Postharvest Quality Exploration of “Crystal” Grapes in Karst Mountainous Area: Regulatory Effect of High Concentration 1-MCP Fumigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Treatment and Storage
2.3. Changes in Appearance Quality during Storage
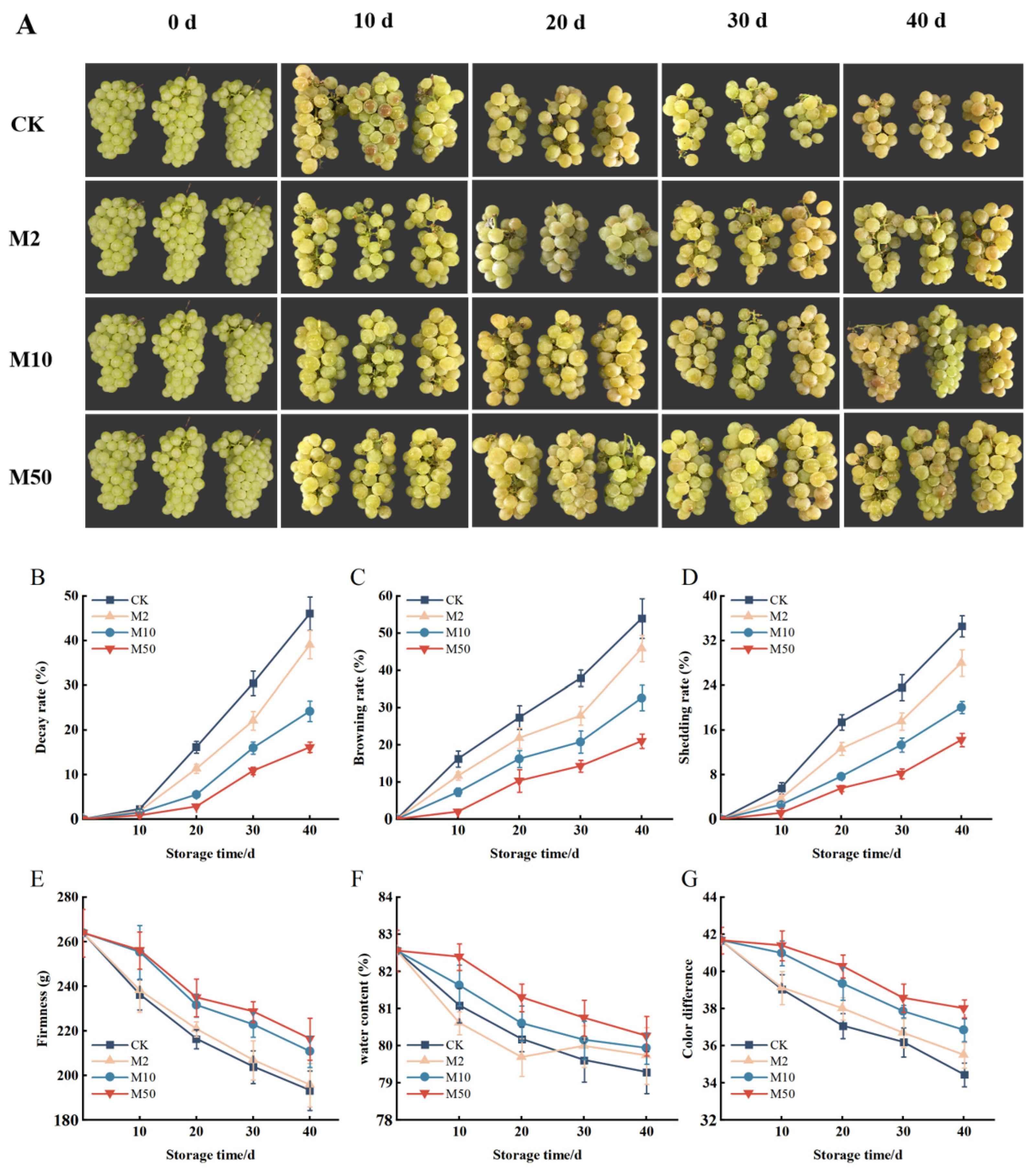
Firmness, Color Difference, Browning Rate, Shedding Rate, Decay Rate, Water Content
2.4. Changes in Physiological and Nutritional Quality during Storage
Respiratory Intensity, Soluble Solids Content (SSC), Soluble Proteins, Free Amino Acids, Titratable Acids, Reducing Sugars
2.5. Changes in Browning-Related Enzyme Activity and Composition during Storage
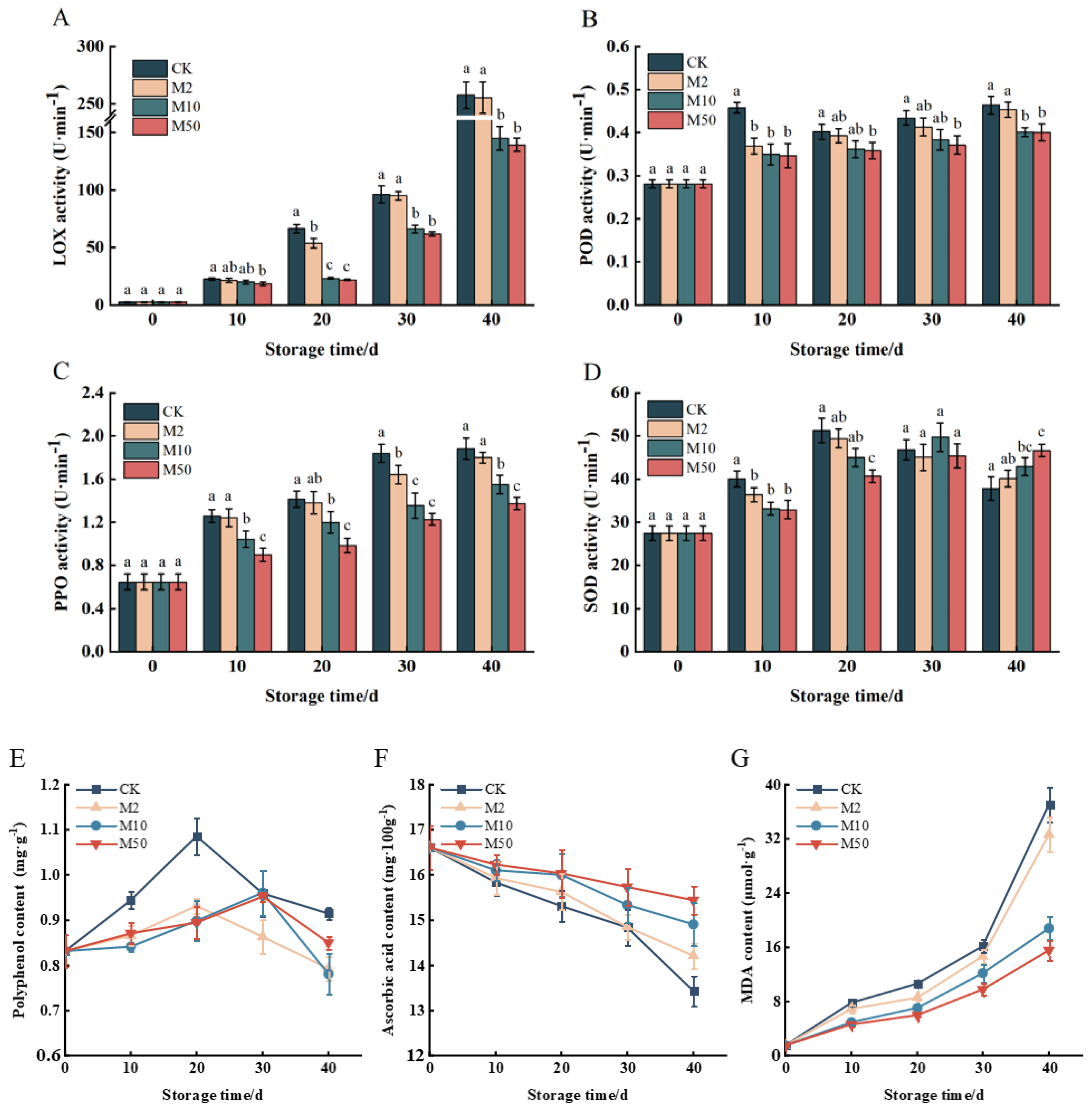
PPO, POD, LOX, SOD, AsA Content, Polyphenols Content, MDA
2.6. Changes in Cell Wall Composition during Storage
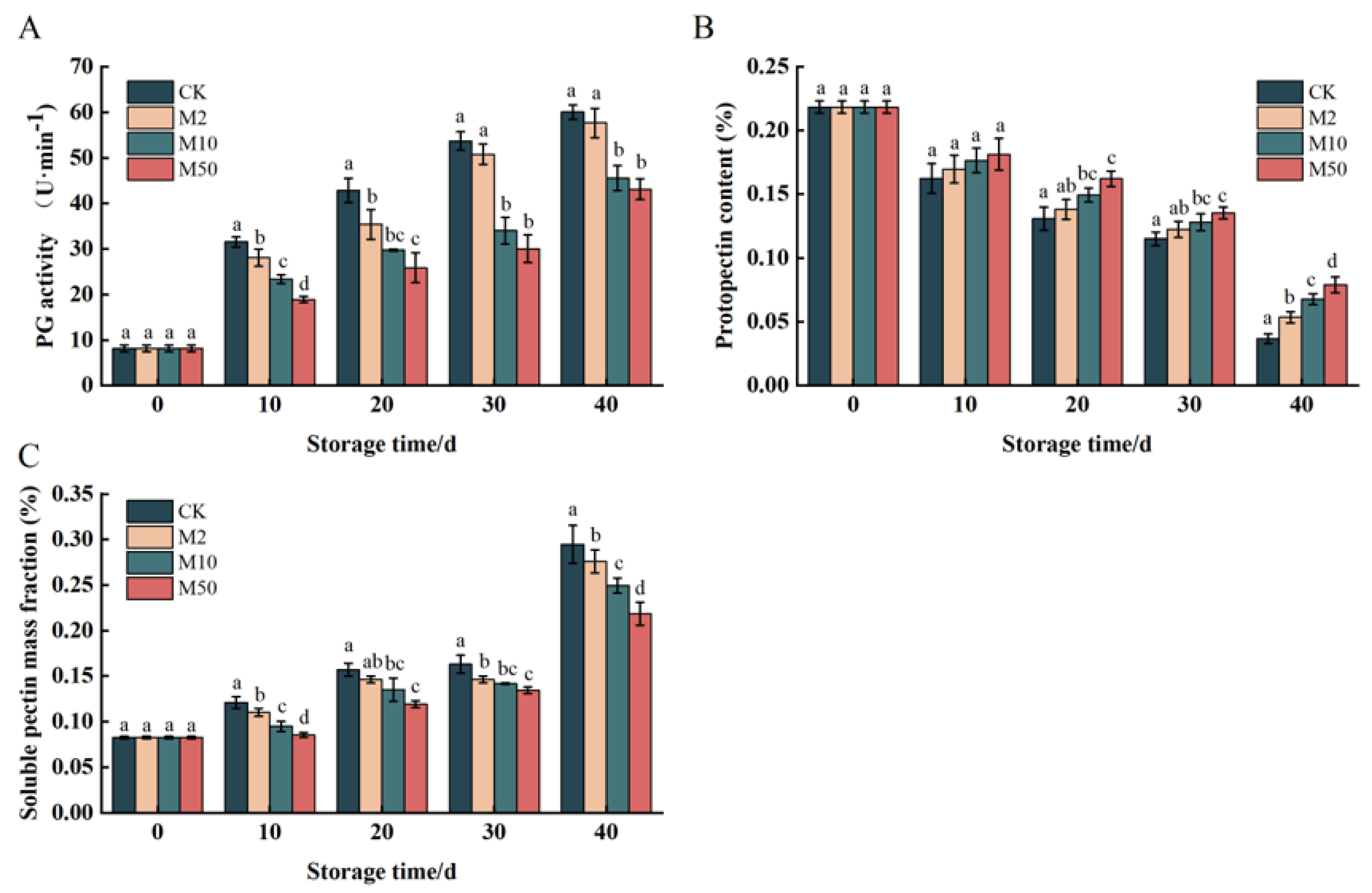
Protopectin, Soluble Pectin Content, PG Activity
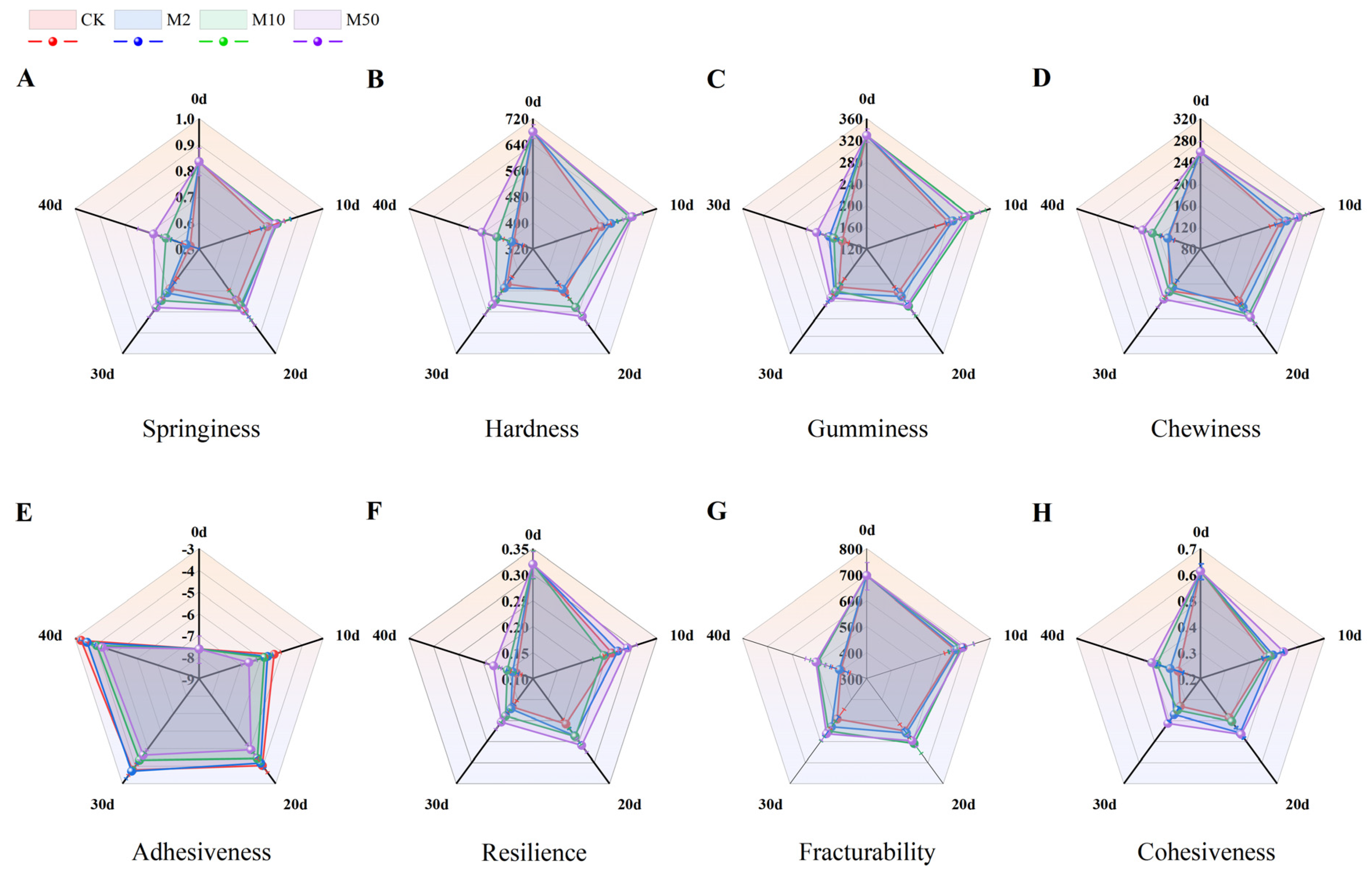
2.7. Changes in Texture Characteristics during Storage
TPA, Tests the Springiness, Hardness, Gumminess, Chewiness, Adhesiveness, Resilience, Cohesiveness Brittleness of the Fruit
2.8. Changes in Aroma Quality during Storage
2.9. Data Analysis
3. Results
3.1. Changes in Appearance Quality such as Firmness, Decay Rate, Browning Rate, Color Difference, and Shedding Rate
3.2. Changes in Respiration Intensity, SSC, Reducing Sugar Content, Titratable Acid Content, Soluble Protein Content, and Free Amino Acid Content
3.3. LOX, POD, PPO, SOD Activity and Changes in Polyphenols, AsA, and MDA Content
3.4. Changes in PG Activity, Protopectin, and Soluble Pectin Content of Fruit Cell Wall
3.5. Effects of 1-MCP Fumigation at Different Concentrations on the Change of Texture Characteristics of “Crystal” Grapes during Storage
3.6. Effects of 1-MCP Fumigation at Different Concentrations on Aroma Quality Changes during Storage of “Crystal” Grapes
4. Discussion
4.1. Effects of Different Concentrations of 1-MCP Treatment on Appearance, Physiological Nutrition, and Aroma Quality of “Crystal” Grapes during Storage
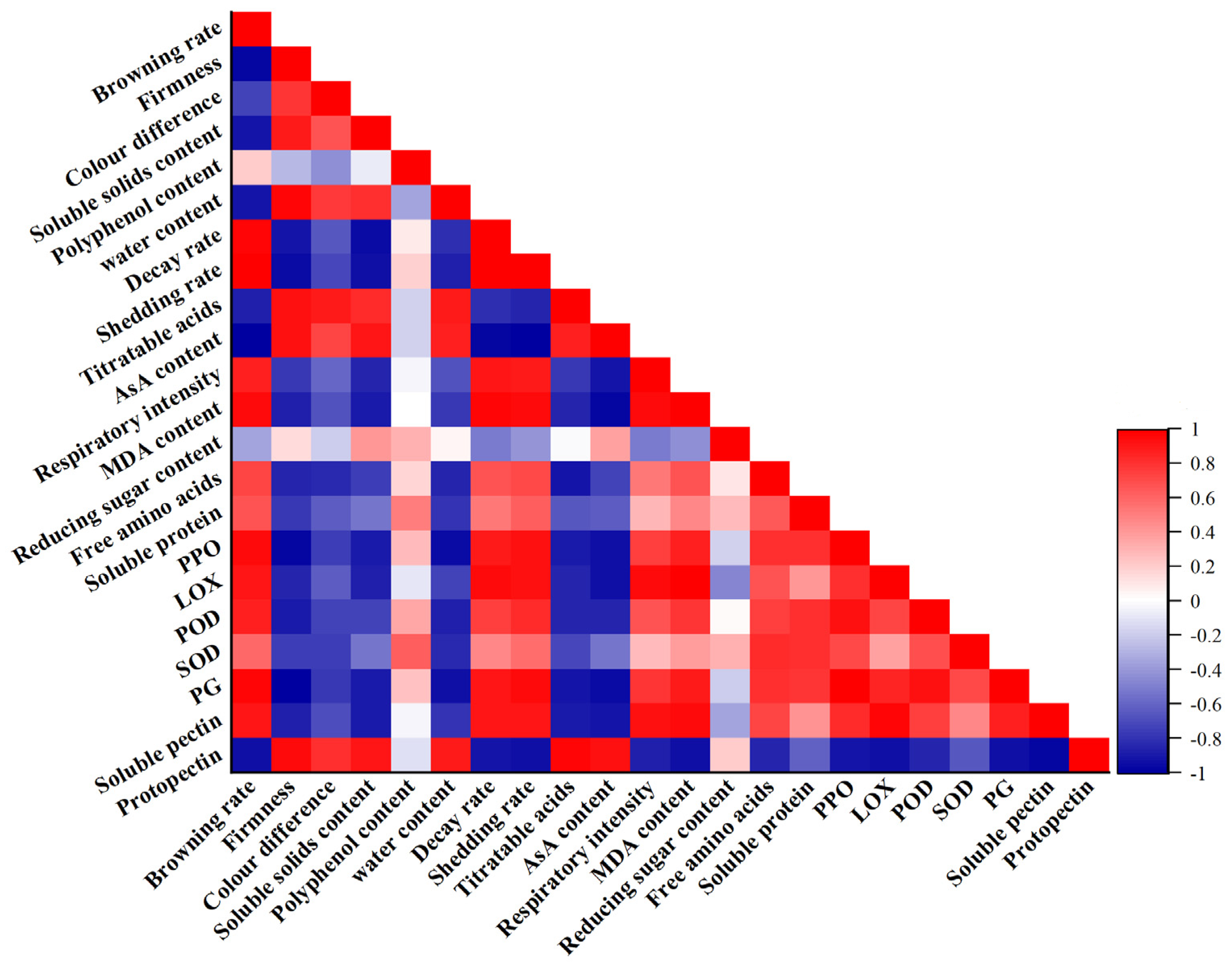
4.2. Relationship between Postharvest Grape Fruit Browning and Browning-Related Enzyme Activity and Antioxidant Content
4.3. Relationship between Postharvest Grape Fruit Softening and Changes in Cell Wall-Related Substances and Texture Characteristics
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wei, X.; Zhou, Q.; Luo, Y.; Cai, M.; Zhou, X.; Yan, W.; Peng, D.; Zhang, J. Vegetation dynamics and its response to driving factors in typical karst regions, Guizhou Province, China. Front. Earth Sci. 2021, 15, 17. [Google Scholar] [CrossRef]
- Petruzzellis, F.; Natale, S.; Bariviera, L.; Calderan, A.; Miheli, A.; Rei, J.; Sivilotti, P.; Uklje, K.; Lisjak, K.; Vanzo, A. High spatial heterogeneity of water stress levels in Refok grapevines cultivated in Classical Karst. Agric. Water Manag. 2022, 260, 107288. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Z.; Li, X.; Wei, J.; Wu, B. Effect of nitrous oxide against Botrytis cinerea and phenylpropanoid pathway metabolism in table grapes. Sci. Hortic. 2019, 254, 99–105. [Google Scholar] [CrossRef]
- Sintuya, P.; Narkprasom, K.; Jaturonglumlert, S.; Whangchai, N.; Peng-Ont, D.; Varith, J. Effect of Gaseous Ozone Fumigation on Organophosphate Pesticide Degradation of Dried Chilies. Ozone Sci. Eng. J. Int. Ozone Assoc. 2018, 40, 473–481. [Google Scholar] [CrossRef]
- Wu, P.; Xin, F.; Xu, H.; Chu, Y.; Zhu, B. Chitosan inhibits postharvest berry abscission of ‘Kyoho’ table grapes by affecting the structure of abscission zone, cell wall degrading enzymes and SO2 permeation. Postharvest Biol. Technol. 2021, 176, 111507. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Palou, L.S.; Garner, D.; Armson, D.A. Concentration by time product and gas penetration after marine container fumigation of table grapes with reduced doses of sulfur dioxide. HortTechnology 2002, 12, 241–245. [Google Scholar] [CrossRef]
- Huerta-Ocampo, J.Á.; Osuna-Castro, J.A.; Lino-López, G.J.; Barrera-Pacheco, A.; Mendoza-Hernández, G.; De León-Rodríguez, A.; de la Rosa, A.P.B. Proteomic analysis of differentially accumulated proteins during ripening and in response to 1-MCP in papaya fruit. J. Proteom. 2012, 75, 2160–2169. [Google Scholar] [CrossRef]
- Ortiz, C.M.; Franceschinis, F.; Grozeff, G.E.G.; Chan, H.; Labavitch, J.M.; Crisosto, C.; Vicente, A.R. Pre-treatment with 1-methylcyclopropene alleviates methyl bromide-induced internal breakdown, softening and wall degradation in blueberry. Postharvest Biol. Technol. 2018, 146, 90–98. [Google Scholar] [CrossRef]
- Serradilla, M.J.; Falagán, N.; Bohmer, B.; Terry, L.A.; Alamar, M.C. The role of ethylene and 1-MCP in early-season sweet cherry ‘Burlat’storage life. Sci. Hortic. 2019, 258, 108787. [Google Scholar] [CrossRef]
- Liu, N.; Xie, G.; Yin, W.; Xinhua, W. Quality characteristics of Niagara grapes and their storage life as affected by 1-MCP combined with sulfur dioxide treatment and modified atmosphere packaging. Int. J. Food Prop. 2022, 25, 159–169. [Google Scholar] [CrossRef]
- Zhu, S.; Liang, Y.; Mu, L.; An, X.; Yin, H. 1-Methylcyclopropene on fruit quality of se-enriched grape (Vitis vinifera L.) during shelf life period. Agronomy 2020, 10, 1411. [Google Scholar] [CrossRef]
- Sabir, F.K.; Agar, I.T. Influence of different concentrations of 1-methylcyclopropene on the quality of tomato harvested at different maturity stages. J. Sci. Food Agric. 2011, 91, 2835–2843. [Google Scholar] [CrossRef] [PubMed]
- Ekman, J.; Clayton, M.; Biasi, W.; Mitcham, E. Interactions between 1-MCP concentration, treatment interval and storage time for ‘Bartlett’pears. Postharvest Biol. Technol. 2004, 31, 127–136. [Google Scholar] [CrossRef]
- Inaba, A.; Liu, X.; Yokotani, N.; Yamane, M.; Lu, W.-J.; Nakano, R.; Kubo, Y. Differential feedback regulation of ethylene biosynthesis in pulp and peel tissues of banana fruit. J. Exp. Bot. 2007, 58, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Wills, R.; Ku, V. Use of 1-MCP to extend the time to ripen of green tomatoes and postharvest life of ripe tomatoes. Postharvest Biol. Technol. 2002, 26, 85–90. [Google Scholar] [CrossRef]
- Jin, P.; Fu, J.; Du, W.; Li, H.; Cui, G. Effects of 1-MCP on Storage Quality and Enzyme Activity of Petals of Edible Rose Cultivar ‘Dianhong’at Low Temperatures. Horticulturae 2022, 8, 954. [Google Scholar] [CrossRef]
- Du, M.; Jia, X.; Li, J.; Li, X.; Jiang, J.; Li, H.; Zheng, Y.; Liu, Z.; Zhang, X.; Fan, J. Regulation effects of 1-MCP combined with flow microcirculation of sterilizing medium on peach shelf quality. Sci. Hortic. 2020, 260, 108867. [Google Scholar] [CrossRef]
- Barbagallo, R.N.; Chisari, M.; Caputa, G. Effects of calcium citrate and ascorbate as inhibitors of browning and softening in minimally processed ‘Birgah’eggplants. Postharvest Biol. Technol. 2012, 73, 107–114. [Google Scholar] [CrossRef]
- Xin, Y.; Jin, Z.; Chen, F.; Lai, S.; Yang, H. Effect of chitosan coatings on the evolution of sodium carbonate-soluble pectin during sweet cherry softening under non-isothermal conditions. Int. J. Biol. Macromol. 2020, 154, 267–275. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, X.; Zhang, Z.; Jiang, G.; Feng, L.; Duan, X.; Jiang, Y. 6-Benzylaminopurine improves the quality of harvested litchi fruit. Postharvest Biol. Technol. 2018, 143, 137–142. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Yan, L.; Zheng, H.; Liu, W.; Liu, C.; Jin, T.; Liu, S.; Zheng, L. UV-C treatment enhances organic acids and GABA accumulation in tomato fruits during storage. Food Chem. 2021, 338, 128126. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, C.; Zhang, L.; Wang, J.; Huang, X.; Zhao, Y.; Liu, Y.; Li, C. Application of chlorine dioxide microcapsule sustained-release antibacterial films for preservation of mangos. J. Food Sci. Technol. 2019, 56, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Galeazzi, M.A.; Sgarbieri, V.C.; Constantinides, S.M. Isolation, purification and physicochemical characterization of polyphenoloxidases (PPO) from a dwarf variety of banana (Musa cavendishii L.). J. Food Sci. 1981, 46, 150–155. [Google Scholar] [CrossRef]
- Wan, C.; Kahramanoğlu, İ.; Chen, J.; Gan, Z.; Chen, C. Effects of hot air treatments on postharvest storage of Newhall navel orange. Plants 2020, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Song, L.; You, Y.; Li, Y.; Duan, X.; Jiang, Y.; Joyce, D.C.; Ashraf, M.; Lu, W. Cold storage duration affects litchi fruit quality, membrane permeability, enzyme activities and energy charge during shelf time at ambient temperature. Postharvest Biol. Technol. 2011, 60, 24–30. [Google Scholar] [CrossRef]
- Ezzat, A.; Szabó, S.; Szabó, Z.; Hegedűs, A.; Berényi, D.; Holb, I.J. Temporal patterns and inter-correlations among physical and antioxidant attributes and enzyme activities of apricot fruit inoculated with Monilinia laxa under salicylic acid and methyl jasmonate treatments under shelf-life conditions. J. Fungi 2021, 7, 341. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Zhang, J.; Xiang, M.; Zeng, J.; Chen, J.; Chen, M. Exogenous melatonin treatment affects ascorbic acid metabolism in postharvest ‘Jinyan’kiwifruit. Front. Nutr. 2022, 9, 1081476. [Google Scholar] [CrossRef]
- Kobori, R.; Yakami, S.; Kawasaki, T.; Saito, A. Changes in the polyphenol content of red raspberry fruits during ripening. Horticulturae 2021, 7, 569. [Google Scholar] [CrossRef]
- Ding, Y.; Sheng, J.; Li, S.; Nie, Y.; Zhao, J.; Zhu, Z.; Wang, Z.; Tang, X. The role of gibberellins in the mitigation of chilling injury in cherry tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2015, 101, 88–95. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, X.; Hou, Y.; Wang, X.; Li, X. Effects of nitric oxide fumigation treatment on retarding cell wall degradation and delaying softening of winter jujube (Ziziphus jujuba Mill. cv. Dongzao) fruit during storage. Postharvest Biol. Technol. 2019, 156, 110954. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, F.; An, H.; Yang, H.; Sun, X.; Guo, X.; Li, L. Physicochemical properties, firmness, and nanostructures of sodium carbonate-soluble pectin of 2 Chinese cherry cultivars at 2 ripening stages. J. Food Sci. 2008, 73, N17–N22. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, Q.; Zhou, X.; Wei, B.; Ji, S. The effect of ethylene absorbent treatment on the softening of blueberry fruit. Food Chem. 2018, 246, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Sun, X.; Lu, H.; Yang, H.; Ruan, Q.; Huang, H.; Chen, M. Detecting and monitoring the flavor of tomato (Solanum lycopersicum) under the impact of postharvest handlings by physicochemical parameters and electronic nose. Sensors 2018, 18, 1847. [Google Scholar] [CrossRef]
- Hossain, M.S.; Ramachandraiah, K.; Hasan, R.; Chowdhury, R.I.; Kanan, K.A.; Ahmed, S.; Ali, M.A.; Islam, M.T.; Ahmed, M. Application of oxalic acid and 1-methylcyclopropane (1-MCP) with low and high-density polyethylene on post-harvest storage of litchi fruit. Sustainability 2021, 13, 3703. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, M.; Bai, L.; Han, X.; Ge, Y.; Wang, W.; Li, J. Effects of 1-methylcyclopropene (1-MCP) on the expression of genes involved in the chlorophyll degradation pathway of apple fruit during storage. Food Chem. 2020, 308, 125707. [Google Scholar] [CrossRef]
- Romanazzi, G.; Feliziani, E.; Baños, S.B.; Sivakumar, D. Shelf life extension of fresh fruit and vegetables by chitosan treatment. Crit. Rev. Food Sci. Nutr. 2017, 57, 579–601. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Rincón, M.; Pulido, R.; Saura-Calixto, F. Guava fruit (Psidium guajava L.) as a new source of antioxidant dietary fiber. J. Agric. Food Chem. 2001, 49, 5489–5493. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Malik, A.U.; Anwar, R.; Anjum, M.A.; Nawaz, A.; Shafique, M.; Naz, S. Combined application of ascorbic and oxalic acids delays postharvest browning of litchi fruits under controlled atmosphere conditions. Food Chem. 2021, 350, 129277. [Google Scholar] [CrossRef]
- Strauss, C.R.; Wilson, B.; Williams, P.J. Novel monoterpene diols and diol glycosides in Vitis vinifera grapes. J. Agric. Food Chem. 1988, 36, 569–573. [Google Scholar] [CrossRef]
- Chiriboga, M.-A.; Bordonaba, J.G.; Schotsmans, W.C.; Larrigaudière, C.; Recasens, I. Antioxidant potential of ‘Conference’pears during cold storage and shelf life in response to 1-methylcyclopropene. LWT-Food Sci. Technol. 2013, 51, 170–176. [Google Scholar] [CrossRef]
- Ali, S.; Anjum, M.A.; Nawaz, A.; Naz, S.; Hussain, S.; Ejaz, S.; Sardar, H. Effect of pre-storage ascorbic acid and Aloe vera gel coating application on enzymatic browning and quality of lotus root slices. J. Food Biochem. 2020, 44, e13136. [Google Scholar] [CrossRef] [PubMed]
- Javed, H.U.; Liu, R.; Li, C.; Zhong, S.; Lai, J.; Hasan, M.; Shu, X.; Zeng, L.-Y. Preparation of Vanillin-Taurine Antioxidant Compound, Characterization, and Evaluation for Improving the Post-Harvest Quality of Litchi. Antioxidants 2023, 12, 618. [Google Scholar] [CrossRef]
- Jimenez, A.; Creissen, G.; Kular, B.; Firmin, J.; Robinson, S.; Verhoeyen, M.; Mullineaux, P. Changes in oxidative processes and components of the antioxidant system during tomato fruit ripening. Planta 2002, 214, 751–758. [Google Scholar] [CrossRef]
- Kanamoto, H.; Takemura, M.; Ohyama, K. Cloning and expression of three lipoxygenase genes from liverwort, Marchantia polymorpha L. in Escherichia coli. Phytochemistry 2012, 77, 70–78. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, H.; Zhang, S.; Chen, Y.; Chen, M.; Lin, Y. The role of active oxygen metabolism in hydrogen peroxide-induced pericarp browning of harvested longan fruit. Postharvest Biol. Technol. 2014, 96, 42–48. [Google Scholar] [CrossRef]
- Di Bella, M.C.; Niklas, A.; Toscano, S.; Picchi, V.; Romano, D.; Lo Scalzo, R.; Branca, F. Morphometric characteristics, polyphenols and ascorbic acid variation in Brassica oleracea L. novel foods: Sprouts, microgreens and baby leaves. Agronomy 2020, 10, 782. [Google Scholar] [CrossRef]
- Huang, H.; Guo, L.; Wang, L.; Wang, H.; Ma, S.; Jiang, Y.; Qu, H. 1-Methylcyclopropene (1-MCP) slows ripening of kiwifruit and affects energy status, membrane fatty acid contents and cell membrane integrity. Postharvest Biol. Technol. 2019, 156, 110941. [Google Scholar] [CrossRef]
- Giménez, M.J.; Valverde, J.M.; Valero, D.; Guillén, F.; Martínez-Romero, D.; Serrano, M.; Castillo, S. Quality and antioxidant properties on sweet cherries as affected by preharvest salicylic and acetylsalicylic acids treatments. Food Chem. 2014, 160, 226–232. [Google Scholar] [CrossRef]
- ROGIERS, S.Y.; Kumar, G.M.; KNOWLES, N.R. Maturation and ripening of fruit of Amelanchier alnifolia Nutt. are accompanied by increasing oxidative stress. Ann. Bot. 1998, 81, 203–211. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Luo, Z. Exogenous application of phytosulfokine α (PSKα) delays senescence in broccoli florets during cold storage by ensuring intracellular ATP availability and avoiding intracellular ROS accumulation. Sci. Hortic. 2021, 276, 109745. [Google Scholar] [CrossRef]
- Tao, D.; Wang, J.; Zhang, L.; Jiang, Y.; Lv, M. 1-Methylcyclopropene alleviates peel browning of ‘Nanguo’pears by regulating energy, antioxidant and lipid metabolisms after long term refrigeration. Sci. Hortic. 2019, 247, 254–263. [Google Scholar] [CrossRef]
- Wang, B.; Wang, J.; Feng, X.; Lin, L.; Zhao, Y.; Jiang, W. Effects of 1-MCP and exogenous ethylene on fruit ripening and antioxidants in stored mango. Plant Growth Regul. 2009, 57, 185–192. [Google Scholar] [CrossRef]
- Alonso, J.; Howell, N.; Canet, W. Purification and characterisation of two pectinmethylesterase from persimmon (Diospyros kaki). J. Sci. Food Agric. 1997, 75, 352–358. [Google Scholar] [CrossRef]
- Ullah, S.; Singh, Z.; Khan, A.S.; Khan, S.A.K.U.; Razzaq, K.; Payne, A.D. Postharvest application of 1-MCP and ethylene influences fruit softening and quality of’Arctic Pride’nectarine at ambient conditions. Aust. J. Crop Sci. 2016, 10, 1257–1265. [Google Scholar] [CrossRef]
- Reddy, S.; Sharma, R.; Barthakur, S. Influence of 1-MCP on texture, related enzymes, quality and their relative gene expression in ‘Amrapali’ mango (Mangifera indica L.) fruits. J. Food Sci. Technol. 2017, 54, 4051–4059. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, R.; Pal, R.; Paul, V.; Dahuja, A. 1-Methylcyclopropene influences biochemical attributes and fruit softening enzymes of ‘Santa Rosa’Japanese plum (Prunus salicina Lindl.). J. Plant Biochem. Biotechnol. 2012, 21, 295–299. [Google Scholar] [CrossRef]
- Jhalegar, M.J.; Sharma, R.; Pal, R.; Sharma, S. Effect of 1-MCP on shelf-life and quality of kiwifruit stored under ambient conditions. Indian J. Hortic. 2012, 69, 258–262. [Google Scholar]


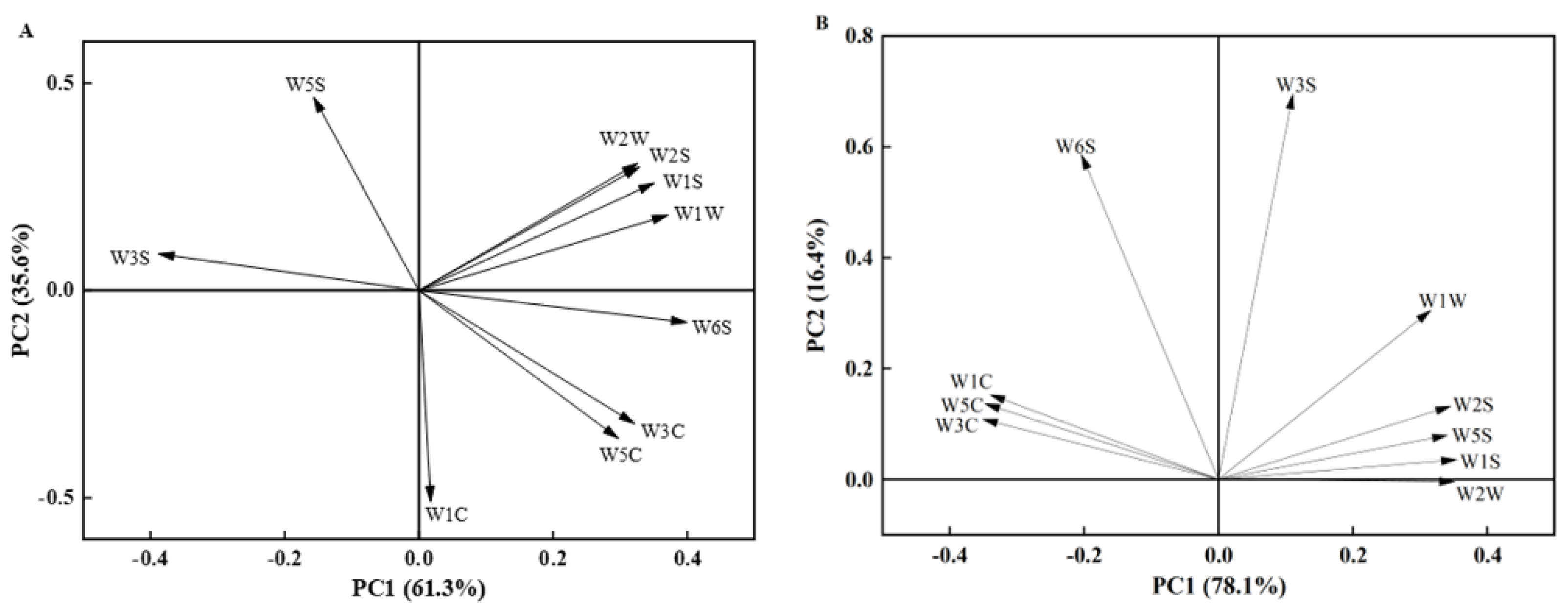


| No. | Sensor | Sensitive Compound |
|---|---|---|
| 1 | W1C | Aromatic compounds |
| 2 | W5S | Nitrogen oxides |
| 3 | W3C | Ammonia, aromatic compounds |
| 4 | W6S | Hydrogen |
| 5 | W5C | Short-chain alkane aromatic compounds |
| 6 | W1S | Methyl aromatics |
| 7 | W1W | Sulfides and terpenes |
| 8 | W2S | Aromatic compounds of alcohols and aldehydes and ketones |
| 9 | W2W | Organic sulfides and aromatic compounds |
| 10 | W3S | Long-chain alkanes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Ji, N.; Zhang, N.; Wang, R.; Li, Y.; Lei, J.; Zhou, R. Postharvest Quality Exploration of “Crystal” Grapes in Karst Mountainous Area: Regulatory Effect of High Concentration 1-MCP Fumigation. Agronomy 2023, 13, 2450. https://doi.org/10.3390/agronomy13102450
Liu R, Ji N, Zhang N, Wang R, Li Y, Lei J, Zhou R. Postharvest Quality Exploration of “Crystal” Grapes in Karst Mountainous Area: Regulatory Effect of High Concentration 1-MCP Fumigation. Agronomy. 2023; 13(10):2450. https://doi.org/10.3390/agronomy13102450
Chicago/Turabian StyleLiu, Renchan, Ning Ji, Ni Zhang, Rui Wang, Yuxin Li, Jiqing Lei, and Renzhang Zhou. 2023. "Postharvest Quality Exploration of “Crystal” Grapes in Karst Mountainous Area: Regulatory Effect of High Concentration 1-MCP Fumigation" Agronomy 13, no. 10: 2450. https://doi.org/10.3390/agronomy13102450
APA StyleLiu, R., Ji, N., Zhang, N., Wang, R., Li, Y., Lei, J., & Zhou, R. (2023). Postharvest Quality Exploration of “Crystal” Grapes in Karst Mountainous Area: Regulatory Effect of High Concentration 1-MCP Fumigation. Agronomy, 13(10), 2450. https://doi.org/10.3390/agronomy13102450






