Woody Biochar Rate and Water Shortage Impact on Early Growth Stages of Chenopodium quinoa Willd.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Setup
2.2. Phenology, Water Relations and Plant Development Measurement
2.3. Statistical Analysis
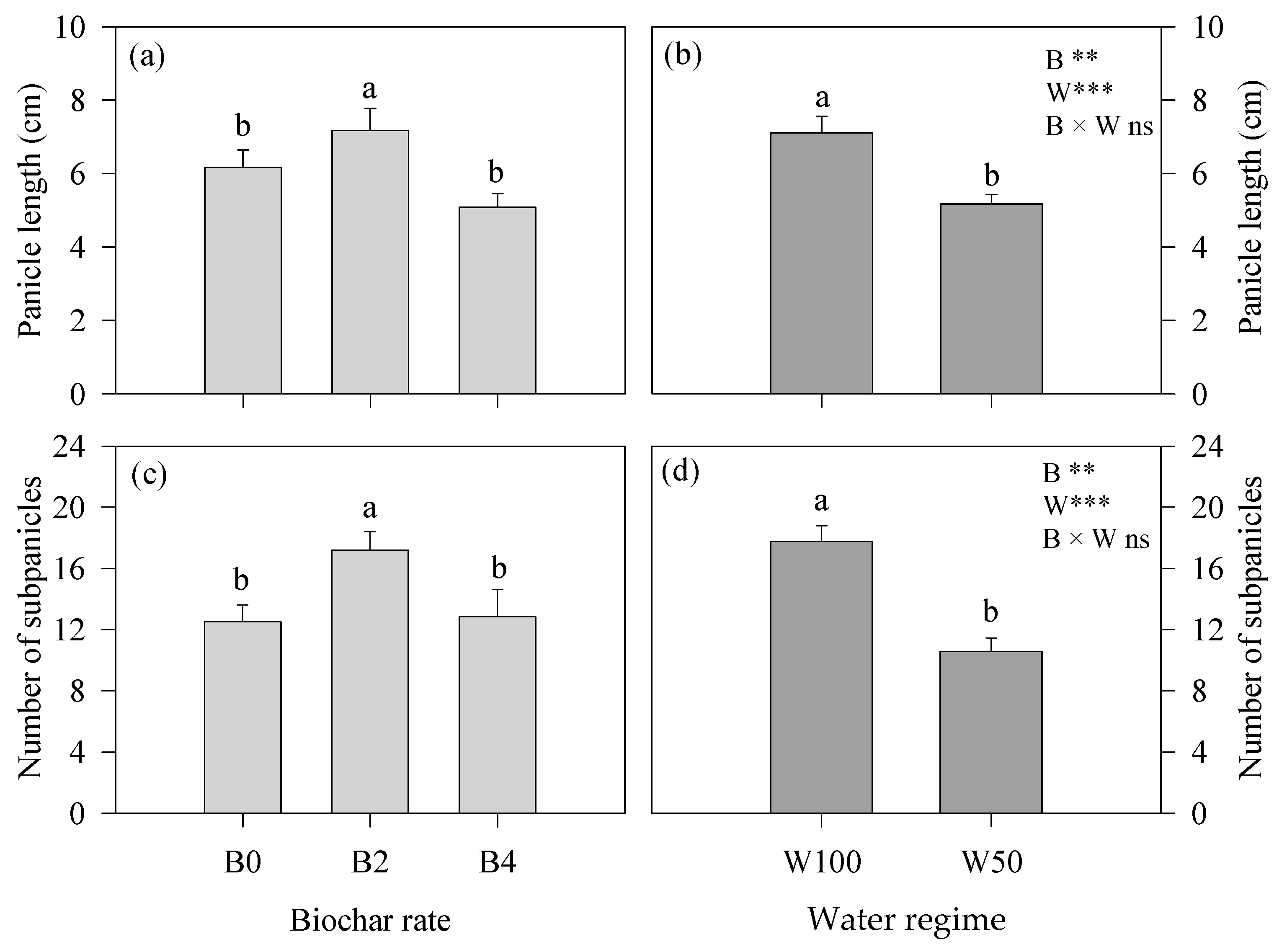
3. Results
3.1. Plant Phenology and Development
3.2. Water- and Growth-Related Parameters and Yield-Contributing Traits at the End of the Experiment
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masson-Delmotte, V. Global Warming of 1.5 °C: An IPCC Special Report on Impacts of Global Warming of 1.5 °C above Pre-industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Contex of Strengthening the Global Response to the Thereat of Blimate Change, Sustainable Development, and Efforts to Eradicate Poverty. 2018. Available online: https://www.ipcc.ch/sr15/ (accessed on 1 October 2023).
- Heydari, M.; Hajinia, S.; Jafarian, N.; Karamian, M.; Mosa, Z.; Asgharzadeh, S.; Rezaei, N.; Guidi, L.; Valkó, O.; Prévosto, B. Synergistic use of biochar and the plant growth-promoting rhizobacteria in mitigating drought stress on oak (Quercus Brantii Lindl.) seedlings. For. Ecol. Manag. 2023, 531, 120793. [Google Scholar] [CrossRef]
- Hamidzadeh, Z.; Ghorbannezhad, P.; Ketabchi, M.R.; Yeganeh, B. Biomass-derived biochar and its application in agriculture. Fuel 2023, 341, 127701. [Google Scholar] [CrossRef]
- Ajayi, A.; Holthusen, D.; Horn, R. Changes in microstructural behaviour and hydraulic functions of biochar amended soils. Soil Tillage Res. 2016, 155, 166–175. [Google Scholar] [CrossRef]
- Kapoor, A.; Sharma, R.; Kumar, A.; Sepehya, S. Biochar as a means to improve soil fertility and crop productivity: A review. J. Plant Nutr. 2022, 45, 2380–2388. [Google Scholar] [CrossRef]
- Verheijen, F.G.; Montanarella, L.; Bastos, A.C. Sustainability, certification, and regulation of biochar. Pesqui. Agropecuária Bras. 2012, 47, 649–653. [Google Scholar] [CrossRef]
- Laird, D.A.; Fleming, P.; Davis, D.D.; Horton, R.; Wang, B.; Karlen, D.L. Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma 2010, 158, 443–449. [Google Scholar] [CrossRef]
- Libutti, A.; Cammerino, A.R.B.; Francavilla, M.; Monteleone, M. Soil amendment with biochar affects water drainage and nutrient losses by leaching: Experimental evidence under field-grown conditions. Agronomy 2019, 9, 758. [Google Scholar] [CrossRef]
- Libutti, A.; Francavilla, M.; Monteleone, M. Hydrological properties of a clay loam soil as affected by biochar application in a pot experiment. Agronomy 2021, 11, 489. [Google Scholar] [CrossRef]
- Ding, X.; Li, G.; Zhao, X.; Lin, Q.; Wang, X. Biochar application significantly increases soil organic carbon under conservation tillage: An 11-year field experiment. Biochar 2023, 5, 28. [Google Scholar] [CrossRef]
- Aborisade, M.A.; Geng, H.; Oba, B.T.; Kumar, A.; Ndudi, E.A.; Battamo, A.Y.; Liu, J.; Chen, D.; Okimiji, O.P.; Ojekunle, O.Z.; et al. Remediation of soil polluted with Pb and Cd and alleviation of oxidative stress in Brassica rapa plant using nanoscale zerovalent iron supported with coconut-husk biochar. J. Plant Physiol. 2023, 287, 154023. [Google Scholar] [CrossRef]
- Anand, A.; Kumar, V.; Kaushal, P. Biochar and its twin benefits: Crop residue management and climate change mitigation in India. Renew. Sustain. Energy Rev. 2022, 156, 111959. [Google Scholar] [CrossRef]
- Manikandan, S.; Vickram, S.; Subbaiya, R.; Karmegam, N.; Chang, S.W.; Ravindran, B.; Awasthi, M.K. Comprehensive review on recent production trends and applications of biochar for greener environment. Bioresour. Technol. 2023, 388, 129725. [Google Scholar] [CrossRef] [PubMed]
- EL Moussaoui, H.; Ainlhout, L.F.Z.; Bouqbis, L. Effect of biochar, biocompost and manure on the growth and productivity of alfalfa (Medicago sativa L.): Field and pots study. Int. J. Recycl. Org. Waste Agric. 2023, 12, 425–440. [Google Scholar] [CrossRef]
- Lebrun, M.; Miard, F.; Drouet, S.; Tungmunnithum, D.; Morabito, D.; Hano, C.; Bourgerie, S. Physiological and molecular responses of flax (Linum usitatissimum L.) cultivars under a multicontaminated technosol amended with biochar. Environ. Sci. Pollut. Res. 2021, 28, 53728–53745. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Liu, S.; Ye, S.; Yang, H.; Song, B.; Qin, F.; Shen, M.; Tan, C.; Zeng, G.; Tan, X. Potential hazards of biochar: The negative environmental impacts of biochar applications. J. Hazard. Mater. 2021, 420, 126611. [Google Scholar] [CrossRef] [PubMed]
- Gale, N.V.; Halim, M.A.; Horsburgh, M.; Thomas, S.C. Comparative responses of early-successional plants to charcoal soil amendments. Ecosphere 2017, 8, e01933. [Google Scholar] [CrossRef]
- Khan, Z.; Khan, M.N.; Zhang, K.; Luo, T.; Zhu, K.; Hu, L. The application of biochar alleviated the adverse effects of drought on the growth, physiology, yield and quality of rapeseed through regulation of soil status and nutrients availability. Ind. Crops Prod. 2021, 171, 113878. [Google Scholar] [CrossRef]
- Lai, W.Y.; Lai, C.M.; Ke, G.R.; Chung, R.S.; Chen, C.T.; Cheng, C.H.; Pai, C.W.; Chen, S.Y.; Chen, C.C. The effects of woodchip biochar application on crop yield, carbon sequestration and greenhouse gas emissions from soils planted with rice or leaf beet. J. Taiwan Inst. Chem. Eng. 2013, 44, 1039–1044. [Google Scholar] [CrossRef]
- Laird, D.; Novak, J.; Collins, H.; Ippolito, J.; Karlen, D.; Lentz, R.; Sistani, K.; Spokas, K.; Van Pelt, R. Multi-year and multi-location soil quality and crop biomass yield responses to hardwood fast pyrolysis biochar. Geoderma 2017, 289, 46–53. [Google Scholar] [CrossRef]
- Gholizadeh, M.; Hu, X.; Liu, Q. A mini review of the specialties of the bio-oils produced from pyrolysis of 20 different biomasses. Renew. Sustain. Energy Rev. 2019, 114, 109313. [Google Scholar] [CrossRef]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An overview on engineering the surface area and porosity of biochar. Sci. Total Environ. 2021, 763, 144204. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, X.; Mao, M.; Wang, X.; Shang, J. Carbon content determines the aggregation of biochar colloids from various feedstocks. Sci. Total Environ. 2023, 880, 163313. [Google Scholar] [CrossRef] [PubMed]
- Greco, G.; González, B.; Manyà, J.J. Operating conditions affecting char yield and its potential stability during slow pyrolysis of biomass: A review. Adv. Carbon Mater. Biomass Overv. Zenodo 2019, 9–19. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, K.; Chen, B. Linking hydrophobicity of biochar to the water repellency and water holding capacity of biochar-amended soil. Environ. Pollut. 2019, 253, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Romdhane, L.; Awad, Y.M.; Radhouane, L.; Dal Cortivo, C.; Barion, G.; Panozzo, A.; Vamerali, T. Wood biochar produces different rates of root growth and transpiration in two maize hybrids (Zea mays L.) under drought stress. Arch. Agron. Soil Sci. 2019, 65, 846–866. [Google Scholar] [CrossRef]
- Kammann, C.I.; Schmidt, H.-P.; Messerschmidt, N.; Linsel, S.; Steffens, D.; Müller, C.; Koyro, H.-W.; Conte, P.; Joseph, S. Plant growth improvement mediated by nitrate capture in co-composted biochar. Sci. Rep. 2015, 5, 11080. [Google Scholar] [CrossRef] [PubMed]
- Akram, Z.; Maqsood Ahmed Basra, S.; Bilal Hafeez, M.; Khan, S.; Nazeer, S.; Iqbal, S.; Sohail Saddiq, M.; Zahra, N. Adaptability and yield potential of new quinoa lines under agro-ecological conditions of Faisalabad-Pakistan. Asian J. Agric. Biol. 2021, 2, 1–8. [Google Scholar] [CrossRef]
- Angeli, V.; Miguel Silva, P.; Crispim Massuela, D.; Khan, M.W.; Hamar, A.; Khajehei, F.; Graeff-Hönninger, S.; Piatti, C. Quinoa (Chenopodium quinoa Willd.): An overview of the potentials of the “golden grain” and socio-economic and environmental aspects of its cultivation and marketization. Foods 2020, 9, 216. [Google Scholar] [CrossRef]
- Jensen, C.; Jacobsen, S.-E.; Andersen, M.; Nunez, N.; Andersen, S.; Rasmussen, L.; Mogensen, V. Leaf gas exchange and water relation characteristics of field quinoa (Chenopodium quinoa Willd.) during soil drying. Eur. J. Agron. 2000, 13, 11–25. [Google Scholar] [CrossRef]
- Kumari, M.; Zinta, G.; Chauhan, R.; Kumar, A.; Singh, S.; Singh, S. Genetic resources and breeding approaches for improvement of amaranth (Amaranthus spp.) and quinoa (Chenopodium quinoa). Front. Nutr. 2023, 10, 1129723. [Google Scholar]
- Razzaghi, F.; Bahadori-Ghasroldashti, M.R.; Henriksen, S.; Sepaskhah, A.R.; Jacobsen, S.E. Physiological characteristics and irrigation water productivity of quinoa (Chenopodium quinoa Willd.) in response to deficit irrigation imposed at different growing stages—A field study from Southern Iran. J. Agron. Crop Sci. 2020, 206, 390–404. [Google Scholar] [CrossRef]
- Fghire, R.; Anaya, F.; Issa, O.A.; Wahbi, S. Physiological and growth response traits to water deficit as indicators of tolerance criteria between quinoa genotypes. J. Mater. Environ. Sci. 2017, 8, 2084–2093. [Google Scholar]
- Nanduri, K.R.; Hirich, A.; Salehi, M.; Saadat, S.; Jacobsen, S.E. Quinoa: A new crop for harsh environments. Sabkha Ecosyst. Vol. VI Asia/Pac. 2019, 49, 301–333. [Google Scholar]
- Issa Ali, O.; Fghire, R.; Anaya, F.; Benlhabib, O.; Wahbi, S. Physiological and Morphological Responses of two Quinoa Cultivars (Chenopodium quinoa Willd.) to Drought Stress. Gesunde Pflanz. 2019, 71, 123–133. [Google Scholar] [CrossRef]
- Hafeez, M.B.; Iqbal, S.; Li, Y.; Saddiq, M.S.; Basra, S.M.; Zhang, H.; Zahra, N.; Akram, M.Z.; Bertero, D.; Curti, R.N. Assessment of phenotypic diversity in the USDA collection of quinoa links genotypic adaptation to germplasm origin. Plants 2022, 11, 738. [Google Scholar] [CrossRef]
- Kammann, C.I.; Linsel, S.; Gößling, J.W.; Koyro, H.-W. Influence of biochar on drought tolerance of Chenopodium quinoa Willd and on soil–plant relations. Plant Soil 2011, 345, 195–210. [Google Scholar] [CrossRef]
- Ramzani, P.M.A.; Shan, L.; Anjum, S.; Ronggui, H.; Iqbal, M.; Virk, Z.A.; Kausar, S. Improved quinoa growth, physiological response, and seed nutritional quality in three soils having different stresses by the application of acidified biochar and compost. Plant Physiol. Biochem. 2017, 116, 127–138. [Google Scholar] [CrossRef]
- Walters, H.; Carpenter-Boggs, L.; Desta, K.; Yan, L.; Matanguihan, J.; Murphy, K. Effect of irrigation, intercrop, and cultivar on agronomic and nutritional characteristics of quinoa. Agroecol. Sustain. Food Syst. 2016, 40, 783–803. [Google Scholar] [CrossRef]
- Geerts, S.; Mamani, R.S.; Garcia, M.; Raes, D. Response of quinoa (Chenopodium quinoa Willd.) to differential drought stress in the Bolivian Altiplano: Towards a deficit irrigation strategy within a water scarce region. In Proceedings of the 1st International Symposium on Land and Water Management for Sustainable Irrigated Agriculture, Adana, Turkey, 4–8 April 2006; pp. 4–8. [Google Scholar]
- Akram, M.Z.; Libutti, A.; Rivelli, A.R. Evaluation of Vegetative Development of Quinoa under Water Stress by Applying Different Organic Amendments. Agronomy 2023, 13, 1412. [Google Scholar] [CrossRef]
- Italian Official Gazette. Ministerial Decree 248 (1999): Approval of “Official Methods of Chemical Soil Analysis”. Ordinary Supplement 185 to the Official Journal of the Italian Republic 248, 21 October 1999, Italy. Available online: www.gazzettaufficiale.it/eli/gu/1999/10/21/248/so/185/sg/pdf (accessed on 29 September 2023).
- Rivelli, A.R.; Libutti, A. Effect of biochar and inorganic or organic fertilizer co-application on soil properties, plant growth and nutrient content in Swiss chard. Agronomy 2022, 12, 2089. [Google Scholar] [CrossRef]
- European Biochar Certificat. Comparison of European Biochar Certificate Version 4. 8 and IBI Biochar Standards Version 2.0 European Biochar Certificate First Publication March 2012b. IBI Biochar Stand First Publ May. 2012. Available online: www.european-biochar.org/en/home (accessed on 15 September 2023).
- International Biochar Initiative. Standardized Product Definition and Product Testing Guidelines for Biochar That Is Used in Soil, Version 2.1; IBI Biochar Standards; International Biochar Initiative: Victor, NY, USA, 2012. [Google Scholar]
- Sosa-Zuniga, V.; Brito, V.; Fuentes, F.; Steinfort, U. Phenological growth stages of quinoa (Chenopodium quinoa) based on the BBCH scale. Ann. Appl. Biol. 2017, 171, 117–124. [Google Scholar] [CrossRef]
- Talebnejad, R.; Sepaskhah, A. Effect of different saline groundwater depths and irrigation water salinities on yield and water use of quinoa in lysimeter. Agric. Water Manag. 2015, 148, 177–188. [Google Scholar] [CrossRef]
- Paneque, M.; José, M.; Franco-Navarro, J.D.; Colmenero-Flores, J.M.; Knicker, H. Effect of biochar amendment on morphology, productivity and water relations of sunflower plants under non-irrigation conditions. Catena 2016, 147, 280–287. [Google Scholar] [CrossRef]
- Stanschewski, C.S.; Rey, E.; Fiene, G.; Craine, E.B.; Wellman, G.; Melino, V.J.; SR Patiranage, D.; Johansen, K.; Schmöckel, S.M.; Bertero, D. Quinoa phenotyping methodologies: An international consensus. Plants 2021, 10, 1759. [Google Scholar] [CrossRef] [PubMed]
- Maestro-Gaitán, I.; Granado-Rodríguez, S.; Orús, M.; Matías, J.; Cruz, V.; Bolaños, L.; Reguera, M. Genotype-dependent responses to long-term water stress in Chenopodium quinoa Willd. bioRxiv 2022. [Google Scholar] [CrossRef]
- Geerts, S.; Raes, D.; Garcia, M.; Mendoza, J.; Huanca, R. Crop water use indicators to quantify the flexible phenology of quinoa (Chenopodium quinoa Willd.) in response to drought stress. Field Crops Res. 2008, 108, 150–156. [Google Scholar] [CrossRef]
- Abrecht, D.; Carberry, P. The influence of water deficit prior to tassel initiation on maize growth, development and yield. Field Crops Res. 1993, 31, 55–69. [Google Scholar] [CrossRef]
- Fukai, S. Phenology in rainfed lowland rice. Field Crops Res. 1999, 64, 51–60. [Google Scholar] [CrossRef]
- Haider, G.; Koyro, H.-W.; Azam, F.; Steffens, D.; Müller, C.; Kammann, C. Biochar but not humic acid product amendment affected maize yields via improving plant-soil moisture relations. Plant Soil 2015, 395, 141–157. [Google Scholar] [CrossRef]
- Gavili, E.; Moosavi, A.A.; Haghighi, A.A.K. Does biochar mitigate the adverse effects of drought on the agronomic traits and yield components of soybean? Ind. Crops Prod. 2019, 128, 445–454. [Google Scholar] [CrossRef]
- Ramlow, M.; Foster, E.; Del Grosso, S.; Cotrufo, M. Broadcast woody biochar provides limited benefits to deficit irrigation maize in Colorado. Agric. Ecosyst. Environ. 2019, 269, 71–81. [Google Scholar] [CrossRef]
- Macdonald, L.M.; Farrell, M.; Zwieten, L.V.; Krull, E.S. Plant growth responses to biochar addition: An Australian soils perspective. Biol. Fertil. Soils 2014, 50, 1035–1045. [Google Scholar] [CrossRef]
- Sandhu, S.S.; Kumar, S. Impact of three types of biochar on the hydrological properties of eroded and depositional landscape positions. Soil Sci. Soc. Am. J. 2017, 81, 878–888. [Google Scholar] [CrossRef]
- Gale, N.V.; Thomas, S.C. Dose-dependence of growth and ecophysiological responses of plants to biochar. Sci. Total Environ. 2019, 658, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Rajkovich, S.; Enders, A.; Hanley, K.; Hyland, C.; Zimmerman, A.R.; Lehmann, J. Corn growth and nitrogen nutrition after additions of biochars with varying properties to a temperate soil. Biol. Fertil. Soils 2012, 48, 271–284. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for environmental management: An introduction. In Biochar for Environmental Management: Science and Technology; Routledge: London, UK, 2012; pp. 1–12. [Google Scholar]
- Rees, F.; Simonnot, M.-O.; Morel, J.-L. Short-term effects of biochar on soil heavy metal mobility are controlled by intra-particle diffusion and soil pH increase. Eur. J. Soil Sci. 2014, 65, 149–161. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.; van der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Rees, F.; Germain, C.; Sterckeman, T.; Morel, J.-L. Plant growth and metal uptake by a non-hyperaccumulating species (Lolium perenne) and a Cd-Zn hyperaccumulator (Noccaea caerulescens) in contaminated soils amended with biochar. Plant Soil 2015, 395, 57–73. [Google Scholar] [CrossRef]
- Hafez, Y.; Attia, K.; Alamery, S.; Ghazy, A.; Al-Doss, A.; Ibrahim, E.; Rashwan, E.; El-Maghraby, L.; Awad, A.; Abdelaal, K. Beneficial effects of biochar and chitosan on antioxidative capacity, osmolytes accumulation, and anatomical characters of water-stressed barley plants. Agronomy 2020, 10, 630. [Google Scholar] [CrossRef]
- Saddiq, M.S.; Wang, X.; Iqbal, S.; Hafeez, M.B.; Khan, S.; Raza, A.; Iqbal, J.; Maqbool, M.M.; Fiaz, S.; Qazi, M.A. Effect of water stress on grain yield and physiological characters of quinoa genotypes. Agronomy 2021, 11, 1934. [Google Scholar] [CrossRef]
- Benaffari, W.; Boutasknit, A.; Anli, M.; Ait-El-Mokhtar, M.; Ait-Rahou, Y.; Ben-Laouane, R.; Ben Ahmed, H.; Mitsui, T.; Baslam, M.; Meddich, A. The native arbuscular mycorrhizal fungi and vermicompost-based organic amendments enhance soil fertility, growth performance, and the drought stress tolerance of quinoa. Plants 2022, 11, 393. [Google Scholar] [CrossRef] [PubMed]
- Gholami, S.; Dehaghi, M.A.; Rezazadeh, A.; Naji, A.M. Seed germination and physiological responses of quinoa to selenium priming under drought stress. Bragantia 2022, 81, e0722. [Google Scholar] [CrossRef]
- Yuan, J.H.; Xu, R.K. The amelioration effects of low temperature biochar generated from nine crop residues on an acidic Ultisol. Soil Use Manag. 2011, 27, 110–115. [Google Scholar] [CrossRef]
- Telahigue, D.C.; Yahia, L.B.; Aljane, F.; Belhouchett, K.; Toumi, L. Grain yield, biomass productivity and water use efficiency in quinoa (Chenopodium quinoa Willd.) under drought stress. J. Sci. Agric. 2017, 1, 222–232. [Google Scholar] [CrossRef]
- Bertero, H.D. Quinoa. In Crop Physiology Case Histories for Major Crops; Elsevier: Amsterdam, The Netherlands, 2021; pp. 250–281. [Google Scholar]
- Rojas, W.; Pinto, M. La diversidad genética de quinua de Bolivia. In Proceedings of the Congreso Científico de la Quinua (Memorias), 14–15 June 2013; Instituto Interamericano de Cooperación para la Agricultura (IICA): La Paz, Bolivia, 2013; pp. 77–92. [Google Scholar]
- Sun, Y.; Liu, F.; Bendevis, M.; Shabala, S.; Jacobsen, S.E. Sensitivity of two quinoa (Chenopodium quinoa Willd.) varieties to progressive drought stress. J. Agron. Crop Sci. 2014, 200, 12–23. [Google Scholar] [CrossRef]
- El Moussaoui, H.; Idardare, Z.; Bouqbis, L. Assessing Alfalfa Productivity and Physiological Parameters: Biochar and Biocompost Versus Conventional Fertilizers with Manure and Chemical Fertilizers. Water Air Soil Pollut. 2023, 234, 1–18. [Google Scholar] [CrossRef]
- Gao, Y.; Shao, G.; Lu, J.; Zhang, K.; Wu, S.; Wang, Z. Effects of biochar application on crop water use efficiency depend on experimental conditions: A meta-analysis. Field Crops Res. 2020, 249, 107763. [Google Scholar] [CrossRef]

| Experimenatal Factor | 28 DAS | 32 DAS | 38 DAS | 45 DAS | |||||
|---|---|---|---|---|---|---|---|---|---|
| LA | SPAD Index | LA | SPAD Index | LA | SPAD Index | LA | SPAD Index | ||
| Biochar (B) | |||||||||
| B0 | 106 b | 44.3 | 201 b | 45.8 ab | 269 b | 43.3 ab | 446 b | 44.6 b | |
| B2 | 121 a | 46.2 | 271 a | 47.0 a | 419 a | 44.5 a | 585 a | 46.4 a | |
| B4 | 76 c | 46.7 | 177 b | 44.6 b | 409 a | 42.3 b | 618 a | 44.3 b | |
| Water regime (W) | |||||||||
| W100 | 106 a | 45.4 | 250 a | 46.9 a | 468 a | 44.3 a | 748 a | 47.7 a | |
| W50 | 96 b | 46.0 | 182 b | 44.7 b | 263 b | 42.4 b | 351 b | 42.5 b | |
| Biochar × water regime | |||||||||
| B0 | W100 | 114 b | 44.0 | 260 ab | 47.3 | 350 b | 44.5 | 613 | 47.4 |
| W50 | 99 bc | 44.5 | 142 d | 44.4 | 189 c | 42.0 | 279 | 41.7 | |
| B2 | W100 | 135 a | 46.5 | 285 a | 47.6 | 517 a | 44.8 | 766 | 48.7 |
| W50 | 106 b | 45.9 | 255 ab | 46.5 | 320 b | 44.2 | 404 | 44.1 | |
| B4 | W100 | 70 d | 45.8 | 205 bc | 45.9 | 538 a | 43.5 | 865 | 47.0 |
| W50 | 83 cd | 47.5 | 148 cd | 43.3 | 281 b | 41.1 | 372 | 41.7 | |
| Significance | |||||||||
| B | *** | ns | *** | * | *** | * | *** | ** | |
| W | ** | ns | *** | ** | *** | * | ** | *** | |
| B × W | *** | ns | * | ns | * | ns | ns | ns | |
| Experimental Factor | 32 DAS | 38 DAS | 45 DAS | ||||
|---|---|---|---|---|---|---|---|
| SD | NB | SD | NB | SD | NB | ||
| Biochar (B) | |||||||
| B0 | 3.5 | 8.3 | 5.5 ab | 13.2 b | 5.9 b | 18.3 b | |
| B2 | 3.8 | 9.5 | 6.1 a | 15.3 a | 6.3 a | 20.3 a | |
| B4 | 3.4 | 8.3 | 5.2 b | 14.0 ab | 5.6 c | 18.5 b | |
| Water regime (W) | |||||||
| W100 | 4.0 a | 9.2 a | 6.5 a | 16.0 a | 6.8 a | 20.9 a | |
| W50 | 3.2 b | 8.2 b | 4.7 b | 12.3 b | 5.1 b | 17.2 b | |
| Biochar × water regime | |||||||
| B0 | W100 | 4.2 a | 9.0 | 6.5 | 15.0 | 6.6 b | 20.0 |
| W50 | 2.9 c | 7.7 | 4.4 | 11.3 | 5.3 c | 16.7 | |
| B2 | W100 | 3.9 ab | 9.7 | 6.8 | 17.3 | 7.1 a | 22.3 |
| W50 | 3.8 ab | 9.3 | 5.5 | 13.3 | 5.4 c | 18.3 | |
| B4 | W100 | 3.8 ab | 9.0 | 6.2 | 15.7 | 6.6 b | 20.3 |
| W50 | 3.0 bc | 7.7 | 4.2 | 12.3 | 4.6 d | 16.7 | |
| Significance | |||||||
| B | ns | ns | * | * | *** | ** | |
| W | *** | * | *** | *** | ** | *** | |
| B × W | * | ns | ns | ns | ** | ns | |
| Experimental Factor | RWC | TW:DW | Ψ | |
|---|---|---|---|---|
| Biochar (B) | ||||
| B0 | 55.9 b | 10.1 a | −3.0 b | |
| B2 | 55.6 b | 9.1 b | −3.1 b | |
| B4 | 61.0 a | 9.5 ab | −2.6 a | |
| Water regime (W) | ||||
| W100 | 61.4 a | 8.9 b | −2.6 a | |
| W50 | 53.6 b | 10.2 a | −3.2 b | |
| Biochar × water regime | ||||
| B0 | W100 | 58.5 ab | 9.6 | −2.8 |
| W50 | 53.3 bc | 10.5 | −3.2 | |
| B2 | W100 | 62.2 a | 8.2 | −2.7 |
| W50 | 49.1 c | 10.1 | −3.6 | |
| B4 | W100 | 63.5 a | 8.9 | −2.3 |
| W50 | 58.5 ab | 10.2 | −2.9 | |
| Significance | ||||
| B | ** | * | *** | |
| W | *** | *** | *** | |
| B × W | * | ns | ns | |
| Experimental Factor | PH | SD | NB | NL | LA | SPAD Index | |
|---|---|---|---|---|---|---|---|
| Biochar (B) | |||||||
| B0 | 47.7 b | 6.5 b | 20.3 b | 79.0 b | 621.1 b | 41.5 b | |
| B2 | 54.8 a | 6.8 a | 24.3 a | 105.0 a | 791.6 a | 44.6 a | |
| B4 | 50.0 b | 6.2 c | 21.2 b | 83.0 b | 674.6 b | 41.3 b | |
| Water regime (W) | |||||||
| W100 | 60.1 a | 7.3 a | 23.8 a | 110.0 a | 955.4 a | 44.5 a | |
| W50 | 41.6 b | 5.7 b | 20.1 b | 68.0 b | 436.2 b | 40.4 b | |
| Biochar × water regime | |||||||
| B0 | W100 | 57.0 | 7.1 a | 21.7 | 96.7 | 862.5 b | 44.2 |
| W50 | 38.3 | 5.8 b | 19.0 | 61.3 | 379.7 c | 38.9 | |
| B2 | W100 | 65.3 | 7.5 a | 26.7 | 128.7 | 1101.8 a | 46.4 |
| W50 | 44.3 | 6.0 b | 22.0 | 81.3 | 481.4 c | 42.8 | |
| B4 | W100 | 58.0 | 7.2 a | 23.0 | 104.7 | 901.8 b | 42.9 |
| W50 | 42.0 | 5.1 c | 19.3 | 61.3 | 447.5 c | 39.6 | |
| Significance | |||||||
| B | ** | *** | *** | *** | *** | ** | |
| W | *** | *** | *** | *** | *** | *** | |
| B × W | ns | ** | ns | ns | * | ns | |
| Experimental Factor | FW | WUE | |||||
|---|---|---|---|---|---|---|---|
| Stem | Leaves | Panicle | Subpanicles | Total | |||
| Biochar (B) | |||||||
| B0 | 9.8 b | 16.2 b | 0.95 b | 0.71 b | 27.6 b | 6.5 ab | |
| B2 | 11.4 a | 19.5 a | 1.38 a | 0.85 a | 33.2 a | 7.2 a | |
| B4 | 9.0 b | 16.1 b | 0.99 b | 0.72 b | 26.8 b | 5.8 b | |
| Water regime (W) | |||||||
| W100 | 14.8 a | 24.2 a | 1.47 a | 1.10 a | 41.5 a | 7.9 a | |
| W50 | 5.4 b | 10.3 b | 0.74 b | 0.41 b | 16.9 b | 5.0 b | |
| Biochar × water regime | |||||||
| B0 | W100 | 14.7 | 23.6 b | 1.35 | 1.01 | 40.7 b | 8.3 a |
| W50 | 4.9 | 8.7 c | 0.55 | 0.40 | 14.6 c | 4.6 c | |
| B2 | W100 | 16.5 | 27.3 a | 1.81 | 1.22 | 46.8 a | 8.7 a |
| W50 | 6.4 | 11.8 c | 0.96 | 0.48 | 19.7 c | 5.7 bc | |
| B4 | W100 | 13.1 | 21.6 b | 1.27 | 1.06 | 37.1 b | 6.9 b |
| W50 | 4.9 | 10.5 c | 0.71 | 0.37 | 16.5 c | 4.8 c | |
| Significance | |||||||
| B | ** | *** | *** | *** | *** | ** | |
| W | *** | *** | *** | *** | *** | *** | |
| B × W | ns | * | ns | ns | * | * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivelli, A.R.; Akram, M.Z.; Libutti, A. Woody Biochar Rate and Water Shortage Impact on Early Growth Stages of Chenopodium quinoa Willd. Agronomy 2024, 14, 53. https://doi.org/10.3390/agronomy14010053
Rivelli AR, Akram MZ, Libutti A. Woody Biochar Rate and Water Shortage Impact on Early Growth Stages of Chenopodium quinoa Willd. Agronomy. 2024; 14(1):53. https://doi.org/10.3390/agronomy14010053
Chicago/Turabian StyleRivelli, Anna Rita, Muhammad Zubair Akram, and Angela Libutti. 2024. "Woody Biochar Rate and Water Shortage Impact on Early Growth Stages of Chenopodium quinoa Willd." Agronomy 14, no. 1: 53. https://doi.org/10.3390/agronomy14010053
APA StyleRivelli, A. R., Akram, M. Z., & Libutti, A. (2024). Woody Biochar Rate and Water Shortage Impact on Early Growth Stages of Chenopodium quinoa Willd. Agronomy, 14(1), 53. https://doi.org/10.3390/agronomy14010053








