Comparison of Aboveground Vegetation and Soil Seed Bank Composition among Three Typical Vegetation Types in the Karst Regions of Southwest China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling of Vegetation and Site Conditions
2.3. Seed Bank Sampling
2.4. Statistical Analyses
- (1)
- Diversity index (H), using the Shannon–Wiener index [21]:
- (2)
- Dominance index (D), using the Simpson dominance index [22]:
- (3)
- Richness index (R):
- (4)
- Pielou index (E) [23]:
- (5)
- Similarity index, using Sørensen’s quotient index (SQ) [24]:
3. Results
3.1. Soil Chemical Properties
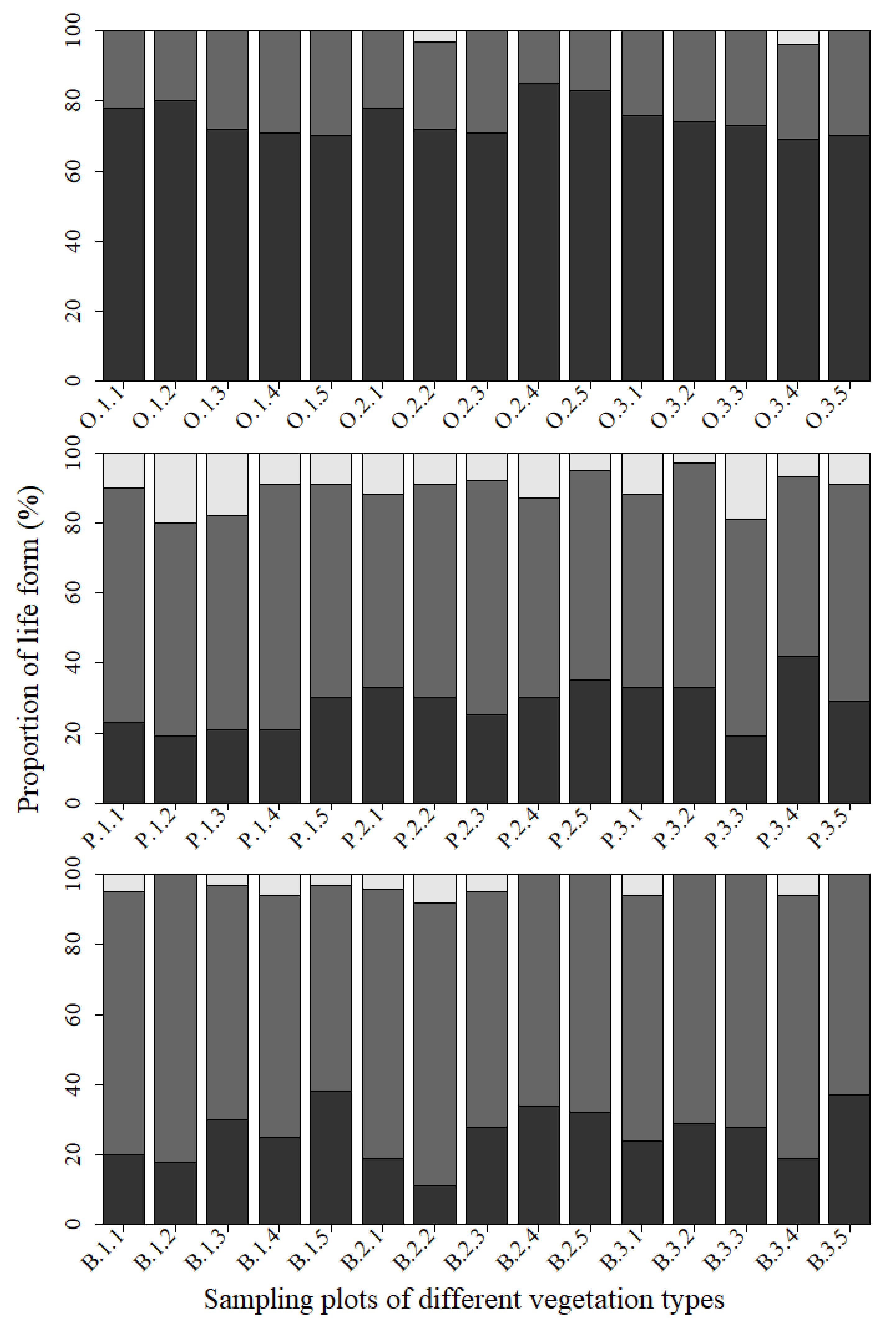
3.2. Species Composition of Aboveground Vegetation
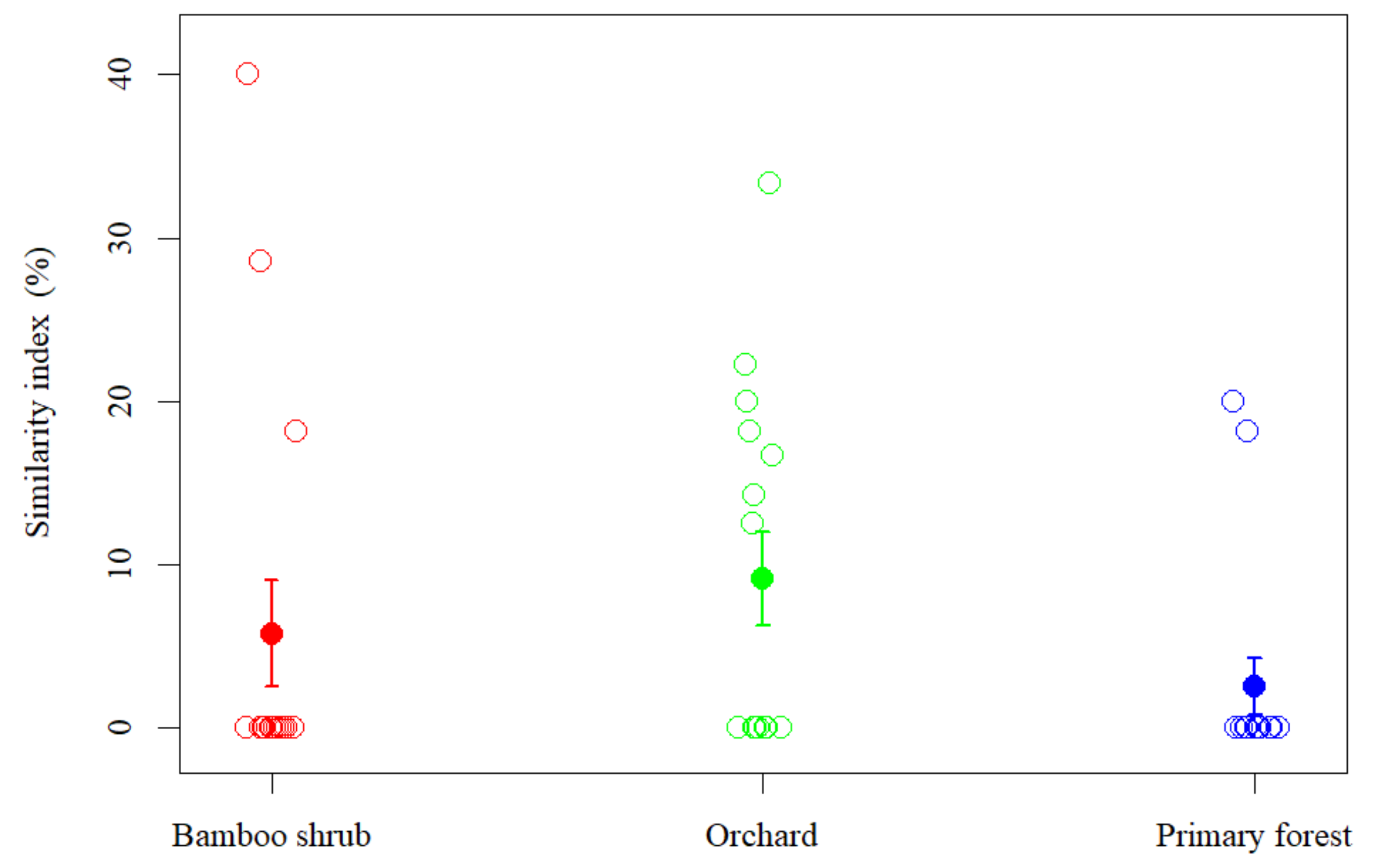
3.3. Species Composition of Soil Seed Bank
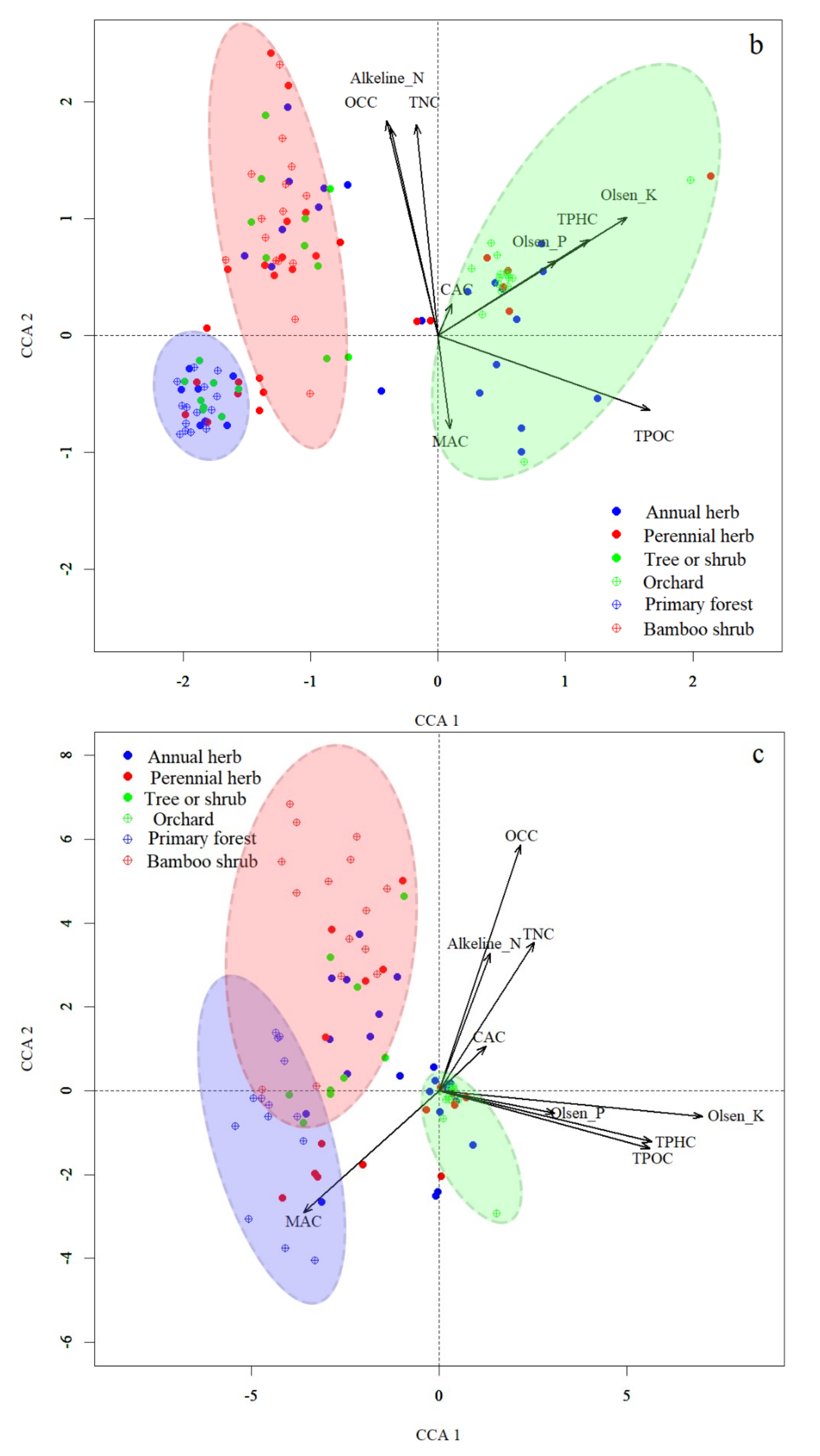
3.4. Correlation between Vegetation, Soil Seed Banks and Soil Properties
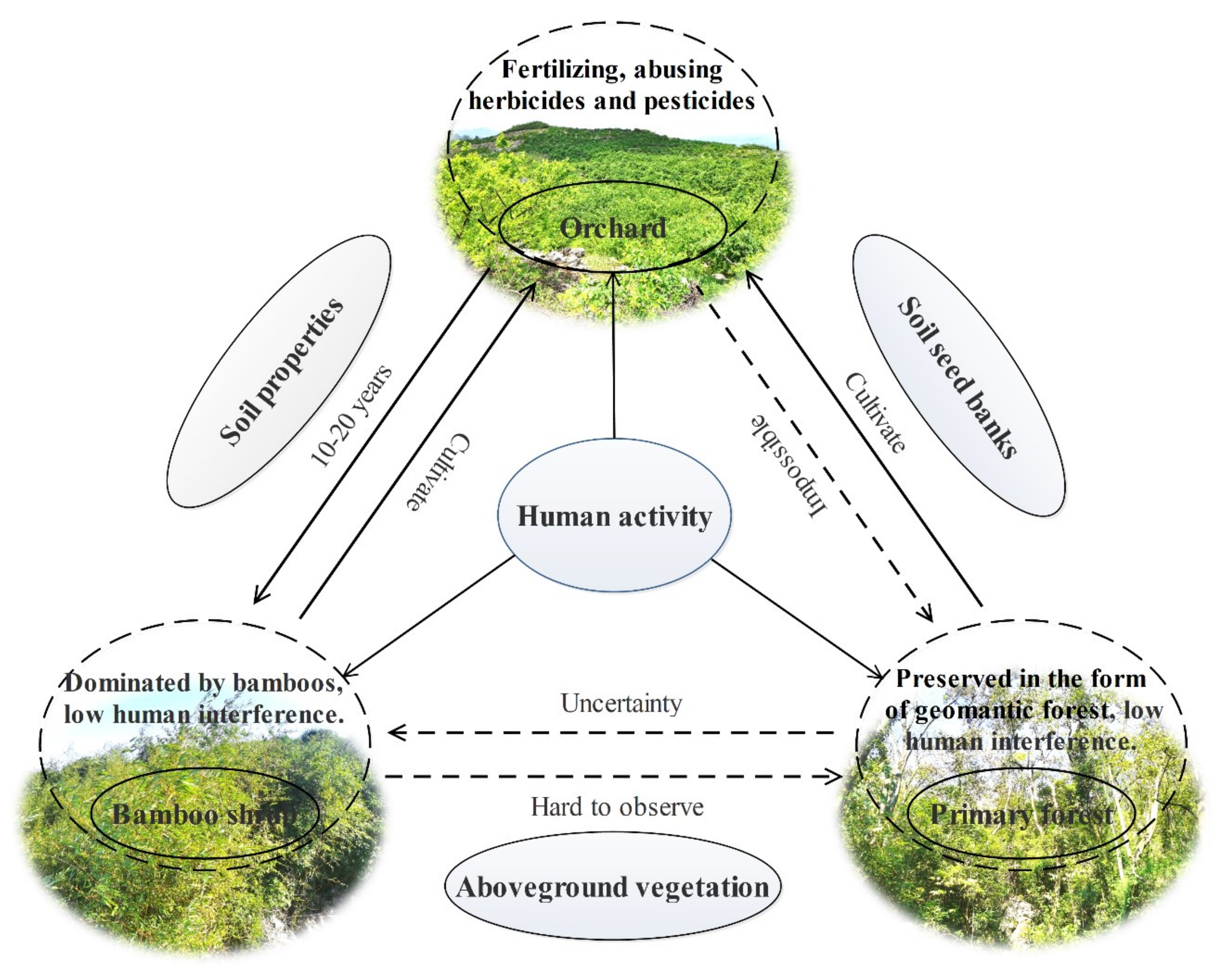
4. Discussion
5. Implications for Ecological Restoration in Karst Rocky Desertification Regions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, K.; Zhang, C.; Chen, H.; Yue, Y.; Zhang, W.; Zhang, M.; Qi, X.; Fu, Z. Karst landscapes of China: Patterns, ecosystem processes and services. Landsc. Ecol. 2019, 34, 2743–2763. [Google Scholar] [CrossRef] [Green Version]
- Yue, Y.; Wang, K.; Zhang, B.; Jiao, Q.; Liu, B.; Zhang, M. Remote sensing of fractional cover of vegetation and exposed bedrock for karst rocky desertification assessment. Procedia Environ. Sci. 2012, 13, 847–853. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Wang, S.; Bai, X.; Luo, G.; Li, Q.; Wu, L.; Yang, Y.; Tian, S.; Li, C.; Deng, Y. Changes in ecosystem service values in karst areas of China. Agric. Ecosyst. Environ. 2020, 301, 107026. [Google Scholar] [CrossRef]
- Liao, C.; Yue, Y.; Wang, K.; Fensholt, R.; Tong, X.; Brandt, M. Ecological restoration enhances ecosystem health in the karst regions of southwest China. Ecol. Indic. 2018, 90, 416–425. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, J.; Pan, F.; Li, D.; Chen, H.; Wang, K. Changes in nitrogen and phosphorus limitation during secondary succession in a karst region in southwest China. Plant Soil 2015, 391, 77–91. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]
- Winbourne, J.B.; Brewer, S.W.; Houlton, B.Z. Iron controls over di-nitrogen fixation in karst tropical forest. Ecology 2017, 98, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Z.; Zheng, H.; Xiao, Y.; Polasky, S.; Liu, J.; Xu, W.; Wang, Q.; Zhang, L.; Xiao, Y.; Rao, E.; et al. Improvements in ecosystem services from investments in natural capital. Science 2016, 352, 1455–1459. [Google Scholar] [CrossRef] [PubMed]
- Green, S.M.; Dungait, J.A.; Tu, C.; Buss, H.L.; Sanderson, N.; Hawkes, S.J.; Xing, K.; Yue, F.; Hussey, V.L.; Peng, J.; et al. Soil functions and ecosystem services research in the Chinese karst Critical Zone. Chem. Geol. 2019, 527, 119107. [Google Scholar] [CrossRef] [Green Version]
- Quine, T.; Guo, D.; Green, S.M.; Tu, C.; Hartley, I.; Zhang, X.; Dungait, J.; Wen, X.; Song, Z.; Liu, H.; et al. Ecosystem service delivery in Karst landscapes: Anthropogenic perturbation and recovery. Acta Geochim. 2017, 36, 416–420. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Zhang, H.; Zhang, Z. Spatially explicit assessment of ecosystem services in China’s Loess Plateau: Patterns, interactions, drivers, and implications. Glob. Planet. Chang. 2018, 161, 41–52. [Google Scholar] [CrossRef]
- Tong, X.; Brandt, M.; Yue, Y.; Horion, S.; Wang, K.; De Keersmaecker, W.; Tian, F.; Schurgers, G.; Xiao, X.; Luo, Y.; et al. Increased vegetation growth and carbon stock in China karst via ecological engineering. Nat. Sustain. 2018, 1, 44–50. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, W.; Gan, X.; Huang, Y.; Guo, Y.; Wen, X. Changes in vegetation and soil properties during recovery of a subtropical forest in South China. J. Mt. Sci. 2018, 15, 46–58. [Google Scholar] [CrossRef]
- Matson, P.A.; Parton, W.J.; Power, A.G.; Swift, M.J. Agricultural Intensification and Ecosystem Properties. Science 1997, 277, 504–509. [Google Scholar] [CrossRef] [Green Version]
- Fisher, J.; Loneragan, W.A.; Dixon, K.; Veneklaas, E.J. Soil seed bank compositional change constrains biodiversity in an invaded species-rich woodland. Biol. Conserv. 2009, 142, 256–269. [Google Scholar] [CrossRef]
- Hopfensperger, K.N. A review of similarity between seed bank and standing vegetation across ecosystems. Oikos 2007, 116, 1438–1448. [Google Scholar] [CrossRef]
- Shang, Z.; Yang, S.; Wang, Y.; Shi, J.; Ding, L.; Long, R. Soil seed bank and its relation with above-ground vegetation along the degraded gradients of alpine meadow. Ecol. Eng. 2016, 90, 268–277. [Google Scholar] [CrossRef]
- Sanou, L.; Savadogo, P.; Zida, D.; Thiombiano, A. Contrasting land use systems influence soil seed bank composition and density in a rural landscape mosaic in West Africa. Flora 2018, 250, 79–90. [Google Scholar] [CrossRef]
- Niknam, P.; Erfanzadeh, R.; Ghelichnia, H.; Cerdà, A.; Erfanzadah, R.; Ghelichhnia, H. Spatial Variation of Soil Seed Bank under Cushion Plants in a Subalpine Degraded Grassland. Land Degrad. Dev. 2017, 29, 4–14. [Google Scholar] [CrossRef]
- Bao, S. Soil Agrochemical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Peet, R.K. The Measurement of Species Diversity. Annu. Rev. Ecol. Syst. 1974, 5, 285–307. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 668. [Google Scholar] [CrossRef]
- Pielou, E.C. An Introduction to Mathematical Ecology; John Wiley: New York, NY, USA, 1969. [Google Scholar]
- Ikeda, A.; Matsuoka, S.; Masuya, H.; Mori, A.; Hirose, D.; Osono, T. Comparison of the diversity, composition, and host recurrence of xylariaceous endophytes in subtropical, cool temperate, and subboreal regions in Japan. Popul. Ecol. 2013, 56, 289–300. [Google Scholar] [CrossRef]
- Jari, O.F.; Guillaume, B.; Michael, F.; Roeland, K.; Pierre, L.; Dan, M.; Peter, R.M.; O’Hara, R.B.; Gavin, L.S.; Peter, S.; et al. Vegan: Community Ecology Package. R package version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 15 December 2021).
- R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2020. Available online: https://www.R-project.org/ (accessed on 11 August 2021).
- Wang, Y.; Chu, L.; Daryanto, S.; Lü, L.; Ala, M.; Wang, L. Sand dune stabilization changes the vegetation characteristics and soil seed bank and their correlations with environmental factors. Sci. Total Environ. 2018, 648, 500–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofmeister, J.; Mihaljevič, M.; Hošek, J.; Sádlo, J. Eutrophication of deciduous forests in the Bohemian Karst (Czech Republic): The role of nitrogen and phosphorus. For. Ecol. Manag. 2002, 169, 213–230. [Google Scholar] [CrossRef]
- Rivera, L.W.; Aide, T. Forest recovery in the karst region of Puerto Rico. For. Ecol. Manag. 1998, 108, 63–75. [Google Scholar] [CrossRef]
- Tackett, N.W.; Craft, C.B. Ecosystem Development on a Coastal Barrier Island Dune Chronosequence. J. Coast. Res. 2010, 264, 736–742. [Google Scholar] [CrossRef]
- Wen, L.; Li, D.; Yang, L.; Luo, P.; Chen, H.; Xiao, K.; Song, T.; Zhang, W.; He, X.; Chen, H.; et al. Rapid recuperation of soil nitrogen following agricultural abandonment in a karst area, southwest China. Biogeochemistry 2016, 129, 341–354. [Google Scholar] [CrossRef]
- Guan, B.; Chen, M.; Elsey-Quirk, T.; Yang, S.; Shang, W.; Li, Y.; Tian, X.; Han, G. Soil seed bank and vegetation differences following channel diversion in the Yellow River Delta. Sci. Total Environ. 2019, 693, 133600. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, W.; Cao, M.; Li, Y. Seasonal variation in density and species richness of soil seed-banks in karst forests and degraded vegetation in central Yunnan, SW China. Seed Sci. Res. 2007, 17, 99–107. [Google Scholar] [CrossRef]
- He, X.; Yuan, L.; Wang, Z.; Zhou, Z.; Wan, L. A study of soil seed banks across one complete chronosequence of secondary succession in a karst landscape. PeerJ 2020, 8, e10226. [Google Scholar] [CrossRef]
- Touzard, B.; Amiaud, B.; Langlois, E.; Lemauviel, S.; Clément, B. The relationships between soil seed bank, aboveground vegetation and disturbances in an eutrophic alluvial wetland of Western France. Flora 2002, 197, 175–185. [Google Scholar] [CrossRef]




| Vegetation Types | Sample Number | Longitude | Latitude | Altitude (m) | Rock Exposed Rate | Slope (°) | Edificator |
|---|---|---|---|---|---|---|---|
| Orchard | O-1 | 110°47′16″ E | 24°55′00″ N | 208 | 75 | 5 | Prunus salicina |
| O-2 | 110°47′13″ E | 24°55′05″ N | 235 | 80 | 2 | ||
| O-3 | 110°47′15″ E | 24°55′06″ N | 234 | 70 | 3 | ||
| Bamboo shrub | B-1 | 110°47′20″ E | 24°55′16″ N | 198 | 85 | 6 | Phyllostachys sulphurea |
| B-2 | 110°47′11″ E | 24°55′07″ N | 229 | 85 | 9 | ||
| B-3 | 110°47′23″ E | 24°55′17″ N | 192 | 80 | 8 | ||
| Primary forest | S-1 | 110°51′50″ E | 24°45′49″ N | 173 | 65 | 10 | Cyclobalanopsis glauca, Pterospermum heterophyllum |
| S-2 | 110°51′49″ E | 24°45′46″ N | 155 | 75 | 17 | ||
| S-3 | 110°52′07″ E | 24°45′18″ N | 150 | 70 | 5 |
| Variable | Comp 1 (36.05%) | Comp 2 (32.58%) |
|---|---|---|
| Organic carbon (OCC) | 0.369 | 0.387 |
| Total nitrogen (TNC) | 0.442 | 0.341 |
| Total phosphorus (TPHC) | 0.392 | −0.379 |
| Total potassium (TPOC) | 0.14 | −0.399 |
| Available nitrogen (Alkeline-N) | 0.417 | 0.361 |
| Available phosphorus (Olsen-P) | 0.353 | −0.356 |
| Rapidly available potassium (Olsen-K) | 0.379 | −0.388 |
| Calcium (CAC) | 0.191 | 0.145 |
| Magnesium (MAC) | −0.132 |
| Latin Name | Family | Life Form | Vegetation Type | |||
|---|---|---|---|---|---|---|
| Orchard | Bamboo Shrub | Primary Forest | ||||
| 1 | Galium aparine | Rubiaceae | Annual herb | 10,733.34 | - | - |
| 2 | Stellaria media | Caryophyllaceae | Annual herb | 6099.99 | 9.99 | - |
| 3 | Oxalis corniculata | Oxalidaceae | Annual herb | 1253.34 | 20.01 | - |
| 4 | Clinopodium chinense | Labiatae | Perennial herb | 860.01 | - | - |
| 5 | Gnaphalium affine | Compositae | Annual herb | 826.68 | - | - |
| 6 | Youngia japonica | Compositae | Annual herb | 346.68 | 69.99 | - |
| 7 | Paspalum thunbergii | Gramineae | Perennial herb | 246.66 | - | - |
| 8 | Cardamine hirsuta | Brassicaceae | Herbs annual | 146.67 | - | - |
| 9 | Capsella bursa-pastoris | Brassicaceae | Annual or biennial herb | 133.32 | - | - |
| 10 | Talinum paniculatum | Portulacaceae | Perennial herb | 60 | - | - |
| 11 | Thyrocarpus sampsonii | Boraginaceae | Annual herb | 33.33 | - | - |
| 12 | Lygodium japonicum | Lygodiaceae | Perennial herbaceous vines | 26.67 | 6.6 | - |
| 13 | Agastache rugosa | Labiatae | Perennial herb | 13.32 | - | - |
| 14 | Centella asiatica | Umbelliferae | Perennial herb | 13.32 | - | - |
| 15 | Eclipta prostrata | Compositae | Annual herb | 13.32 | - | - |
| 16 | Dendranthema indicum | Compositae | Perennial herb | 13.32 | 110.01 | - |
| 17 | Solanum lyratum | Solanaceae | Perennial herbaceous vines | 6.66 | 39.99 | - |
| 18 | Parathelypteris glanduligera | Thelypteridaceae | Perennial herb | 6.66 | - | - |
| 19 | Cichorium endivia | Compositae | Annual herb | 6.66 | - | - |
| 20 | Clematis florida | Ranunculaceae | Perennial herbaceous vines | 6.66 | - | - |
| 21 | Selaginella uncinata | Selaginellaceae | Perennial herb | - | 20.01 | - |
| 22 | Conandron ramondioides | Gesneriaceae | Perennial herb | - | 200.01 | - |
| 23 | Botrychium ternatum | Botrychiaceae | Perennial herb | 279.99 | - | 530.01 |
| 24 | Woodsia ilvensis | Woodsiaceae | Perennial herb | 33.33 | 30 | 279.99 |
| 25 | Broussonetia papyifera | Moraceae | Trees or shrubs | 33.33 | 50.01 | 270 |
| 26 | Solanum nigrum | Solanaceae | Annual herb | 53.34 | 80.01 | 60 |
| 27 | Carex doniana | Cyperaceae | Annual herb | 6.66 | 129.99 | 50.01 |
| 28 | Oplismenus compositus | Gramineae | Annual herb | - | - | 50.01 |
| 29 | Stephania tetrandra | Menispermaceae | Perennial herbaceous vines | - | 39.99 | 39.99 |
| 30 | Arthraxon hispidus | Gramineae | Annual herb | - | - | 39.99 |
| 31 | Achyranthes bidentata | Amaranthaceae | Perennial herb | - | - | 39.99 |
| 32 | Setaria viridis | Gramineae | Annual herb | 20.01 | 170.01 | 30 |
| 33 | Erigeron acer | Compositae | Biennial herb | 13.32 | 39.99 | 20.01 |
| 34 | Commelina bengalensis | Commelinaceae | Perennial herb | 1113.33 | 9.99 | 9.99 |
| 35 | Pilea notate | Urticaceae | Perennial herb | 26.67 | 9.99 | 9.99 |
| 36 | Artemisia carvifolia | Compositae | Annual or biennial herb | 13.32 | 9.99 | 9.99 |
| 37 | Fatoua villosa | Moraceae | Annual herb | 6.66 | - | 9.99 |
| 38 | Mallotus paniculatus | Euphorbiaceae | Trees or shrubs | - | - | 9.99 |
| 39 | Vernonia esculenta | Compositae | Trees or shrubs | - | 20.01 | 9.99 |
| 40 | Pistacia chinensis | Anacardiaceae | Trees or shrubs | - | - | 9.99 |
| 41 | Praxelis clematidea | Compositae | Annual | - | 20.01 | 9.99 |
| 42 | Celtis sinensis | Ulmaceae | Trees or shrubs | - | - | 9.99 |
| 43 | Cyclobalanopsis glauca | Fagaceae | Trees or shrubs | - | - | 9.99 |
| 44 | Cayratia japonica | Vitaceae | Perennial herbaceous vines | - | - | 9.99 |
| Soil Depth | Orchard | Bamboo Shrub | Primary Forest |
|---|---|---|---|
| 0–5 cm | 12,906.67 ± 8995.6 | 406.67 ± 55.87 | 826.67 ± 197.66 |
| 5–10 cm | 6033.33 ± 5357.29 | 353.33 ± 68.24 | 493.33 ± 106.77 |
| 10–15 cm | 3526.67 ± 1398.33 | 326.6 ± 67.21 | 200 ± 43.38 |
| Total | 22,446.67 ± 14,315.18 | 1086.6 ± 213.22 | 1519.89 ± 383.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Li, Y.; Li, J.; Li, J.; Wen, S.; Huang, F.; He, W.; Wang, B.; Lu, S.; Li, D.; et al. Comparison of Aboveground Vegetation and Soil Seed Bank Composition among Three Typical Vegetation Types in the Karst Regions of Southwest China. Agronomy 2022, 12, 1871. https://doi.org/10.3390/agronomy12081871
Guo Y, Li Y, Li J, Li J, Wen S, Huang F, He W, Wang B, Lu S, Li D, et al. Comparison of Aboveground Vegetation and Soil Seed Bank Composition among Three Typical Vegetation Types in the Karst Regions of Southwest China. Agronomy. 2022; 12(8):1871. https://doi.org/10.3390/agronomy12081871
Chicago/Turabian StyleGuo, Yili, Yufei Li, Jianxing Li, Jiaqi Li, Shujun Wen, Fuzhao Huang, Wen He, Bin Wang, Shuhua Lu, Dongxing Li, and et al. 2022. "Comparison of Aboveground Vegetation and Soil Seed Bank Composition among Three Typical Vegetation Types in the Karst Regions of Southwest China" Agronomy 12, no. 8: 1871. https://doi.org/10.3390/agronomy12081871
APA StyleGuo, Y., Li, Y., Li, J., Li, J., Wen, S., Huang, F., He, W., Wang, B., Lu, S., Li, D., Xiang, W., & Li, X. (2022). Comparison of Aboveground Vegetation and Soil Seed Bank Composition among Three Typical Vegetation Types in the Karst Regions of Southwest China. Agronomy, 12(8), 1871. https://doi.org/10.3390/agronomy12081871







