Understanding the Role of Physiological and Agronomical Traits during Drought Recovery as a Determinant of Differential Drought Stress Tolerance in Barley
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
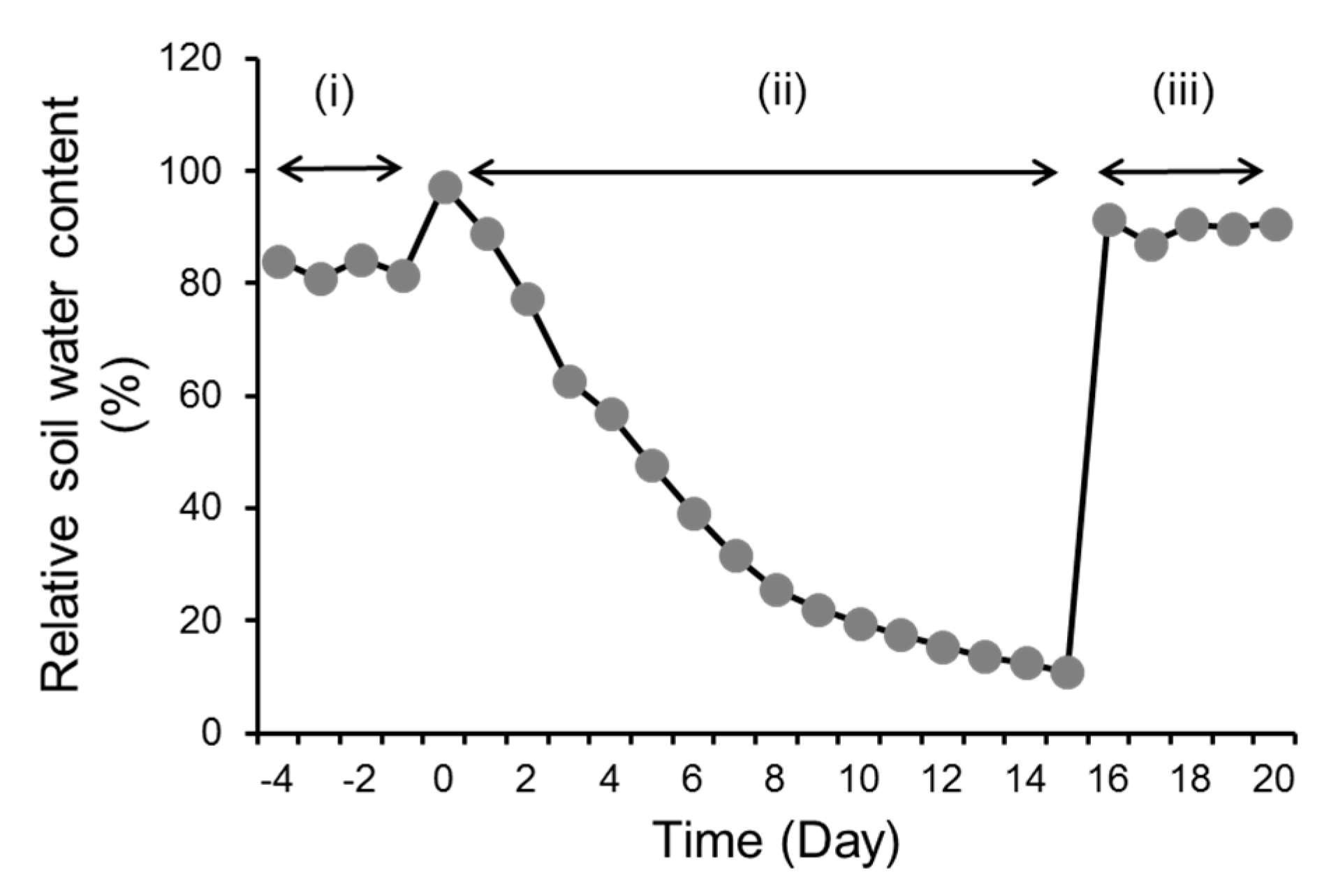
2.2. Scoring for Drought Stress Tolerance
2.3. Scoring for Drought Recovery Index
2.4. Scoring for Salinity Stress Tolerance
2.5. Traits Tested for Association with Drought Tolerance
2.5.1. Stomatal Density
2.5.2. Seedling Tests in Polyethylene Glycol (PEG) Media and Control Conditions
2.5.3. Chlorophyll Fluorescence
2.5.4. SPAD Values
2.5.5. Residual Transpiration
2.5.6. Statistical Analysis
3. Results
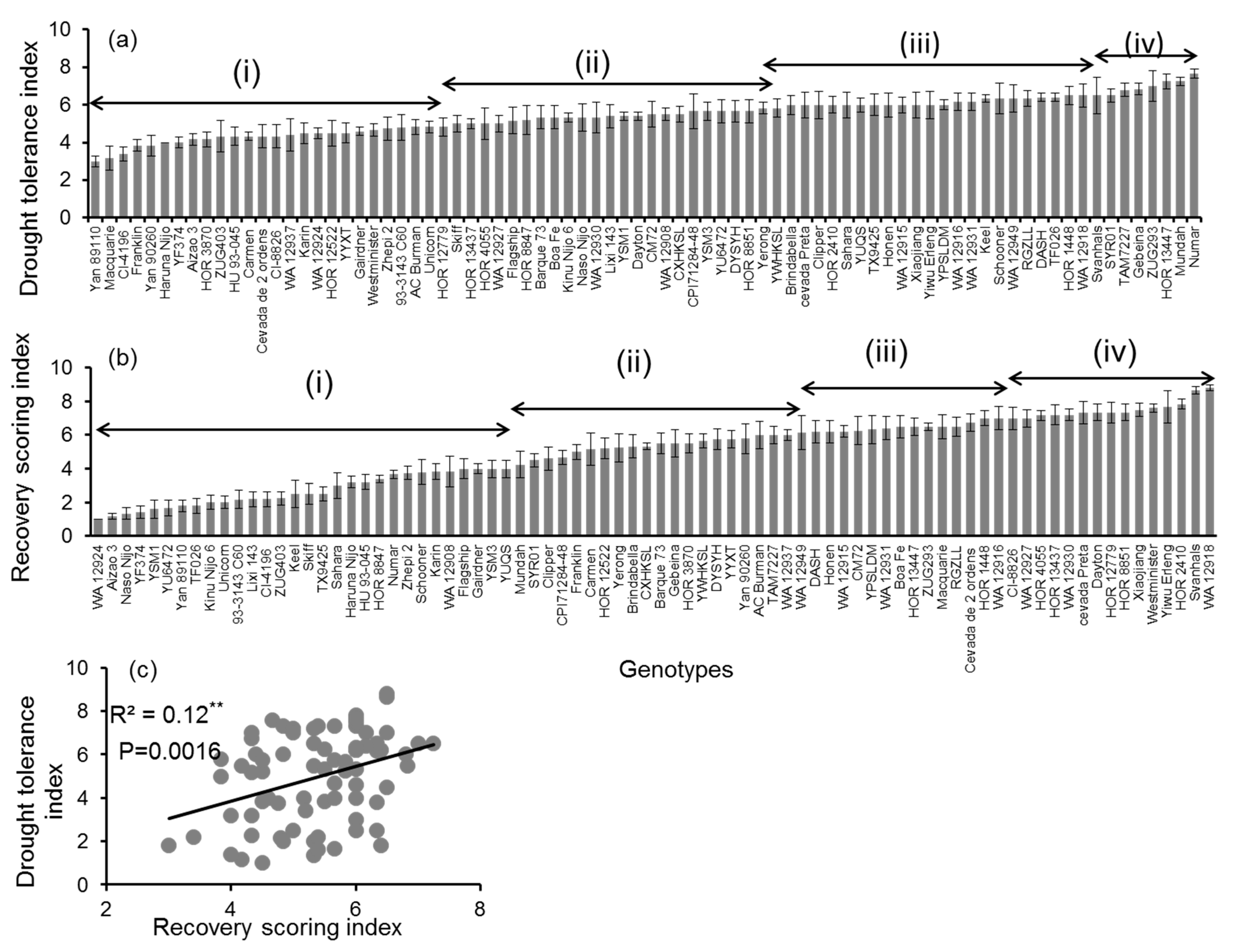
3.1. Genotypic Variation in Progressive Drought Stress and Recovery
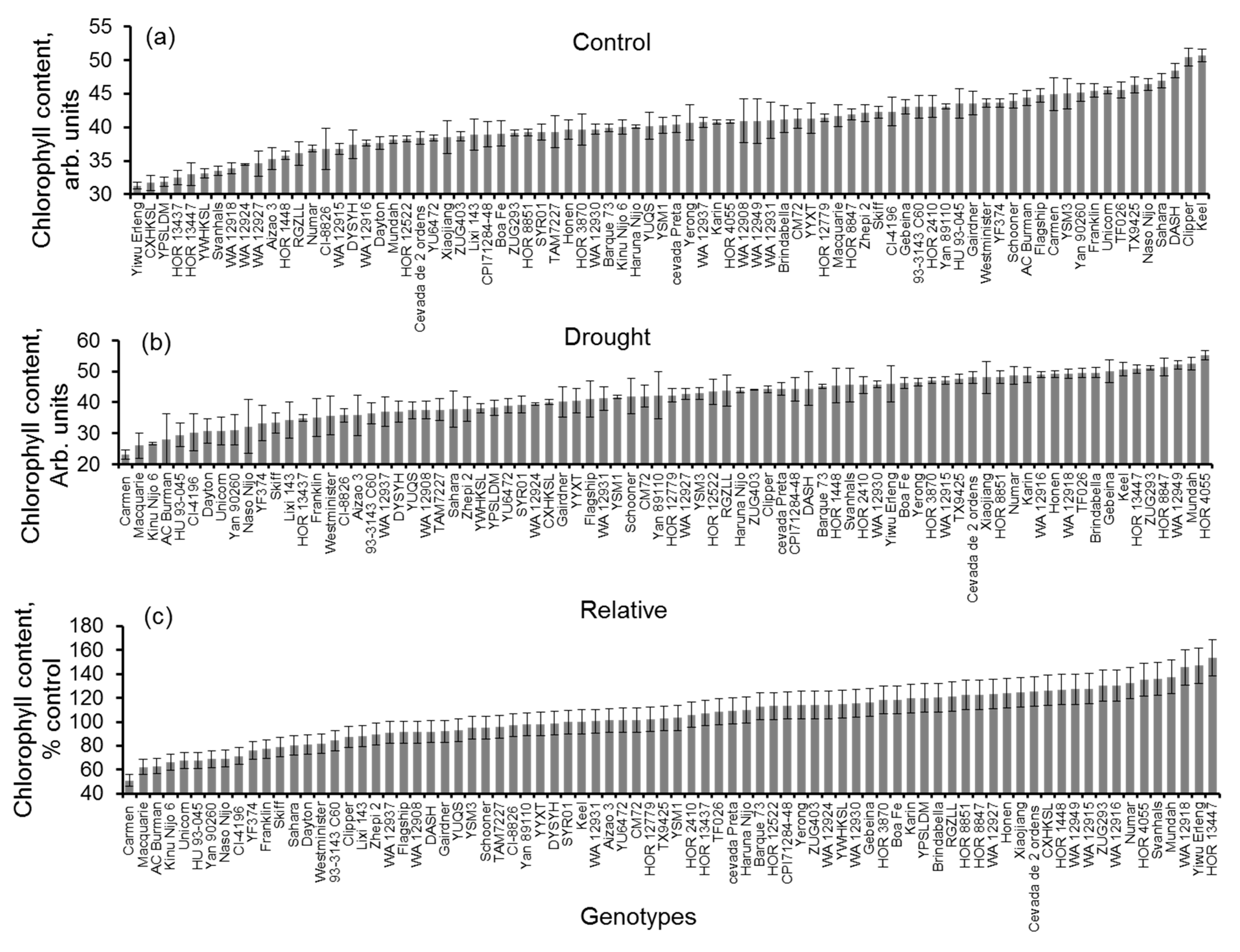
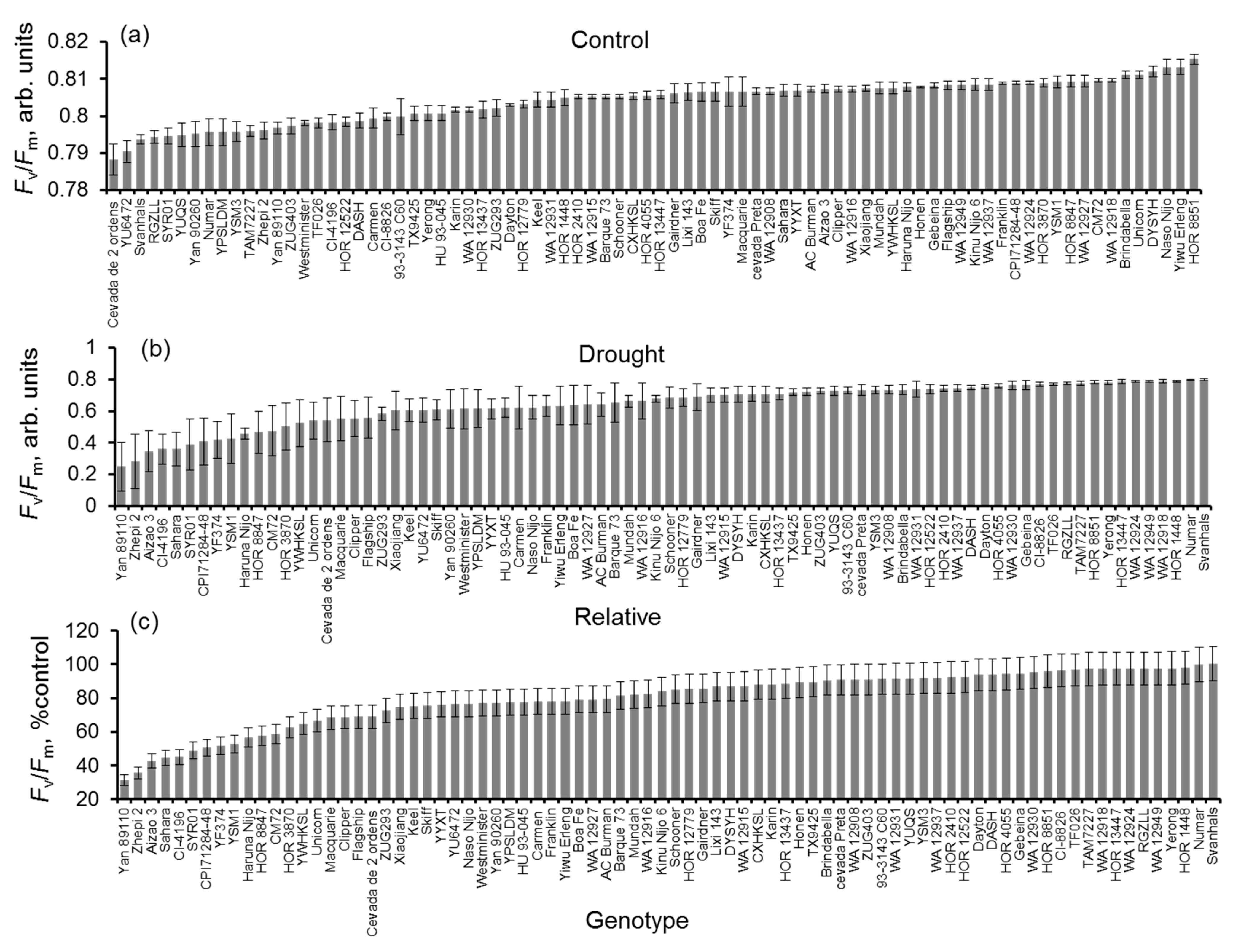
3.2. Impact of Drought Stress on Chlorophyll Content and Chlorophyll Fluorescence
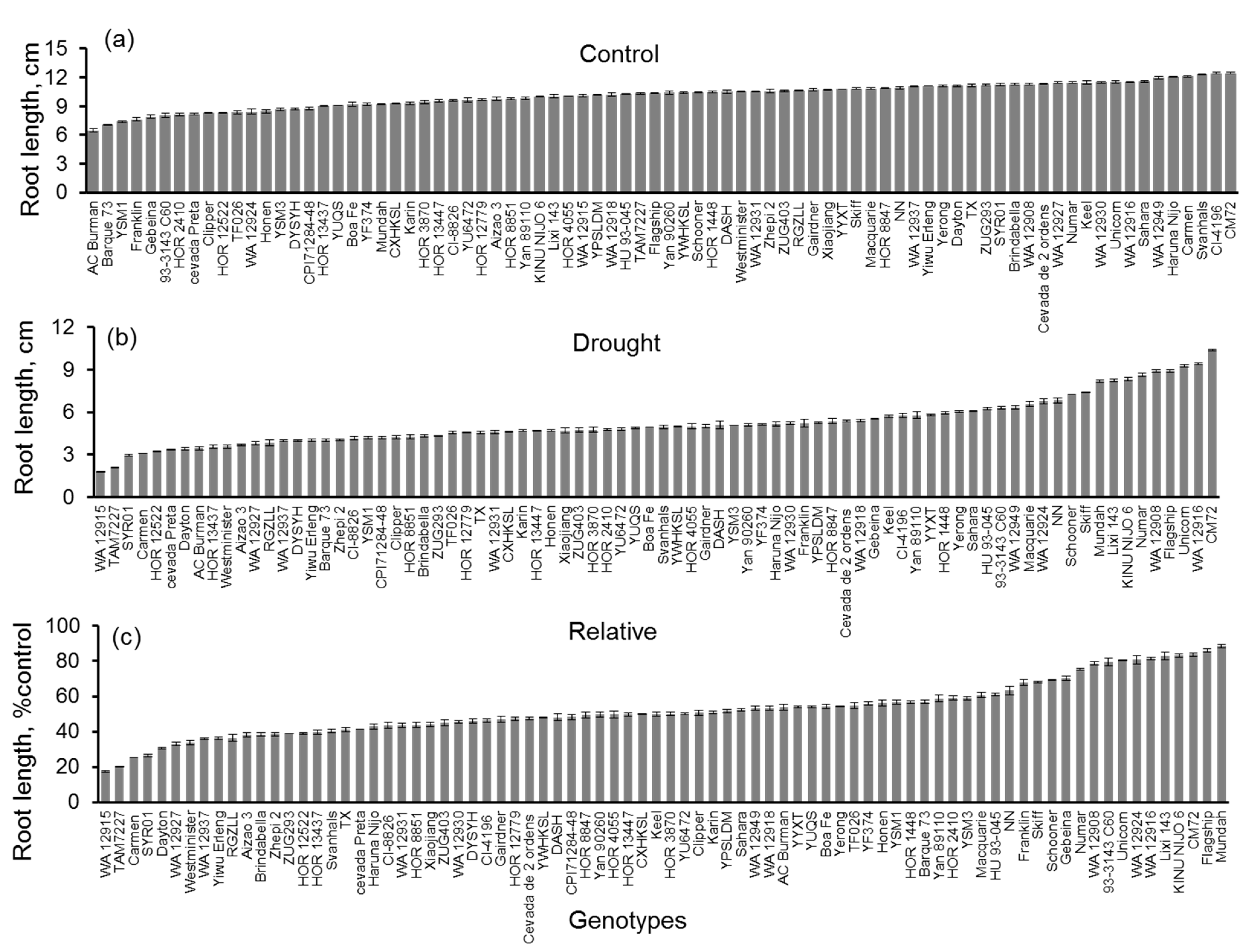
3.3. Seedling Tests in Control and PEG Solution
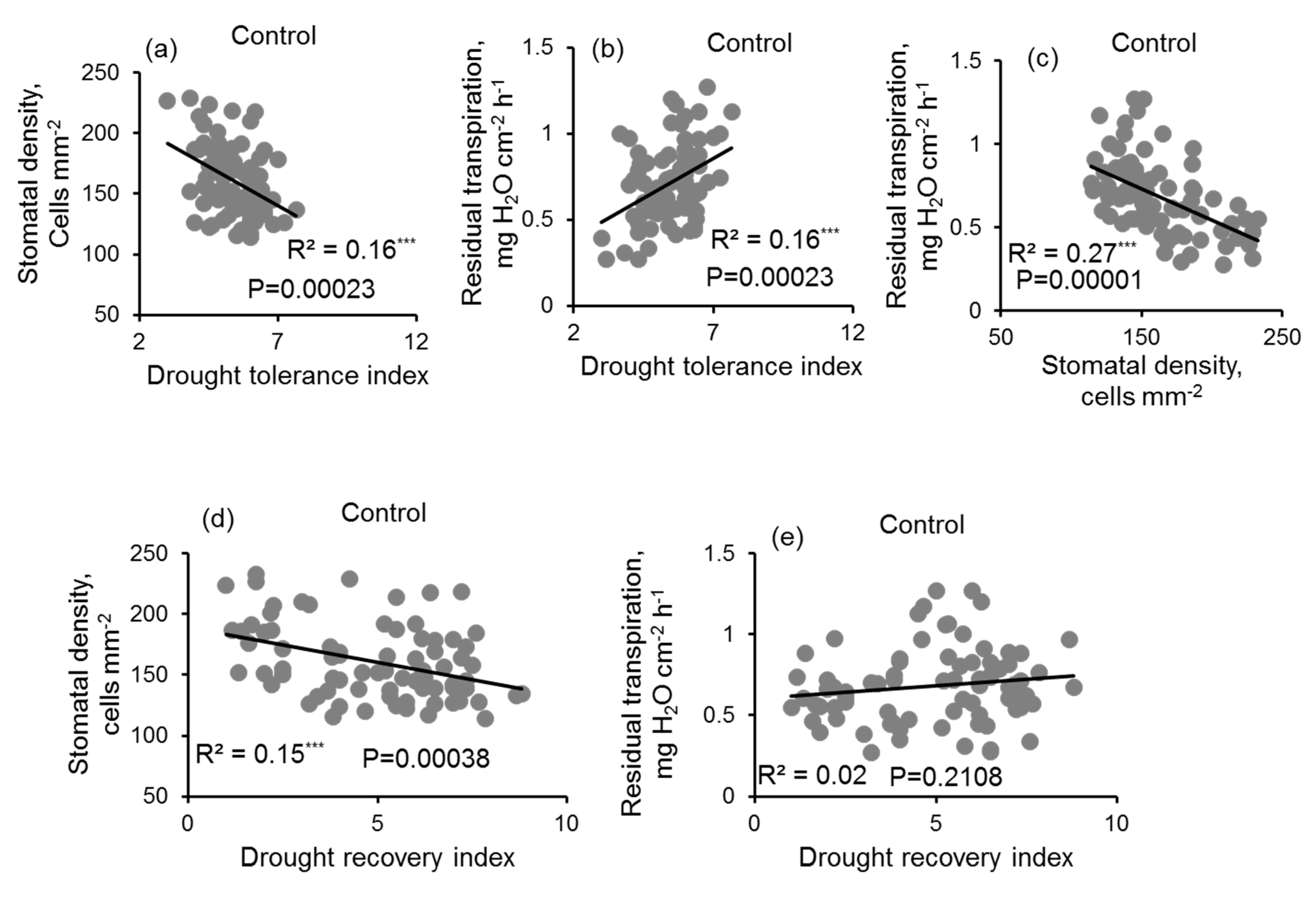
3.4. Correlation Analysis
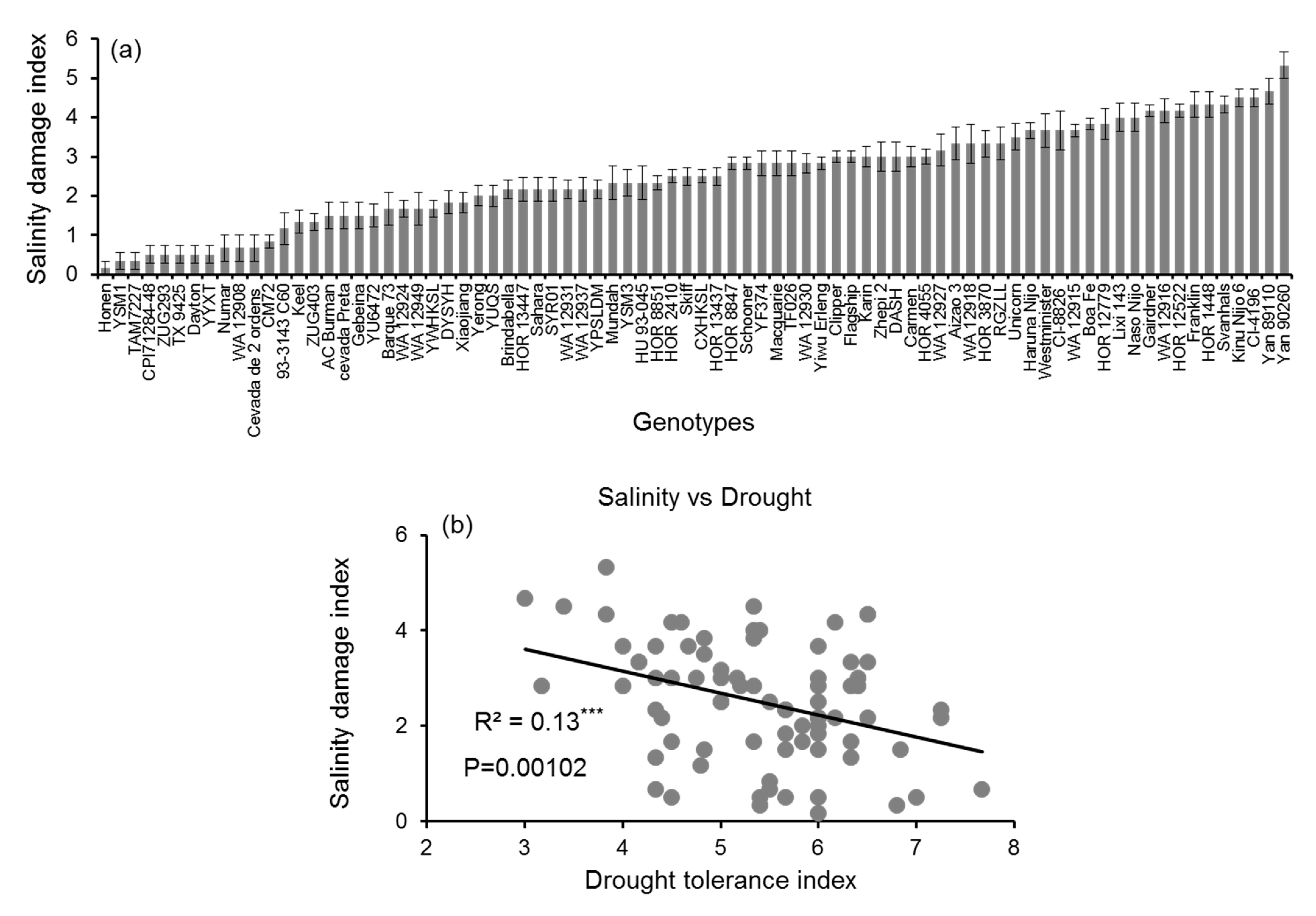
3.5. Drought Stress vs. Salinity Stress
4. Discussion
4.1. Drought Tolerance and Recovery Indices Were Correlated with One Another
4.2. SPAD Values and Fv/Fm Ratios Showed a Strong Correlation with the Drought Tolerance Index and Drought Recovery Index
4.3. PEG-Induced Drought Stress and Root Length
4.4. Drought Stress Tolerance Index Was Negatively Correlated with Stomatal Density but Positively Correlated with the Residual Transpiration of Irrigated Plants
4.5. Correlation between Drought and Salinity Stress Tolerance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, M.; Liu, P.; Guo, Y.; Wang, Y.; Geng, X.; Nie, Z.; Yu, Y. Change-point analysis of precipitation and drought extremes in china over the past 50 years. Atmosphere 2020, 11, 11. [Google Scholar] [CrossRef]
- Yu, C.; Huang, X.; Chen, H.; Huang, G.; Ni, S.; Wright, J.S.; Hall, J.; Ciais, P.; Zhang, J.; Xiao, Y.; et al. Assessing the impacts of extreme agricultural droughts in China under climate and socioeconomic changes. Earths Future 2018, 6, 689–703. [Google Scholar] [CrossRef]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Razzaq, A.; Wani, S.H.; Saleem, F.; Yu, M.; Zhou, M.; Shabala, S. Rewilding crops for climate resilience: Economic analysis and de novo domestication strategies. J. Exp. Bot. 2021, 72, 6123–6139. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Cao, B.; Cao, D.; Leng, G.; Li, H.; Yin, L.; Shan, L.; Deng, X. Genotypic variation in growth and physiological response to drought stress and re-watering reveals the critical role of recovery in drought adaptation in maize seedlings. Front. Plant Sci. 2016, 6, 1241. [Google Scholar] [CrossRef]
- Sallam, A.; Alqudah, A.M.; Dawood, M.F.; Baenziger, P.S.; Börner, A. Drought stress tolerance in wheat and barley: Advances in physiology, breeding and genetics research. Int. J. Mol. Sci. 2019, 20, 3137. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Al-Juburi, H.J.; Somasundaram, R.; Panneerselvam, R. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Arunyanark, A.; Jogloy, S.; Akkasaeng, C.; Vorasoot, N.; Kesmala, T.; Nageswara Rao, R.C.; Wright, G.C.; Patanothai, A. Chlorophyll stability is an indicator of drought tolerance in peanut. J. Agron. Crop Sci. 2008, 194, 113–125. [Google Scholar] [CrossRef]
- Filek, M.; Łabanowska, M.; Kościelniak, J.; Biesaga-Kościelniak, J.; Kurdziel, M.; Szarejko, I.; Hartikainen, H. Characterization of barley leaf tolerance to drought stress by chlorophyll fluorescence and electron paramagnetic resonance studies. J. Agron. Crop Sci. 2015, 201, 228–240. [Google Scholar] [CrossRef]
- Baker, N.R.; Rosenqvist, E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comas, L.H.; Becker, S.R.; Von Mark, V.C.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.A.; Brodribb, T.J.; Lucani, C.J.; DaMatta, F.M.; McAdam, S.A. Coordinated plasticity maintains hydraulic safety in sunflower leaves. Plant Cell Environ. 2018, 41, 2567–2576. [Google Scholar] [CrossRef] [PubMed]
- Blatt, M.R.; Brodribb, T.J.; Torii, K.U. Small Pores with a Big Impact. Am. Soc. Plant Biol. 2017, 174, 467–469. [Google Scholar] [CrossRef]
- Chen, Z.H.; Chen, G.; Dai, F.; Wang, Y.; Hills, A.; Ruan, Y.L.; Zhang, G.; Franks, P.J.; Nevo, E.; Blatt, M.R. Molecular evolution of grass stomata. Trends Plant Sci. 2017, 22, 124–139. [Google Scholar] [CrossRef]
- Zhang, C.; Preece, C.; Filella, I.; Farré-Armengol, G.; Peñuelas, J. Assessment of the Response of Photosynthetic Activity of Mediterranean Evergreen Oaks to Enhanced Drought Stress and Recovery by Using PRI and R690/R630. Forests 2017, 8, 386. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant. 2017, 162, 2–12. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 516. [Google Scholar] [CrossRef]
- Huang, X.; Tanveer, M.; Min, Y.; Shabala, S. Melatonin as a regulator of plant ionic homeostasis: Implications for abiotic stress tolerance. J. Exp. Bot. 2022, erac224. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.Y.; Chang, C. Toward a smart skin: Harnessing cuticle biosynthesis for crop adaptation to drought, salinity, temperature, and ultraviolet stress. Front. Plant Sci. 2022, 13, 961829. [Google Scholar] [CrossRef]
- Ketehouli, T.; Quoc, V.H.N.; Dong, J.Y.; Do, H.; Li, X.W.; Wang, F.W. Overview of the roles of calcium sensors in plants’ response to osmotic stress signalling. Funct. Plant Biol. 2022, 49, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Boulc’h, P.N.; Caullireau, E.; Faucher, E.; Gouerou, M.; Guerin, A.; Miray, R.; Couee, I. Abiotic stress signalling in extremophile land plants. J. Exp. Bot. 2020, 71, 5771–5785. [Google Scholar] [CrossRef] [PubMed]
- Franks, P.J.; Doheny-Adams, T.W.; Britton-Harper, Z.J.; Gray, J.E. Increasing water-use efficiency directly through genetic manipulation of stomatal density. New Phytol. 2015, 207, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.; Hepworth, C.; Dutton, C.; Dunn, J.A.; Hunt, L.; Stephens, J.; Waugh, R.; Cameron, D.D.; Gray, J.E. Reducing stomatal density in barley improves drought tolerance without impacting on yield. Plant Physiol. 2017, 174, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Morales-Navarro, S.; Pérez-Díaz, R.; Ortega, A.; De Marcos, A.; Mena, M.; Fenoll, C.; González-Villanueva1, E.; Ruiz-Lara, S. Overexpression of a SDD1-like gene from wild tomato decreases stomatal density and enhances dehydration avoidance in Arabidopsis and cultivated tomato. Front. Plant Sci. 2018, 9, 940. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Shabala, L.; Brodribb, T.J.; Zhou, M.; Shabala, S. Understanding physiological and morphological traits contributing to drought tolerance in barley. J. Agron. Crop Sci. 2019, 205, 129–140. [Google Scholar] [CrossRef]
- Caird, M.A.; Richards, J.H.; Donovan, L.A. Nighttime stomatal conductance and transpiration in C3 and C4 plants. Plant Physiol. 2007, 143, 4–10. [Google Scholar] [CrossRef]
- Even, M.; Sabo, M.; Meng, D.; Kreszies, T.; Schreiber, L.; Fricke, W. Night-time transpiration in barley (Hordeum vulgare) facilitates respiratory carbon dioxide release and is regulated during salt stress. Ann. Bot. 2018, 122, 569–582. [Google Scholar] [CrossRef]
- Lynch, J.P. Steep, cheap and deep: An ideotype to optimize water and N acquisition by maize root systems. Ann. Bot. 2013, 112, 347–357. [Google Scholar] [CrossRef]
- Lynch, J.P.; Chimungu, J.G.; Brown, K.M. Root anatomical phenes associated with water acquisition from drying soil: Targets for crop improvement. J. Exp. Bot. 2014, 65, 6155–6166. [Google Scholar] [CrossRef]
- Ji, H.; Liu, L.; Li, K.; Xie, Q.; Wang, Z.; Zhao, X.; Li, X. PEG-mediated osmotic stress induces premature differentiation of the root apical meristem and outgrowth of lateral roots in wheat. J. Exp. Bot. 2014, 65, 4863–4872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasanuzzaman, M.; Shabala, L.; Brodribb, T.J.; Zhou, M.; Shabala, S. Assessing the suitability of various screening methods as a proxy for drought tolerance in barley. Funct. Plant Biol. 2016, 44, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Davies, N.W.; Shabala, L.; Zhou, M.; Brodribb, T.J.; Shabala, S. Residual transpiration as a component of salinity stress tolerance mechanism: A case study for barley. BMC Plant Biol. 2017, 17, 107. [Google Scholar] [CrossRef]
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.; Jiang, D.; Dai, T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 4615. [Google Scholar] [CrossRef]
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.O.; Rosenqvist, E. Wheat cultivars selected for high Fv/Fm under heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and dry matter. Physiol. Plant. 2015, 153, 284–298. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence-a practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Szira, F.; Bálint, A.; Börner, A.; Galiba, G. Evaluation of drought-Related traits and screening methods at different developmental stages in spring barley. J. Agron. Crop Sci. 2008, 194, 334–342. [Google Scholar] [CrossRef]
- Ilyas, M.; Nisar, M.; Khan, N.; Hazrat, A.; Khan, A.H.; Hayat, K.; Fahad, S.; Khan, A.; Ullah, A. Drought tolerance strategies in plants: A mechanistic approach. J. Plant Growth Regul. 2021, 40, 926–944. [Google Scholar] [CrossRef]
- Wasaya, A.; Zhang, X.; Fang, Q.; Yan, Z. Root phenotyping for drought tolerance: A review. Agronomy 2018, 8, 241. [Google Scholar] [CrossRef]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef]
- Franks, P.J.; Beerling, D.J. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc. Natl. Acad. Sci. USA 2009, 106, 10343–10347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasanuzzaman, M.; Shabala, L.; Zhou, M.; Brodribb, T.J.; Corkrey, R.; Shabala, S. Factors determining stomatal and non-stomatal (residual) transpiration and their contribution towards salinity tolerance in contrasting barley genotypes. Environ. Exp. Bot. 2018, 153, 10–20. [Google Scholar] [CrossRef]
- Zhu, M.; Zhou, M.; Shabala, L.; Shabala, S. Linking osmotic adjustment and stomatal characteristics with salinity stress tolerance in contrasting barley accessions. Funct. Plant Biol. 2015, 42, 252–263. [Google Scholar] [CrossRef]
- González, A.; Ayerbe, L. Effect of terminal water stress on leaf epicuticular wax load, residual transpiration and grain yield in barley. Euphytica 2010, 172, 341–349. [Google Scholar] [CrossRef]
- Clarke, J.; Richards, R.; Condon, A. Effect of drought stress on residual transpiration and its relationship with water use of wheat. Can. J. Plant Sci. 1991, 71, 695–702. [Google Scholar] [CrossRef]
- Riederer, M.; Schreiber, L. Protecting against water loss: Analysis of the barrier properties of plant cuticles. J. Exp. Bot. 2001, 52, 2023–2032. [Google Scholar] [CrossRef]
- Bi, H.; Kovalchuk, N.; Langridge, P.; Tricker, P.J.; Lopato, S.; Borisjuk, N. The impact of drought on wheat leaf cuticle properties. BMC Plant Biol. 2017, 17, 85. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Zhang, X.; Lu, X.; Chen, G.; Chen, Z.H. Molecular and Evolutionary Mechanisms of Cuticular Wax for Plant Drought Tolerance. Front. Plant Sci. 2017, 8, 621. [Google Scholar] [CrossRef]
- Seo, P.J.; Lee, S.B.; Suh, M.C.; Park, M.J.; Go, Y.S.; Park, C.M. The MYB96 transcription factor regulates cuticular wax biosynthesis under drought conditions in Arabidopsis. Plant Cell 2011, 23, 1138–1152. [Google Scholar] [CrossRef]
- Seo, P.J.; Park, C.M. Cuticular wax biosynthesis as a way of inducing drought resistance. Plant Signal Behav. 2011, 6, 1043–1045. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Y.; Xie, Y.; Wang, Y.; Duan, L.; Zhang, M.; Li, Z. Ethephon improved drought tolerance in maize seedlings by modulating cuticular wax biosynthesis and membrane stability. J. Plant Physiol. 2017, 214, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Grünhofer, P.; Herzig, L.; Sent, S.; Zeisler-Diehl, V.V.; Schreiber, L. Increased cuticular wax deposition does not change residual foliar transpiration. Plant Cell Environ. 2022, 45, 1157–1171. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.R.; Gabr, A.M.; AL-Sayed, H.M.; Smetanska, I. Effect of drought and salinity stress on total phenolic, flavonoids and flavonols contents and antioxidant activity in in vitro sprout cultures of garden cress (Lepidium sativum). Res. J. Appl. Sci. 2012, 8, 3934–3942. [Google Scholar]
| Scale | Descriptions | Tolerance Index Categories |
|---|---|---|
| 1 | Whole plant is dead | Highly sensitive |
| 2 | Plant is mostly dead | Sensitive |
| 3 | More than 75% of all leaves dried | Sensitive |
| 4 | More than 50% of all leaves dried | Sensitive |
| 5 | More than 25% of all leaves dried | Moderately sensitive |
| 6 | About 1/2 of the leaf length is dry | Moderately tolerant |
| 7 | About ¼ of the leaf length is dry | Tolerant |
| 8 | Slight drying at the tips of lower leaves | Tolerant |
| 9 | No drought stress symptoms | Highly tolerant |
| Scale | Descriptions | Tolerance Index Categories |
|---|---|---|
| 1 | Whole plant is dead | Highly sensitive |
| 2 | No regrowth (plants are almost dead) | Sensitive |
| 3 | All leaves dried but half of the stem still green | Sensitive |
| 4 | All leaves almost dried but whole stem still green | Sensitive |
| 5 | Regrowth started from auxiliary buds (stem still green, new shoot started from auxiliary bud) | Moderately sensitive |
| 6 | Regrowth stated from apical meristem (growing new leaves from the shoot) | Moderately tolerant |
| 7 | Regrowth started from both auxiliary bud and apical meristem | Tolerant |
| 8 | New leaf started from apical meristem, new shoot started from auxiliary bud, and new tillering started from the lower node | Tolerant |
| 9 | No drought stress symptoms (fully recovery) | Highly tolerant |
| Scale | Descriptions | Tolerance Index Categories |
|---|---|---|
| 0 | No visual symptoms; healthy looking plants | Highly Tolerant |
| 1 | Some initial signs of chlorosis | Tolerant |
| 2 | One necrotic leaf | Tolerant |
| 3 | One to two necrotic leaves | Tolerant |
| 4 | Two necrotic leaves of all leaves dried | Moderately tolerant |
| 5 | Two to three necrotic leaves | Moderately sensitive |
| 6 | Three necrotic leaves; the whole plant is losing its green color | Sensitive |
| 7 | All of the plants are looking very unhealthy, with most starting to die | Sensitive |
| 8 | Most leaves have died; plant is dry | Sensitive |
| 9 | Completely dead plants | Highly sensitive |
| Parameters | Index | R2 Value | ||
|---|---|---|---|---|
| Control | Drought | Relative | ||
| Chlorophyll content | Drought tolerance index | 0.03 | 0.29 *** | 0.28 *** |
| Fv/Fm ratio | 0.00 | 0.19 *** | 0.19 *** | |
| Root length | 0.00 | 0.00 | 0.00 | |
| Chlorophyll content | Drought recovery index | 0.14 *** | 0.16 *** | 0.18 *** |
| Fv/Fm ratio | 0.00 | 0.17 *** | 0.16 *** | |
| Root length | 0.01 | 0.17 *** | 0.14 *** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasanuzzaman, M.; Shabala, L.; Brodribb, T.J.; Zhou, M.; Shabala, S. Understanding the Role of Physiological and Agronomical Traits during Drought Recovery as a Determinant of Differential Drought Stress Tolerance in Barley. Agronomy 2022, 12, 2136. https://doi.org/10.3390/agronomy12092136
Hasanuzzaman M, Shabala L, Brodribb TJ, Zhou M, Shabala S. Understanding the Role of Physiological and Agronomical Traits during Drought Recovery as a Determinant of Differential Drought Stress Tolerance in Barley. Agronomy. 2022; 12(9):2136. https://doi.org/10.3390/agronomy12092136
Chicago/Turabian StyleHasanuzzaman, Md., Lana Shabala, Timothy J. Brodribb, Meixue Zhou, and Sergey Shabala. 2022. "Understanding the Role of Physiological and Agronomical Traits during Drought Recovery as a Determinant of Differential Drought Stress Tolerance in Barley" Agronomy 12, no. 9: 2136. https://doi.org/10.3390/agronomy12092136
APA StyleHasanuzzaman, M., Shabala, L., Brodribb, T. J., Zhou, M., & Shabala, S. (2022). Understanding the Role of Physiological and Agronomical Traits during Drought Recovery as a Determinant of Differential Drought Stress Tolerance in Barley. Agronomy, 12(9), 2136. https://doi.org/10.3390/agronomy12092136








