Synergistic Effects of Bicarbonate and Selenium on Cadmium Transport in Karst-Adaptable Plants Based on Plant Electrical Signals
Abstract
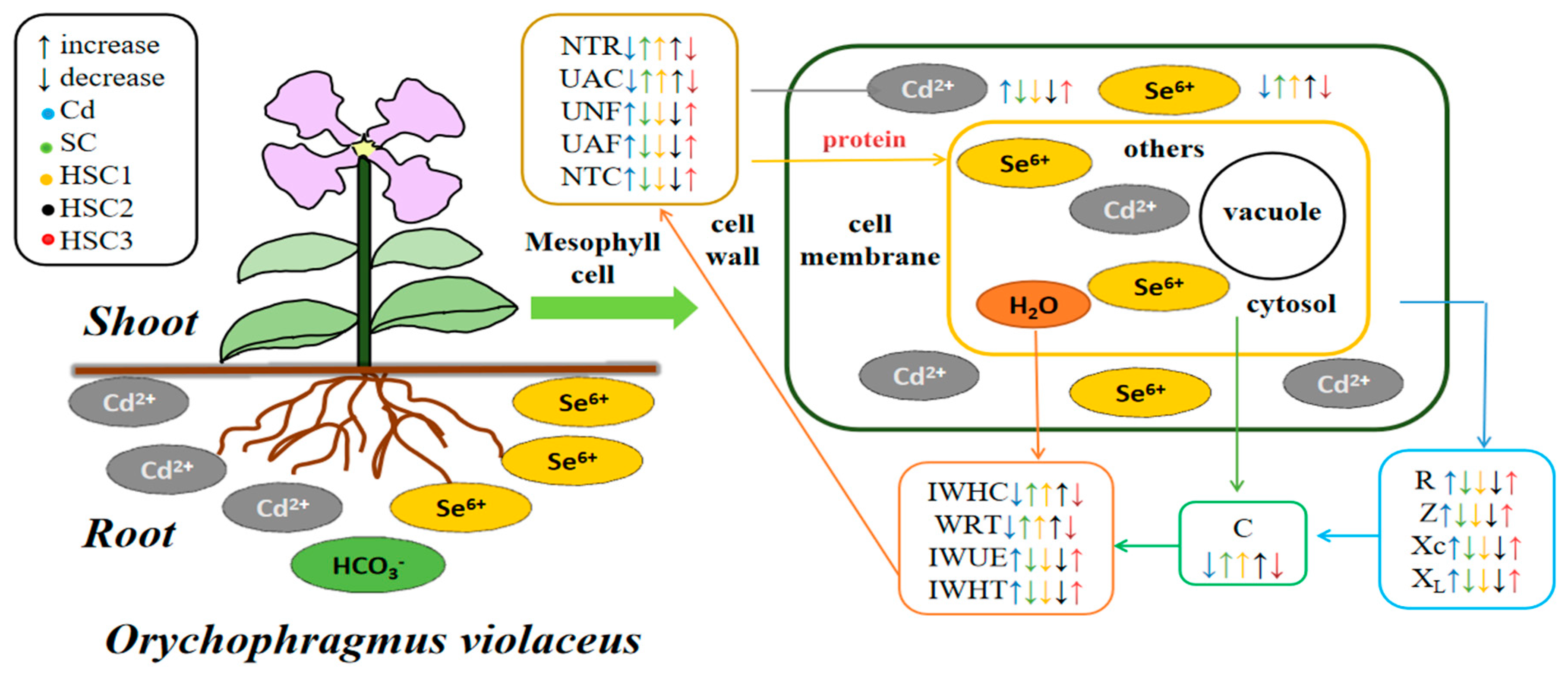
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Bicarbonate–Selenium–Cadmium Treatment
2.3. Plant Growth Conditions
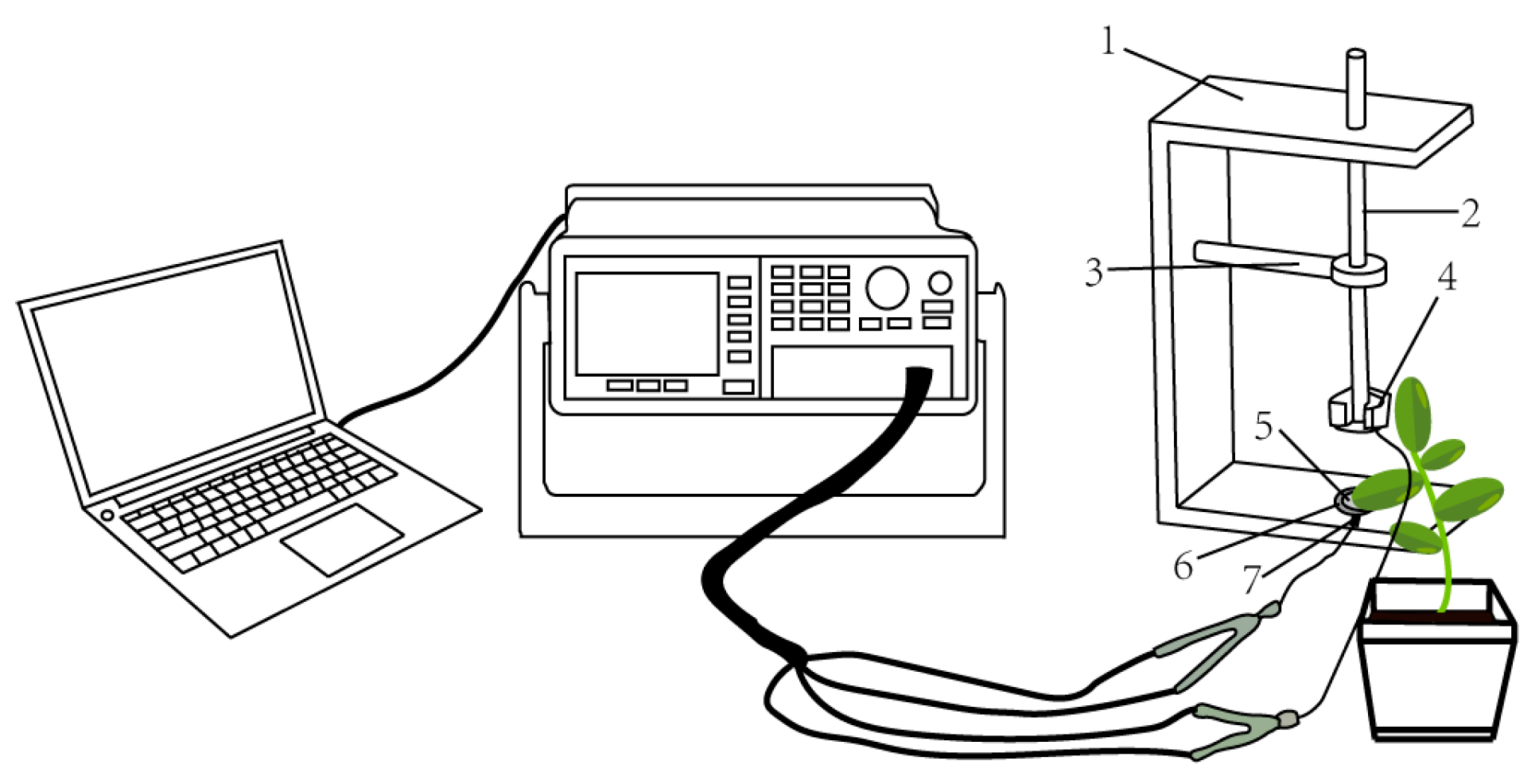
2.4. Measurement of Electrophysiological Information of Plants
2.5. Calculation of Plant Electrophysiological Parameters
2.6. Estimation of Intracellular Water Metabolism Capacity Based on Electrical Signals of Plant Species
2.7. Characterization of Nutrient Translocation Capacity BASED on Electrophysiological Signals of Plant Species
2.8. Selenium and Cadmium Content of Plants
2.9. Selenium–Cadmium Bio Transfer/Bioconcentration Factor
2.10. Statistical Analysis
3. Results
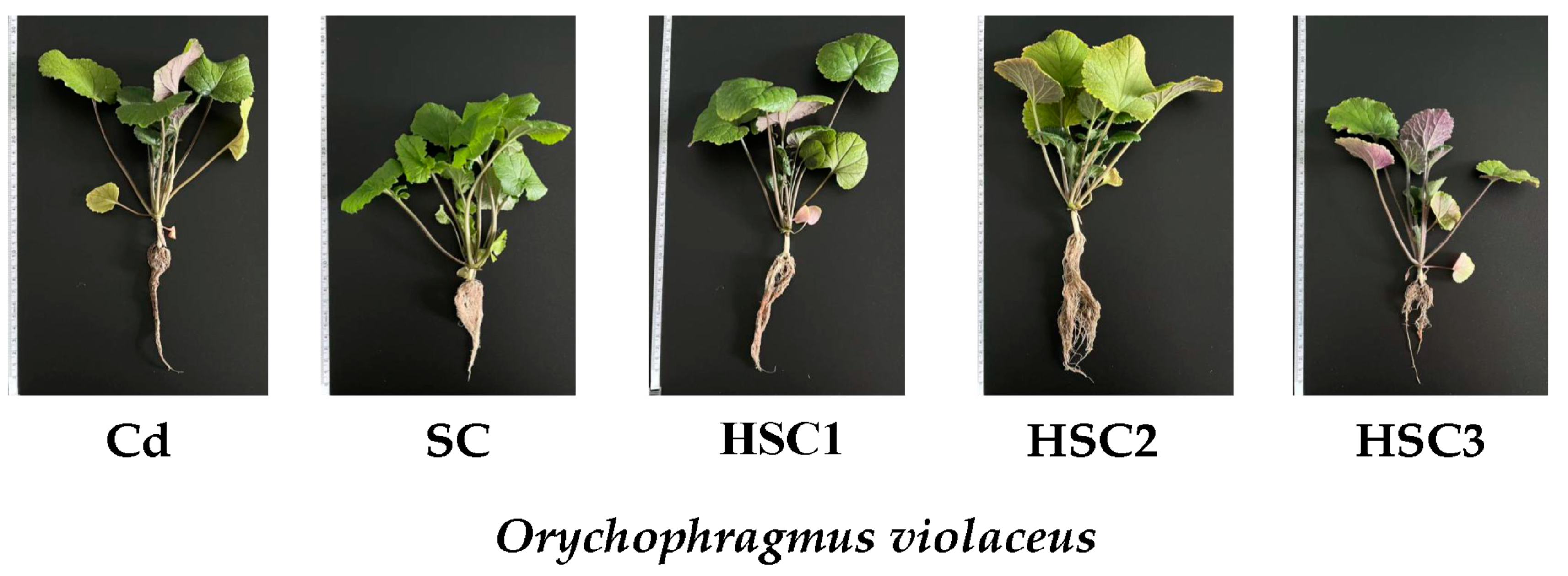
3.1. Plant Growth Features
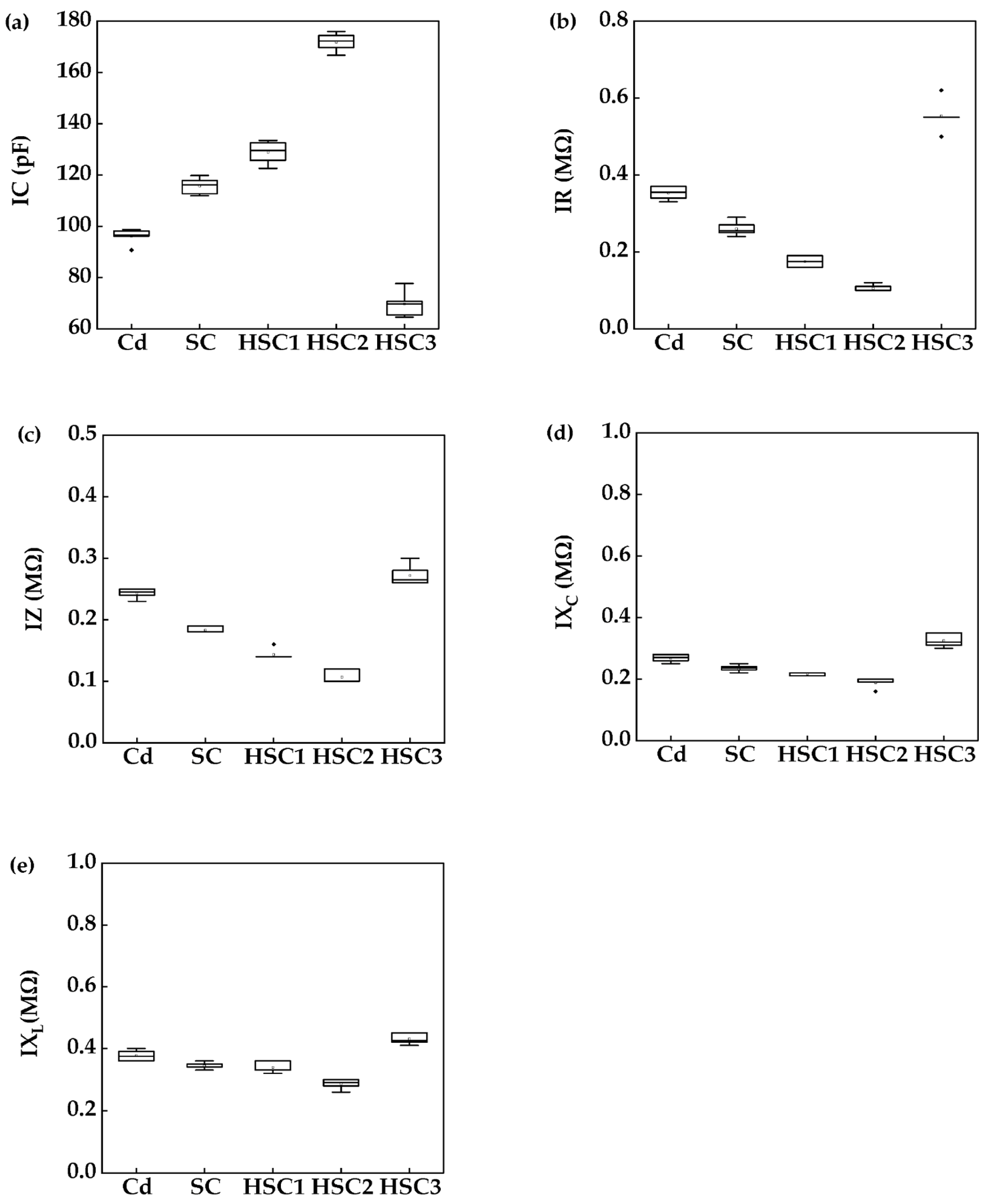
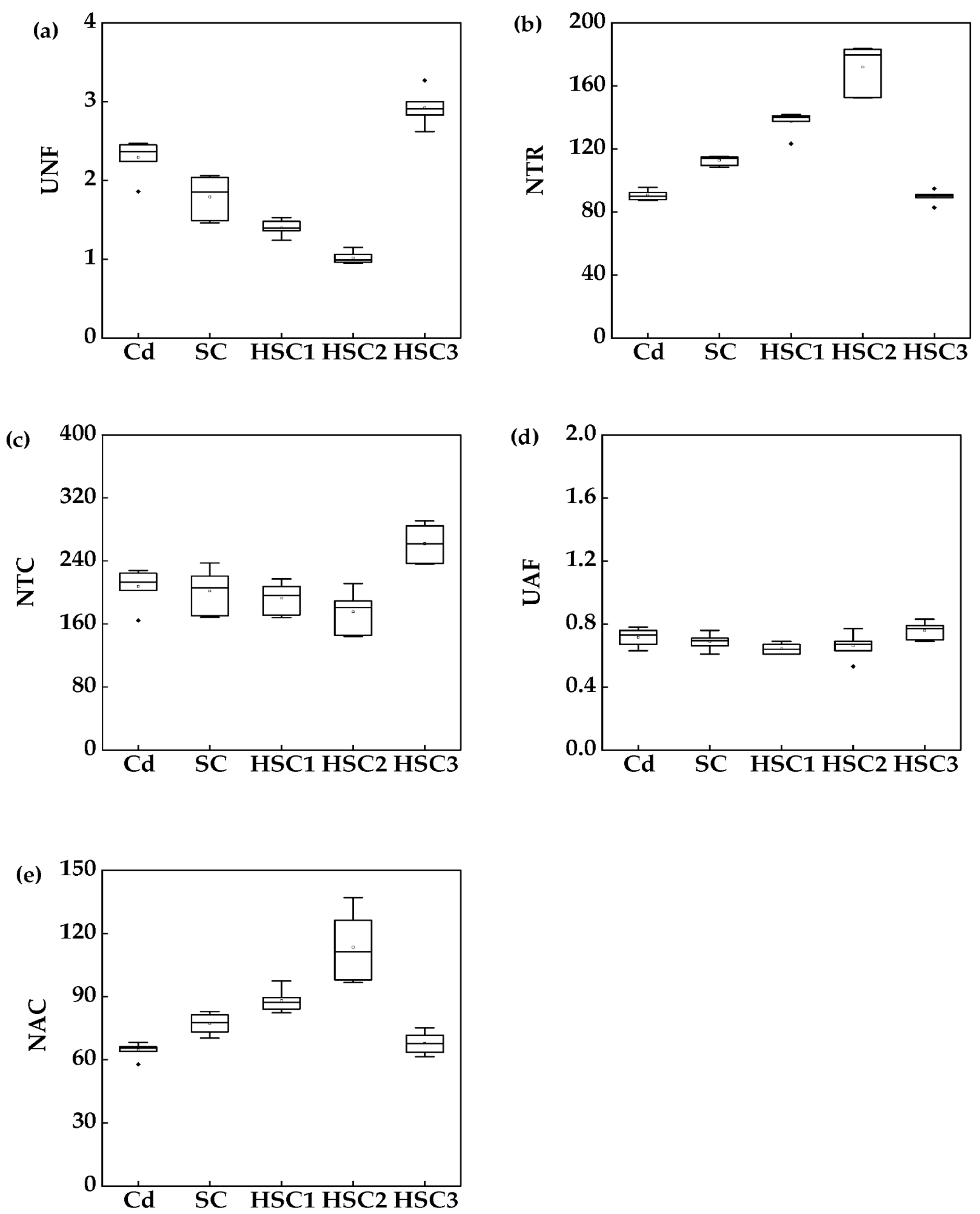
3.2. Electrophysiological Parameters of the Plants
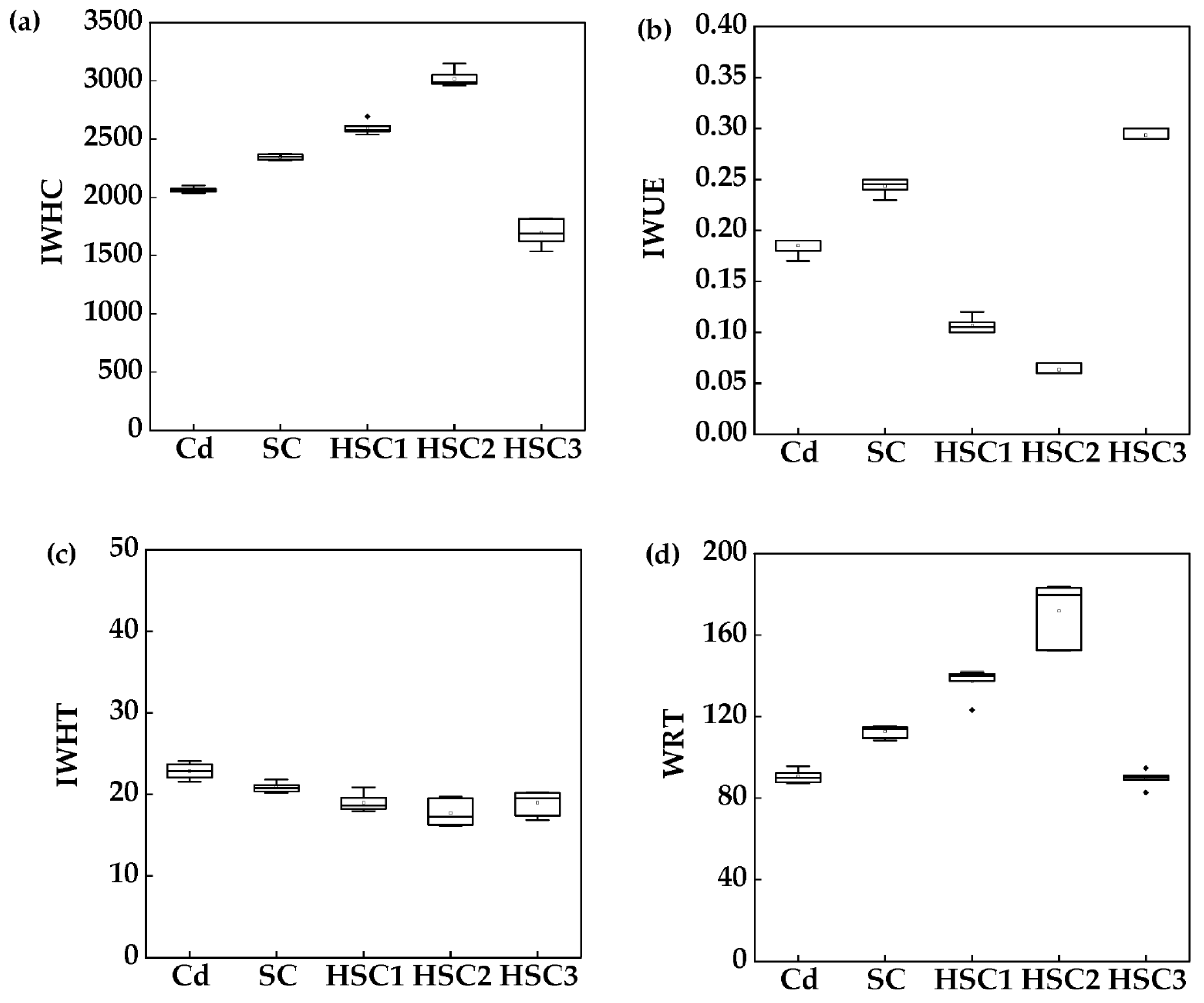
3.3. Intracellular Water Metabolism Capacity
3.4. Nutrient Translocation Capacity
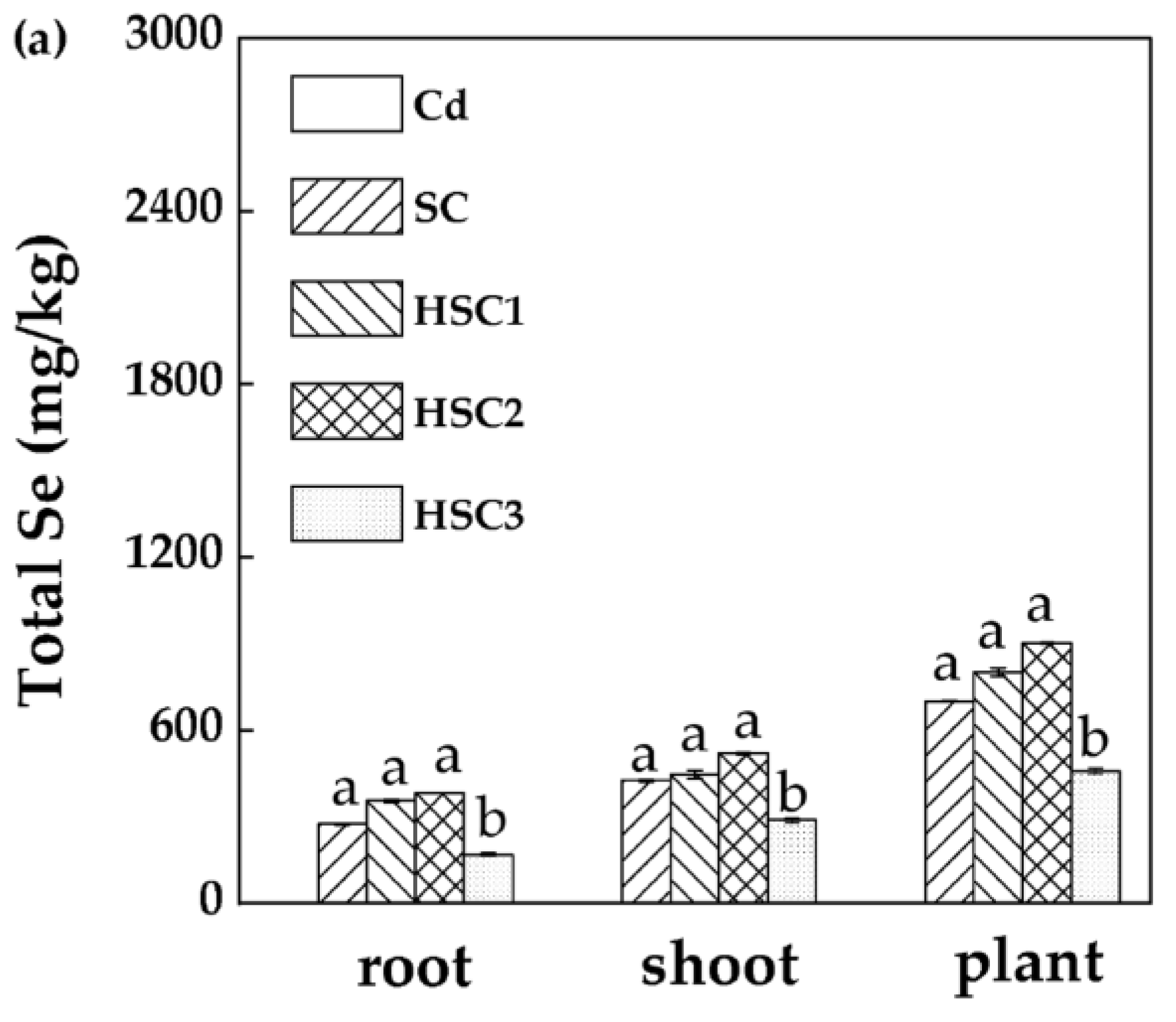
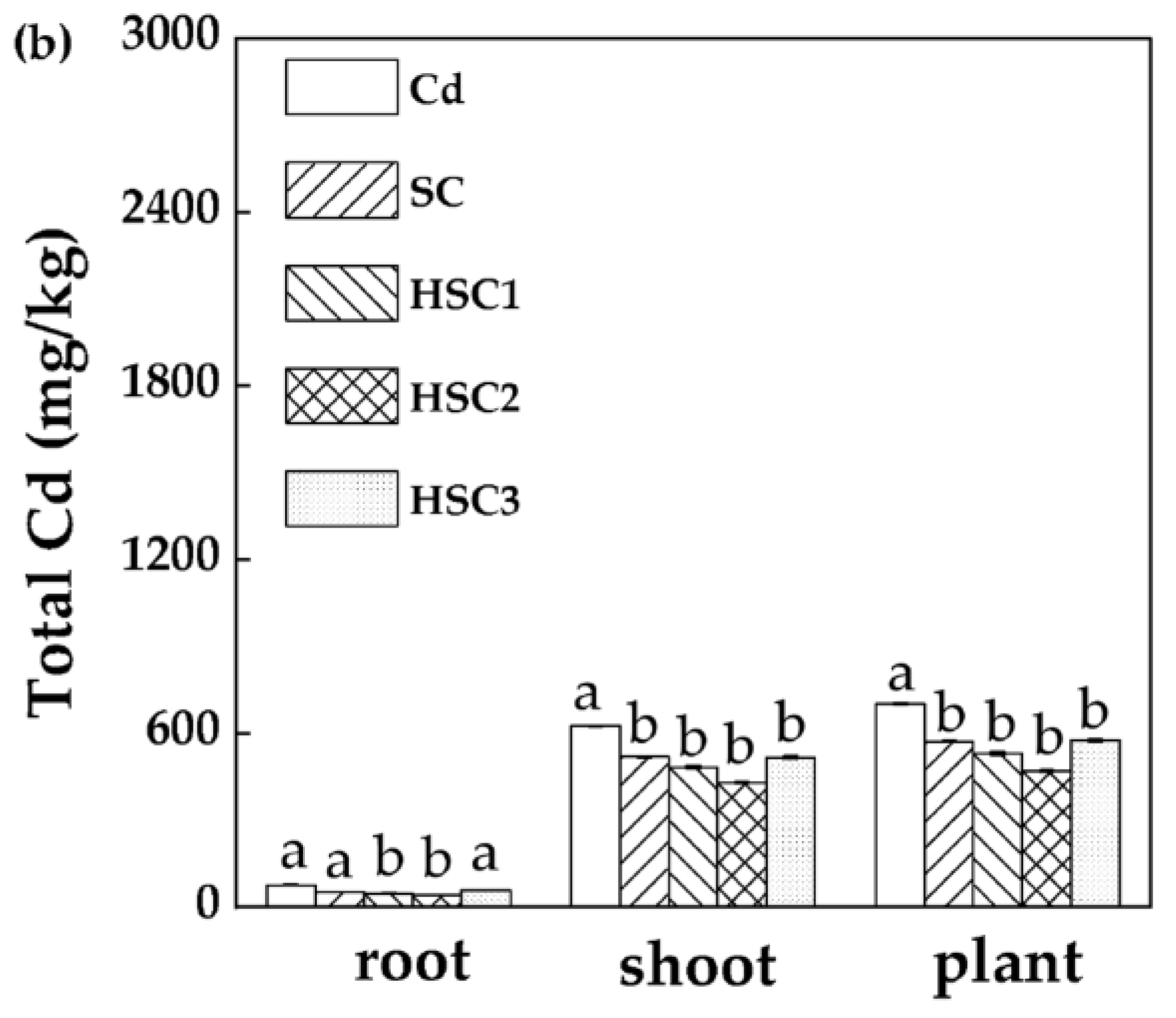
3.5. Characterization of the Selenium–Cadmium Translocation in Plants under the Influence of Bicarbonate
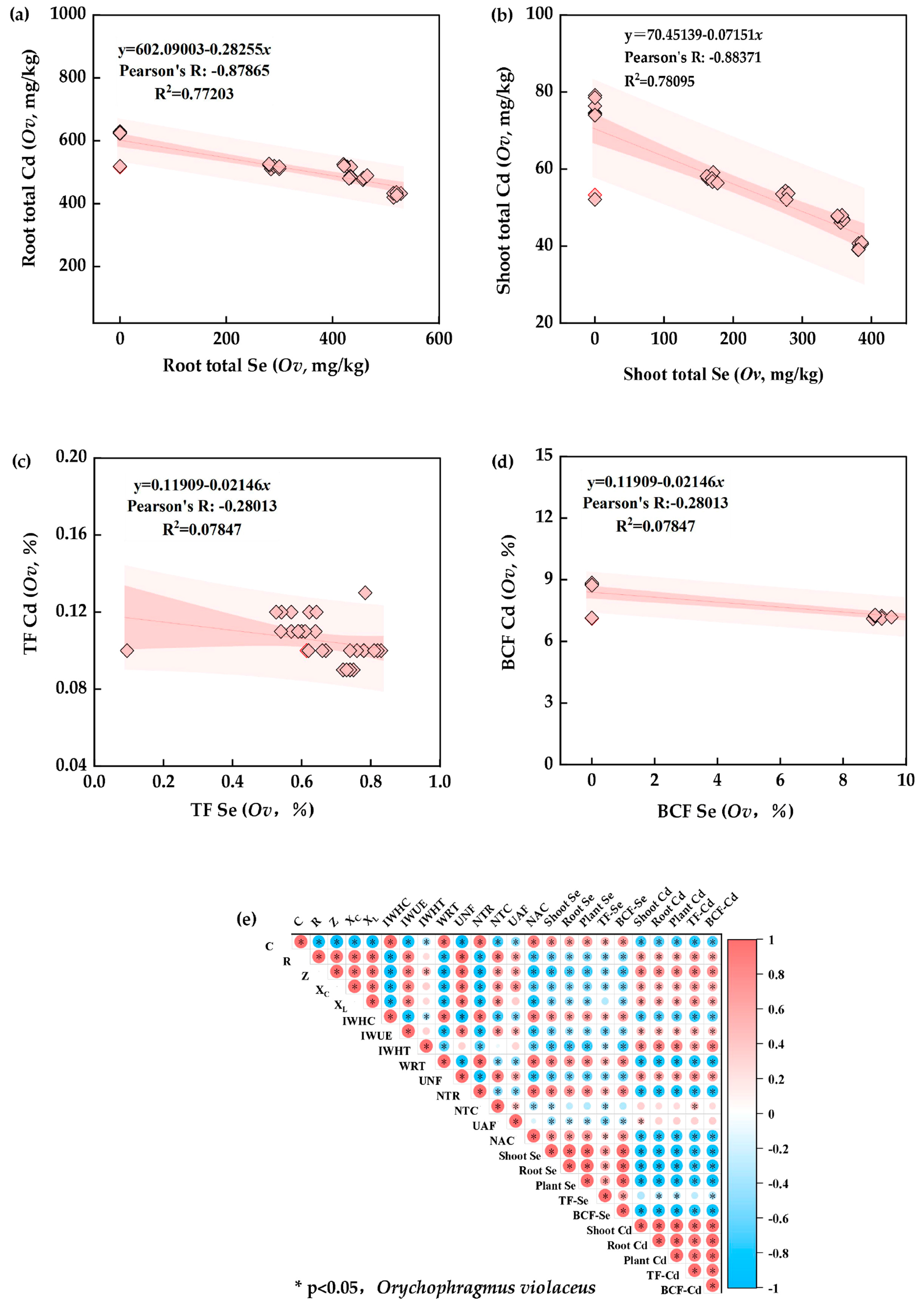
3.6. Correlation between Plant Electrical Signals and Selenium–Cadmium Translocation
4. Discussion
4.1. Characteristics in the Electrical Signals of Ov
4.2. Responses of Intracellular Water Capacity by Electrical Signal Analyses in Ov
4.3. Nutrient Translocation Capacity as Characterized by Plant Electrical Signals
4.4. Effect of Bicarbonate on Selenium–Cadmium Interactions in Plants
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, Y.; Zhang, Y.; Peng, C.; Wan, X.; Guo, Z.; Xiao, X. Distribution characteristics and risk assessment of heavy metals in soil and street dust with different land uses, a case in Changsha, China. Int. J. Environ. Res. Public Health 2021, 18, 10733. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, C.C.; Yu, T.; Liu, X.; Xia, X.; Hou, Q.; Yang, Y.; Yang, Z.; Wang, L. Annual net input fluxes of cadmium in paddy soils in karst and non-karst areas of Guangxi, China. J. Geochem. Explor. 2022, 241, 107072. [Google Scholar] [CrossRef]
- Schneeweiss, S.; Avorn, J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J. Clin. Epidemiol. 2005, 58, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Soubasakou, G.; Cavoura, O.; Damikouka, I. Phytoremediation of cadmium-contaminated soils: A review of new cadmium hyperaccumulators and factors affecting their efficiency. Bull. Environ. Contam. Toxicol. 2022, 109, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Abdallah, E.; Hashem, A.; Sarwat, M.; Gucel, S. Exogenous application of selenium mitigates cadmium toxicity in Brassica juncea L. (Czern & Cross) by up-regulating antioxidative system and secondary metabolites. J. Plant Growth Regul. 2016, 35, 936–950. [Google Scholar] [CrossRef]
- Huang, Q.; Xu, Y.; Liu, Y.; Qin, X.; Huang, R.; Liang, X. Selenium application alters soil cadmium bioavailability and reduces its accumulation in rice grown in Cd-contaminated soil. Environ. Sci. Pollut. Res. Int. 2018, 25, 31175–31182. [Google Scholar] [CrossRef]
- Kyriaki, P.; Mertzanis, A.; Dimitris, V.; Evripides, V.; Vasileios, K. The contribution of karstic rocks to soil quality, Ioannina plain (Epirus, Hellas). J. Geochem. Explor. 2015, 154, 224–237. [Google Scholar] [CrossRef]
- Reza, K.; Santos, R. Natural and human-induced factors on the accumulation and migration of pedogenic carbonate in soil: A review. Land 2022, 11, 1448. [Google Scholar] [CrossRef]
- Dubey, S.; Shri, M.; Gupta, A.; Rani, V.; Chakrabarty, D. Toxicity and detoxification of heavy metals during plant growth and metabolism. Environ. Chem. Lett. 2018, 16, 1169–1192. [Google Scholar] [CrossRef]
- Koselski, M.; Wasko, P.; Derylo, K.; Tchorzewski, M.; Trebacz, K. Glutamate-induced electrical and calcium signals in the moss physcomitrella patens. Plant Cell Physiol. 2020, 61, 1807–1817. [Google Scholar] [CrossRef]
- Volkov, A.; Nyasani, E.; Tuckett, C.; Scott, J.; Jackson, M.; Greeman, E.; Greenidge, A.; Cohen, D.; Volkova, M.; Shtessel, Y. Electrotonic potentials in Aloe vera L.: Effects of intercellular and external electrodes arrangement. Bioelectrochemistry 2017, 113, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Ekaterina, S.; Vladimir, S. Electrical signals, plant tolerance to actions of stressors, and programmed cell death: Is interaction possible? Plants 2021, 10, 1704. [Google Scholar] [CrossRef]
- Canales, J.; Henriquez, V.; Brauchi, S. The integration of electrical signals originating in the root of vascular plants. Front. Plant Sci. 2018, 8, 2173. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Mao, R.; Li, Z.; Wu, Y.; Qin, X.; Fu, W. Leaf intracellular water transport rate based on physiological impedance: A possible role of leaf internal retained water in photosynthesis and growth of tomatoes. Front. Plant Sci. 2022, 13, 845628. [Google Scholar] [CrossRef] [PubMed]
- Novacky, A.; Karr, A.; Van, J. Using electrophysiology to study plant disease development. Bioscience 1976, 26, 499–504. [Google Scholar] [CrossRef]
- Kashif, A.; Wu, Y.; Chen, Q.; Qureshi, W.; Xing, D.; Mazhar, H.; Sher, A. The differential responses of Aegiceras corniculatum and Kandelia candel under salt stress and re-watering phase. A study of leaf electrophysiological and growth parameters. J. Plant Interact. 2021, 16, 307–320. [Google Scholar] [CrossRef]
- Xie, J.; Wu, Y.; Xing, D.; Li, Z.; Chen, T.; Duan, R.; Zhu, X. A comparative study on the circadian rhythm of the electrical signals of Broussonetia papyrifera and Morus alba. Plant Signal Behav. 2021, 16, 1950899. [Google Scholar] [CrossRef]
- Xing, D.; Chen, X.; Wu, Y.; Xu, X.; Chen, Q.; Li, L.; Zhang, C. Rapid prediction of the re-watering time point of Orychophragmus violaceus L. based on the online monitoring of electrophysiological indexes. Sci. Hortic. 2019, 256, 108642. [Google Scholar] [CrossRef]
- Palmer, C.; Warwick, S.; Keller, W. Brassicaceae (Cruciferae) family, plant biotechnology, and phytoremediation. Int. J. Phytoremediation 2001, 3, 245–287. [Google Scholar] [CrossRef]
- Kovács, Z.; Soós, Á.; Kovács, B.; Kaszás, L.; Elhawat, N.; Bákonyi, N.; Razem, M.; Fári, M.; Prokisch, J.; Domokos, S.; et al. Uptake dynamics of ionic and elemental selenium forms and their metabolism in multiple-harvested alfalfa (Medicago sativa L.). Plants 2021, 10, 1277. [Google Scholar] [CrossRef]
- Kong, W.; Hou, X.; Wei, L.; Chen, W.; Liu, J.; Schnoor, J.; Jiang, G. Accumulation, translocation, and transformation of two CdSe/ZnS quantum dots in rice and pumpkin plants. Sci. Total Environ. 2023, 864, 161156. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, C.; Wu, Z.; Liu, X.; Cai, M.; Jia, W.; Zhao, X. Selenium reduces cadmium accumulation in seed by increasing cadmium retention in root of oilseed rape (Brassica napus L.). Environ. Exp. Bot. 2019, 158, 161–170. [Google Scholar] [CrossRef]
- Grybos, M.; Davranche, M.; Gruau, G.; Petitjean, P.; Pédrot, M. Increasing pH drives organic matter solubilization from wetland soils under reducing conditions. Geoderma 2009, 154, 13–19. [Google Scholar] [CrossRef]
- Wang, F.; Wang, X.; Chen, Q.; Song, N. Extension of a biotic ligand model for predicting the toxicity of metalloid selenate to wheat: The effects of pH, phosphate and sulphate. Chemosphere 2021, 264, 128424. [Google Scholar] [CrossRef] [PubMed]
- Camara, A.; Wan, Y.; Yu, Y.; Wang, Q.; Li, H. Effect of selenium on uptake and translocation of arsenic in rice seedlings (Oryza sativa L.). Ecotox. Environ. Saf. 2018, 148, 869–875. [Google Scholar] [CrossRef]
- Sakouhi, L.; Mahjoubi, Y.; Labben, A.; Kharbech, O.; Chaoui, A.; Djebali, W. Effects of cadmium-selenium interaction on glyoxalase and antioxidant systems of pisum sativum germinating seeds. J. Plant Growth Regul. 2022, 42, 3084–3099. [Google Scholar] [CrossRef]
- Chrispeels, M.; Crawford, N.; Schroeder, J. Proteins for transport of water and mineral nutrients across the membranes of plant cells. Plant Cell 1999, 11, 661. [Google Scholar] [CrossRef]
- Ghosh, U.; Chatterjee, A.; Bremer, E. Determining the moisture and plant effect on nutrient release, and plant nutrient uptake using ion exchange resin membrane. Commun. Soil Sci. Plant Anal. 2018, 49, 782–790. [Google Scholar] [CrossRef]
- Dadmohammadi, Y.; Datta, A. Prediction of effective moisture diffusivity in plant tissue food materials over extended moisture range. Dry. Technol. 2019, 38, 1–15. [Google Scholar] [CrossRef]
- Li, H.; Lv, J.; Su, Y.; Wu, Y. Appropriate sodium bicarbonate concentration enhances the intracellular water metabolism, nutrient transport and photosynthesis capacities of Coix Iacryma-jobi L. Agronomy 2023, 13, 1790–1804. [Google Scholar] [CrossRef]
- Dreyer, I. Nutrient cycling is an important mechanism for homeostasis in plant cells. Plant Physiol. 2021, 187, 2246–2261. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, F.; Markgren, J.; Hedenqvist, M.; Johansson, E. Modeling to understand plant protein structure-function relationships-implications for seed storage proteins. Molecules 2020, 25, 873. [Google Scholar] [CrossRef] [PubMed]
- Decaroli, M.; Furini, A.; Dalcorso, G.; Rojas, M.; Di, S. Endomembrane reorganization induced by heavy metals. Plants 2020, 9, 482. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Wang, H.; Chen, J.; Chang, J.; Zhao, F. Molecular mechanisms underlying the toxicity and detoxification of trace metals and metalloids in plants. J. Integr. Plant Biol. 2023, 65, 570–593. [Google Scholar] [CrossRef]
- Li, D.; He, T.; Saleem, M.; He, G. Metalloprotein-specific or critical amino acid residues: Perspectives on plant-precise detoxification and recognition mechanisms under cadmium stress. Int. J. Mol. Sci. 2022, 23, 1734. [Google Scholar] [CrossRef]
- Miyoshi, Y.; Soma, F.; Yin, Y.; Nobuo, S.; Enomoto, K.; Nagao, Y.; Yamaguchi, M.; Kawachi, N.; Yoshida, E.; Tashima, H.; et al. Rice immediately adapts the dynamics of photosynthates translocation to roots in response to changes in soil water environment. Front. Plant Sci. 2023, 13, 1024144. [Google Scholar] [CrossRef]
| Treatment | Reagent | Substance Content |
|---|---|---|
| HCO3− | NaHCO3 | 1/5/15 mM |
| Se6+ | Na2Se2O4 | 0.46 mM |
| Cd2+ | CdSO4 | 0.27 mM |
| Cd | Cd2+ | 0.27 mM |
| SC | Se6+:Cd2+ | 0.46 mM:0.27 mM |
| HSC1 | HCO3−:Se6+:Cd2+ | 1 mM:0.46 mM:0.27 mM |
| HSC2 | HCO3−:Se6+:Cd2+ | 5 mM:0.46 mM:0.27 mM |
| HSC3 | HCO3−:Se6+:Cd2+ | 15 mM:0.46 mM:0.27 mM |
| pH | KOH | 7.9 ± 0.35 |
| Treatment | Root Dw, mg/Plant | Stem Dw, mg/Plant | Leaves Dw, mg/Plant | Plant Dw, mg/Plant | Root/Shoot Ratio R/S, % |
|---|---|---|---|---|---|
| Cd | 70.85 ± 7.83 b | 119.92 ± 10.23 c | 138.53 ± 10.76 d | 329.3 ± 16.86 d | 27.48 ± 3.31 a |
| SC | 76.71 ± 4.88 b | 148.51 ± 6.14 b | 190.87 ± 4.31 c | 416.08 ± 12.24 c | 22.6 ± 1.29 c |
| HSC1 | 117.42 ± 10.93 a | 213.5 ± 6.78 a | 278.99 ± 8.56 b | 609.91 ± 14.56 b | 23.87 ± 2.44 b |
| HSC2 | 129.2 ± 5.68 a | 222.75 ± 8.46 a | 333.3 ± 10.13 a | 685.25 ± 18.47 a | 23.24 ± 0.95 b |
| HSC3 | 37.64 ± 6.85 c | 60.3 ± 6.74 d | 82.3 ± 3.22 e | 180.24 ± 7.96 e | 26.53 ± 5.47 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, A.; Wu, Y.; Qin, Z.; Zhu, Y.; Li, L.; Xiao, J.; Aboueldahab, M.; Wan, H.; Ming, J.; Xiang, J. Synergistic Effects of Bicarbonate and Selenium on Cadmium Transport in Karst-Adaptable Plants Based on Plant Electrical Signals. Agronomy 2024, 14, 218. https://doi.org/10.3390/agronomy14010218
Xia A, Wu Y, Qin Z, Zhu Y, Li L, Xiao J, Aboueldahab M, Wan H, Ming J, Xiang J. Synergistic Effects of Bicarbonate and Selenium on Cadmium Transport in Karst-Adaptable Plants Based on Plant Electrical Signals. Agronomy. 2024; 14(1):218. https://doi.org/10.3390/agronomy14010218
Chicago/Turabian StyleXia, Antong, Yanyou Wu, Zhanghui Qin, Yunfen Zhu, Lin Li, Juyue Xiao, Mohamed Aboueldahab, Haiying Wan, Jiajia Ming, and Jiqian Xiang. 2024. "Synergistic Effects of Bicarbonate and Selenium on Cadmium Transport in Karst-Adaptable Plants Based on Plant Electrical Signals" Agronomy 14, no. 1: 218. https://doi.org/10.3390/agronomy14010218
APA StyleXia, A., Wu, Y., Qin, Z., Zhu, Y., Li, L., Xiao, J., Aboueldahab, M., Wan, H., Ming, J., & Xiang, J. (2024). Synergistic Effects of Bicarbonate and Selenium on Cadmium Transport in Karst-Adaptable Plants Based on Plant Electrical Signals. Agronomy, 14(1), 218. https://doi.org/10.3390/agronomy14010218







