Production of Black Cumin via Somatic Embryogenesis, Chemical Profile of Active Compounds in Callus Cultures and Somatic Embryos at Different Auxin Supplementations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Seed Germination
2.2. Callus Induction and Differentiation, and Somatic Embryo Formation and Conversion to Plantlets
| Callus percentage (%) = (No. of explant gave callus/total No. of explants) × 100 |
| Somatic embryo percentage (%) = (No. of explant gave embryo/total No. of explants) × 100 |
| Callus differentiation percentage (%) = (No. of callus gave shoot/total No. of callus) × 100 |
| Embryo conversion to plantlets percentage (%) = (No. of embryo conversion to plantlets/Total No. of embryo) × 100 |
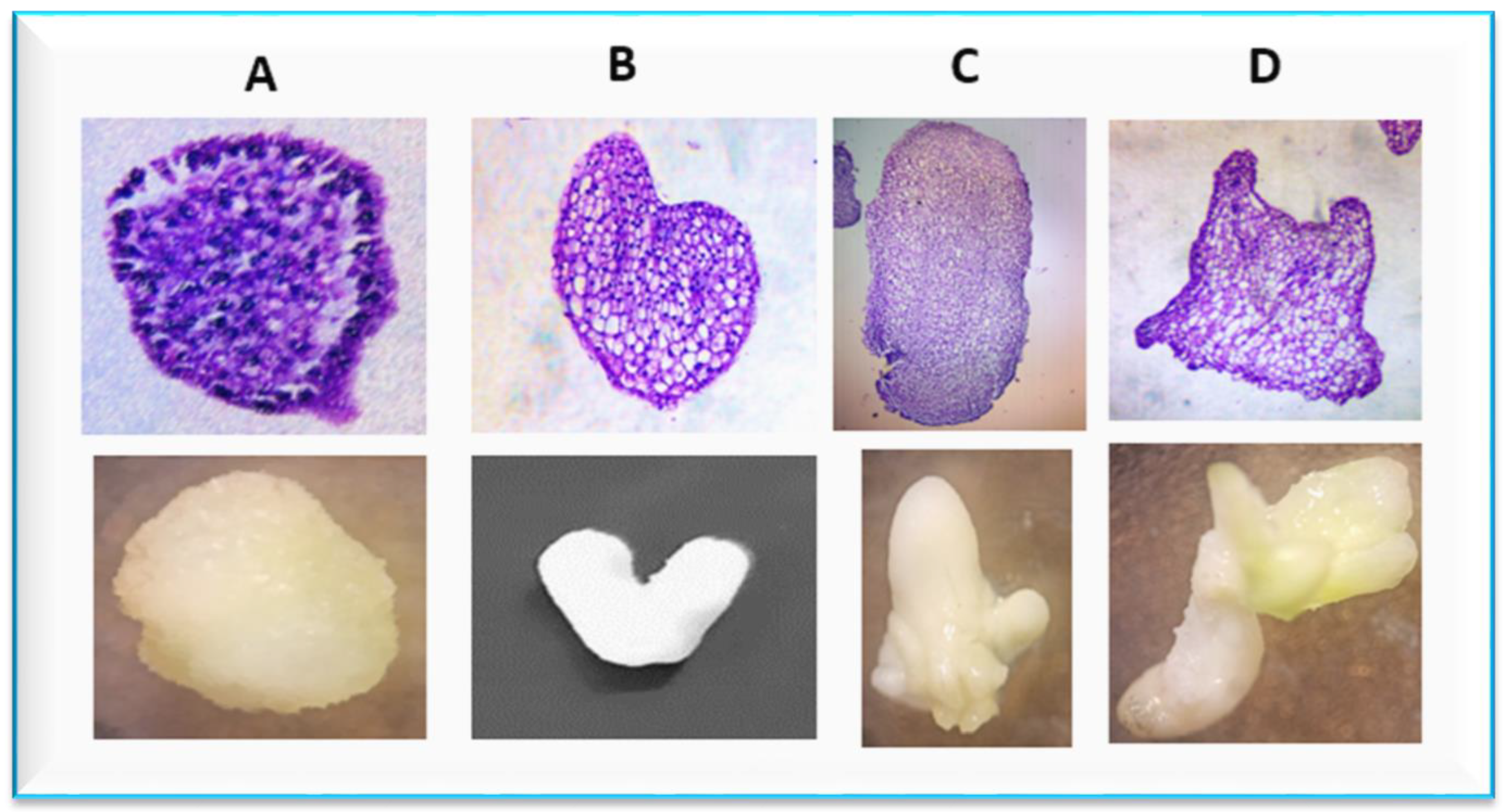
2.3. Histological Study of the Embryogenic Callus
2.4. Gas Chromatography Analysis of Extracts from Seeds, Calli, and Embryos
2.5. Statistical Analyses
3. Results
3.1. Callus Induction and Somatic Embryo Formation
3.2. Differentiation of Calli into Shoots/Plants and Embryo Conversion to Plantlets
3.3. Histological Analysis of the Embryogenic Calli
3.4. Gas Chromatography Analyses of Callus, Embryo, and Seed Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Telci, İ.; Özek, T.; Demirtaş, İ.; Özek, G.; Yur, S.; Ersoy, S.; Yasak, S.; Gül, F.; Karakurt, Y. Studies on black cumin genotypes of Turkiye: Agronomy, seed and thymoquinone yields. J. Appl. Res. Med. Aromat. Plants 2023, 35, 100494. [Google Scholar] [CrossRef]
- Golkar, P.; Nourbakhsh, V. Analysis of genetic diversity and population structure in Nigella sativa L. using agronomic traits and molecular markers (SRAP and SCoT). Ind. Crops Prod. 2019, 130, 170–178. [Google Scholar] [CrossRef]
- Dalli, M.; Azizi, S.; Kandsi, F.; Gseyra, N. Evaluation of the in vitro antioxidant activity of different extracts of Nigella sativa L. seeds, and the quantification of their bioactive compounds. Mater. Today Proc. 2021, 45, 7259–7263. [Google Scholar] [CrossRef]
- Golkar, P.; Bakhshi, G.; Vahabi, M.R. Phytochemical, biochemical, and growth changes in response to salinity in callus cultures of Nigella sativa L. Vitr. Cell. Dev. Biol. Plant 2020, 56, 247–258. [Google Scholar] [CrossRef]
- Hannan, A.; Rahman, A.; Sohag, A.A.M.; Uddin, J.; Dash, R.; Sikder, M.H.; Rahman, S.; Timalsina, B.; Munni, Y.A.; Sarker, P.P.; et al. Black Cumin (Nigella sativa L.): A Comprehensive Review on Phytochemistry, Health Benefits, Molecular Pharmacology, and Safety. Nutrients 2021, 13, 1784. [Google Scholar] [CrossRef] [PubMed]
- Nazari, A.; Zarringhalami, S.; Asghari, B. Influence of germinated black cumin (Nigella sativa L.) seeds extract on the physicochemical, antioxidant, antidiabetic, and sensory properties of yogurt. Food Biosci. 2023, 53, 102437. [Google Scholar] [CrossRef]
- Kemal, M.; Esertaş, Ü.Z.Ü.; Kanbur, E.D.; Kara, Y.; Özçelik, A.E.; Can, Z.; Kolaylı, S. Characterization of the black cumin (Nigella sativa L.) honey from Türkiye. Food Biosci. 2023, 53, 102760. [Google Scholar] [CrossRef]
- Burdock, G.A. Assessment of black cumin (Nigella sativa L.) as a food ingredient and putative therapeutic agent. Regul. Toxicol. Pharmacol. 2022, 128, 105088. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Hosayni, M.; Alizadeh, S.; Zandi, R.; Rahmati, S.; Rezaee, V. Effects of black cumin (Nigella sativa) seed meal on growth performance, blood and biochemical indices, meat quality and cecal microbial load in broiler chickens. Livest. Sci. 2023, 274, 105272. [Google Scholar] [CrossRef]
- Kazmi, A.; Khan, M.A.; Ali, H. Biotechnological approaches for production of bioactive secondary metabolites in Nigella sativa: An up-to-date review. Int. J. Sec. Metab. 2019, 6, 172–195. [Google Scholar] [CrossRef]
- Zaky, A.A.; Shim, J.H.; Abd El-Aty, A.M. A Review on Extraction, Characterization, and Applications of Bioactive Peptides from Pressed Black Cumin Seed Cake. Front. Nutr. 2021, 8, 743909. [Google Scholar] [CrossRef]
- Sakdasri, W.; Sila-ngam, P.; Chummengyen, S.; Sukruay, A.; Ngamprasertsith, S.; Supang, W.; Sawangkeaw, R. Optimization of yield and thymoquinone content of screw press-extracted black cumin seed oil using response surface methodology. Ind. Crops Prod. 2023, 191, 115901. [Google Scholar] [CrossRef]
- Alkhatib, H.; Mawazi, S.M.; Al-Mahmood, S.M.A.; Zaiter, A.; Doolaanea, A.A. Thymoquinone content in marketed black seed oil in Malaysia. J. Pharm. Bioallied Sci. 2020, 12, 284–288. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, S.A.E.-S.; Rizk, M.A.; Yokoyama, N.; Igarashi, I. Evaluation of the in vitro and in vivo inhibitory effect of thymoquinone on piroplasm parasites. Parasites Vectors 2019, 12, 37. [Google Scholar] [CrossRef]
- Khurshid, Y.; Syed, B.; Simjee, S.U.; Beg, O.; Ahmed, A. Antiproliferative and apoptotic effects of proteins from black seeds (Nigella sativa) on human breast MCF-7 cancer cell line. BMC Complement. Med. Ther. 2020, 20, 5. [Google Scholar] [CrossRef]
- Abd-Rabou, A.A.; Edris, A.E. Cytotoxic, apoptotic, and genetic evaluations of Nigella sativa essential oil nanoemulsion against human hepatocellular carcinoma cell lines. Cancer Nanotechnol. 2021, 12, 28. [Google Scholar] [CrossRef]
- Mohaddab, M.; El Goumi, Y.; Gallo, M.; Montesano, D.; Zengin, G.; Bouyahya, A.; Fakiri, M. Biotechnology and In Vitro Culture as an Alternative System for Secondary Metabolite Production. Molecules 2022, 27, 8093. [Google Scholar] [CrossRef] [PubMed]
- Bibi, A.; Khan, M.A.; Adil, M.; Mashwani, Z.U.R. Production of callus biomass and antioxidant secondary metabolites in black cumin. J. Anim. Plant Sci. 2018, 28, 1321–1328. [Google Scholar]
- Al-Ani, N.K. Thymol Production from Callus Culture of Nigella sativa L. Plant Tissue Cult. Biotech. 2009, 18, 181–185. [Google Scholar] [CrossRef]
- Alemi, M.; Sabouni, F.; Sanjarian, F.; Haghbeen, K.; Ansari, S. Anti-inflammatory effect of seeds and callus of Nigella sativa L. extracts on mix glial cells with regard to their thymoquinone content. AAPS PharmSciTech. 2013, 14, 160–167. [Google Scholar] [CrossRef]
- Chaudhry, H.; Fatima, N.; Ahmad, I.Z. Establishment of callus and cell suspension cultures of Nigella sativa L. for thymol production. Int. J. Pharm. Pharm. Sci. 2014, 6, 788–794. [Google Scholar]
- Landa, P.; Maršík, P.; Vaněk, T.; Rada, V.; Kokoška, L. In vitro antimicrobial activity of extracts from the callus cultures of some Nigella species. Biologia 2006, 61, 285–288. [Google Scholar] [CrossRef]
- Hoseinpanahi, S.; Majdi, M.; Mirzaghaderi, G. Effects of growth regulators on in vitro callogenesis and regeneration of black cumin (Nigella sativa L.). Iran. J. Rangel. Forest Plant Breed. 2016, 24, 232–242. [Google Scholar]
- Elhag, H.; El-Olemy, M.M.; Al-Said, M.S. Enhancement of Somatic Embryogenesis and Production of Developmentally Arrested Embryos in Nigella sativa L. Hort. Sci. 2004, 39, 321–323. [Google Scholar] [CrossRef]
- Kim, D.H.; Kang, K.W.; Sivanesan, I. Influence of auxins on somatic embryogenesis in Haworthia retusa Duval. Biologia 2019, 74, 25–33. [Google Scholar] [CrossRef]
- Vondráková, Z.; Krajňáková, J.; Fischerová, L.; Vágner, M.; Eliášová, K. Physiology and role of plant growth regulators in somatic embryogenesis. In Vegetative Propagation of Forest Trees; Park, Y.S., Bonga, J.M., Moon, H.K., Eds.; National Institute of Forest Science: Seoul, Republic of Korea, 2016; pp. 123–169. [Google Scholar]
- Simon, S.; Petrášek, J. Why plants need more than one type of auxin. Plant Sci. 2011, 180, 454–460. [Google Scholar] [CrossRef]
- Hazubska-Przybył, T.; Ratajczak, E.; Obarska, A.; Pers-Kamczyc, E. Different Roles of Auxins in Somatic Embryogenesis Efficiency in Two Picea Species. Int. J. Mol. Sci. 2020, 21, 3394. [Google Scholar] [CrossRef] [PubMed]
- de Morais Oliveira, J.P.; da Silva, A.J.; Catrinck, M.N.; Clarindo, W.R. Embryonic abnormalities and genotoxicity induced by 2,4-dichlorophenoxyacetic acid during indirect somatic embryogenesis in Coffea. Sci. Rep. 2023, 13, 9689. [Google Scholar]
- Bhusare, B.P.; John, C.K.; Bhatt, V.P.; Nikam, T.D. Induction of somatic embryogenesis in leaf and root explants of Digitalis lanata Ehrh.: Direct and indirect method. S. Afr. J. Bot. 2020, 130, 356–365. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- El-Mahrouk, M.E.; Maamoun, M.K.; Dewir, Y.H.; Omran, S.A.; EL-Banna, A.N. Morphological and molecular characterization of induced mutants in Nigella sativa L. using irradiation and chemical mutagens. In The 9th Plant Breeding International Conference; Faculty of Agriculture, Benha University: Benha, Egypt, 2015. [Google Scholar]
- El-Mahrouk, M.E.; Maamoun, M.K.; Dewir, Y.H.; El-Banna, A.N.; Rihan, H.Z.; Salamh, A.; Al-Aizari, A.A.; Fuller, M.P. Synchronized Seed Germination and Seedling Growth of Black Cumin. HortTechnology 2022, 32, 182–190. [Google Scholar] [CrossRef]
- Boissot, N.; Valdez, M.; Guiderdoni, E. Plant regeneration from leaf and seed-derived calli and suspension cultures of the African perennial wild rice, Oryza longistaminata. Plant Cell Rep. 1990, 9, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Mahmmoud, Y.A.; Christensen, S.B. Oleic and linoleic acids are active principles in Nigella sativa and stabilize an E(2)P conformation of the Na,K-ATPase. Fatty acids differentially regulate cardiac glycoside interaction with the pump. Biochim. Biophys. Acta 2011, 1808, 2413–2420. [Google Scholar] [CrossRef]
- Hassanien, M.F.; Assiri, A.M.; Alzohairy, A.M.; Oraby, H.F. Health-promoting value and food applications of black cumin essential oil: An overview. J. Food Sci. Technol. 2015, 52, 6136–6142. [Google Scholar] [CrossRef]
- Banerjee, S.; Gupta, S. Morphogenesis in tissue cultures of different organs of Nigella sativa. Physiol. Plant 1975, 33, 185–187. [Google Scholar] [CrossRef]
- Chand, S.; Roy, S.C. Study of callus tissues from different parts of Nigella sativa (Ranunculaceae). Experientia 1980, 36, 305–306. [Google Scholar] [CrossRef]
- Klimek-Chodacka, M.; Kadluczka, D.; Lukasiewicz, A.; Malec-Pala, A.; Baranski, R.; Grzebelus, E. Effective callus induction and plant regeneration in callus and protoplast cultures of Nigella damascena L. Plant Cell Tissue Organ. Cult. 2020, 143, 693–707. [Google Scholar] [CrossRef]
- Khan, M.A.; Abbasi, B.H.; Ali, H.; Ali, M.; Adil, M.; Hussain, I. Temporal variations in metabolite profiles at different growth phases during somatic embryogenesis of Silybum marianum L. Plant Cell Tissue Organ. Cult. 2015, 120, 127–139. [Google Scholar] [CrossRef]
- Khan, M.S.; Tabrez, S.; Rabbani, N.; Oves, M.; Shah, A.; Alsenaidy, M.A.; Al-Senaidy, A.M. Physico-chemical stress induced amyloid formation in insulin: Amyloid characterization, cytotoxicity analysis against human neuroblastoma cell lines and its prevention using black seeds (Nigella sativa L.). Chin. J. Integr. Med. 2015. [Google Scholar] [CrossRef]
- Abouzid, S.F.; El-Bassuon, A.A.; Nasib, A.; Khan, S.; Qureshi, J.; Choudhary, M.I. Withaferin A production by root cultures of Withania coagulans. Int. J. Appl. Res. Nat. Prod. 2010, 3, 23–27. [Google Scholar]
- Majid, A. The Chemical Constituents and Pharmacological Effects of Nigella sativa—A Review. J. Biosci. Appl. Res. 2018, 4, 389–400. [Google Scholar] [CrossRef]
- Forouzanfar, F.; Bazzaz, B.S.; Hosseinzadeh, H. Black cumin (Nigella sativa L.) and its constituent (thymoquinone): A review on antimicrobial effects. Iran. J. Basic. Med. Sci. 2014, 17, 929–938. [Google Scholar] [PubMed]
- Radusheva, P.; Pashev, A.; Uzunova, G.; Nikolova, K.; Gentscheva, G.; Perifanova, M.; Maria Marudova, M. Comparative physicochemical analysis of oils derived from Nigella sativa L. and Coriandrum sativum L. J. Chem. Technol. Metall. 2021, 56, 1175–1180. [Google Scholar]
- Albakry, Z.; Karrar, E.; Ahmed, I.A.M.; Oz, E.; Proestos, C.; El Sheikha, A.F.; Oz, F.; Wu, G.; Wang, X. Nutritional Composition and Volatile Compounds of Black Cumin (Nigella sativa L.) Seed, Fatty Acid Composition and Tocopherols, Polyphenols, and Antioxidant Activity of Its Essential Oil. Horticulturae 2022, 8, 575. [Google Scholar] [CrossRef]
- Hernandez, E.M. Specialty Oils: Functional and Nutraceutical Properties. In Woodhead Publishing Series in Food Science, Technology and Nutrition, Functional Dietary Lipids; Sanders, T.A.B., Ed.; Woodhead Publishing: Sawston, UK, 2016; pp. 69–101. [Google Scholar] [CrossRef]

| Auxins (mg L−1) | Callus (%) | Callus Diameter (cm) | Callus Fresh Weight (g) | Embryo (%) | Number of Embryos | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hyp. | Cot. | Hyp. | Cot. | Hyp. | Cot. | Hyp. | Cot. | Hyp. | Cot. | |
| IBA | ||||||||||
| 0.0 | 50 c | 40 c | 0.3 i | 0.4 i | 1.3 i | 0.65 i | 6.6 f | 3.3 f | 0.33 c | 0.33 c |
| 1.0 | 99 a | 99 a | 2.2 de | 1.5 g | 3.1 g | 2.3 hi | 67 d | 77 c | 1.9 b | 2.8 a |
| 2.0 | 99 a | 100 a | 1.7 f | 1.6 fg | 3.2 fg | 3.3 ef | 98 a | 98 a | 1.9 b | 2.9 a |
| 3.0 | 97 a | 99 a | 1.7 f | 2.2 de | 3.2 fg | 3.2 fg | 98 a | 98 a | 2.8 a | 2.8 a |
| NAA | ||||||||||
| 0.0 | 50 c | 40 c | 0.3 i | 0.4 i | 1.3 i | 0.65 i | 6.6 f | 3.3 f | 0.33 c | 0.33 c |
| 1.0 | 98 a | 100 a | 2.7 a | 2.4 b | 4.4 a | 4.5 a | 89 b | 67 d | 2.8 a | 1.9 b |
| 2.0 | 99 a | 98 a | 1.3 h | 1.6 fg | 2.4 h | 3.5 cd | 98 a | 98 a | 2.7 a | 1.9 b |
| 3.0 | 98 a | 98 a | 2.4 b | 2.3 bcd | 3.4 de | 4.2 b | 78 c | 88 b | 2.9 a | 1.9 b |
| 2,4-D | ||||||||||
| 0.0 | 50 c | 40 c | 0.3 i | 0.4 i | 1.3 i | 0.65 i | 6.6 f | 3.3 f | 0.33 c | 0.33 c |
| 1.0 | 97 a | 89 b | 2.3 bcd | 1.3 h | 4.2 b | 3.6 c | 0 g | 0 g | 1.9 b | 1.9 b |
| 2.0 | 98 a | 89 b | 2.4 bc | 1.2 h | 3.5 cd | 2.2 i | 0 g | 0 g | 1.9 b | 1.9 b |
| 3.0 | 97 a | 99 a | 2.1 e | 2.1 e | 3.4 de | 2.2 i | 57 e | 68 d | 1.8 b | 1.9 b |
| Significance | ||||||||||
| A | *** | *** | *** | *** | *** | |||||
| E | ** | *** | *** | *** | ** | |||||
| C | *** | *** | *** | *** | N.S | |||||
| A × E | *** | *** | *** | *** | *** | |||||
| A × C | *** | *** | *** | *** | *** | |||||
| C × E | *** | *** | *** | *** | *** | |||||
| A × C × E | *** | *** | *** | *** | *** | |||||
| Auxins (mg L−1) | Embryo Conversion to Plantlets (%) | Callus Differentiation (%) | ||
|---|---|---|---|---|
| Hyp. | Cot. | Hyp. | Cot. | |
| IBA | ||||
| 0.0 | 3.3 g | 3.3 g | 10.0 g | 3.3 h |
| 1.0 | 37.7 a | 29.4 c | 47.0 c | 8.4 f |
| 2.0 | 23.0 d | 35.0 ab | 97.7 a | 77.7 b |
| 3.0 | 23.0 de | 37.7 a | 79.7 b | 77.7 b |
| NAA | ||||
| 0.0 | 3.3 g | 3.3 g | 10.0 g | 3.3 h |
| 1.0 | 24.0 d | 9.33 f | 47.7 c | 13.0 e |
| 2.0 | 37.7 a | 27.4 c | 49.4 c | 50.0 c |
| 3.0 | 33.7 b | 27.7 cd | 77.7 b | 27.7 d |
| 2,4-D | ||||
| 0.0 | 3.3 g | 3.3 g | 10.0 g | 3.3 h |
| 1.0 | 0 h | 0 h | 0 g | 0 g |
| 2.0 | 0 h | 0 h | 0 g | 0 g |
| 3.0 | 8.4 f | 9.4 f | 0 g | 0 g |
| Significance | ||||
| A | *** | *** | ||
| E | * | *** | ||
| C | *** | *** | ||
| A × E | *** | *** | ||
| A × C | *** | *** | ||
| C × E | *** | *** | ||
| A × C × E | *** | *** | ||
| No. | Compound | Molecular Weight | RT (min) | Area (%) | |
|---|---|---|---|---|---|
| 1 mg L−1 NAA | 3 mg L−1 NAA | ||||
| 1 | Thymoquinone | 164 | 7.18 | 1.58 | 2.76 |
| 2 | Onamine 12 | 213 | 11.96 | 8.80 | 8.14 |
| 3 | Anastrozole | 293 | 15.05 | 1.28 | - |
| 4 | Myristamine oxide | 241 | 15.76 | 2.98 | - |
| 5 | Sulfobetaine 14 | 363 | 15.77 | - | 2.86 |
| 6 | Methyl Palmitate | 270 | 19.61 | 5.98 | 7.22 |
| 7 | Hexadecanoic Acid (Palmitic acid) | 256 | 20.30 | 4.00 | 4.32 |
| 8 | 2,3-Dehydro methyl linoleate | 294 | 22.29 | 10.09 | 14.62 |
| 9 | Oleic acid methyl ester | 296 | 22.38 | 9.2 | 13.07 |
| 10 | Elaidic acid methyl ester | 296 | 22.48 | 2.11 | 2.75 |
| 11 | Benzyl amine | 234 | 22.56 | 2.18 | 1.87 |
| 12 | Methyl stearate | 298 | 22.80 | 1.72 | 2.41 |
| 13 | Octadecadienoic acid (Linoleic acid) | 280 | 22.98 | 5.49 | 6.85 |
| 14 | Oleic Acid (cis-9-Octadecenoic acid) | 282 | 23.05 | 8.21 | - |
| 15 | Linoleol chloride | 298 | 23.06 | - | 11.16 |
| 16 | 2,2′-methylenebis [4-methyl-6-tert-butylphenol] | 340 | 27.02 | 1.17 | 1.33 |
| 17 | Oleic acid | 282 | 27.10 | 1.17 | - |
| 18 | Diisooctyl phthalate | 390 | 28.66 | 30.63 | 20.64 |
| 19 | Oleic acid, 3-(octadecyloxy) propyl ester | 529 | 34.29 | 1.57 | - |
| 20 | 4H-1-Benzopyran-4-One,2-(3,4 Dimethoxyphenyl)-3,5-Dihydroxy-7-Methoxy | 344 | 35.25 | 1.83 | - |
 |  | 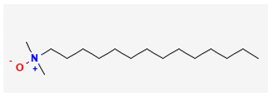 | |||
| Thymoquinone (C10H12O2) | Onamine 12 (C14H31N) | Myristamine oxide (C16H35NO) | |||
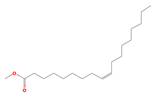 | 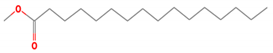 | 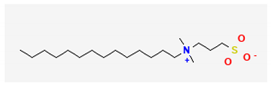 | |||
| Oleic acid methyl ester (C19H36O2) | Methyl Palmitate (C17H34O2) | Sulfobetaine 14 (C19H41NO3S) | |||
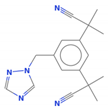 | 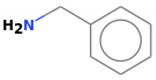 |  | |||
| Anastrozole (C17H19N5) | Benzyl amine (C7H9N) | Hexadecanoic Acid (Palmitic acid) (C16H32O2) | |||
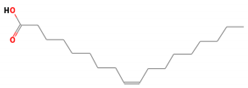 |  |  | |||
| Oleic acid (C18H34O2) | Octadecadienoic acid (Linoleic acid) (C18H32O2) | Elaidic acid methyl ester (C19H36O2) | |||
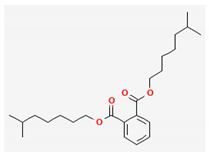 |  |  | |||
| Diisooctyl phthalate (C24H38O4) | Oleic Acid (cis-9-Octadecenoic acid) (C18H34O2) | Methyl stearate (C19H38O2) | |||
 |  | 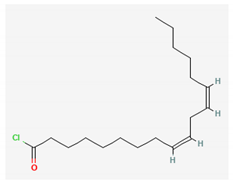 | |||
| Oleic acid, 3-(octadecyloxy) propyl ester (C39H76O3) | 2,2′-methylenebis [4-methyl-6-tert-butylphenol] (C23H32O2) | Linoleoyl chloride (C18H31ClO) | |||
| No. | Compound | Molecular Weight | RT (min) | Area (%) | |
|---|---|---|---|---|---|
| 2 mg L−1 IBA | 3 mg L−1 IBA | ||||
| 1 | Nizatidine | 331 | 11.96 | 6.42 | 5.59 |
| 2 | Bisabolol oxide II | 238 | 14.89 | 5.66 | - |
| 3 | Bisabolone oxide A | 236 | 15.40 | 4.91 | - |
| 4 | Myristamine oxide | 241 | 15.76 | 2.12 | 1.89 |
| 5 | alpha Bisabolol oxide A | 238 | 16.56 | 38.10 | 1.79 |
| 6 | Methyl Palmitate | 270 | 19.61 | 4.58 | 7.15 |
| 7 | Palmitic Acid | 256 | 20.30 | 2.72 | 3.75 |
| 8 | Octadecadienoic acid, methyl ester | 294 | 22.29 | 8.08 | 14.94 |
| 9 | Elaidic acid methyl ester | 296 | 22.38 | 8.72 | 14.72 |
| 10 | 11-Octadecenoic acid, methyl ester | 296 | 22.48 | - | 3.20 |
| 11 | 1-Morpholino-2-(Benzylamino) Propane | 234 | 22.56 | - | 1.20 |
| 12 | Methyl stearate | 298 | 22.79 | - | 2.86 |
| 13 | Linoelaidic acid | 280 | 22.98 | 3.77 | 6.85 |
| 14 | Oleic acid -1,2,3,7,8- | 282 | 23.05 | 6.31 | 8.72 |
| 15 | Trans-13-Octadecenoic acid | 282 | 27.10 | - | 1.50 |
| 16 | Diisooctyl phthalate | 390 | 28.66 | 8.61 | 25.84 |
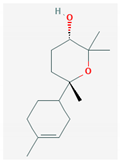 |  | 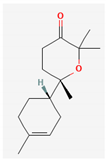 | |||
| Alpha Bisabolol oxide A (C15H26O2) | Bisabolol oxide II (C15H26O2) | Bisabolone oxide A (C15H24O2) | |||
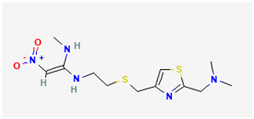 |  | 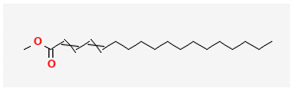 | |||
| Nizatidine (C12H21N5O2S2) | Methyl Palmitate (C17H34O2) | Octadecadienoic acid, methyl ester (C19H34O2) | |||
 |  | 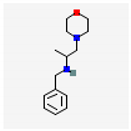 | |||
| 11-Octadecenoic acid, methyl ester (C19H36O2) | Elaidic acid methyl ester (C19H36O2) | 1-Morpholino-2-(Benzylamino) Propane | |||
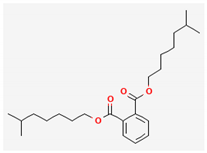 |  |  | |||
| Diisooctyl phthalate (C24H38O4) | Trans-13-Octadecenoic acid(C18H34O2) | Oleic acid-1,2,3,7,8-(C18H34O2) | |||
| No. | Compound | Molecular Weight | RT (min) | Area (%) |
|---|---|---|---|---|
| 1 | Thymol | 134 | 5.25 | 0.37 |
| 2 | Thymoquinone | 164 | 7.37 | 0.98 |
| 3 | Methyl myristate | 242 | 22.09 | 0.30 |
| 4 | Palmitoleic acid | 268 | 25.77 | 0.55 |
| 5 | Methyl palmitate | 270 | 26.27 | 16.00 |
| 6 | Methyl Heptadecanoate | 284 | 28.16 | 0.17 |
| 7 | Methyl linoleate | 294 | 29.42 | 38.38 |
| 8 | Methyl oleate | 296 | 29.60 | 25.03 |
| 9 | Methyl stearate | 298 | 30.04 | 8.01 |
| 10 | 8,11-Octadecadienoic acid, methyl ester | 294 | 30.33 | 0.26 |
| 11 | Methyl octadecadienoate | 294 | 31.11 | 0.31 |
| 12 | Methyl Eicosadienoic | 322 | 32.92 | 7.09 |
| 13 | Methyl gadoleate | 324 | 33.02 | 1.45 |
| 14 | Heneicosanoic acid | 326 | 33.49 | 0.73 |
| 15 | Pentacosylic acid | 382 | 39.74 | 0.37 |
 |  |  | ||
| Thymol (C10H14O) | Thymoquinone (C10H12O2) | Methyl myristate (C15H30O2) | ||
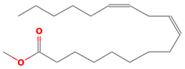 |  | 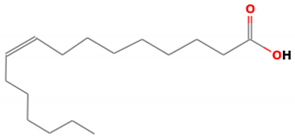 | ||
| Methyl linoleate(C19H34O2) | Methyl Heptadecanoate (C18H36O2) | Palmitoleic acid (C16H30O2) | ||
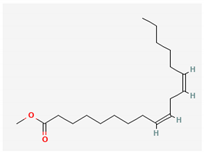 |  |  | ||
| Methyl octadecadienoate (C19H34O2) | Methyl oleate (C19H36O2) | 8,11-Octadecadienoic acid, methyl ester (C19H34O2) | ||
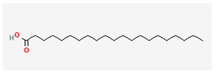 |  |  | ||
| Heneicosanoic acid (C21H42O2) | Methyl gadoleate (C21H40O2) | Methyl Eicosadienoic (C21H38O2) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higazy, A.E.; El-Mahrouk, M.E.; El-Banna, A.N.; Maamoun, M.K.; El-Ramady, H.; Abdalla, N.; Dobránszki, J. Production of Black Cumin via Somatic Embryogenesis, Chemical Profile of Active Compounds in Callus Cultures and Somatic Embryos at Different Auxin Supplementations. Agronomy 2023, 13, 2633. https://doi.org/10.3390/agronomy13102633
Higazy AE, El-Mahrouk ME, El-Banna AN, Maamoun MK, El-Ramady H, Abdalla N, Dobránszki J. Production of Black Cumin via Somatic Embryogenesis, Chemical Profile of Active Compounds in Callus Cultures and Somatic Embryos at Different Auxin Supplementations. Agronomy. 2023; 13(10):2633. https://doi.org/10.3390/agronomy13102633
Chicago/Turabian StyleHigazy, Ahmed E., Mohammed E. El-Mahrouk, Antar N. El-Banna, Mosaad K. Maamoun, Hassan El-Ramady, Neama Abdalla, and Judit Dobránszki. 2023. "Production of Black Cumin via Somatic Embryogenesis, Chemical Profile of Active Compounds in Callus Cultures and Somatic Embryos at Different Auxin Supplementations" Agronomy 13, no. 10: 2633. https://doi.org/10.3390/agronomy13102633
APA StyleHigazy, A. E., El-Mahrouk, M. E., El-Banna, A. N., Maamoun, M. K., El-Ramady, H., Abdalla, N., & Dobránszki, J. (2023). Production of Black Cumin via Somatic Embryogenesis, Chemical Profile of Active Compounds in Callus Cultures and Somatic Embryos at Different Auxin Supplementations. Agronomy, 13(10), 2633. https://doi.org/10.3390/agronomy13102633










