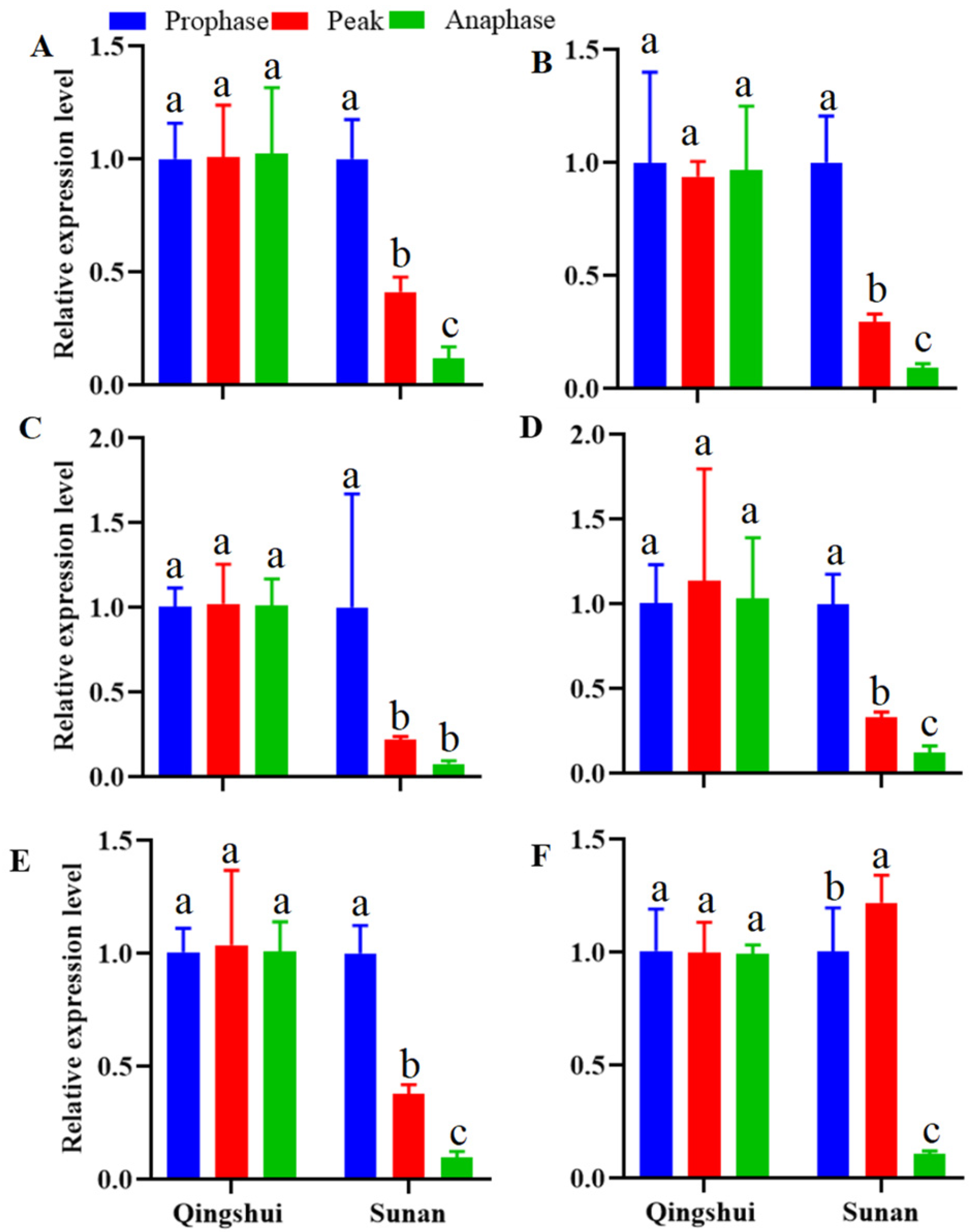
3.5. Expression Analysis of Hormone Synthesis-Related Genes in the Stems and Roots of Kentucky Bluegrass at Different Tillering Stages
3.5.1. Differential Expression of Genes Related to Auxin Signal Transduction
3.5.2. Differential Expression of CK Signal Transduction-Related Genes
3.5.3. Differential Expression of SL Signal Transduction-Related Genes
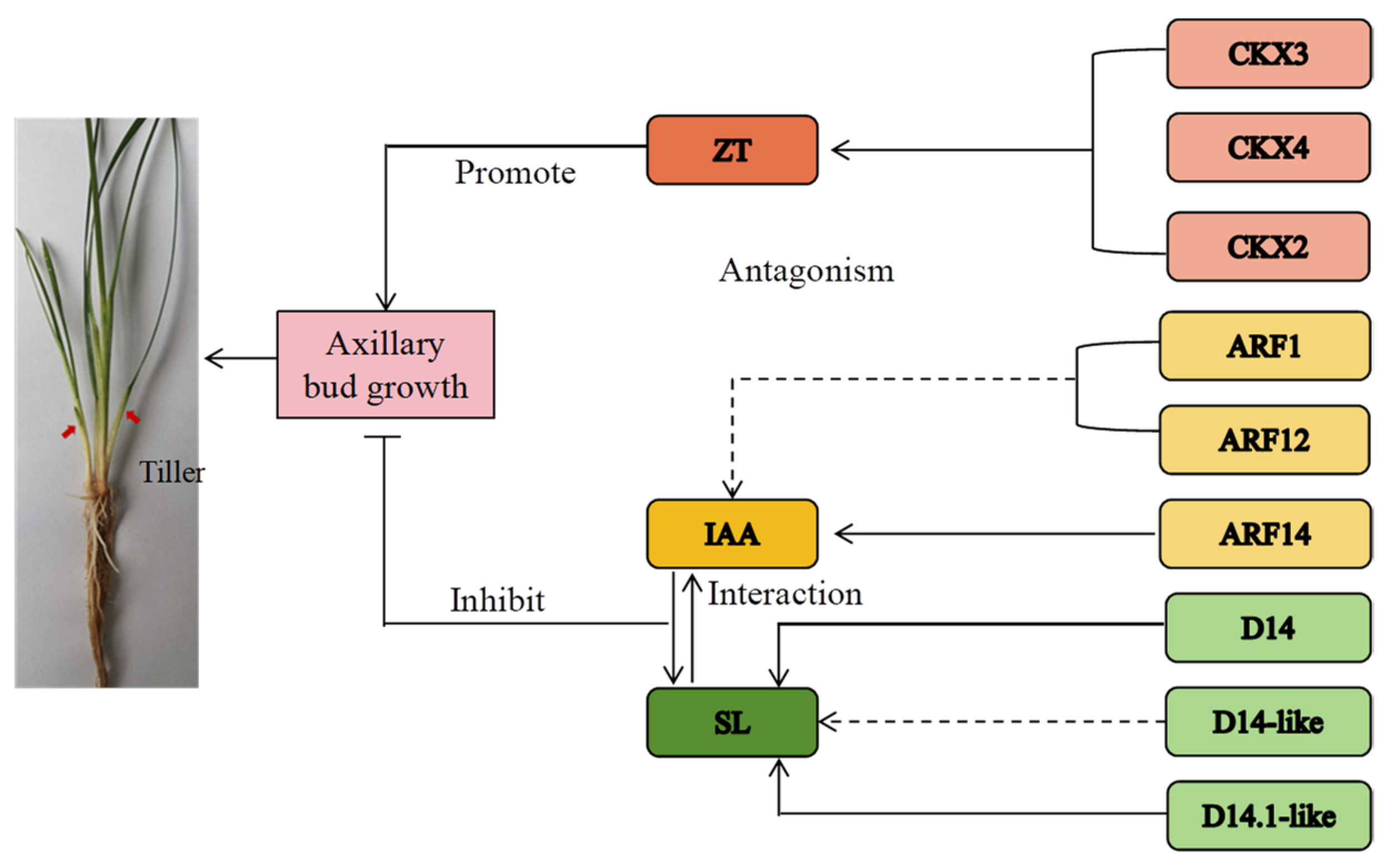
4. Discussion
4.1. Tillering Characteristics and Research Significance of Wild Kentucky Bluegrass in Gansu Province
4.2. Relationship between Endogenous Hormone Content and Related Gene Expression in the Stems and Roots Kentucky Bluegrass and Tillering Formation at Different Developmental Stages
4.2.1. Relationship between IAA and Related Gene Expression and Tillering Formation and Development of Kentucky Bluegrass
4.2.2. Relationship between CK and Related Gene Expression and Tillering Formation and Development of Kentucky Bluegrass
4.2.3. Relationship between SL and Related Gene Expression and Tillering Formation and Development of Kentucky Bluegrass
4.3. Effects of Dynamic Equilibrium of Endogenous Hormones of Kentucky Bluegrass on Tillering
4.4. Strategies of Applying Plant Hormone to Improve Tillering of Kentucky Bluegrass
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, L.; Li, W.; Miura, K.; Ashikari, M.; Kyozuka, J. Control of tiller growth of rice by OsSPL14 and strigolactones, which work in two independent pathways. Plant Cell Physiol. 2012, 53, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, N.; Taylor, C.; Leyser, O. Strigolactone can promote or inhibit shoot branching by triggering rapid depletion of the auxin efflux protein PIN1 from the plasma membrane. PLoS Biol. 2013, 11, e1001474. [Google Scholar] [CrossRef] [PubMed]
- Brewer, P.B.; Dun, E.A.; Ferguson, B.J.; Rameau, C.; Beveridge, C.A. Strigolactone acts downstream of auxin to regulate bud outgrowth in Pea and Arabidopsis. Plant Physiol. 2009, 150, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Zha, M.; Zhao, Y.; Wang, Y.; Chen, B.; Tan, Z. Strigolactones and cytokinin interaction in buds in the control of rice tillering. Front. Plant Sci. 2022, 13, 837136. [Google Scholar] [CrossRef]
- Wang, B.; Smith, S.M.; Li, J. Genetic regulation of shoot architecture. Annu. Rev. Plant Biol. 2018, 69, 437–468. [Google Scholar] [CrossRef] [PubMed]
- Duan, E.; Wang, Y.; Li, X.; Lin, Q.; Zhang, T.; Wang, Y.; Zhou, C.; Zhang, H.; Jiang, L.; Wang, J.; et al. OsSHI1 regulates plant architecture through modulating the transcriptional activity of IPA1 in Rice. Plant Cell 2019, 31, 1026–1042. [Google Scholar] [CrossRef]
- Li, X.; Qian, Q.; Fu, Z.; Wang, Y.; Xiong, G.; Zeng, D.; Wang, X.; Liu, X.; Teng, S.; Hiroshi, F.; et al. Control of tillering in rice. Nature 2003, 422, 618–621. [Google Scholar] [CrossRef]
- Jian, L.C.; Li, J.H.; Li, P.H. Seasonal alteration in amount of Ca(2+) in apical bud cells of mulberry (Morus bombciz Koidz): An electron microscopy cytochemical study. Tree Physiol. 2000, 20, 623. [Google Scholar] [CrossRef][Green Version]
- Wang, C.T.; Wang, G.X.; Liu, W.; Wang, Y.; Hu, L.; Ma, L. Effects of establishing an artificial grassland on vegetation characteristics and soil quality in a degraded meadow. Isr. J. Ecol. Evol. 2013, 59, 141–153. [Google Scholar] [CrossRef]
- Doust, A.N.; Kellogg, E.A. Effect of genotype and environment on branching in weedy green millet (Setaria viridis) and domesticated foxtail millet (Setaria italica) (Poaceae). Mol. Ecol. 2010, 15, 1335–1349. [Google Scholar] [CrossRef]
- Guo, J.W.; Li, L.Y.; Tian, X.H.; Du, W.H. Study on the production performance and quality of forage triticale line indifferent areas of Gansu Province. Acta Agrestia Sin. 2019, 27, 1250–1258. [Google Scholar]
- Song, C.Y.; Wan, Y.F.; Li, Y.E. Relationships between tiller dynamic, earbearing tiller rate and yield of double cropping rice under elevated temperature and CO concentration. Crop J. 2023, 1–10, 3–13. [Google Scholar]
- Domagalska, M.A.; Leyser, O. Signal integration in the control of shoot branching. Nat. Rev. Mol. Cell Biol. 2011, 12, 211–221. [Google Scholar] [CrossRef]
- Ongaro, V.; Leyser, O. Hormonal control of shoot branching. J. Exp. Bot. 2008, 59, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, C.A.; Kyozuka, J. New genes in the strigolactone related shoot branching pathway. Curr. Opin. Plant Biol. 2010, 13, 34–39. [Google Scholar] [CrossRef]
- Liu, H.; Jia, S.; Shen, D.; Liu, J.; Li, J.; Zhao, H.; Han, S.; Wang, Y. Four AUXIN RESPONSE FACTOR genes downregulated by microRNA167 are associated with growth and development in Oryza sativa. Funct. Plant Biol. 2012, 39, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Cline, M.G. Exogenous auxin effects on lateral bud outgrowth in decapitated shoots. Ann. Bot. 1996, 78, 255–266. [Google Scholar] [CrossRef]
- Wang, R.F.; Zhang, J.W.; Lü, P.; Dong, S.T.; Liu, P.; Zhao, B. Tillering characteristics of multi-tiller maize and influence of plant density and sowing date. Acta Agron. Sin. 2012, 38, 322–332. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, D.D.; Xu, J.X.; Ding, Y.F.; Wang, Q.S.; Wang, Q.S.; Li, G.; Liu, Z.; Wang, S. Effect of cytokinins on the growth of rice tiller buds and the expression of the genes regulating rice tillering. Sci. Agric. Sin. 2012, 45, 44–51. [Google Scholar]
- Cai, T.; Meng, X.; Liu, X.; Liu, T.; Wang, H.; Jia, Z.; Yang, D.; Ren, X. Exogenous hormonal application regulates the occurrence of wheat tillers by changing endogenous hormones. Front. Plant Sci. 2018, 9, 1886. [Google Scholar] [CrossRef]
- Shang, X.L.; Xie, R.R.; Tian, H.; Wang, Q.L.; Guo, F.Q. Putative zeatin O-glucosyltransferase OscZOG1 regulates root and shoot development and formation of agronomic traits in rice. J. Integr. Plant Biol. 2016, 58, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Xi, C.; Carolien, R.S.; Harro, B. The interaction between strigolactones and other plant hormones in the regulation of plant development. Front. Plant Sci. 2013, 4, 199. [Google Scholar] [CrossRef]
- Minakuchi, K.; Kameoka, H.; Yasuno, N.; Umehara, M.; Luo, L.; Kobayashi, K.; Hanada, A.; Ueno, K.; Asami, T.; Yamaguchi, S.; et al. CULM1 (FC1) works downstream of strigolactones to inhibit the outgrowth of axillary buds in rice. Plant Cell Physiol. 2010, 51, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Xu, Y.; Liu, H.; Mao, Z.; Zhang, C.; Ma, Y.; Zhang, Q.; Meng, Z.; Chong, K. The interaction between OsMADS57 and OsTB1 modulates rice tillering via DWARF14. Nat. Commun. 2013, 4, 1566. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Ji, Y.; Hu, J.; Guo, R.; Sun, S.; Wang, X. Strigolactones and brassinosteroids antagonistically regulate the stability of the D53-OsBZR1 complex to determine FC1 expression in rice tillering. Mol. Plant 2020, 13, 586–597. [Google Scholar] [CrossRef]
- Dun, E.; Hanan, J.; Beveridge, C. Computational modeling and molecular physiology experiments reveal new insights into shoot branching in pea. Plant Cell 2009, 21, 3459–3472. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Beveridge, C.A. Roles for auxin, cytokinin, and strigolactone in regulating shoot branching. Plant Physiol. 2009, 149, 1929–1944. [Google Scholar] [CrossRef]
- Sitbon, F.; Hennion, S.; Sundberg, B.; Little, C.H.; Olsson, O.; Sandberg, G. Transgenic tobacco plants coexpressing the agrobacterium tumefaciens iaaM and iaaH genes display altered growth and indoleacetic acid metabolism. Plant Physiol. 1992, 99, 1062–1069. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, D.P.; Zhang, D.C. Advances in molecular mechanism of rice tillering. Biot. Resour. 2022, 44, 26–35. [Google Scholar]
- Duan, J.; Yu, H.; Yuan, K.; Liao, Z.; Meng, X.; Jing, Y.; Liu, G.; Chu, J.; Li, J. Strigolactone promotes cytokinin degradation through transcriptional activation of cytokinin oxidase/dehydrogenase 9 in rice. Proc. Natl. Acad. Sci. USA 2019, 116, 14319–14324. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, H. The Female Gametophyte Characteristics and Gene Expression Analysis Involved in Apomixis of Wild Germplasm Materials of Kentucky Bluegrass in Gansu Province of China. J. Plant Growth Regul. 2022, 42, 2283–2304. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows, version 21.0; IBM Corp.: Armonk, NY, USA, 2012.
- Cao, X.W.; Cui, H.M.; Yao, Y.; Xiong, A.S.; Hou, X.L.; Li, Y. Effects of endogenous hormones on variation of shoot branching in a variety of non-heading Chinese cabbage and related gene expression. J. Plant Biol. 2017, 60, 343–351. [Google Scholar] [CrossRef]
- Campbell, B.T.; Baenziger, P.S.; Gill, K.S.; KSGill Eskridge, K.M.; Budak, H.; Erayman, M.; Dweikat, I.; Yen, Y. Identification of QTLs and environmental interactions associated with agronomic traits on chromosome 3A of wheat. Crop Sci. 2003, 43, 1493–1505. [Google Scholar] [CrossRef]
- Spielmeyer, W.; Richards, R.A. Comparative mapping of wheat chromosome 1AS which contains the tiller inhibition gene (tin) with rice chromosome 5S. Theor. Appl. Genet. 2004, 109, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.C.; Hwa, C.M. Genetic modification of plant architecture and variety improvement in rice. Heredity 2008, 101, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Ljung, K.; Bhalerao, R.P.; Sandberg, G. Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant J. 2001, 28, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Schachtman, D.; Reid Rayling, S. Phosphorus uptake by plants:From soil to cell. Plant Physiol. 1998, 116, 447–453. [Google Scholar] [CrossRef]
- Agusti, J.; Greb, T. Going with the wind adaptive dynamics of plant secondary meristems. Mech. Dev. 2013, 130, 34–44. [Google Scholar] [CrossRef]
- Cline, M.G. The role of hormones in apical dominance. New approaches to an old problem in plant development. Physiol. Plant. 2010, 90, 230–237. [Google Scholar] [CrossRef]
- Verma, S.; Negi, N.P.; Pareek, S.; Mudgal, G.; Kumar, D. Auxin response factors in plant adaptation to drought and salinity stress. Physiol. Plant 2022, 174, e13714. [Google Scholar] [CrossRef]
- Li, J.; Jiang, Y.; Zhang, J.; Ni, Y.; Jiao, Z.; Li, H.; Wang, T.; Zhang, P.; Guo, W.; Li, L.; et al. Key auxin response factor (ARF) genes constraining wheat tillering of mutant dmc. PeerJ 2021, 9, e12221. [Google Scholar] [CrossRef]
- Uzair, M.; Long, H.; Zafar, S.A.; Patil, S.B.; Chun, Y.; Li, L.; Fang, J.; Zhao, J.; Peng, L.; Yuan, S.; et al. Narrow Leaf 21, encoding ribosomal protein RPS3A, controls leaf development in rice. Plant Physiol. 2021, 186, 497–518. [Google Scholar] [CrossRef]
- Simonini, S.; Deb, J.; Moubayidin, L.; Stephenson, P.; Valluru, M.; Freire-Rios, A.; Sorefan, K.; Weijers, D.; Friml, J.; Østergaard, L. A noncanonical auxin-sensing mechanism is required for organ morphogenesis in Arabidopsis. Genes Dev. 2016, 30, 2286–2296. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, X.; Song, W.; Zhang, Y.; Xu, G. Over-expression of OsPIN2 leads to increased tiller numbers, angle and shorter plant height through suppression of OsLAZY1. Plant Biotechnol. J. 2012, 10, 139–149. [Google Scholar] [CrossRef]
- Huang, D.; Wang, S.; Zhang, B.; Shang-Guan, K.; Shi, Y.; Zhang, D.; Liu, X.; Wu, K.; Xu, Z.; Fu, X.; et al. A gibberellin-mediated DELLA -NAC signaling cascade regulates cellulose synthesis in rice. Plant Cell 2015, 27, 1681–1696. [Google Scholar] [CrossRef] [PubMed]
- Ekamber, K.P.K.M. Hormonal regulation of tiller dynamics in differentially-tillering rice cultivars. Plant Growth Regul. 2007, 53, 215–223. [Google Scholar] [CrossRef]
- Yuan, J.C.; Liu, Y.H. Advancing in the control of lateral buds and branching initiation in high plants. Hebei North Univ. 2007, 23, 18–23. [Google Scholar]
- Li, C.X.; Zhao, G.C.; Dai, X.M.; Jiang, L.N.; Shang, Y.L. Research on the relationship between wheat tillering dynamics and endogenous hormone. Acta Agron. Sin. 2000, 26, 963–968. [Google Scholar] [CrossRef]
- Werner, T.; Motyka, V.; Strnad, M.; Schmülling, T. Regulation of plant growth by cytokinin. Proc. Natl. Acad. Sci. India. Sect. B 2001, 98, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Fang, J.; Xu, F.; Wang, W.; Sun, X.; Chu, J.; Cai, B.; Feng, Y.; Chu, C. CYTOKININ OXIDASE/DEHYDROGENASE4 Integrates Cytokinin and Auxin Signaling to Control Rice Crown Root Formation. Plant Physiol. 2014, 165, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Szala, K.; Ogonowska, H.; Lugowska, B.; Zmijewska, B.; Wyszynska, R.; Dmochowska-Boguta, M.; Orczyk, W.; Nadolska-Orczyk, A. Different sets of TaCKX genes affect yield-related traits in wheat plants grown in a controlled environment and in field conditions. BMC Plant Biol. 2020, 20, 496. [Google Scholar] [CrossRef]
- Takeda, T.; Suwa, Y.; Suzuki, M.; Kitano, H.; Ueguchi-Tanaka, M.; Ashikari, M.; Matsuoka, M.; Ueguchi, C. The OsTB1 gene negatively regulates lateral branching in rice. Plant J. 2003, 33, 513–520. [Google Scholar] [CrossRef]
- David, Z. Strigolactones are a new-defined class of plant hormones which inhibit shoot branching and mediate the interaction of plant-AM fungi and plant-parasitic weeds. Sci. China 2009, 52, 693–700. [Google Scholar] [CrossRef]
- Gomez-Roldan, V.; Fermas, S.; Brewer, P.B.; Puech-Pagès, V.; Dun, E.A.; Pillot, J.P.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J.C.; et al. Strigolactone inhibition of shoot branching. Nature 2008, 455, 189–19415. [Google Scholar] [CrossRef]
- Umehara, M.; Hanada, A.; Yoshida, S.; Akiyama, K.; Arite, T.; Takeda-Kamiya, N.; Magome, H.; Kamiya, Y.; Shirasu, K.; Yoneyama, K.; et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature 2008, 455, 195200. [Google Scholar] [CrossRef]
- Xu, J.; Ding, C.; Ding, Y.; Tang, S.; Zha, M.; Luo, B.; Wang, S. A Proteomic approach to analyze differential regulation of proteins during bud outgrowth under apical dominance based on the auxin transport canalization model in rice (Oryza sativa L). J. Plant Growth Regul. 2015, 34, 122–136. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, T.; Wang, M.; Liu, Y.; Yuan, S.; Gao, Y.; Yin, L.; Sun, W.; Peng, L.; Zhang, W.; et al. DWARF3 participates in an SCF complex and associates with DWARF14 to suppress rice shoot branching. Plant Cell Physiol. 2014, 55, 1096–1109. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, X.; Zhang, H.; Cheng, Z.; Liu, J.; Zhou, C.; Luo, S.; Luo, W.; Li, S.; Xing, X.; et al. Dwarf and High Tillering1 represses rice tillering through mediating the splicing of D14 pre-mRNA. Plant Cell 2022, 34, 3301–3318. [Google Scholar] [CrossRef]
- Zhang, Q.; Xie, J.; Zhu, X.; Ma, X.; Yang, T.; Khan, N.U.; Zhang, S.; Liu, M.; Li, L.; Liang, Y.; et al. Natural variation in Tiller Number 1 affects its interaction with TIF1 to regulate tillering in rice. Plant Biotechnol. J. 2023, 21, 1044–1057. [Google Scholar] [CrossRef] [PubMed]
- Kameoka, H.; Kyozuka, J. Downregulation of rice DWARF 14 like suppress mesocotyl elongation via a strigolactone independent pathway in the dark. J. Genet. Genom. 2015, 42, 119–124. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, H. Identification and expression analysis of the MADS-box genes of Kentucky bluegrass during inflorescence development. Physiol. Mol. Biol. Plants 2022, 28, 1359–1374. [Google Scholar] [CrossRef]
- Zha, M.; Imran, M.; Wang, Y.; Xu, J.; Ding, Y.; Wang, S. Transcriptome analysis revealed the interaction among strigolactones, auxin, and cytokinin in controlling the shoot branching of rice. Plant Cell Rep. 2019, 38, 279–293. [Google Scholar] [CrossRef]
- Brenner, M.L. The role of hormones in photosynthate partitioning and seed filling. In Plant Hormones and Their Role in Plant Growth and Development; Springer: Dordrecht, The Netherlands, 1987; pp. 474–493. [Google Scholar] [CrossRef]
- Shimizu-Sato, S.; Mori, H. Control of outgrowth and dormancy in axillary buds. Plant Physiol. 2001, 127, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.E.; Cox, M.C.; Ross, J.J.; Krisantini, S.; Beveridge, C.A. Auxin dynamics after decapitation are not correlated with the initial growth of axillary buds. Plant Physiol. 2005, 138, 1665–1672. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Bangerth, F. Stimulatory effect of cytokinins and interaction with IAA on the release of lateral buds of pea plants from apical dominance—ScienceDirect. J. Plant Physiol. 2003, 160, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.L. Functional Analysis of Cytokinin Oxidase/Dehydrogenase 3 in Rice (Oryza sativa L.); Zhejiang Normal University: Jinhua, China, 2020. [Google Scholar]
- Johnson, X.; Brcich, T.; Dun, E.A.; Goussot, M.; Haurogné, K.; Beveridge, C.A.; Rameau, C. Branching genes are conserved across species. Genes controlling a novel signal in pea are coregulated by other long-distance signals. Plant Physiol. 2006, 142, 1014–1026. [Google Scholar] [CrossRef]
- Emery RJ, N.; Longnecker, N.E.; Atkins, C.A. Branch development in Lupinus angustifolius L. II. Relationship with endogenous ABA, IAA and cytokinins in axillary and main stem buds. J. Exp. Bot. 1998, 49, 555–562. [Google Scholar] [CrossRef]
- Doebley, J.F.; Gaut, B.S.; Smith, B.D. The molecular genetics of crop domestication. Cell 2006, 127, 1309–1321. [Google Scholar] [CrossRef]
- Li, L.; Xie, C.; Zong, J.; Guo, H.; Li, D.; Liu, J. Physiological and comparative transcriptome analyses of the high-tillering mutant mtn1 reveal regulatory mechanisms in the tillering of centipede grass (Eremochloa ophiuroides (Munro) Hack.). Int. J. Mol. Sci. 2022, 23, 11580. [Google Scholar] [CrossRef]
- Gou, J.; Debnath, S.; Sun, L.; Flanagan, A.; Tang, Y.; Jiang, Q.; Wen, J.; Wang, Z.Y. From model to crop: Functional characterization of SPL8 in M. truncatula led to genetic improvement of biomass yield and abiotic stress tolerance in alfalfa. Plant Biotechnol. J. 2018, 16, 951–962. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, Y.; Wang, Q.; Meng, D.; Wang, S. Effects of nitrogen and 6-benzylaminopurine on rice tiller bud growthand changes in endogenous hormones and nitrogen. Crop Sci. 2011, 51, 786–792. [Google Scholar] [CrossRef]
- Harrison, M.A.; Kaufman, P.B. Does ethylene play a role in the release of lateral buds (tillers) from apical dominance in oats? Plant Physiol. 1982, 70, 811–814. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, X.; Zhang, J.; Chen, F.; Li, Y.; Ma, H. The Relationship between Endogenous Hormone Content and Related Gene Expression and Tillering in Wild Kentucky Bluegrass. Agronomy 2023, 13, 2899. https://doi.org/10.3390/agronomy13122899
Ha X, Zhang J, Chen F, Li Y, Ma H. The Relationship between Endogenous Hormone Content and Related Gene Expression and Tillering in Wild Kentucky Bluegrass. Agronomy. 2023; 13(12):2899. https://doi.org/10.3390/agronomy13122899
Chicago/Turabian StyleHa, Xue, Jinqing Zhang, Fenqi Chen, Yajun Li, and Huiling Ma. 2023. "The Relationship between Endogenous Hormone Content and Related Gene Expression and Tillering in Wild Kentucky Bluegrass" Agronomy 13, no. 12: 2899. https://doi.org/10.3390/agronomy13122899
APA StyleHa, X., Zhang, J., Chen, F., Li, Y., & Ma, H. (2023). The Relationship between Endogenous Hormone Content and Related Gene Expression and Tillering in Wild Kentucky Bluegrass. Agronomy, 13(12), 2899. https://doi.org/10.3390/agronomy13122899



