Abstract
Chinese cherries, with their delightful blend of sourness and sweetness, are highly favored for their taste and nutritional benefits. However, they mature in conditions of high temperatures and rainfall, making them vulnerable to fungal infections which compromise their post-harvest quality. Our research aimed to study the effects of pre-harvest spraying with salicylic acid (SA) and sodium nitroprusside (SNP) on the pathogenic fungi in Manaohong cherries and their subsequent storage quality. We discovered that using SA and SNP at varying fruit development stages preserved fruit hardness, texture, appearance, and respiratory rate during storage, although it did not significantly alter the fruit’s dimensions. Furthermore, this pre-harvest treatment preserved levels of titratable acids, total phenols, and other antioxidants in the cherries, bolstered the activities of certain antioxidant enzymes (SOD, APX), and inhibited the activity of PPO and POD enzymes.Notably, the SA treatment alone demonstrated superior storage performance compared to combined treatments. Our research also identified Alternaria alternata and Colletotrichum godetiae as the primary pathogens in Manaohong cherries. In in vitro experiments, neither SA nor SNP inhibited these fungi’s growth. Consequently, we evaluated 12 pesticides and determined that 5% hexaconazole and 50% benomyl were most effective against these pathogens. Thus, to enhance the shelf life of Manaohong cherries and ensure their post-harvest quality, we recommend a pre-harvest spray of a SA, and combined with 5% hexaconazole and 50% benomyl. This approach not only promises enhanced cherry longevity but also lays a foundational strategy for the flourishing Manaohong Cherry industry.
1. Introduction
The cherry plant (Prunus avium L.) belongs to the Rosaceae family and is renowned for its juicy, delicious, and delicate fruit. There are currently four main types of cherries: Chinese cherries, Hair cherries, European sweet cherries, and European sour cherries. Despite variations in growth conditions and environments, these cherry varieties share similar functional characteristics and contain active substances that offer excellent health benefits. Research has suggested that cherries can potentially reduce the risk of various diseases, such as cancer, cardiovascular disease, and diabetes [1]. Among the four primary cherry types in China, Chinese cherry (Cerasus pseudocerasus) is a non-respiratory climacteric fruit [2]. A notable variant, Manaohong Cherry (Prunus pseudocerasu L.), a variant found in Nayong County, Guizhou Province, falls into this category. It is highly valued for its early maturity, high yield, adaptability, attractive color, delightful taste, and good storage characteristics. Due to its economic benefits and market competitiveness, Manaohong Cherry trees are extensively cultivated in Guizhou Province [3]. However, the high transpiration and respiration rate of cherry fruits make fresh sweet cherries highly susceptible to decay after harvest, causing huge economic losses. Pathogen infection and fruit aging, microbial community, storage temperature, and environmental conditions during transportation are all important factors that affect decay, and they have a significant impact on market acceptability [4]. Numerous studies have demonstrated the potential of utilizing various compounds to enhance the post-harvest longevity and resilience of fruits and vegetables. Salicylic acid (SA), a naturally occurring phenolic molecule, plays a crucial role in controlling plant growth. It enhances plant resistance to invasive pathogens by activating defense-related genes and responding to pathogenic infections. In relation to citrus green mold, it was discovered by Hamss [5] that the bacterium Bacillus amyloliquefaciens (SF14), when combined with an optimal amount of SA, exerted control over the disease and altered the cell wall structure in citrus peel. Additionally, SA has shown potential as a foliar spray for crop protection against diseases, fungi, and pests, thereby enhancing crop resilience [6]. The application of SA has proven effective in managing post-harvest decay and extending the shelf life of various fruits, including strawberry [7], grape [8], pear [9], sweet cherry [10], winter jujube [11], among others. Consequently, SA has gained popularity as an alternative approach for fruit preservation and fungal disease management in recent years. Sodium nitroprusside (SNP) is commonly used as an exogenous nitric oxide (NO) donor in response to various biotic and abiotic stresses. Researchers have found that NO releasing agents can enhance stress resistance and reduce disease incidence in fruits [12]. Moreover, NO plays a significant role in reducing damage caused by reactive oxygen species and regulating physiological and biochemical reactions within cells [13]. The application of external NO, such as SNP, triggers the activation of antioxidant enzymes, increases levels of antioxidant compounds, suppresses the accumulation of reactive oxygen species, mitigates membrane lipid peroxidation, preserves membrane integrity, and enhances cold tolerance in litchi fruit [14]. Spraying SNP solution before harvest has been shown to promote wound healing in post-harvest melon epidermis by activating phenylpropanoid metabolism [15]. These findings highlight the potential of using NO and SA treatments to enhance stress resistance, disease management, and post-harvest quality preservation in various fruits.
Pathogenic fungi pose a significant threat to fresh plant products, contributing to approximately 50% of storage losses and negatively impacting the shelf life and sales of fruits [16]. These fungi primarily infect fruits during their growth, with conidia adhering to the fruit’s surface, leading to the destruction of epidermal cells and rapid decay [17]. Fungicides remain one of the main methods for disease prevention and control; however, prolonged use of a single agent can result in pathogen resistance [18]. Therefore, there is an urgent need for a green, safe, efficient, and synergistic approach to crop disease prevention and control. The utilization of pre-harvest fungicides combined with resistance stimulants has shown promising results in various fruits, ensuring post-harvest preservation of fruit and vegetable quality while eliminating harmful pathogens [19]. For example, a study demonstrated that applying a specific dosage of mancozeb-45 (0.25%) and carbendazim (0.15%) before harvesting, followed by the application of salicylic acid (2.0 mM), enhanced the mango’s antioxidant system, and increased its resistance against anthracnose [20]. Although limited research exists on the topic, combining low-temperature storage with bactericide treatment has potential to extend the storage period of cherries.
This experiment aimed to investigate the effects of preharvest spraying of salicylic acid (SA) and sodium nitroprusside (SNP) on the storage quality and pathogenic fungi of Manaohong Cherry. We conducted a comprehensive analysis of antioxidant enzymes and related physiological indicators to determine the optimal spraying treatment and identify the main pathogenic fungi that cause fruit rot. In addition, indoor toxicity tests were conducted to evaluate the efficacy of fungicides with antibacterial properties. This study provides valuable insights into the potential of using SA, SNP, and biopesticides in combination to enhance biological management of postharvest fruit diseases.
2. Materials and Methods
2.1. Preharvest Spraying Treatments and Fruit Harvest
Twelve Manaohong Cherry fruit trees were randomly selected and identified. The experiment spanned from the fruit setting stage to the maturity stage, which occurred between 17 March 2022, and 27 April 2022. Four different growth stages were considered: fruit setting stage (17 March), expansion stage (13 April), color conversion stage (20 April), and maturity stage (27 April). At each stage, a specific volume and concentration of salicylic acid (SA) and sodium nitroprusside (SNP) solution were sprayed on the trees. The experiment consisted of four treatments: a control group (CK) treated with distilled water, an SA treatment group (A) treated with 1 mmol∙L−1 SA solution, an SNP treatment group (N) treated with 0.25 mmol∙L−1 SNP solution, and a combined treatment group (AN) treated with 1 mmol∙L−1 SA + 0.25 mmol∙L−1 SNP. Each treatment was applied to three trees, with clear markings for identification. For each spraying, 1 L of solution was evenly sprayed on each tree. The final spraying was conducted 7 days before the harvest during the mature period, which was 45 days after flowering. Cherries of the same size, without mechanical damage, and at the same level of maturity were carefully selected. Put the cherries in a designated basket that has undergone thorough washing and disinfection procedures. Within 3 h, they were transported back to the laboratory, where field heat was eliminated by standing at room temperature for 2 h. Subsequently, the cherries were packed in a cold storage at 1 ± 0.5 °C. Approximately 0.5 kg of cherries were weighed using an electronic balance and placed in small frames. Each treatment had three parallel samples. Once all the cherries were properly packed, they were sealed in polyethylene fresh-keeping bags (PE20) with a thickness of 0.02 mm. The bags had an O2 permeability coefficient of 6571 mL/(m2·d), a CO2 permeability coefficient of 21,880 mL/(m2·d), and a moisture permeability rate of 4.82 g/(m2·d). The bags, pre-cooled for 24 h at (1 ± 0.5 °C), were provided by National Engineering Technology Research Center for Preservation of Agriculture Product (Tianjin). Corresponding physiological and biochemical measurements were taken at intervals of 0, 7, 14, 21, and 28 days to assess the cherries’ stored quality.
2.2. Isolation, Purification, and Identification of Pathogenic Fungi
Sampling was conducted at multiple stages, specifically after 7 days of spraying during the fruit setting stage (17 March), expansion stage (6 April and 13 April), color conversion stage (20 April), and maturity stage (27 April). Ten healthy cherries are randomly selected from each treatment and separated and purified using tissue isolation. Small pieces of cherry tissues measuring 4 × 4 × 3 mm was sliced and treated with 75% ethanol for 30 s for initial disinfection. Subsequently, they were disinfected with 1% sodium chlorate for 3 min. After rinsing with sterile water at least twice, the tissues were dried using sterile filter paper and transferred onto Potato Dextrose Agar (PDA) medium. The plates were then incubated in a controlled environment at a constant temperature and humidity of 28 °C for a period of 3–7 days. The growth of mycelium and the characteristics of the colonies were closely monitored. Purification of strains was achieved by repeatedly isolating and purifying the musing the plate streaking method. The pathogen hyphae were cultured for 5–7 days. Colony traits of each pathogenic variant were observed on the PDA medium, and the mycelium structure and conidia traits were examined using the BX-53 Optical Microscope (Olympus corporation, Tokyo, Japan). Preliminary identification of the pathogenic fungus was also conducted. After a certain level of purification, the pathogen hyphae were cultured for 5–7 days. Subsequently, the hyphae were ground into powder using liquid nitrogen, and DNA extraction was performed using a plant genomic DNA extraction kit (Sangon Biotech Co., Ltd., Shanghai, China). The rDNA-ITS sequence universal primers (ITS1:5′-TCCGTAGGTGAACCTGCGG-3′ and ITS4:5′-TCCTCCGCTTATTGATATGC-3′) were employed. The sequenced samples were sent to Bioengineering (Shanghai) Co., Ltd. for further sequencing analysis. The obtained sequencing data was analyzed using BLAST in the NCBI database to identify the ITS sequences of closely related strains. The phylogenetic tree was constructed using MEGA 11 software based on the neighbor-joining (NJ) method. In the reverse connection experiment, healthy cherry fruits were selected and soaked in 75% ethanol for 30 s for disinfection. Then, they were treated with 1% sodium chlorate for 3 min and washed at least twice with sterile water. A certain concentration of spore solution was prepared using purified strains. In a sterile environment, 9 healthy cherry fruits were inoculated through the equatorial part of each cherry fruit with an inoculation needle. The fruit was transferred to a sterile culture dish, sealed with a sealing film, and placed in a constant temperature and humidity incubator at 25 °C and 95% humidity to observe and record the occurrence rate of the fruit.
2.3. In Vitro Inhibitory Effects of Different Concentrations of SA and SNP on the Pathogenic Fungus
Concentrations of 0.1, 0.5, and 1 mmol∙L−1 for salicylic acid (SA) and 0.025, 0.25, and 0.5 mmol∙L−1 for sodium nitroprusside (SNP) were selected for the experiment. Sterilized Potato Dextrose Agar (PDA) medium was prepared with different concentrations of SA and SNP: 0.1, 0.5, and 1 mmol∙L−1 for SA, and 0.025, 0.25, and 0.5 mmol∙L−1 for SNP. The control group consisted of PDA medium treated with distilled water. Use a sterile hole punch to extract a 5 mm disc from the periphery of the culture of pathogenic fungus, which has been cultured for 5 days. For each pathogen, three parallel cultures were established and incubated at 28 °C for a duration of five to seven days. The colony diameters were recorded daily using a cross method.
2.4. Fungicide Sensitivity of Isolates
The antifungal activity of fungicides was assessed using the mycelial growth rate method [21]. Under sterile conditions, the experimental drugs were prepared as a stock solution with an effective concentration of 10,000 μg∙mL−1 using sterile water. Different volumes of the stock solution were used to prepare the desired mass concentrations, which were then UV-sterilized in an ultra-clean bench for 0.5 h and kept for backup. The Potato Dextrose Agar (PDA) medium was sterilized and cooled to approximately 55 °C. Five different levels of mass concentration were created by adding 5 mL of the respective concentrations to 45 mL of PDA medium. The liquid medium was poured into four culture dishes (90 mm diameter) for each concentration level. As a control group, sterile water was substituted for the fungicidal substance. A 5 mm activated fungal cake was placed at the center of each medium plate using a sterilized puncturing tool. Place the Petri dish (including the control) in the HSP-360BE constant temperature and humidity biochemical incubator for culture until the colony diameter of the control group reaches 40 to 60 mm. The colony diameter was measured using the cross method, and the growth inhibition rate of each agent on the four pathogenic fungi was calculated using the provided formula. The virulence equation was obtained by taking the logarithm of the mass density of the agent as the independent variable and the corresponding probability of inhibition rate as the dependent variable.
2.5. Effects of Preharvest SA and SNP Application on Cherry Fruit Size and Appearance
At the time of harvest, a random sample of thirty undamaged cherries was selected from various treatment groups. The diameter of each individual cherry was measured using a vernier caliper with a precision of 0.01 cm. The mass of each cherry was recorded by weighing it on an electronic balance.
2.6. Determination of Decay Rate, Soluble Solids, Respiration Rate and MDA Content
Rotten cherries were identified based on the presence of cracking, brown spots, and running water on their surface. To determine the decay rate of cherry quality, the weight of each rotten cherry was divided by the total weight of all cherries. The soluble solids content was measured using a PAL-1 handheld data display refractometer (PAL-1, ATAGO, Tokyo, Japan) [22]. Determination of the rate of respiration was carried out using a PBL checkpoint O2/CO2 headspace analyze [23]. For the analysis of malondialdehyde (MDA) content, 3.0 g of cherry fruit samples were weighed and ground with 5 mL of a cold solution containing 5% (w/v) trichloroacetic acid, The mixture was centrifuged, and the supernatant was collected and mixed with thiobarbituric acid. The reaction took place in a boiling water bath for 20 min. The optical density (OD) values at 420 nm, 532 nm, and 600 nm were recorded, and the MDA content was expressed as μmol∙g−1 [24].
2.7. Determination of Texture
Fifteen cherries were randomly selected for each treatment and determination of fruit hardness using the TA.XT plus texture analyzer (Stable Micro Systems., London, UK) Determination of fruit firmness: During the measurement process, all fruit stalks were aligned in a consistent direction, and they were punctured using the P/2N probe. The firmness test was conducted with parameters including a 5 mm puncture depth, a pre-test speed of 2 mm/s, a test speed of 1 mm/s, and a post-test speed of 1 mm/s.
To evaluate the texture of the pulp, the cherry fruits were positioned on the test board of the EZ-SX texture analyzer (manufactured by Stable Micro Systems, UK). A cylindrical P100 probe with a diameter of 100 mm was used for the Texture Profile Analysis (TPA) test. The test parameters included an initial speed of 2 mm/s, a test speed of 1 mm/s, an upward speed of 1 mm/s after the test, a compression deformation of 25% in the cherry pulp, a 2 s pause time between compressions, and a trigger force of 5 g. The texture parameters derived from the texture characteristic curve were hardness, elasticity, cohesiveness, adhesion, chewiness, resilience, and brittleness. A total of nine fruits were tested from each treatment, and the averaged results were obtained [25].
2.8. Determination of Titratable Acid, Soluble Protein, Vc, and Total Phenol Content
To determine the titratable acid (TA) in cherry juice, 25 mL of the juice was taken and titrated to pH 8.2 using a 0.1 mmol·L−1 sodium hydroxide solution. The TA result is expressed as a percentage of malic acid [26]. For the determination of soluble protein, each fruit extract was ground in a 50 mM potassium phosphate buffer (pH 7.0). The supernatant of the fruit extract was mixed with 1.0 mL of Coomassie brilliant blue dye and measured at 595 nm. The content was calculated using bovine serum albumin as the standard [27]. The total phenolic content in cherry fruit was determined using the Folin–Ciocalteu method [28]. The Vc (Vitamin C) content was determined using the 2,6-dichlorophenol indophenol method. In this method, 5 mL of decolorized extract was measured in a 50 mL conical flask and titrated with oxidized 2,6-dichlorophenol indophenol. The endpoint was indicated by the appearance of a red color, and the volume consumed was recorded to calculate the Vc content [29].
2.9. Determination of Antioxidant Enzyme Activity
To isolate antioxidant enzymes, start by obtaining 1 g of frozen sample and grind with 2 mL of 100 mmol∙L−1 phosphate buffer (pH 7). Centrifuge the homogenate at 12,000× g for 30 min at 4 °C, and collect the resulting liquid as a raw enzyme solution. The activity of SOD enzyme was determined using the nitrogen blue tetrazolium reduction method. After grinding with buffer and centrifugation, the supernatant was combined with nitrogen blue tetrazolium solution for determination [30]. The absorbance value was measured at 560 nm, and 50% of the inhibition of nitrogen blue tetrazolium photochemical reduction was 1 enzyme activity unit (expressed as U/g FW), using a UV-2550 ultraviolet spectrophotometer. Polyphenol oxidase (PPO) activity was measured at 420 nm by monitoring the change in absorbance value. The measurement unit used was the enzyme quantity required to increase the absorbance by 0.01 per minute, with the results reported as U∙g−1 protein [31]. To determine Peroxidase (POD) activity, mix 3.0 mL of a 25 mmol∙L−1 guaiacol solution with 0.5 mL of enzyme extract. Then, add 200 μL of a 0.5 mol∙L−1 hydrogen peroxide solution and measure the absorbance at 470 nm [32]. For Ascorbate peroxidase (APX) activity, start by homogenizing 3 g of tissue with 7 mL of a 50 mmol∙L−1 sodium phosphate buffer (pH 7.0). The reaction mixture should contain 0.2 mL of enzyme solution, 0.1 mL of a 9 mmol∙L−1 ascorbic acid (AsA) solution, 50 μL of 30% (v/v) H2O2, and 3 mL of a 100 mmol∙L−1 sodium phosphate buffer solution (pH 7.0). An APX unit is defined as the amount of enzyme that oxidizes 1 μmol of ascorbic acid per minute [33].
2.10. Data Analysis
All experiments and analyses used three biological replicates, plotted using Origin 2021, one-way ANOVA (ANOVA) with SPSS19.0 software, and data difference significance analysis (p < 0.05, significant difference) using Duncan’s new complex range method.
3. Results
3.1. Effects of Pre-Harvest Spraying of SA and SNP on the Size of Cherry Fruit at Maturity and the Appearance of Fruit during Storage
The quality of cherry fruit appearance is assessed based on the weight of individual fruits. Table 1 indicates that pre-harvest spraying treatments had no significant impact on the weight, size, or shape index of ripened cherries. Based on the results presented in Figure 1, there were no significant differences observed in the visual appearance of cherry fruits between the treatment and control groups at the beginning of storage. However, as the storage period progressed, notable changes started to occur on the fruit surface, including browning, depressions, damage, and signs of rot. These changes became evident after 14 d of storage in all experimental groups. Compared with other treatment groups, it was observed that the fruit appearance in the CK group was noticeably worse compared to the other three treatment groups. The cherry fruits treated with A and AN exhibited better visual appearance after storage, while the A single treatment group showed the most favorable fruit appearance among all treatments.

Table 1.
Effects of pre-harvest SA and SNP spraying on cherry fruit size.

Figure 1.
Effects of pre-harvest SA and SNP spraying on appearance quality of cherries.
3.2. Effects of SA and SNP Spraying Pre-Harvest on Decay Rate, Firmness, Respiration Rate and Malondialdehyde Content of Manaohong Cherries during Storage
Determining the rate of decay is a valuable technique for evaluating the effects of storage on fruits and vegetables [34]. Figure 2A illustrates notable differences in decay rates among the three experimental groups and the control group throughout the storage period. All treatments experienced a certain degree of increase in decay rate starting 7 days before storage. From day 14 to day 21, two distinct patterns emerged: the decay rate of the CK and N treatment groups significantly increased, while the decay rate of the A and AN treatment group increased more slowly, and there was no significant difference between the A and AN treatment group. At the end of storage, compared with the AN treatment group, the decay rate of the A treatment group slightly increased, but the overall difference was not significant. The decay rate of the AN treatment group was 30.32%, 7.59% lower than that of the A treatment group. These results highlight the good effects of AN and A treatments in reducing decay rate and maintaining fruit quality during storage.

Figure 2.
Effects of SA and SNP spraying before harvest on Decay rate (A), Firmness (B), Respiration rate (C) and Malondialdehyde content (D) of stored cherries.
The acceptance of cherries by consumers is heavily influenced by their firmness, which is a significant quality characteristic. Firmness serves as an indicator of fruit texture and its ability to withstand storage [35]. As shown in Figure 2B, puncture firmness initially increased and then declined, reaching its peak on the 7th day of storage before gradually decreasing. This pattern could be attributed to the positive effect of pre-cooling treatment, which enhanced fruit firmness. By the end of the storage period, the A treatment group displayed the highest level of firmness, indicating superior firmness. In contrast, the N treatment group exhibited the lowest level of firmness among the treatments. These results highlight the effectiveness of the A treatment in maintaining fruit firmness throughout the storage duration.
The respiration rate is a fundamental physiological metabolic activity that occurs during the ripening process of fresh horticultural products, and it serves as a key indicator for assessing their suitable storage life [36]. As depicted in Figure 2C, the respiration rate of each treatment group initially increased and then decreased during storage. Throughout the storage period, the respiration rate of the AN treatment group remained relatively constant, showing no significant increase or decrease compared to the rate observed on day 0. The changes in SA treatment were significant, but the respiratory rate of SA treatment was slightly lower than that of AN treatment group on the day of picking, during the middle and late storage periods.
MDA (malondialdehyde) content serves as an indicator of lipid peroxidation in cellular membranes and indirectly reflects the level of cellular damage in fruits [37]. Figure 2D demonstrates that MDA content varied throughout the storage period, showing a correlation with the rate of decay. Furthermore, as the duration of storage increased, there was a gradual elevation in MDA content. On the day of picking, both the A treatment group and the CK group exhibited significantly higher MDA levels compared to the N and AN group. However, by the end of storage, the respective increases in MDA content for the CK, A, N, and AN group were reported as 13.77, 12.19, 13.61, and 11.95 mol∙g−1. The above results indicate that SA treatment and composite treatment effectively inhibited the increase in MDA content in cherry fruits during storage, but the difference between the two was not significant.
3.3. Effects of Pre-Harvest SA and SNP Spraying on the Texture of Manaohong Cherries during Storage
Texture profile analysis (TPA) is a reliable method for objectively assessing fruit texture by simulating oral chewing movement and determining texture parameters of solid and semi-solid samples [38]. The trend in TPA hardness alteration aligns with that of puncture firmness, suggesting that treatments A and AN can delay the decline in hardness during storage and preserve fruit firmness. Referring to Figure 3, the storage duration negatively influenced resilience, brittleness, and elasticity. Throughout the storage period, both the A and AN treatment groups exhibited significantly lower brittleness compared to the CK and N treatment groups. Examining the trend of chewing degree (Figure 3E), we observe an initial increase followed by a decrease and then another increase. The rise in chewing intensity during the initial 7 days of storage can be attributed to improved fruit chewing quality through refrigeration. In the later phase of storage, both A and AN treatment enhance the cherry fruit’s chewiness. Figure 3F,G represent adhesion and cohesion, respectively. These two trends exhibit similarities, with an overall pattern of initially decreasing and then increasing. At the beginning of storage, treatment N shows notably elevated adhesion and cohesion. By the end of the storage period, the highest adherence is observed in the A treatment group, measuring 0.42 mJ. This represents an increase of 42.9%, 47.6%, and 60.0% compared to the adherence levels of the CK, N, and AN group, respectively. Throughout the storage duration, the alteration pattern of cohesion in cherry fruits subjected to treatments A and AN is similar, with no significant distinction observed between the two.

Figure 3.
Effects of pre-harvest SA and SNP spraying on the texture of cherry fruit ((A) Hardness, (B) Restorative, (C) Brittleness, (D) Elasticity, (E) Chewiness, (F) Adhesivity, (G) Cohesiveness).
3.4. Effects of Pre-Harvest SA and SNP Spraying on the Contents of Titratable Acid, Soluble Solids, Soluble Protein, Free Amino Acids, Vc and Total Phenols of Manaohong Cherries during Storage
The flavor quality of fruits is influenced by their titratable acid content [39]. As shown in Figure 4A, the titratable acid content of the A treatment group did not exhibit a significant decrease during the storage period and remained like the levels observed on the day of harvesting. In contrast, the other three experimental groups displayed a pattern of initial decline, followed by an increase, and then another decline in titratable acid content, indicating that the application of SA solution effectively preserved the titratable acid content of cherries throughout the storage period. As depicted in Figure 4B, there were no significant variations in the soluble solids content among the different treatment groups at the time of harvest. However, during the storage period, there was an initial increase followed by a subsequent decline in the observed changes. In the later stage of storage, the Soluble solid content in the A and AN treatment group were higher than those in the CK and N treatment groups. According to the data presented in Figure 4C, On the day of picking, the soluble protein content in the A treatment group was the highest, but during storage, the changes in soluble protein content in the A and N treatments were similar, and at the end of storage, it was still significantly higher than the CK and AN treatment groups, indicating that SA and SNP single treatments effectively delayed the decrease in soluble protein content during the later stages of storage. The amount of free amino acids in fruit significantly influences its taste and nutritional quality [40]. As shown in Figure 4D, the free amino acid content exhibited a consistent upward trend throughout the storage period. On the day of harvest, the levels of free amino acids in each treatment group were notably lower compared to the CK control group. However, by the end of storage, the levels of unbound amino acids in the A and N treatment groups were significantly reduced compared to the CK and AN treatment group. This observation suggests that SA and SNP may function as signaling molecules, slowing down the increase in free amino acid content by inhibiting the decomposition of related enzymes or proteins. As illustrated in Figure 4E, the overall change in VC content showed a downward trend, and the VC content of the A treatment group remained relatively high throughout the entire storage period. It was significantly higher than the other three treatment groups 14 days before storage, but by the 28th day of storage, there was no significant difference in Vc content between the A treatment group and the AN treatment group. However, overall, SA single treatment can effectively delay the decrease in VC content. Phenolic compounds serve as secondary metabolites in fruits, contributing to oxidative processes and playing a key role in fruit browning and peel color darkening. These compounds significantly impact the appearance and commercial value of fruits [41]. As demonstrated in Figure 4F, the content of phenolic compounds exhibited an initial increase followed by a subsequent decline throughout the storage period. The treatment group displayed a significantly higher total phenolic content at the end of storage compared to the control group. This suggests that the Manaohong Cherry fruits treated with salicylic acid and sodium nitroprusside had lower levels of browning. These findings are in line with the results reported by Kerc [42].

Figure 4.
Effects of pre-harvest SA and SNP spraying on the contents of Titratable acid (TA, (A)), Soluble solid content (SSC, (B)), Soluble protein (C), Free amino acids (D), Vc (E) and Total phenols (F) in cherry fruits.
3.5. Effects of Pre-Harvest SA and SNP Spraying on Enzyme Activity of Manaohong Cherries during Storage
The antioxidant enzyme system in plants includes Superoxide dismutase (SOD), which acts as the initial defense mechanism. SOD rapidly converts O2− into H2O2 and O2, effectively eliminating O2− and protecting oxygen-metabolizing cells from its harm. As shown in Figure 5A, the SOD activity in the fruits of various treatment groups followed a pattern of initial decline, subsequent increase, and then another decline as the storage time extended. This decrease in SOD activity observed in all treatment groups on the 7th day may be attributed to the inhibitory effects of low temperature. The peak SOD activity was observed on the 14th day of storage for all treatments. At the end of storage, there were no significant differences in SOD activity among the three treatments (p > 0.05). However, the CK group exhibited the lowest enzyme activity with a measurement of only 5.52 U∙g−1. During the entire storage period, the A and CK treatment groups showed an overall trend of first increasing and then decreasing. The POD enzyme activity of the AN treatment group showed a decreasing trend 14 days before storage, but began to recover after 14 days of storage, possibly due to a sudden decrease in temperature leading to a decrease in POD enzyme activity. From the 21st day of storage, the enzyme activity of the A treatment group was lower than that of the other three treatment groups (p < 0.05), indicating that the SA treatment group had a significant inhibitory effect on POD enzyme activity. On the day of harvest, the CK and A treatment groups showed a slight increase in PPO enzyme activity compared to the N and AN group. During storage, cherry fruits treated with A demonstrated significantly reduced PPO activity, except on the day of harvest and the 28th day, compared to the other three treatment groups. The combined treatment (AN) accelerated the increase in PPO enzyme activity after storage, indicating that a single SA treatment can inhibit the decline of enzyme activity during the initial storage phase and post-pone the escalation of PPO activity in the subsequent storage phase. Fluctuations in APX activity were observed under different treatments, as depicted in Figure 5D. Group A exhibited significantly higher APX activity compared to the other groups (p < 0.05), At the end of storage, the APX activity of group A was 1.04, 2.30, and 1.16 times higher than that of CK, N, and AN, respectively.
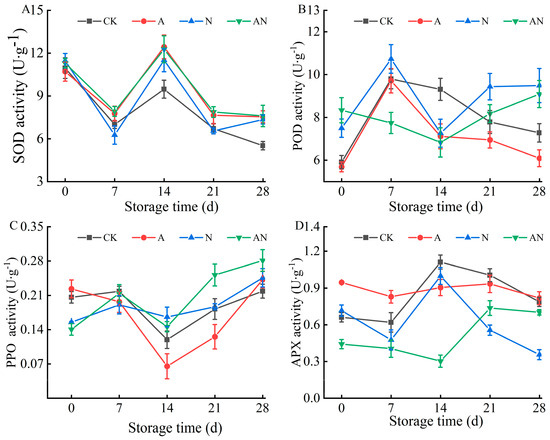
Figure 5.
Effects of pre-harvest SA and SNP spraying on enzyme activity of cherry fruit ((A) SOD activity, (B) POD activity, (C) PPO activity, (D) APX activity).
3.6. Based on Principal Component Analysis and Correlation Analysis, the Fruit Quality of Manaohong Cherry Was Comprehensively Evaluated
Taking the contribution rate of each principal component as the weight, the comprehensive evaluation function is obtained by the weighted sum of the corresponding principal component scores and weights, that is, Z = 0.391 × Y1 + 0.175 × Y2 + 0.140 × Y3 + 0.064 × Y4 + 0.053 × Y5. The comprehensive score and ranking results of fruit quality of the four treatments were calculated by the function. As shown in Table 2, the higher the comprehensive score, the better the comprehensive quality of the variety in the measured multiple indicators. As shown in Figure 6a, during the whole storage period, the comprehensive score of A treatment was higher than that of its three treatment groups in the first 14 days, and its comprehensive score was slightly lower than that of AN treatment group at the end of storage. Overall, the storage effect of A treatment group was the best.

Table 2.
Composite component score.
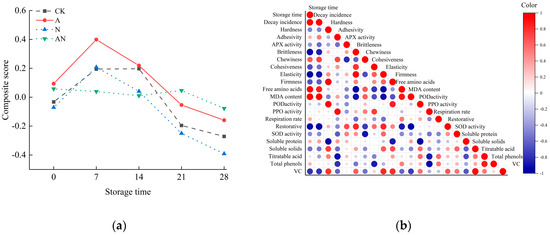
Figure 6.
Principal component analysis (a) and correlation analysis ((b), Correlation analysis of A treatment).
Figure 6b is the correlation heat map of the A treatment group. As shown in the figure, the storage time was significantly positively correlated with the decay rate, free amino acid, MDA content, and respiration rate, and was significantly negatively correlated with APX enzyme, POD enzyme, SOD enzyme and other related antioxidant enzymes, indicating that pre-harvest SA spraying treatment maintained the storage quality of the fruit by activating the antioxidant system of the plant.
3.7. Isolation and Purification of Pathogenic Fungus
Table 3 presents the isolation and purification of cherry fruits at five different growth stages, ranging from 17 March 2022 to 27 April 2022. Variations were observed in the types and quantities of isolated pathogens at different stages of growth. Spraying was performed during the first 7 days of each growth period, with samples taken 7 days later. A total of eight strains were isolated and purified from the fruit setting period for identification of the pathogenic fungus. Additionally, 12 strains were isolated during the mature period. Through morphological and molecular biological identification, the 12 strains were identified as Fusarium fujikuroi, Diaporthe, Colletotrichum godetiae, Alternaria alternata, Aspergillus flavus, Nigrospora oryzae, Sordariales, Schizophyllum commune, Irpex lacteus, Xylaria, Cladosporium, and Bjerkandera adusta. Koch’s rule verification revealed that Aspergillus flavus, Nigrospora oryzae, Sphaerotheca, Schizophyllum commune, Irpex lacteus, Cladosporium, and Bjerkandera adusta were not significantly pathogenic during the reverse inoculation process [43]. Therefore, these pathogens were determined not to be the primary causes of Manaohong Cherry decay. Although Diaporthe and Fusarium fujikuroi can cause cherry disease under certain conditions, their disease rates and severity are considerably lower compared to Colletotrichum godetiae and Alternaria alternata.

Table 3.
Effects of pre-harvest SA and SNP spraying on pathogen species in different growth stages of cherries.
We found a specific correlation mechanism between pathogen species and fruit ripening process. The number of pathogen species found in cherry fruits in each treatment group was significantly lower than that in CK treatment group. This indicates that pre-harvest spraying of SA and SNP, along with increased spraying frequency, enhanced the fruit’s disease resistance. It is worth noting that Colletotrichum godetiae was present in all four treatments, suggesting that its spores had already adhered to the surface of Manaohong Cherry fruits during the fruit setting period and remained dormant until the later stages of post-harvest storage, impacting the fruit’s storage quality. Alternaria is a common latent pathogen that infects fruits after harvest, particularly mature fruits harvested after rainfall, leading to the development of black spots on the fruit. The table shows no isolations of Alternaria during the fruit setting and expansion Period. However, Alternaria alternata was found during the color transition period in both the CK and SNP treatments. The pathogen was not isolated in the SA and composite treatments. However, as the fruit ripens, the natural resistance of the fruit peel gradually decreases, allowing dormant Alternaria to proliferate within the fruit. Thus, Alternaria isolated from all four treatments. This suggests that the solution containing SA in the pre-harvest spraying treatment could delay the emergence of Alternaria, but it could not inhibit the attachment of Alternaria conidia from the environment, leading to damage of host cells. Based on the analysis of physiological indicators, it is speculated that pre-harvest SA and SNP treatments may enhance the disease resistance of cherry fruits, prolong their shelf life, and maintain storage quality. However, it does not have a notable suppressive impact on anthracnose fungus. These findings align with previous research on the effects of salicylic acid on sweet cherries [44]. Pre-harvest treatment with 2 mM salicylic acid (SA) significantly improved disease resistance and reduced the incidence rate of fruits.
3.8. Pathogen Identification
Through morphology, biology, and Koch’s rule verification, YT1 (Alternaria alternata) and YT2 (Colletotrichum godetiae) were identified as the main pathogenic fungi affecting cherry fruits. Morphologically, YT1 colonies appear relatively dense, with a dark black inner layer and a dark gray outer layer. The back of the culture medium exhibits a yellow-brown color, and under a microscope, a single spore is observed to be short and elliptical. Preliminary morphological identification suggests it belongs to Alternaria alternata. On the other hand, YT2 colonies display an orange color at the front, with well-developed aerial hyphae. The conidia are long oval to cylindrical, straight, and have a blunt round top. Based on preliminary morphological identification, it is suggested that YT2 is Colletotrichum, commonly known as anthrax fungus. To amplify the rDNA-ITS sequence, amplification using primers, the sequencing results were analyzed using BLAST and MEGA 11 software against the NCBI database.A phylogenetic tree was constructed, as depicted in Figure 7. Both strains clustered together with high homology and similarity to Alternaria alternate (OM319502) and Colletotrichum godetiae (MN429214), showing 93% and 95%, respectively. Thus, based on the biological identification results, YT1 and YT2 were identified as Alternaria alternata and Colletotrichum godetiae, respectively.
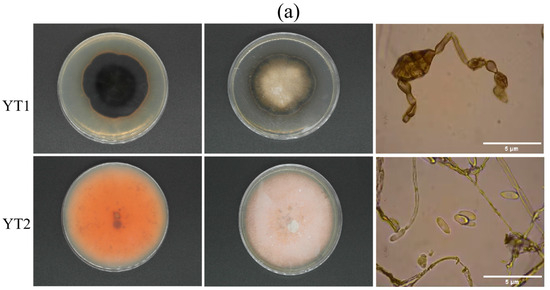
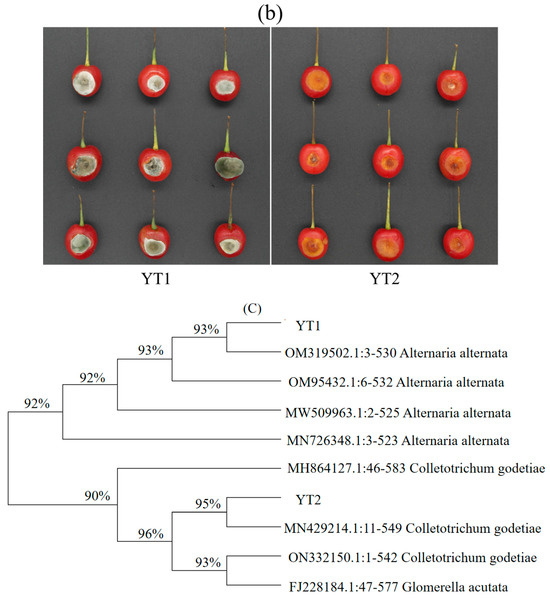
Figure 7.
Isolation and identification of pathogenic bacteria (a) Morphological identification of pathogenic fungus (YT1: Alternaria alternata, YT2: Colletotrichum godetiae); (b) Pathogenicity analysis of pathogens on cherry fruits (YT1: Alternaria alternata, YT2: Colletotrichum godetiae); (c) Phylogenetic tree of rDNA-ITS sequence.
3.9. Inhibitory Effects of Salicylic Acid and Sodium Nitroprusside at Different Concentrations on YT1 and YT2 In Vitro
As illustrated in Figure 8A, the colony diameter of each treatment group progressively increased with the cultivation time. There were no significant differences in the colony diameter of Alternaria alternata among the different concentration A treatment groups on the initial day of cultivation (p > 0.05). On the fifth day, the low concentration A treatment enhanced the growth of colony diameter, while there was no notable difference in colony diameter between the 1 mmol∙L−1 SA treatment and the CK group. Similarly, as seen in Figure 8B, the trend of changes for Colletotrichum showed similarities to Alternaria. During the early stage of cultivation, the incidence diameter of anthracnose fungus treated with low concentration A was significantly lower than that in the CK and 1 mmol∙L−1 A treatment groups. However, by the 5th day of cultivation, there was no significant difference between the CK treatment and the 1 mmol∙L−1 A treatment groups. In terms of Alternaria growth (Figure 8C), the culture medium containing SNP solution promoted colony diameter during the cultivation period. On the 5th day of cultivation, the colony diameter of the 0.25 mmol∙L−1 N treatment group was 31.62 ± 0.46 mm, which was 16.81% higher than that of the CK group. Furthermore, as shown in Figure 8D, the inhibition rate of different concentrations of N treatment groups on anthracnose fungus exhibited a positive correlation with the concentration. Higher concentrations resulted in larger colony diameters. By the 5th day of cultivation, the colony diameter of the anthracnose fungus in the 0.25 mmol∙L−1 N treatment group was 8.61% lower than that in the CK group.

Figure 8.
Effect of SA and SNP on colony growth of Alternaria alternata (A,C) and Colletotrichum godetiae (B,D), The lowercase English letters (a, b, c, d) indicate significant differences between different treatment groups.
3.10. Fungicide Sensitivity of Isolates
The indoor toxicity of the two strains was assessed using the mycelium growth rate method, employing a total of 12 fungicides. The logarithm of the agent concentration served as the abscissa, while the probability of the relative inhibition rate was used as the ordinate. Through this evaluation, the toxicity regression equation, EC50 values, and correlation coefficients for the 12 fungicides against the two cherry strains were obtained. Table 4 displays the results of the indoor toxicity assessment of the 12 fungicides against Alternaria alternata. Among them, 5% hexaconazole exhibited the lowest EC50 value of 0.0001 μg∙mL−1, followed closely by 40% difenoconazole and 50% fludioxonil, with EC50 values of 0.0054 and 0.570318 g∙mL−1, respectively. Regarding the indoor toxicity test conducted on Colletotrichum godetiae, 50% benomyl and 80% mancozeb displayed higher toxicity, with EC50 values of 0.0018 and 0.01084 μg∙mL−1. After careful evaluation, it is suggested that a combination of 5% hexaconazole and 50% benomyl can be utilized as effective substances for controlling Manaohong Cherry in the field. These substances could also serve experimental purposes in controlling cherry pathogen infections, providing a theoretical basis for extending the post-harvest shelf life of cherries and minimizing economic losses.

Table 4.
Fungicide sensitivity of isolates.
4. Discussion
4.1. Effects of Pre-Harvest SA and SNP Spray on Postharvest Storage Quality
The assessment of the commercial value of fruits considers both their appearance and nutritional composition [45]. Cherry, as a highly popular fresh fruit, is prone to mechanical damage and microbial infection due to high temperatures and rainy picking. Consequently, its storage period is often short, which does not fully meet consumer demand. Fruit hardness and appearance are crucial factors in determining the fruit’s edibility. The results demonstrate that the SA treatment exhibits notable reductions in decay rate and respiration rate, preserving the integrity of cell membranes and slowing down the fruit’s softening process compared to the control treatment. Throughout the storage period, measuring the soluble solids content serves as an important indicator of cherries’ physical and chemical properties. There is a gradual decrease in dissolved substance concentration, indicating a decline in soluble solids content. This decline can be attributed to physiological factors such as fruit respiration. Similar findings were observed in a study on salicylic acid applied to winter jujube [46]. Cherries are rich in organic acids and phenols, both of which have significant impacts on human health [47]. The application of salicylic acid before harvesting effectively delays the decrease in titratable acid, soluble solids, and total phenol content during storage. It contributes to maintaining the fruit’s quality throughout storage, highlighting the notable preserving effect of pre-harvest spraying with salicylic acid on cherries.
Fruit texture plays a vital role in determining the quality characteristics of fruits. During storage, fruits undergo various physiological and biochemical changes, such as an increase in soluble substances and the breakdown of organic acids. These transformations significantly impact the fruit’s texture modifications. In this study, the pre-harvest SA and AN treatment exhibited significantly higher levels of hardness, chewiness, and brittleness compared to the CK and N treatments during the later stages of storage. Both the A and AN treatment were found to enhance the texture of cherry fruits during later storage. However, the cohesiveness of cherry fruits treated with A and AN showed a similar trend, with no notable distinction between the two. These results suggest that the SA treatment effectively preserves the texture parameters of cherries prior to harvest. Similar findings have been observed in grapes, where measures of pulp hardness, elasticity, cohesiveness, chewiness, and resilience are used to assess grape pulp texture [48].
Fruits and vegetables naturally contain a variety of enzymes such as SOD, PPO, POD, APX, as well as non-enzymatic antioxidant systems including total phenols. Both NO and SA have been found to enhance the activity of antioxidant enzymes and substances, while mitigating oxidative damage to cells. SOD serves as a primary defense mechanism in cells against highly harmful superoxide [49]. Pre-harvest spraying of salicylic acid increased SOD activity in cherries and delayed the accumulation of MDA during storage. PPO plays a crucial role in the discoloration of fruits and vegetables affected by cold damage. In the early stages of storage, there was a notable increase in PPO activity in the CK, N, and AN group, which could be attributed to chilling injury (CI). However, the application of SA treatment effectively suppressed the rise in PPO activity throughout the entire storage period. APX enzyme catalyzes the reaction between ascorbic acid and H2O2 to produce H2O, thereby eliminating H2O2 generated under plant stress and reducing its damaging effects [50]. This research revealed that SA sustained comparatively higher levels of APX activity compared to the other treatment groups. This allowed SA to convert H2O2 into H2O more efficiently, minimizing its detrimental impact. Research has shown that SA and composite treatment groups effectively delayed the decrease in antioxidant enzyme (SOD and APX) activity during cherry fruit storage and effectively inhibited the increase in PPO and POD enzyme activities. Through the analysis of principal component comprehensive score, it is found that the effect of the SA single treatment was found to be significant when compared to the other three treatment groups.
4.2. Effects of Salicylic Acid and Sodium Nitroprusside Spray on Pathogen Species and Inhibition In Vitro
The significant issue currently faced in the post-harvest storage of fruits and vegetables is the substantial loss of fruit due to microbial infection. If disease-causing microorganisms are not effectively controlled during the growth period of the Guizhou Manaohong Cherry industry, it could negatively impact the industry’s future progress. Anthracnose is a prevalent and significant plant infection [51]. Dormant anthracnose infections become active only during storage or when fruits are displayed on market shelves, resulting in substantial financial losses and compromising product quality. This study identified Alternaria alternata and Colletotrichum godetiae as the main pathogens affecting Manaohong Cherries. However, Alternaria alternata was not found during the initial stages of cherry fruit development but was instead detected and isolated during the veraison and maturity stages. This occurrence could be attributed to fruit ripeness and weather conditions. As fruit maturity increased, both Colletotrichum and Alternaria were isolated from the surface of each treated cherry fruit. This suggests that pre-harvest treatments with SA and SNP could delay the occurrence of pathogenic fungi but had limited effects on latent pathogens, subsequently affecting fruit quality. Previous studies have identified Alternaria alternata and Colletotrichum godetiae as the main pathogens responsible for sweet cherry leaf spot in Beijing [52], which aligns with the species of pathogens isolated from Manaohong Cherry fruits in this study.
To verify the direct inhibitory effects of pre-harvest spraying with SA and SNP on the two strains, we conducted in vitro verification experiments. The results revealed that neither SA nor SNP had significant inhibitory effects on the mycelial growth of Colletotrichum godetiae or Alternaria alternata. However, considering the analysis of physiological indices, pathogen isolation and identification, and the in vitro inhibitory effect of SA and SNP treatment on the pathogenic fungi, As shown in Figure 9, it is speculated that spraying a certain concentration of SA and SNP solution before harvest can help maintain fruit quality during storage. It is important to note that although this treatment did not suppress the growth of pathogenic fungi in vitro, it induced a defense response in the fruit. Therefore, it may not effectively reduce the occurrence of fungal infections caused by pathogenic organisms. Previous research has shown that SA treatment reduces anthracnose incidence, enhances mango fruit resistance to pathogens, and inhibits the growth of mango anthracnose mycelium [53]. The findings of our research differ from this conclusion, which could be attributed to various external factors prompting different hosts to combat specific diseases at varying optimal levels. Additionally, the interaction pattern between external substances, hosts, and pathogens may also contribute to these contrasting results.
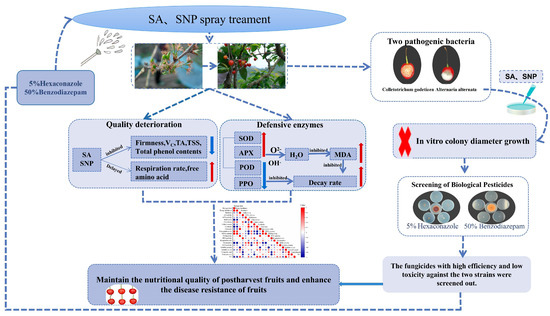
Figure 9.
Illustrates the mechanism model of exogenous salicylic acid (SA) and sodium nitroprusside (SNP) in delaying post-harvest decay of Manaohong Cherry.
4.3. Fungicide Sensitivity of Isolates
The increasing public concern for environmental preservation and the pursuit of sustainable and eco-friendly practices have made the utilization of biological pesticides for managing plant diseases and pests inevitable. In various regions of Guizhou Province, research has been conducted to isolate and identify decayed sweet cherry fruits, which has revealed Colletotrichum godetiae as the fungus responsible for sweet cherry anthracnose. Furthermore, inhibitory effects on anthracnose have been observed with difenoconazole, triclosan, and tebuconazole [54]. The pathogenic fungus identified in this study aligns with these findings, despite the different sweet cherry varieties examined. The aim of this study was to establish a scientific foundation for the prevention and control of post-harvest diseases in Manaohong Cherry. For our indoor toxicity experiments, we selected 12 pesticides that showed inhibitory effects on the growth of Colletotrichum and Alternaria. Based on the EC50 values obtained, it is recommended to prioritize the use of biological pesticides such as 5% hexaconazole and 50% benomyl for effective control in actual production.
5. Conclusions
In summary, our findings indicate that the application of 1 mmol·L−1 SA and the combined treatment effectively maintained the appearance quality of the fruit, slowing down the occurrence of softening and decay. However, SA treatment overall showed greater effectiveness. By applying SA before harvest, it is possible to prevent nutrient depletion of Vc, total phenols, soluble solids, and titratable acids throughout the entire storage process. In addition, SA treatment significantly delayed the decrease in SOD and APX enzyme activities during cherry fruit storage, while also inhibiting the increase in PPO and POD enzyme activities, thereby maintaining good storage quality. This was achieved by enhancing the content of antioxidant substances and the activity of antioxidant enzymes in the fruit. In addition, correlation analysis further confirms that SA treatment can effectively inhibit decay, MDA, and respiratory rate, thereby maintaining fruit quality during storage. In this research, Alternaria alternata and Colletotrichum godetiae were identified as the primary pathogens affecting Manaohong Cherry fruits. It was observed that the in vitro growth of these pathogens was not suppressed by SA and SNP treatments. However, these treatments effectively preserved post-harvest storage quality. To address this issue further, indoor toxicity experiments revealed that 5% hexaconazole and 50% benomyl exhibited the highest toxicity against these two fungi. This study provides a sound scientific basis for reducing and precisely applying pesticides in the chemical control of post-harvest decay caused by local Alternaria alternata and Colletotrichum godetiae in Manaohong Cherry. Moreover, it offers valuable insights into the combined application of SA, SNP, and biological pesticides to enhance the biological control potential for post-harvest fruit diseases.
Author Contributions
N.Z.: investigation, material collection, data curation, formal analysis, software, experimental operation, methodology, and writing—original draft. N.J.: Funding acquisition, supervision, project administration, and writing—review and editing. R.L.: methodology, software, and experimental operation. R.W.: methodology and software. C.C.: methodology and software. H.N.: experimental operation. C.M. and J.L.: methodology and software. Q.T.: investigation, material cultivation and management, and material collection. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Guiyang Science and Technology Talent Plan Project (ZhuKeHeTong[2023]48-20), the Guiyang Science and Technology Plan Project (ZhuKeHeTong[2021]3-21), the Guizhou Provincial Science and Technology Plan Project (QianKeZhongYinDi[2020]4018), the Guizhou Province College Student Innovation and Entrepreneurship Training Program Project (S202210976002), and this achievement is also supported by the Academic Seedling Cultivation and Free Exploration Innovation Special Project of the Guizhou Provincial Department of Science and Technology.
Data Availability Statement
The data used to support the findings of this study can be madeavailable by the corresponding author upon request.
Conflicts of Interest
The all authors declare no conflict of interest. Qiuyun Tao is from Company Guizhou Colorful Rural Agriculture Development Co., Ltd., Company Guizhou Colorful Rural Agriculture Development Co., Ltd. had no role in the design of the study; in the collection, analyses, or interpretation of date; in the writing of manuscript, and in the decision to publish the results.
References
- Berta, G.B.; Silva, A.P.; Moutinho-Pereira, J.; Bacelar, E.; Rosa, E.; Meyer, A.S. Effect of ripeness and postharvest storage on the evolution of colour and anthocyanins in cherries (Prunus avium L.). Food Chem. 2007, 103, 976–984. [Google Scholar]
- Habib, M.; Bhat, M.; Dar, B.; Wani, A. Sweet cherries from farm to table: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Song, C.M.; Wen, X.P. Exploring the original causes of cherry cultivar Manaohong originated from Nayong County, Guizhou Province using ISSR markers. Beijing Linye Daxue Xuebao/J. Beijing For. Univ. 2011, 33, 94–97. [Google Scholar]
- Zhang, Q.; Shi, W.; Zhou, B.; Du, H.; Xi, L.; Zou, M.; Zou, H.; Xin, L.; Gao, Z.; Chen, Y. Variable characteristics of microbial communities on the surface of sweet cherries under different storage conditions. Postharvest Biol. Technol. 2021, 173, 111408. [Google Scholar]
- El Hamss, H.; Kajad, N.; Belabess, Z.; Lahlali, R. Enhancing bioefficacy of Bacillus amyloliquefaciens SF14 with salicylic acid for the control of the postharvest citrus green mould. Plant Stress 2023, 7, 100144. [Google Scholar] [CrossRef]
- Asghari, M.; Aghdam, M.S. Impact of salicylic acid on post-harvest physiology of horticultural crops. Trends Food Sci. Technol. 2010, 21, 502–509. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Deng, M.; Gui, R.; Liu, Y.; Chen, X.; Lin, Y.; Li, M.; Wang, Y.; He, W. Blue light combined with salicylic acid treatment maintained the postharvest quality of strawberry fruit during refrigerated storage. Food Chem. X 2022, 15, 100384. [Google Scholar] [CrossRef]
- Nia, A.E.; Taghipour, S.; Siahmansour, S. Effects of salicylic acid preharvest and Aloe vera gel postharvest treatments on quality maintenance of table grapes during storage. S. Afr. J. Bot. 2022, 147, 1136–1145. [Google Scholar]
- Luo, M.; Ge, W.; Sun, H.; Yang, Q.; Sun, Y.; Zhou, X.; Zhou, Q.; Ji, S. Salicylic acid treatment alleviates diminished ester production in cold-stored ‘Nanguo’ pear by promoting the transcription of PuAAT. Postharvest Biol. Technol. 2022, 187, 111849. [Google Scholar] [CrossRef]
- Giménez, M.J.; Valverde, J.M.; Valero, D.; Guillén, F.; Martínez-Romero, D.; Serrano, M.; Castillo, S. Quality and antioxidant properties on sweet cherries as affected by preharvest salicylic and acetylsalicylic acids treatments. Food Chem. 2014, 160, 226–232. [Google Scholar] [CrossRef]
- Cao, J.; Yan, J.; Zhao, Y.; Jiang, W. Effects of postharvest salicylic acid dipping on Alternaria rot and disease resistance of jujube fruit during storage. J. Sci. Food Agric. 2013, 93, 3252–3258. [Google Scholar] [CrossRef]
- Romanazzi, G.; Sanzani, S.M.; Bi, Y.; Tian, S.; Gutiérrez Martínez, P.; Alkan, N. Induced resistance to control postharvest decay of fruit and vegetables. Postharvest Biol. Technol. 2016, 122, 82–94. [Google Scholar] [CrossRef]
- Rabiei, V.; Kakavand, F.; Zaare-Nahandi, F.; Razavi, F.; Aghdam, M.S. Nitric oxide and γ-aminobutyric acid treatments delay senescence of cornelian cherry fruits during postharvest cold storage by enhancing antioxidant system activity. Sci. Hortic. 2019, 243, 268–273. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, W.; Hu, M.; Pan, Y.; Jiang, Y.; Zhang, Z.; Jiang, G. Nitric oxide is involved in melatonin-induced cold tolerance in postharvest litchi fruit. Postharvest Biol. Technol. 2023, 196, 112157. [Google Scholar] [CrossRef]
- Wang, B.; Jiang, H.; Bi, Y.; He, X.; Wang, Y.; Li, Y.; Zheng, X.; Prusky, D. Preharvest multiple sprays with sodium nitroprusside promote wound healing of harvested muskmelons by activation of phenylpropanoid metabolism. Postharvest Biol. Technol. 2019, 158, 110988. [Google Scholar] [CrossRef]
- Lahlali, R.; Aksissou, W.; Lyousfi, N.; Ezrari, S.; Blenzar, A.; Tahiri, A.; Amiri, S. Biocontrol activity and putative mechanism of Bacillus amyloliquefaciens (SF14 and SP10), Alcaligenes faecalis ACBC1, and Pantoea agglomerans ACBP1 against brown rot disease of fruit. Microb. Pathog. 2020, 139, 103914. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Munster, M.; Johnson, C.; Louws, F.J. First Report of Anthracnose Caused by Colletotrichum fragariae on Cyclamen in North Carolina. Plant Dis. 2011, 95, 1480. [Google Scholar] [CrossRef] [PubMed]
- Massi, F.; Torriani, S.F.F.; Borghi, L.; Toffolatti, S.L. Fungicide Resistance Evolution and Detection in Plant Pathogens: Plasmopara viticola as a Case Study. Microorganisms 2021, 9, 119. [Google Scholar] [CrossRef]
- Amin, M.; Malik, A.U.; Sattar, A.; And, K.; Javed, N. Potential of fungicides and plant activator for postharvest disease management in mangoes. Int. J. Agric. Biol. 2011, 13, 671–676. [Google Scholar]
- Majumdar, N.; Mandal, N.C.; Nath, R. Management of quiescent pathogens rots of mango with preharvest spraying of true fungicides and salicylic acid. Int. J. Chem. Stud. 2020, 8, 2809–2813. [Google Scholar] [CrossRef]
- Edwards, S.G.; Seddon, B. Mode of antagonism of Brevibacillus brevis against Botrytis cinerea in vitro. J. Appl. Microbiol. 2001, 4, 91. [Google Scholar] [CrossRef]
- Han, C.; Zuo, J.; Wang, Q.; Xu, L.; Wang, Z.; Dong, H.; Gao, L. Effects of 1-MCP on postharvest physiology and quality of bitter melon (Momordica charantia L.). Sci. Hortic. 2015, 182, 86–91. [Google Scholar] [CrossRef]
- Bhan, C.; Asrey, R.; Meena, N.K.; Rudra, S.G.; Chawla, G.; Kumar, R.; Kumar, R. Guar gum and chitosan-based composite edible coating extends the shelf life and preserves the bioactive compounds in stored Kinnow fruits. Int. J. Biol. Macromol. 2022, 222 Pt B, 2922–2935. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.; Li, J.; Gu, X.; Zhao, L.; Li, B.; Wang, K.; Yang, Q.; Zhang, H. Pichia caribbica improves disease resistance of cherry tomatoes by regulating ROS metabolism. Biol. Control 2022, 169, 104870. [Google Scholar] [CrossRef]
- Giacosa, S.; Torchio, F.; Segade, S.R.; Giust, M.; Tomasi, D.; Gerbi, V.; Rolle, L. Selection of a mechanical property for the flesh firmness of table grapes in accordance with an OIV ampelographic descriptor. Am. J. Enol. Vitic. 2014, 65, 206–214. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, B.; Zhang, W.; Cao, J.; Jiang, W. Enhancement of quality and antioxidant metabolism of sweet cherry fruit by near-freezing temperature storage. Postharvest Biol. Technol. 2019, 147, 113–122. [Google Scholar] [CrossRef]
- Hussain, T.; Kalhoro, D.H.; Yin, Y.L. Identification of nutritional composition and antioxidant activities of fruit peels as a potential source of nutraceuticals. Front. Nutr. 2023, 9, 1065698. [Google Scholar] [CrossRef]
- Kaulmann, A.; Jonville, M.C.; Schneider, Y.J.; Hoffmann, L.; Bohn, T. Carotenoids, polyphenols and micronutrient profiles of Brassica oleraceae and plum varieties and their contribution to measures of total antioxidant capacity. Food Chem. 2014, 155, 240–250. [Google Scholar] [CrossRef]
- Khan, M.M.R.; Rahman, M.M.; Islam, M.S.; Begum, S.A. A Simple UV-spectrophotometric Method for the Determination of Vitamin C Content in Various Fruits and Vegetables at Sylhet Area in Bangladesh. J. Biol. Sci. 2006, 6, 2238. [Google Scholar] [CrossRef]
- Saba, M.K.; Arzani, K.; Barzegar, M. Postharvest Polyamine Application Alleviates Chilling Injury and Affects Apricot Storage Ability. Food Chem. 2012, 60, 8947–8953. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.F.; Hu, Y.H.; Lin, H.T.; Liu, X.; Chen, Y.H.; Zhang, S.; Chen, Q.X. Inhibitory Effects of Propyl Gallate on Tyrosinase and Its Application in Controlling Pericarp Browning of Harvested Longan Fruits. J. Agric. Food Chem. 2013, 61, 2889–2895. [Google Scholar] [CrossRef]
- Pan, L.; Chen, X.; Xu, W.; Fan, S.; Wan, T.; Zhang, J.; Cai, Y. Methyl jasmonate induces postharvest disease resistance to decay caused by Alternaria alternata in sweet cherry fruit. Sci. Hortic. 2022, 292, 110624. [Google Scholar] [CrossRef]
- Zhao, J.J.; Wang, B.; Cui, K.; Cao, J.; Jiang, W. Improving postharvest quality and antioxidant capacity of sweet cherry fruit by storage at near-freezing temperature. Sci. Hortic. 2019, 246, 68–78. [Google Scholar] [CrossRef]
- Bai, J.; Plotto, A.; Spotts, R.; Rattanapanone, N. Ethanol vapor and saprophytic yeast treatments reduce decay and maintain quality of intact and fresh-cut sweet cherries. Postharvest Biol. Technol. 2011, 62, 204–212. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Cui, Q.-L.; Wang, Y.; Shi, F.; Fan, H.; Zhang, Y.-Q.; Lai, S.-T.; Li, Z.-H.; Li, L.; Sun, Y.-K. Effect of Edible Carboxymethyl Chitosan-Gelatin Based Coating on the Quality and Nutritional Properties of Different Sweet Cherry Cultivars during Postharvest Storage. Coatings 2021, 11, 396. [Google Scholar] [CrossRef]
- Brizzolara, S.; Manganaris, G.A.; Fotopoulos, V.; Watkins, C.B.; Tonutti, P. Primary metabolism in fresh fruits during storage. Front. Plant Sci. 2020, 11, 80. [Google Scholar] [CrossRef]
- Li, Y.; Song, W.; Gao, H.; Li, L. Properties of antimicrobial polylactic acid-based film and its effect on cherry quality preservation. Shipin Kexue/Food Sci. 2020, 41, 216–222. [Google Scholar]
- Bianchi, T.; Guerrero, L.; Gratacos-Cubarsi, M.; Claret, A.; Argyris, J.; Garcia-Mas, J.; Hortos, M. Textural properties of different melon (Cucumis melo L.) fruit types: Sensory and physical-chemical evaluation. Sci. Hortic. 2016, 201, 201. [Google Scholar] [CrossRef]
- Zhang, J.; Nie, J.Y.; Jing, L.I.; Zhang, H.; Wang, J. Evaluation of sugar and organic acid composition and their levels in highbush blueberries from two regions of China. J. Integr. Agric. 2020, 19, 2352–2361. [Google Scholar] [CrossRef]
- Mandrioli, R.; Mercolini, L.; Raggi, M.A. Recent trends in the analysis of amino acids in fruits and derived foodstuffs. Anal. Bioanal. Chem. 2013, 405, 7941–7956. [Google Scholar] [CrossRef]
- Mendes-Oliveira, G.; Gu, G.; Luo, Y.; Zografos, A.; Minas, I.; Nou, X. Edible and water-soluble corn zein coating impregnated with nisin for Listeria monocytogenes reduction on nectarines and apples. Postharvest Biol. Technol. 2022, 185, 111811. [Google Scholar] [CrossRef]
- Kerch, G.; Sabovics, M.; Kruma, Z.; Kampuse, S.; Straumite, E. Effect of chitosan and chitooligosaccharide on vitamin C and polyphenols contents in cherries and strawberries during refrigerated storage. Eur. Food Res. Technol. 2011, 233, 351–358. [Google Scholar] [CrossRef]
- Li, H.H.; Tang, W.; Liu, K.; Zhang, L.; Tang, X.F.; Miao, M.; Liu, Y.S. First Report of Fusarium fujikuroi Causing Brown Leaf Spot on Kiwifruit. Plant Dis. 2020, 104, 1560–1561. [Google Scholar] [CrossRef]
- Yao, H.; Tian, S. Effects of pre- and post-harvest application of salicylic acid or methyl jasmonate on inducing disease resistance of sweet cherry fruit in storage. Postharvest Biol. Technol. 2005, 35, 253–262. [Google Scholar] [CrossRef]
- Li, X.; Peng, S.; Yu, R.; Li, P.; Zhou, C.; Qu, Y.; Li, H.; Luo, H.; Yu, L. Co-Application of 1-MCP and Laser Microporous Plastic Bag Packaging Maintains Postharvest Quality and Extends the Shelf-Life of Honey Peach Fruit. Foods 2022, 11, 1733. [Google Scholar] [CrossRef]
- Yang, W.; Kang, J.; Liu, Y.; Guo, M.; Chen, G. Effect of salicylic acid treatment on antioxidant capacity and endogenous hormones in winter jujube during shelf life. Food Chem. 2022, 397, 133788. [Google Scholar] [CrossRef]
- Brozdowski, J.; Waliszewska, B.; Loffler, J.; Hudina, M.; Veberic, R.; Mikulic-Petkovsek, M. Composition of Phenolic Compounds, Cyanogenic Glycosides, Organic Acids and Sugars in Fruits of Black Cherry (Prunus serotina Ehrh.). Forests 2021, 12, 762. [Google Scholar] [CrossRef]
- Rolle, L.; Giacosa, S.; Gerbi, V.; Novello, V. Comparative Study of Texture Properties, Color Characteristics, and Chemical Composition of Ten White Table-Grape Varieties. Am. J. Enol. Vitic. 2011, 62, 49–56. [Google Scholar] [CrossRef]
- Mondal, K.; Sharma, N.S.; Malhotra, S.P.; Dhawan, K.; Singh, R. Antioxidant Systems in Ripening Tomato Fruits. Biol. Plant. 2004, 48, 49–53. [Google Scholar] [CrossRef]
- Chen, H.; Ling, J.; Wu, F.; Zhang, L.; Sun, Z.; Yang, H. Effect of hypobaric storage on flesh lignification, active oxygen metabolism and related enzyme activities in bamboo shoots. LWT-Food Sci. Technol. 2013, 51, 190–195. [Google Scholar] [CrossRef]
- Dean, R.; Kan, J.A.L.V.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Pietro, A.D.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Thomidis, T.; Tsipouridis, C. First Report of Alternaria Leaf Spot on Cherry Trees in Greece. Plant Dis. 2006, 90, 680. [Google Scholar] [CrossRef] [PubMed]
- Junyu, H.E.; Ren, Y.; Chen, C.; Liu, J.; Liu, H. Defense Responses of Salicylic Acid in Mango Fruit Against Postharvest Anthracnose, Caused by Colletotrichum gloeosporioides and its Possible Mechanism. J. Food Saf. 2016, 37, e12294. [Google Scholar]
- Peng, K.Q.; Pan, Y.T.; Tan, T.J.; Zeng, X.Y.; Lin, M.L.; Jiang, S.; Zhao, Z.B.; Tian, F.H.; Zhao, X.S. Characterization and fungicide sensitivity of Colletotrichum godetiae causing sweet cherry fruit anthracnose in Guizhou, China. Front. Microbiol. 2022, 13, 923181. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).