New Flowering and Architecture Traits Mediated by Multiplex CRISPR-Cas9 Gene Editing in Hexaploid Camelina sativa
Abstract
:1. Introduction
2. Materials and Methods
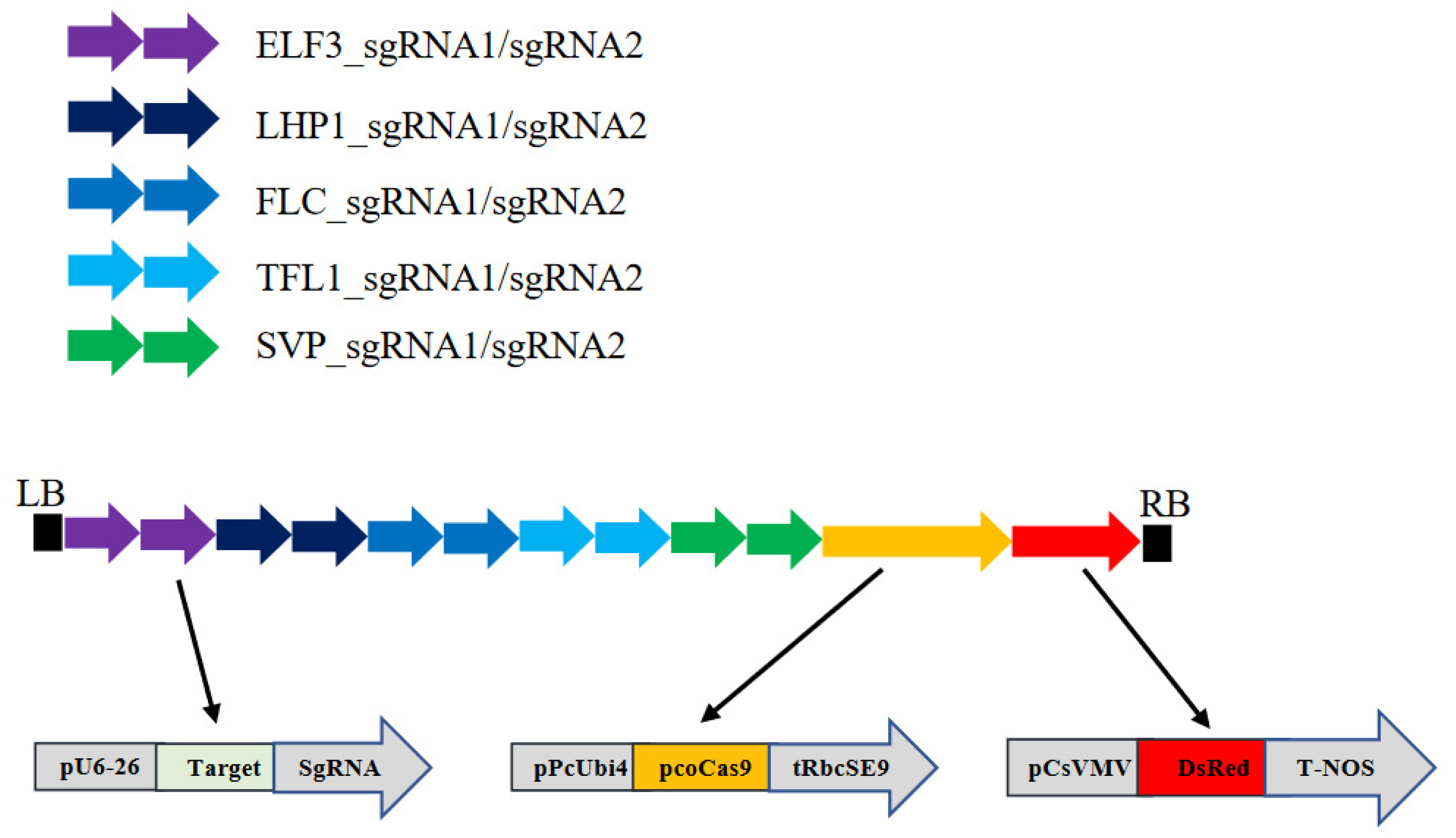
2.1. Construction of Binary Vector pFlo6 Bearing the Genes Necessary for CRISPR-Cas9 Mutation of the Five Target Genes
2.2. Plant Growth Conditions and Plant Transformation
2.3. Plant Nomenclature
2.4. Phenotype Analysis
2.5. Genotype Analysis
3. Results
3.1. Overall Experimental Strategy
3.2. Genetic Transformation and Production of Construct-Free Segregants
3.3. High Plant Density Phenotype Screen
3.4. Selection of Early Flowering Mutants
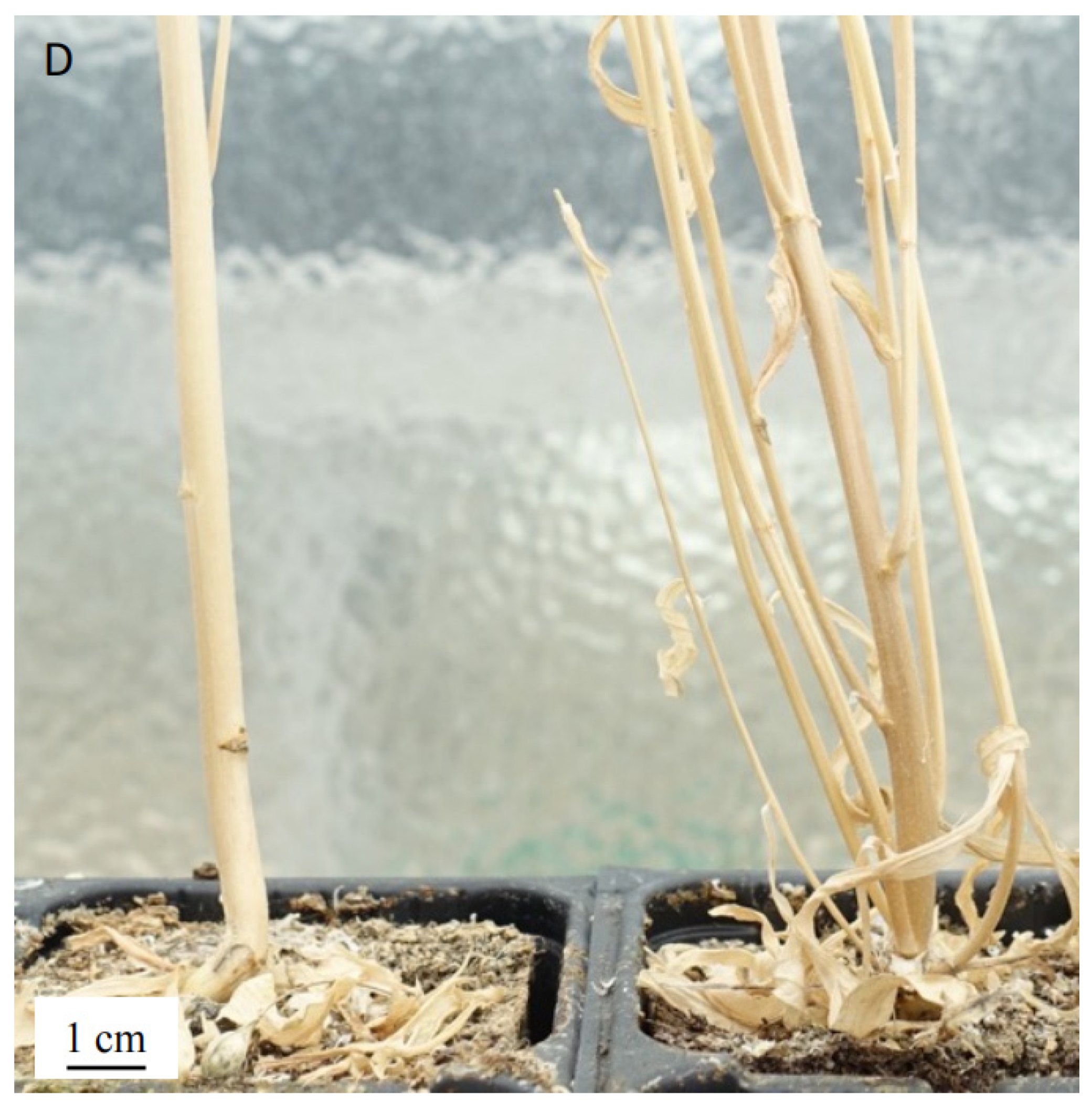
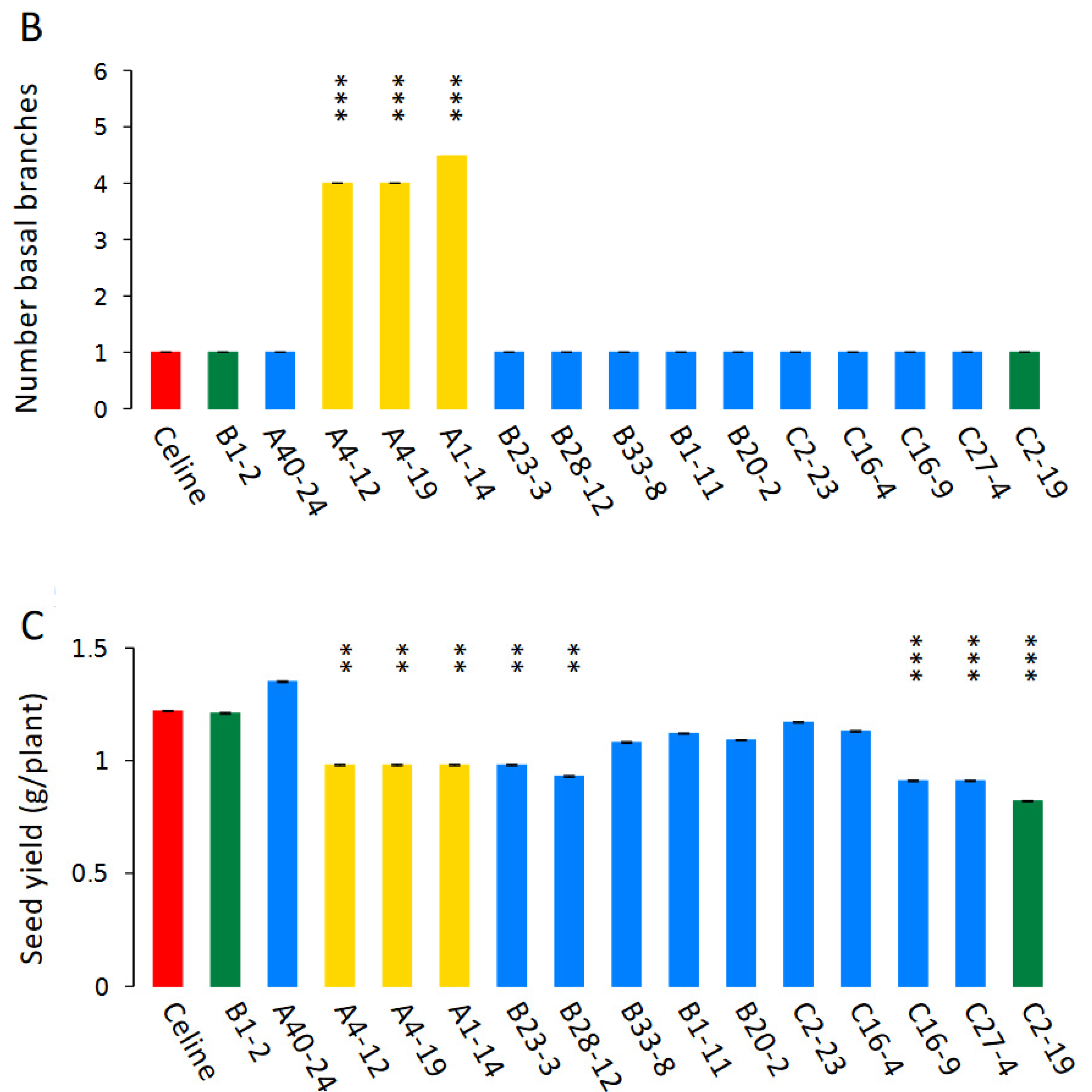
3.5. Phenotyping of T5 Mutants
3.6. Genotypes of T5 Lines
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Vollmann, J.; Eynck, C. Camelina as a Sustainable Oilseed Crop: Contributions of Plant Breeding and Genetic Engineering. Biotechnol. J. 2015, 10, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Berti, M.; Gesch, R.; Eynck, C.; Anderson, J.; Cermak, S. Camelina Uses, Genetics, Genomics, Production, and Management. Ind. Crops Prod. 2016, 94, 690–710. [Google Scholar] [CrossRef]
- Faure, J.D.; Tepfer, M. Camelina, a Swiss Knife for Plant Lipid Biotechnology. OCL—Oilseeds Fats Crops Lipids 2016, 43, D503. [Google Scholar] [CrossRef]
- Sainger, M.; Jaiwal, A.; Sainger, P.A.; Chaudhary, D.; Jaiwal, R.; Jaiwal, P.K. Advances in Genetic Improvement of Camelina Sativa for Biofuel and Industrial Bio-Products. Renew. Sustain. Energy Rev. 2017, 68, 623–637. [Google Scholar] [CrossRef]
- Al-Shehbaz, I.A.; Beilstein, M.A.; Kellogg, E.A. Systematics and Phylogeny of the Brassicaceae (Cruciferae): An Overview. Plant Syst. Evol. 2006, 259, 89–120. [Google Scholar] [CrossRef]
- Neupane, D.; Lohaus, R.H.; Solomon, J.K.Q.; Cushman, J.C. Realizing the Potential of Camelina Sativa as a Bioenergy Crop for a Changing Global Climate. Plants 2022, 11, 772. [Google Scholar] [CrossRef]
- Crowley, J.G.; Fröhlich, A. Factors Affecting the Composition and Use of Camelina; Teagasc: Dublin, Ireland, 1998; Volume 4, pp. 1–23. [Google Scholar]
- Gesch, R.W.; Archer, D.W.; Berti, M.T. Dual Cropping Winter Camelina with Soybean in the Northern Corn Belt. Agron. J. 2014, 106, 1735–1745. [Google Scholar] [CrossRef]
- Berti, M.; Samarappuli, D.; Johnson, B.L.; Gesch, R.W. Integrating Winter Camelina into Maize and Soybean Cropping Systems. Ind. Crops Prod. 2017, 107, 595–601. [Google Scholar] [CrossRef]
- Groeneveld, J.H.; Klein, A.M. Pollination of Two Oil-Producing Plant Species: Camelina (Camelina sativa L. Crantz) and Pennycress (Thlaspi arvense L.) Double-Cropping in Germany. GCB Bioenergy 2014, 6, 242–251. [Google Scholar] [CrossRef] [Green Version]
- Leclère, M.; Loyce, C.; Jeuffroy, M.H. Growing Camelina as a Second Crop in France: A Participatory Design Approach to Produce Actionable Knowledge. Eur. J. Agron. 2018, 101, 78–89. [Google Scholar] [CrossRef]
- Lu, C.; Kang, J. Generation of Transgenic Plants of a Potential Oilseed Crop Camelina Sativa by Agrobacterium-Mediated Transformation. Plant Cell Rep. 2008, 27, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Kagale, S.; Koh, C.; Nixon, J.; Bollina, V.; Clarke, W.E.; Tuteja, R.; Spillane, C.; Robinson, S.J.; Links, M.G.; Clarke, C.; et al. The Emerging Biofuel Crop Camelina Sativa Retains a Highly Undifferentiated Hexaploid Genome Structure. Nat. Commun. 2014, 5, 3706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Hu, X.; Lovell, J.T.; Grabowski, P.P.; Mamidi, S.; Chen, C.; Amirebrahimi, M.; Kahanda, I.; Mumey, B.; Barry, K.; et al. Genetic Dissection of Natural Variation in Oilseed Traits of Camelina by Whole-Genome Resequencing and QTL Mapping. Plant Genome 2021, 14, e20110. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lopez, N.; Haslam, R.P.; Napier, J.A.; Sayanova, O. Successful High-Level Accumulation of Fish Oil Omega-3 Long-Chain Polyunsaturated Fatty Acids in a Transgenic Oilseed Crop. Plant J. 2014, 77, 198–208. [Google Scholar] [CrossRef] [Green Version]
- Petrie, J.R.; Shrestha, P.; Belide, S.; Kennedy, Y.; Lester, G.; Liu, Q.; Divi, U.K.; Mulder, R.J.; Mansour, M.P.; Nichols, P.D.; et al. Metabolic Engineering Camelina Sativa with Fish Oil-like Levels of DHA. PLoS ONE 2014, 9, e85061. [Google Scholar] [CrossRef]
- Morineau, C.; Bellec, Y.; Tellier, F.; Gissot, L.; Kelemen, Z.; Nogué, F.; Faure, J.-D. Selective Gene Dosage by CRISPR-Cas9 Genome Editing in Hexaploid Camelina Sativa. Plant Biotechnol. J. 2016, 15, 729–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augustin, J.M.; Brock, J.R.; Augustin, M.M.; Wellinghoff, R.L.; Shipp, M.; Higashi, Y.; Kumssa, T.T.; Cahoon, E.B.; Kutchan, T.M. Field Performance of Terpene-Producing Camelina Sativa. Ind. Crops Prod. 2019, 136, 50–58. [Google Scholar] [CrossRef]
- Gao, C. Genome Engineering for Crop Improvement and Future Agriculture. Cell 2021, 184, 1621–1635. [Google Scholar] [CrossRef]
- Rodríguez-Leal, D.; Lemmon, Z.H.; Man, J.; Bartlett, M.E.; Lippman, Z.B. Engineering Quantitative Trait Variation for Crop Improvement by Genome Editing. Cell 2017, 171, 470–480.e8. [Google Scholar] [CrossRef] [Green Version]
- Varkonyi-Gasic, E.; Wang, T.; Voogd, C.; Jeon, S.; Drummond, R.S.M.; Gleave, A.P.; Allan, A.C. Mutagenesis of Kiwifruit CENTRORADIALIS-like Genes Transforms a Climbing Woody Perennial with Long Juvenility and Axillary Flowering into a Compact Plant with Rapid Terminal Flowering. Plant Biotechnol. J. 2019, 17, 869–880. [Google Scholar] [CrossRef] [Green Version]
- Ramadan, M.; Alariqi, M.; Ma, Y.; Li, Y.; Liu, Z.; Zhang, R.; Jin, S. Efficient CRISPR/Cas9 Mediated Pooled—SgRNAs Assembly Accelerates Targeting Multiple Genes Related to Male Sterility in Cotton. Plant Methods 2021, 17, 16. [Google Scholar] [CrossRef] [PubMed]
- Armario Najera, V.; Twyman, R.M.; Christou, P.; Zhu, C. Applications of Multiplex Genome Editing in Higher Plants. Curr. Opin. Biotechnol. 2019, 59, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Nogué, F.; Mara, K.; Collonnier, C.; Casacuberta, J.M. Genome Engineering and Plant Breeding: Impact on Trait Discovery and Development. Plant Cell Rep. 2016, 35, 1475–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koornneef, M.; Hanhart, C.J.; van der Veen, J.H. A Genetic and Physiological Analysis of Late Flowering Mutants in Arabidopsis Thaliana. MGG Mol. Gen. Genet. 1991, 229, 57–66. [Google Scholar] [CrossRef]
- Leijten, W.; Koes, R.; Roobeek, I.; Frugis, G. Translating Flowering Time from Arabidopsis Thaliana to Brassicaceae and Asteraceae Crop Species. Plants 2018, 7, 111. [Google Scholar] [CrossRef] [Green Version]
- Corbesier, L.; Coupland, G. The Quest for Florigen: A Review of Recent Progress. J. Exp. Bot. 2006, 57, 3395–3403. [Google Scholar] [CrossRef] [Green Version]
- Koornneef, M.; Blankestijn-de Vries, H.; Hanhart, C.; Soppe, W.; Peeters, T. The Phenotype of Some Late-flowering Mutants Is Enhanced by a Locus on Chromosome 5 That Is Not Effective in the Landsberg Erecta Wild-type. Plant J. 1994, 6, 911–919. [Google Scholar] [CrossRef]
- Jeong, H.L.; Seong, J.Y.; Soo, H.P.; Hwang, I.; Jong, S.L.; Ji, H.A. Role of SVP in the Control of Flowering Time by Ambient Temperature in Arabidopsis. Genes Dev. 2007, 21, 397–402. [Google Scholar] [CrossRef] [Green Version]
- Gaudin, V.; Libault, M.; Pouteau, S.; Juul, T.; Zhao, G.; Lefebvre, D.; Grandjean, O. Mutations in LIKE HETEROCHROMATIN PROTEIN 1 Affect Flowering Time and Plant Architecture in Arabidopsis. Development 2001, 128, 4847–4858. [Google Scholar] [CrossRef]
- Takada, S.; Goto, K. Terminal Flower2, an Arabidopsis Homolog of Heterochromatin Protein1, Counteracts the Activation of Flowering Locus T by Constans in the Vascular Tissues of Leaves to Regulate Flowering Time. Plant Cell 2003, 15, 2856–2865. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Teo, Z.W.N.; Bi, Y.; Song, S.; Xi, W.; Yang, X.; Yin, Z.; Yu, H. A Conserved Genetic Pathway Determines Inflorescence Architecture in Arabidopsis and Rice. Dev. Cell 2013, 24, 612–622. [Google Scholar] [CrossRef] [Green Version]
- Shannon, S.; Meeks-Wagner, D.R. A Mutation in the Arabidopsis TFL1 Gene Affects Inflorescence Meristem Development. Plant Cell 1991, 3, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Zagotta, M.T.; Hicks, K.A.; Jacobs, C.I.; Young, J.C.; Hangarter, R.P.; Meeks-Wagner, D.R. The Arabidopsis ELF3 Gene Regulates Vegetative Photomorphogenesis and the Photoperiodic Induction of Flowering. Plant J. 1996, 10, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Raschke, A.; Ibañez, C.; Ullrich, K.K.; Anwer, M.U.; Becker, S.; Glöckner, A.; Trenner, J.; Denk, K.; Saal, B.; Sun, X.; et al. Natural Variants of ELF3 Affect Thermomorphogenesis by Transcriptionally Modulating PIF4-Dependent Auxin Response Genes. BMC Plant Biol 2015, 15, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Box, M.S.; Huang, B.E.; Domijan, M.; Jaeger, K.E.; Khattak, A.K.; Yoo, S.J.; Sedivy, E.L.; Jones, D.M.; Hearn, T.J.; Webb, A.A.R.; et al. ELF3 Controls Thermoresponsive Growth in Arabidopsis. Curr. Biol. 2015, 25, 194–199. [Google Scholar] [CrossRef] [Green Version]
- Sarrion-Perdigones, A.; Vazquez-Vilar, M.; Palaci, J.; Castelijns, B.; Forment, J.; Ziarsolo, P.; Blanca, J.; Granell, A.; Orzaez, D. GoldenBraid 2.0: A Comprehensive DNA Assembly Framework for Plant Synthetic Biology. Plant Physiol. 2013, 162, 1618–1631. [Google Scholar] [CrossRef] [Green Version]
- Vazquez-Vilar, M.; Bernabé-Orts, J.M.; Fernandez-del-Carmen, A.; Ziarsolo, P.; Blanca, J.; Granell, A.; Orzaez, D. A Modular Toolbox for GRNA–Cas9 Genome Engineering in Plants Based on the GoldenBraid Standard. Plant Methods 2016, 12, 10. [Google Scholar] [CrossRef] [Green Version]
- Concordet, J.-P.; Haeussler, M. CRISPOR: Intuitive Guide Selection for CRISPR/Cas9 Genome Editing Experiments and Screens. Nucleic Acids Res. 2018, 46, W242–W245. [Google Scholar] [CrossRef]
- Julié-Galau, S.; Bellec, Y.; Faure, J.D.; Tepfer, M. Evaluation of the Potential for Interspecific Hybridization between Camelina Sativa and Related Wild Brassicaceae in Anticipation of Field Trials of GM Camelina. Transgenic Res. 2014, 23, 67–74. [Google Scholar] [CrossRef]
- Martinelli, T.; Galasso, I. Phenological Growth Stages of Camelina Sativa According to the Extended BBCH Scale. Ann. Appl. Biol. 2011, 158, 87–94. [Google Scholar] [CrossRef]
- Legland, D.; Arganda-Carreras, I.; Andrey, P. MorphoLibJ: Integrated Library and Plugins for Mathematical Morphology with ImageJ. Bioinformatics 2016, 32, 3532–3534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biot, E.; Crowell, E.; Burguet, J.; Höfte, H.; Vernhettes, S.; Andrey, P. Strategy and Software for the Statistical Spatial Analysis of 3D Intracellular Distributions. Plant J. Cell Mol. Biol. 2016, 87, 230–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutcheon, C.; Ditt, R.F.; Beilstein, M.; Comai, L.; Schroeder, J.; Goldstein, E.; Shewmaker, C.K.; Nguyen, T.; De Rocher, J.; Kiser, J. Polyploid Genome of Camelina Sativa Revealed by Isolation of Fatty Acid Synthesis Genes. BMC Plant Biol. 2010, 10, 233. [Google Scholar] [CrossRef] [Green Version]
- Kagale, S.; Nixon, J.; Khedikar, Y.; Pasha, A.; Provart, N.J.; Clarke, W.E.; Bollina, V.; Robinson, S.J.; Coutu, C.; Hegedus, D.D.; et al. The Developmental Transcriptome Atlas of the Biofuel Crop Camelina Sativa. Plant J. Cell Mol. Biol. 2016, 88, 879–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, J.V.; Horvath, D.P.; Doğramaci, M.; Dorn, K.M.; Chao, W.S.; Watkin, E.E.; Hernandez, A.G.; Marks, M.D.; Gesch, R. Expression of FLOWERING LOCUS C and a Frameshift Mutation of This Gene on Chromosome 20 Differentiate a Summer and Winter Annual Biotype of Camelina Sativa. Plant Direct 2018, 2, e00060. [Google Scholar] [CrossRef] [Green Version]
- Hotton, S.K.; Kammerzell, M.; Chan, R.; Hernandez, B.T.; Young, H.A.; Tobias, C.; McKeon, T.; Brichta, J.; Thomson, N.J.; Thomson, J.G. Phenotypic Examination of Camelina sativa (L.) Crantz Accessions from the USDA-ARS National Genetics Resource Program. Plants 2020, 9, 642. [Google Scholar] [CrossRef]
- Walsh, K.D.; Hills, M.J.; Martin, S.L.; Hall, L.M. Pollen-Mediated Gene Flow in Camelina sativa (L.) Crantz. Crop Sci. 2015, 55, 196–202. [Google Scholar] [CrossRef]
- Walsh, K.D.; Puttick, D.M.; Hills, M.J.; Yang, R.C.; Topinka, K.C.; Hall, L.M. Short Communication: First Report of Outcrossing Rates in Camelina [Camelina sativa (L.) Crantz], a Potential Platform for Bioindustrial Oils. Can. J. Plant Sci. 2012, 92, 681–685. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Li, C.; Li, Y.; Yu, H. Mobile TERMINAL FLOWER1 Determines Seed Size in Arabidopsis. Nat. Plants 2020, 6, 1146–1157. [Google Scholar] [CrossRef]
- Luo, Z.; Brock, J.; Dyer, J.M.; Kutchan, T.; Schachtman, D.; Augustin, M.; Ge, Y.; Fahlgren, N.; Abdel-Haleem, H. Genetic Diversity and Population Structure of a Camelina Sativa Spring Panel. Front. Plant Sci. 2019, 10, 184. [Google Scholar] [CrossRef] [Green Version]



| T5 Line | Early Flowering | Shorter Stature | Determinate Growth | Basal Branching |
|---|---|---|---|---|
| Céline | ||||
| B1-2 * | +++ | +++ | +++ | − |
| A40-24 * | ++ | ++ | − | − |
| A4-12 * | ++ | ++ | −/++ | +++ |
| A4-19 * | ++ | ++ | −/++ | +++ |
| A1-14 * | + | ++ | − | +++ |
| B23-3 * | ++ | ++ | − | − |
| B28-12 | ++ | ++ | − | − |
| B33-8 | ++ | ++ | ++ | − |
| B1-11 | ++ | ++ | −/++ | − |
| B20-2 | ||||
| C2-23 | ++ | ++ | ++ | − |
| C16-4 | ++ | ++ | ++ | − |
| C16-9 | ++ | ++ | ++ | − |
| C27-4 | ++ | ++ | ++ | − |
| C2-19 * | +++ | +++ | +++ | − |
| Gene | Accession | sgRNA | T5 Plant | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A40-24-25 | A4-12-25 | A4-12-26 | A4-19-25 | A4-19-26 | A1-14-25 | B23-3-25 | B1-2-25 | C2-19-25 | |||
| SVP | Csa16g044290 | 1 | WT | WT | WT | WT | WT | WT | WT | WT | WT |
| 2 | WT | WT | WT | −7/WT | WT | WT | WT | +T | −41 | ||
| Csa07g052630 | 1 | WT | WT | WT | WT | WT | WT | WT | WT | WT | |
| 2 | WT | WT | WT | WT | WT | +C | WT | +C | WT | ||
| Csa09g086860 | 1 | WT | WT | WT | WT | WT | WT | WT | WT | WT | |
| 2 | +C | −7/WT | WT | WT | WT | WT | −34 | −5 | −4 | ||
| FLC | Csa13g011890 | 1 | WT | WT | WT | WT | WT | WT | WT | WT | WT |
| 2 | WT | WT | WT | WT | WT | WT | WT | WT | WT | ||
| Csa08g054450 | 1 | WT | WT | WT | WT | WT | WT | WT | WT | WT | |
| 2 | +T | WT | WT | WT | WT | WT | WT | WT | WT | ||
| Csa20g015400 | 1 | WT | WT | WT | WT | WT | WT | WT | WT | WT | |
| 2 | WT | WT | WT | WT | WT | WT | WT | WT | WT | ||
| LHP1 | Csa13g020480 | 1 | −20 | +C | +C | +C | +C | WT | WT | +A | +C |
| 2 | WT | −ACG | −ACG | −ACG | −ACG | 5 | +A | WT | WT/−1 | ||
| Csa08g009910 | 1 | WT | WT | WT | WT | WT | WT | +A | +A | +A | |
| 2 | WT | −4 | −4 | −4 | −4 | −4 | WT | −10/snp | +T | ||
| Csa20g025860 | 1 | WT | WT | WT | WT | WT | −123 | WT | +C | -G | |
| 2 | WT | WT | WT | WT | WT | −123 | −A | WT | WT | ||
| TFL1 | Csa13g003940 | 1 | +T | +T | +T | +T | +T | +A | WT | +A | +T |
| 2 | +A | WT/−2 | WT/−2 | WT/−2 | WT | WT | WT | +C | +T | ||
| Csa08g060650 | 1 | WT | WT | WT | WT | WT | WT | +A | +T | +C | |
| 2 | WT | WT/+T | +A | WT/+T | WT/+T | +A | WT | −4 | −4 | ||
| Csa20g005030 | 1 | +G | WT | WT | WT | WT | WT | WT | WT | CD | |
| 2 | WT | −4 & 2snp | −4 & 2snp | −4 & 2snp | −4 & 2snp | WT | WT | −4 | CD | ||
| ELF3 | Csa16g047550 | 1 | WT | WT | WT | WT | WT | WT | WT | −T & snp | −C |
| 2 | WT | WT | WT | WT | WT | WT | WT | WT | WT | ||
| Csa07g056950 | 1 | WT | WT | WT | WT | WT | WT | WT | WT | WT | |
| 2 | WT | WT | WT | WT | WT | WT | WT | WT | WT | ||
| Csa09g092140 | 1 | WT | WT | WT | WT | WT | WT | WT | WT | WT | |
| 2 | WT | WT | WT | WT | WT | WT | WT | WT | WT | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellec, Y.; Guyon-Debast, A.; François, T.; Gissot, L.; Biot, E.; Nogué, F.; Faure, J.-D.; Tepfer, M. New Flowering and Architecture Traits Mediated by Multiplex CRISPR-Cas9 Gene Editing in Hexaploid Camelina sativa. Agronomy 2022, 12, 1873. https://doi.org/10.3390/agronomy12081873
Bellec Y, Guyon-Debast A, François T, Gissot L, Biot E, Nogué F, Faure J-D, Tepfer M. New Flowering and Architecture Traits Mediated by Multiplex CRISPR-Cas9 Gene Editing in Hexaploid Camelina sativa. Agronomy. 2022; 12(8):1873. https://doi.org/10.3390/agronomy12081873
Chicago/Turabian StyleBellec, Yannick, Anouchka Guyon-Debast, Tracy François, Lionel Gissot, Eric Biot, Fabien Nogué, Jean-Denis Faure, and Mark Tepfer. 2022. "New Flowering and Architecture Traits Mediated by Multiplex CRISPR-Cas9 Gene Editing in Hexaploid Camelina sativa" Agronomy 12, no. 8: 1873. https://doi.org/10.3390/agronomy12081873
APA StyleBellec, Y., Guyon-Debast, A., François, T., Gissot, L., Biot, E., Nogué, F., Faure, J.-D., & Tepfer, M. (2022). New Flowering and Architecture Traits Mediated by Multiplex CRISPR-Cas9 Gene Editing in Hexaploid Camelina sativa. Agronomy, 12(8), 1873. https://doi.org/10.3390/agronomy12081873






