Biostimulants Promote Plant Development, Crop Productivity, and Fruit Quality of Protected Strawberries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Greenhouse Experiment
2.2. High-Tunnel Experiment
2.3. Treatments
2.4. Measured Parameters
2.4.1. Soil Biological Activity
2.4.2. Chlorophyll Fluorescence
2.4.3. Chlorophyll Content and Photosynthetic Parameters
2.4.4. Non-Destructive Growth Parameters
2.4.5. Foliar Mineral Analysis
2.4.6. Yield
2.4.7. Total Sugar Level (°Brix)
2.4.8. Total Phenolic Content (TPC)
2.4.9. Total Anthocyanin Content (TAC)
2.5. Statistical Analysis
3. Results
3.1. Microbial Activity
3.2. Photosynthetic Parameters and Leaf Chlorophyll Content
3.3. Growth Parameters
3.4. Foliar Mineral Content
3.5. Yield and Fruit Size
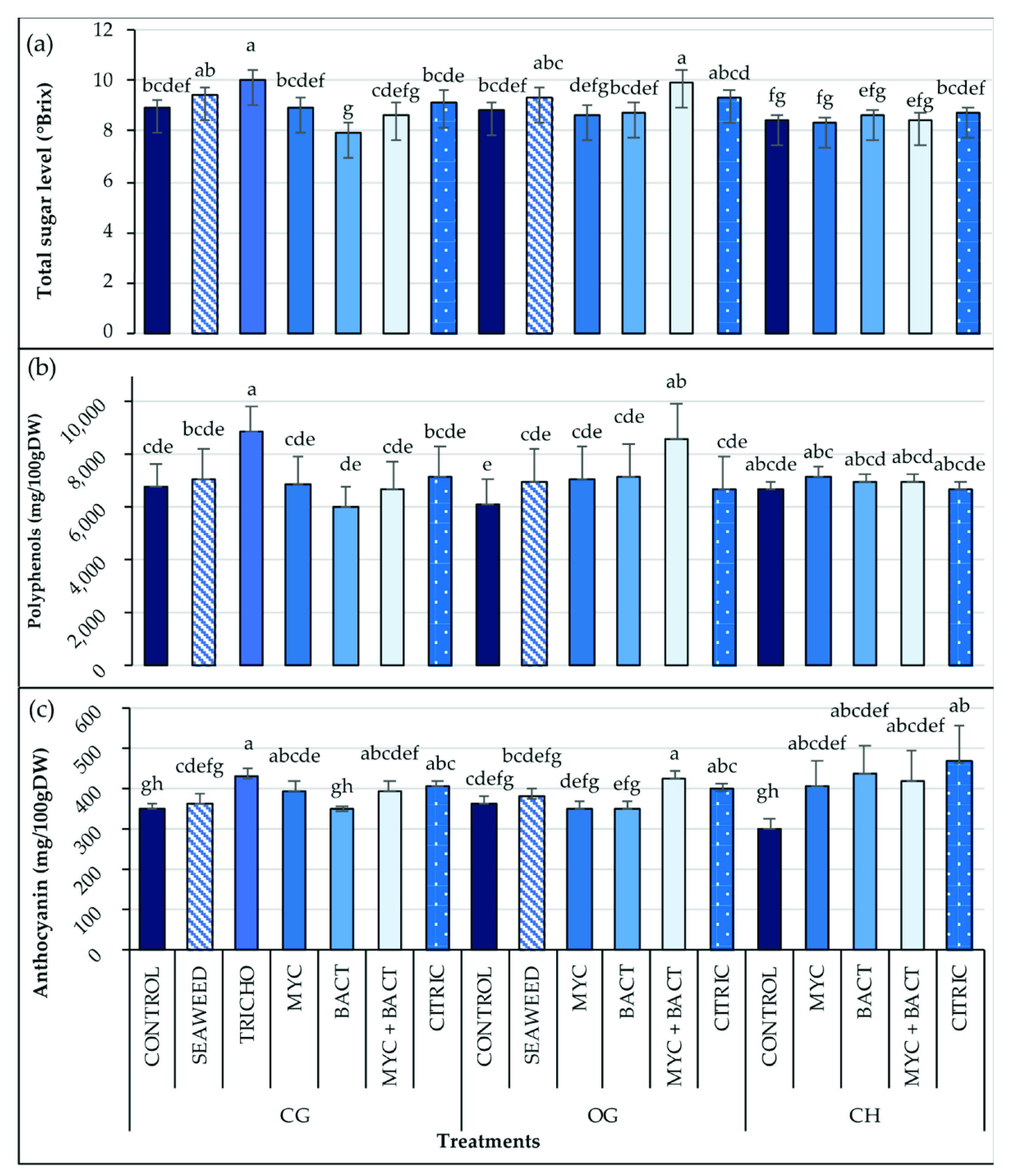
3.6. Fruit Quality
3.7. Principal Component Analysis (PCA)
4. Discussion
4.1. Impact of the Biostimulants
4.2. Impact of the Growing Systems
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statistics Canada. 2022. Available online: https://agriculture.canada.ca/en/canadas-agriculture-sectors/horticulture/horticulture-sector-reports/statistical-overview-canadian-fruit-industry-2020 (accessed on 5 May 2022).
- Habauzit, V.; Milenkovic, D.; Morand, C. Vascular protective effects of fruit polyphenols. In Polyphenols in Human Health and Disease; Elsevier: Amsterdam, The Netherlands, 2014; pp. 875–893. [Google Scholar]
- Vendrame, S.; Del Bo’, C.; Ciappellano, S.; Riso, P.; Klimis-Zacas, D. Berry fruit consumption and metabolic syndrome. Antioxidants 2016, 5, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peña-Sanhueza, D.; Inostroza-Blancheteau, C.; Ribera-Fonseca, A.; Reyes-Díaz, M. Anthocyanins in berries and their potential use in human health. In Superfood and Functional Food-The Development of Superfoods and Their Roles as Medicine; Shiomi, N., Waisundara, V., Eds.; IntechOpen: London, UK, 2017; pp. 155–172. [Google Scholar]
- Shennan, C.; Krupnik, T.J.; Baird, G.; Cohen, H.; Forbush, K.; Lovell, R.J.; Olimpi, E.M. Organic and conventional agriculture: A useful framing? Annu. Rev. Environ. Resour. 2015, 42, 1–30. [Google Scholar] [CrossRef]
- Willer, H.; Trávníček, J.; Meier, C.; Schlatter, B. The World of Organic Agriculture 2022, Statistics and Emerging Trends; FiBL, IFOAM—Organics International: Bonn, Germany, 2022; p. 342. [Google Scholar]
- Dorais, M. Organic strawberry production: A new sustainable paradigm. In Proceedings of the 8th edition of the International Strawberry Symposium, Québec city, QC, Canada, 14–17 August 2016; ISHS: Leuven, Belgium, 2016. [Google Scholar]
- Dorais, M.; Alsanius, B. Advances and trends in organic fruit and vegetable farming research. Hortic. Rev. 2015, 43, 185–268. [Google Scholar]
- De Pascale, S.; Rouphael, Y.; Colla, G. Plant biostimulants: Innovative tool for enhancing plant nutrition in organic farming. Eur. J. Hortic. Sci. 2017, 82, 277–285. [Google Scholar] [CrossRef]
- Rouphael, Y.; Rouphael, Y.; Colla, G. Biostimulants for Sustainable Crop Production; Burleigh Dodds Science Publishing: Cambridge, UK, 2020. [Google Scholar]
- Stevens, C.V. The Chemical Biology of Plant Biostimulants; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Ramawat, N.; Bhardwaj, V. Biostimulants: Exploring Sources and Applications; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Tarafdar, J. Biostimulants for sustainable crop production. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 299–313. [Google Scholar]
- Storer, K.; Kendall, S.; White, C.; Roques, S.; Berry, P.A. Review of the Function, Efficacy and Value of Biostimulant Products Available for UK Cereals and Oilseeds; Agriculture and Horticulture Development Board (ADHB): Warwickshire, UK, 2016. [Google Scholar]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Cataldo, E.; Fucile, M.; Mattii, G.B. Biostimulants in viticulture: A sustainable approach against biotic and abiotic stresses. Plants 2022, 11, 162. [Google Scholar] [CrossRef]
- Monteiro, E.; Gonçalves, B.; Cortez, I.; Castro, I. The Role of Biostimulants as Alleviators of Biotic and Abiotic Stresses in Grapevine: A Review. Plants 2022, 11, 396. [Google Scholar] [CrossRef]
- Mrid, R.B.; Benmrid, B.; Hafsa, J.; Boukcim, H.; Sobeh, M.; Yasri, A. Secondary metabolites as biostimulant and bioprotectant agents: A review. Sci. Total Environ. 2021, 777, 146204. [Google Scholar] [CrossRef]
- Kunicki, E.; Grabowska, A.; Sękara, A.; Wojciechowska, R. The effect of cultivar type, time of cultivation, and biostimulant treatment on the yield of spinach (Spinacia oleracea L.). Folia Hortic. 2010, 22, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Kocira, A.; Świeca, M.; Kocira, S.; Złotek, U.; Jakubczyk, A. Enhancement of yield, nutritional and nutraceutical properties of two common bean cultivars following the application of seaweed extract (Ecklonia maxima). Saudi J. Biol. Sci. 2018, 25, 563–571. [Google Scholar] [CrossRef] [Green Version]
- Gu, D.; Wang, X.-F.; Ding, F.-J. Plant biostimulants: A review on categories, effects and application. In Proceedings of the Chinese Society of Plant Nutrition and Fertilizer Science 2014 Academic Annual Conference, Harbin, China, 13–15 August 2014; Available online: https://www.researchgate.net/publication/290019169 Plant biostimulants a review on categories effects and application (accessed on 2 February 2019).
- Luziatelli, F.; Ficca, A.G.; Colla, G.; Švecová, E.B.; Ruzzi, M. Foliar application of vegetal-derived bioactive compounds stimulates the growth of beneficial bacteria and enhances microbiome biodiversity in lettuce. Front. Plant Sci. 2019, 10, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants, and crop responses: A review. Biol. Agric. Hortic. 2015, 31, 1–17. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant Action of Protein Hydrolysates: Unraveling Their Effects on Plant Physiology and Microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parađiković, N.; Teklić, T.; Zeljković, S.; Lisjak, M.; Špoljarević, M. Biostimulants research in some horticultural plant species—A review. Food Energy Secur. 2019, 8, e00162. [Google Scholar] [CrossRef]
- Marfà, O.; Cáceres, R.; Polo, J.; Ródenas, J. Animal protein hydrolysate as a biostimulant for transplanted strawberry plants subjected to cold stress. In Proceedings of the VI International Strawberry Symposium 842, Huelva, Spain, 3 March 2008; pp. 315–318. [Google Scholar]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- de Vasconcelos, A.C.F.; Chaves, L.H.G. Biostimulants and Their Role in Improving Plant Growth under Abiotic Stresses. In Biostimulants in Plant Science; IntechOpen: London, UK, 2019. [Google Scholar]
- El-Miniawy, S.; Ragab, M.; Youssef, S.; Metwally, A. Influence of foliar spraying of seaweed extract on growth, yield and quality of strawberry plants. J. Appl. Sci. Res. 2014, 10, 88–94. [Google Scholar]
- Brown, P.H.; Amaral, D.C.; Park, M.; Schmidt, J.; Gaudin, A. Plant biostimulants and their influence on nutrient use efficiency (NUE). In Biostimulants for Sustainable Crop Production; Burleigh Dodds Science Publishing: Cambridge, UK, 2020; pp. 297–336. [Google Scholar]
- Raynal Lacroix, C.; Carmentran, M. Fertilization of strawberry crops: Yield and fruit quality. Infos Ctifl 2001, 170, 41–44. [Google Scholar]
- Adam, G.; Duncan, H. Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol. Biochem. 2001, 33, 943–951. [Google Scholar] [CrossRef] [Green Version]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Hansen, P.; McRoberts, R.; Isebrands, J.; Dixon, R. Determining CO2 exchange rate as a function of photosynthetic photon flux density. Photosynthetica 1987, 21, 98–101. [Google Scholar]
- Conseil des Productions Végétales du Québec, Canada. Méthodes d’Analyses des Sols, des Fumiers et des Tissus Végétaux. 1988. Available online: https://www.agrireseau.net/documents/96351/methoded_analyse-des-sols-des-fumiers-et-des-tissus-vegetaux-agdex-533-mai-1988 (accessed on 27 March 2019).
- Watanabe, F.S.; Olsen, S.R. Test of an Ascorbic Acid Method for Determining Phosphorus in Water and NaHCO3 Extracts from Soil. Soil Sci. Soc. Am. J. 1965, 29, 677–678. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Collaborators. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Prior, R.L. Systematic Identification and Characterization of Anthocyanins by HPLC-ESI-MS/MS in Common Foods in the United States: Fruits and Berries. J. Agric. Food Chem. 2005, 53, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.; Landi, L.; Pietramellara, G.; Renella, G. Microbial diversity and soil functions. Eur. J. Soil Sci. 2003, 54, 655–670. [Google Scholar] [CrossRef]
- Araújo, A.S.; Leite, L.F.; Santos, V.B.; Carneiro, R.F. Soil Microbial Activity in Conventional and Organic Agricultural Systems. Sustainability 2009, 1, 268–276. [Google Scholar] [CrossRef] [Green Version]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed Extracts as Biostimulants of Plant Growth and Development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Alam, M.Z.; Braun, G.; Norrie, J.; Hodges, D.M. Effect of Ascophyllum extract application on plant growth, fruit yield and soil microbial communities of strawberry. Can. J. Plant Sci. 2013, 93, 23–36. [Google Scholar] [CrossRef]
- Spinelli, F.; Fiori, G.; Noferini, M.; Sprocatti, M.; Costa, G. A novel type of seaweed extract as a natural alternative to the use of iron chelates in strawberry production. Sci. Hortic. 2010, 125, 263–269. [Google Scholar] [CrossRef]
- Hosseini, S.; Lakzian, A.; Halajnia, A. The effect of EDTA and citric acid on soil enzymes activity, substrate induced respiration and Pb availability in a contaminated soil. J. Water Soil 2016, 30, 2032–2045. [Google Scholar]
- Drouillon, M.; Merckx, R. The role of citric acid as a phosphorus mobilization mechanism in highly P-fixing soilsPapel del ácido cítrico como mecanismo de movilización de fósforo en suelos con alta capacidad de retención del fósforo. Gayana Bot 2003, 60, 55–62. [Google Scholar] [CrossRef]
- Fan, D.; Hodges, D.M.; Critchley, A.T.; Prithiviraj, B. A Commercial Extract of Brown Macroalga (Ascophyllum nodosum) Affects Yield and the Nutritional Quality of Spinach In Vitro. Commun. Soil Sci. Plant Anal. 2013, 44, 1873–1884. [Google Scholar] [CrossRef]
- Karlidag, H.; Yildirim, E.; Turan, M.; Pehluvan, M.; Donmez, F. Plant Growth-promoting Rhizobacteria Mitigate Deleterious Effects of Salt Stress on Strawberry Plants (Fragaria × ananassa). HortScience 2013, 48, 563–567. [Google Scholar] [CrossRef] [Green Version]
- Zare-Maivan, H.; Khanpour-Ardestani, N.; Ghanati, F. Influence of mycorrhizal fungi on growth, chlorophyll content, and potassium and magnesium uptake in maize. J. Plant Nutr. 2017, 40, 2026–2032. [Google Scholar] [CrossRef]
- Islam, M.R.; Sultana, T.; Joe, M.M.; Yim, W.; Cho, J.C.; Sa, T. Nitrogen—fixing bacteria with multiple plant growth—promoting activities enhance growth of tomato and red pepper. J. Basic Microbiol. 2013, 53, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Talebi, M.; Hadavi, E.; Jaafari, N. Foliar Sprays of Citric Acid and Malic Acid Modify Growth, Flowering, and Root to Shoot Ratio of Gazania (Gazania rigens L.): A Comparative Analysis by ANOVA and Structural Equations Modeling. Adv. Agric. 2014, 2014, 1–6. [Google Scholar]
- Hajreza, M.; Hadavi, E.; Zeynanlou, A.; Mirzapour, M.; Naeini, M. Effect of different levels of citric acid and salicylic acid at pre-harvesting stage on vase-life of rose (Rosa hybrida L.) cut flower. J. Sci. Technol. Greenh. Cult. Isfahan Univ. Technol. 2013, 4, 99–109. [Google Scholar]
- Jafari, N.; Hadavi, E. Growth and essential oil yield of dill (Anethum graveolens) as affected by foliar sprays of citric acid and malic acid. In Proceedings of the I International Symposium on Medicinal, Aromatic and Nutraceutical Plants from Mountainous Areas (MAP-Mountain 2011) 955, Saas-Fee, Switzerland, 6–9 July 2011. [Google Scholar]
- El-Yazal, M.A.S. The application of citric acid in combination with some micronutrients increases the growth, productivity and a few chemical constituents of maize (Zea mays) plants. Int. Lett. Nat. Sci. 2019, 76, 87. [Google Scholar] [CrossRef] [Green Version]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Wang, L.; Cui, D.; Zhao, X.; He, M. The Important Role of the Citric Acid Cycle in Plants. Genom. Appl. Biol. 2017, 8, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Basak, A. Biostimulators–definitions, classification and legislation. In Biostimulators In Modern Agriculture. General Aspects; Gawrońska, H., Ed.; Editorial House Wieś Jutra: Warszawa, Poland, 2008; pp. 7–17. [Google Scholar]
- Esitken, A.; Karlidag, H.; Ercisli, S.; Turan, M.; Sahin, F. The effect of spraying a growth promoting bacterium on the yield, growth and nutrient element composition of leaves of apricot (Prunus armeniaca L. cv. Hacihaliloglu). Aust. J. Agric. Res. 2003, 54, 377–380. [Google Scholar] [CrossRef]
- Esitken, A.; Pirlak, L.; Turan, M.; Sahin, F. Effects of floral and foliar application of plant growth promoting rhizobacteria (PGPR) on yield, growth, and nutrition of sweet cherry. Sci. Hortic. 2006, 110, 324–327. [Google Scholar] [CrossRef]
- Egamberdiyeva, D. The effect of plant growth promoting bacteria on growth and nutrient uptake of maize in two different soils. Appl. Soil Ecol. 2007, 36, 184–189. [Google Scholar] [CrossRef]
- Oliveira, I.J.; Fontes, J.R.A.; Pereira, B.F.F.; Muniz, A.W. Inoculation with Azospirillum brasiliense increases maize yield. Chem. Biol. Technol. Agric. 2018, 5, 6. [Google Scholar] [CrossRef]
- Canbolat, M.Y.; Bilen, S.; Çakmakçı, R.; Şahin, F.; Aydın, A. Effect of plant growth-promoting bacteria and soil compaction on barley seedling growth, nutrient uptake, soil properties and rhizosphere microflora. Biol. Fertil. Soils 2006, 42, 350–357. [Google Scholar] [CrossRef]
- Abd-Allah, E.; Issa, M.; El-Kader, S.A.; El-Salam, H.A.; El-Hakim, W.A. Effect of some antioxidants treatments on yield, some chemical constituents and antinutrional factors of some vegetable legumes. In Proceedings of the 1st International Conference on Desert Cultivation: Future of Desert Cultivation Problems and Solutions, Ramadi, Iraq, 27–29 March 2007. [Google Scholar]
- Abido, W.A.; Ibrah, M.E.; El-Zeny, M.M. Growth, Productivity and Quality of Sugar Beet as Affected by Antioxidants Foliar Application and Potassium Fertilizer Top Dressing. Asian J. Crop Sci. 2015, 7, 113–127. [Google Scholar] [CrossRef] [Green Version]
- Fawy, H.A.; Attia, M.F. Effect of Some Antioxidants and Micronutrients Foliar Application on Yield and Quality of Wheat Grown in Siwa Oasis. Agric. Res. 2013, 38, 997–1007. [Google Scholar]
- Lingua, G.; Bona, E.; Manassero, P.; Marsano, F.; Todeschini, V.; Cantamessa, S.; Copetta, A.; D’Agostino, G.; Gamalero, E.; Berta, G. Arbuscular Mycorrhizal Fungi and Plant Growth-Promoting Pseudomonads Increases Anthocyanin Concentration in Strawberry Fruits (Fragaria × ananassa var. Selva) in Conditions of Reduced Fertilization. Int. J. Mol. Sci. 2013, 14, 16207–16225. [Google Scholar] [CrossRef]
- Copetta, A.; Bardi, L.; Bertolone, E.; Berta, G. Fruit production and quality of tomato plants (Solanum lycopersicum L.) are affected by green compost and arbuscular mycorrhizal fungi. Plant Biosyst. 2011, 145, 106–115. [Google Scholar] [CrossRef]
- Todeschini, V.; AitLahmidi, N.; Mazzucco, E.; Marsano, F.; Gosetti, F.; Robotti, E.; Bona, E.; Massa, N.; Bonneau, L.; Marengo, E. Impact of Beneficial Microorganisms on Strawberry Growth, Fruit Production, Nutritional Quality, and Volatilome. Front. Plant Sci. 2018, 9, 1611. [Google Scholar] [CrossRef]
- Pascale, A.; Vinale, F.; Manganiello, G.; Nigro, M.; Lanzuise, S.; Ruocco, M.; Marra, R.; Lombardi, N.; Woo, S.; Lorito, M. Trichoderma and its secondary metabolites improve yield and quality of grapes. Crop Prot. 2017, 92, 176–181. [Google Scholar] [CrossRef] [Green Version]
- Ferrini, F.; Nicese, F. Response of English Oak (Quercus Robur L.) Trees to Biostimulants Application in the Urban Environment. Arboric. Urban For. 2002, 28, 70–75. [Google Scholar] [CrossRef]
- Amanda, A.; Ferrante, A.; Valagussa, M.; Piaggesi, A. Effect of biostimulants on quality of baby leaf lettuce grown under plastic tunnel. In Proceedings of the International Symposium on Strategies Towards Sustainability of Protected Cultivation in Mild Winter Climate 807, Leuven, Belgium, 7 April 2008; ISHS: Leuven, Belgium, 2008. [Google Scholar]
- Dinesh, R.; Srinivasan, V.; Hamza, S.; Manjusha, A. Short-term incorporation of organic manures and biofertilizers influences biochemical and microbial characteristics of soils under an annual crop [Turmeric (Curcuma longa L.)]. Bioresour. Technol. 2010, 101, 4697–4702. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Hu, F.; Chen, X.; Huang, Q.; Jiao, J.; Zhang, B.; Li, H. Organic amendments with reduced chemical fertilizer promote soil microbial development and nutrient availability in a subtropical paddy field: The influence of quantity, type, and application time of organic amendments. Appl. Soil Ecol. 2009, 42, 166–175. [Google Scholar] [CrossRef]
- Macit, I.; Koc, A.; Guler, S.; Deligoz, I. Yield, quality and nutritional status of organically and conventionally-grown strawberry cultivars. Asian J. Plant Sci. 2007, 6, 1131–1136. [Google Scholar]
- Conti, S.; Villari, G.; Faugno, S.; Melchionna, G.; Somma, S.; Caruso, G. Effects of organic vs. conventional farming system on yield and quality of strawberry grown as an annual or biennial crop in southern Italy. Sci. Hortic. 2014, 180, 63–71. [Google Scholar] [CrossRef]
- Dorais, M.; Schwarz, D. Organic Tomato. In Tomato; Crop Production Science in Horticulture Series; CABI Intern: Boston, MA, USA, 1 August 2018; Chapter 11. [Google Scholar]
- Desjardins, Y. Are organically grown fruits and vegetables nutritionally better than conventional ones? Revisiting the question with new eyes. In Proceedings of the International Symposium on Innovation in Integrated and Organic Horticulture (INNOHORT) 1137, Leuven, Belgium, 8 January 2015; ISHS: Leuven, Belgium, 2015. [Google Scholar]
- Andrade, C.A.W.; Miguel, A.C.A.; Spricigo, P.C.; Dias, C.T.d.S.; Jacomino, A.P. Comparison of quality between organic and conventional strawberries from multiple farms. Rev. Bras. De Frutic. 2017, 39, 2. [Google Scholar]
- Kobi, H.; Martins, M.; Silva, P.; Souza, J.; Carneiro, J.; Heleno, F.; Queiroz, M.; Costa, N. Organic, and conventional strawberries: Nutritional quality, antioxidant characteristics and pesticide residues. Fruits 2018, 73, 39–47. [Google Scholar] [CrossRef]
- Krolow, A.C.R.; Schwengber, J.E.; Ferri, N.L. Avaliações físicas e químicas de morango cv. Aromas produzidos em sistema orgânico e convencional. Rev. Bras. Agroecol. 2007, 2, 1732–1735. [Google Scholar]
- Dorais, M.; Ehret, D. Agronomy and the nutritional quality of fruit. In Improving the Health-Promoting Properties of Fruit and Vegetable Products; CRC: Boca Raton, FL, USA, 2008; pp. 346–391. [Google Scholar]
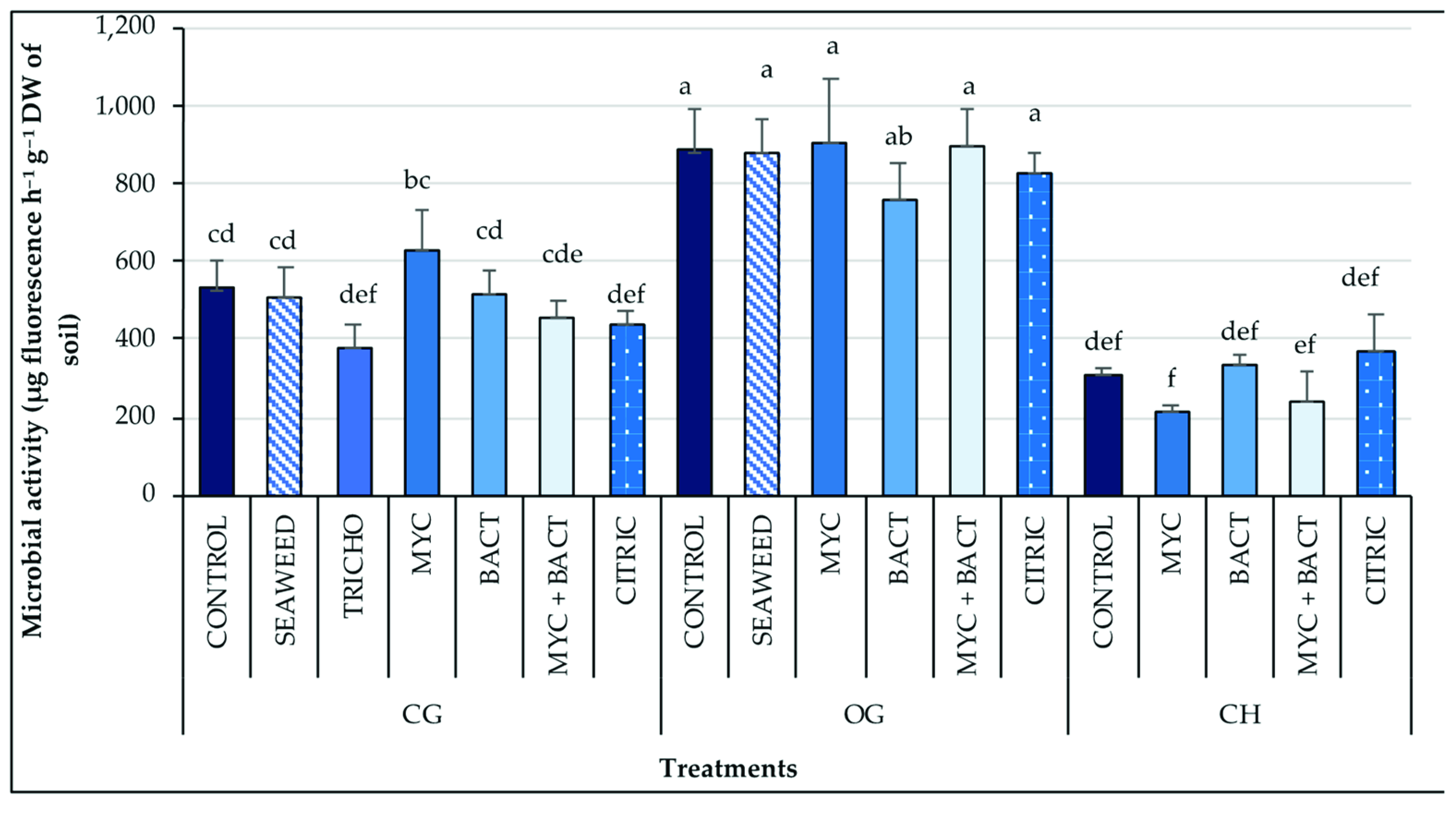
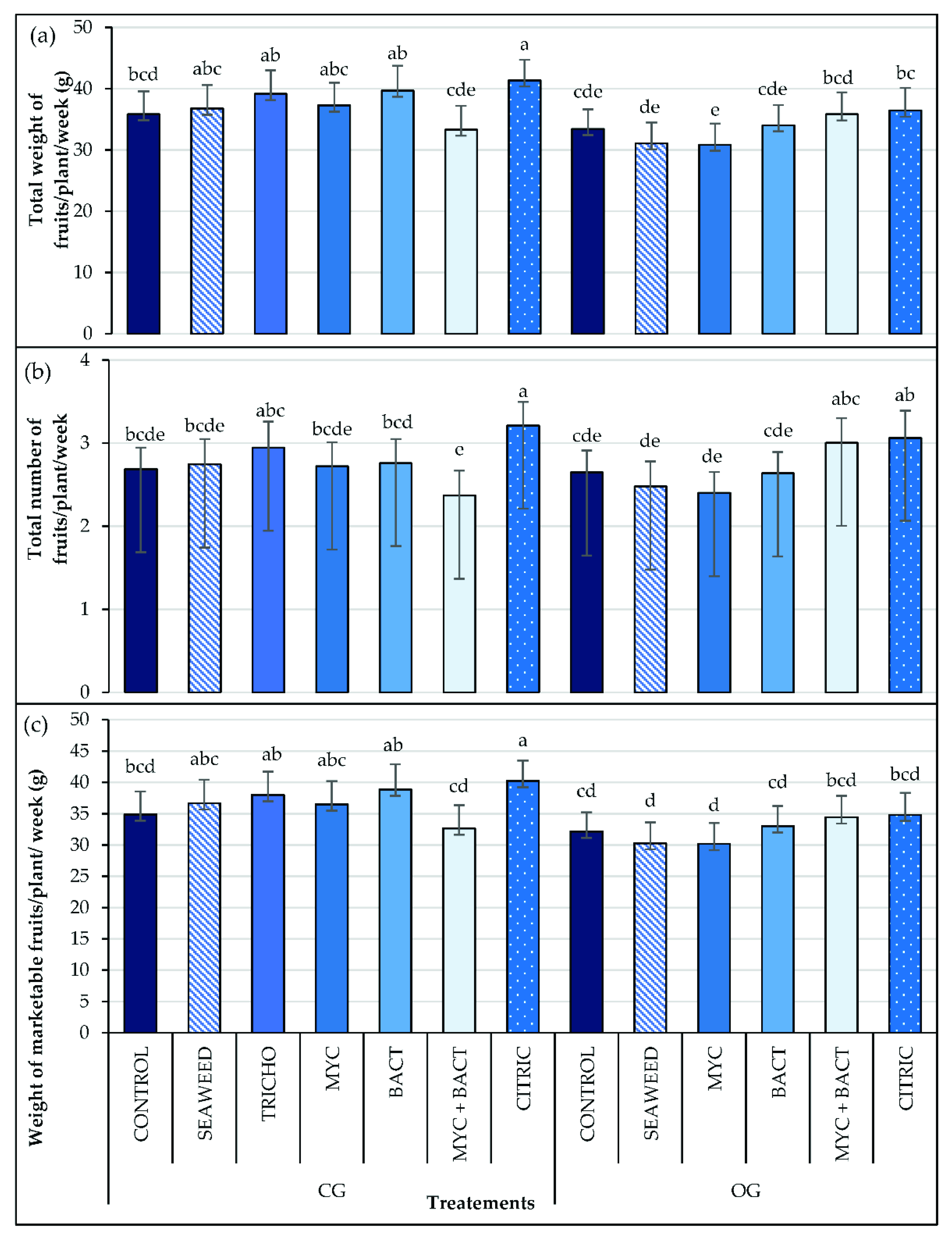
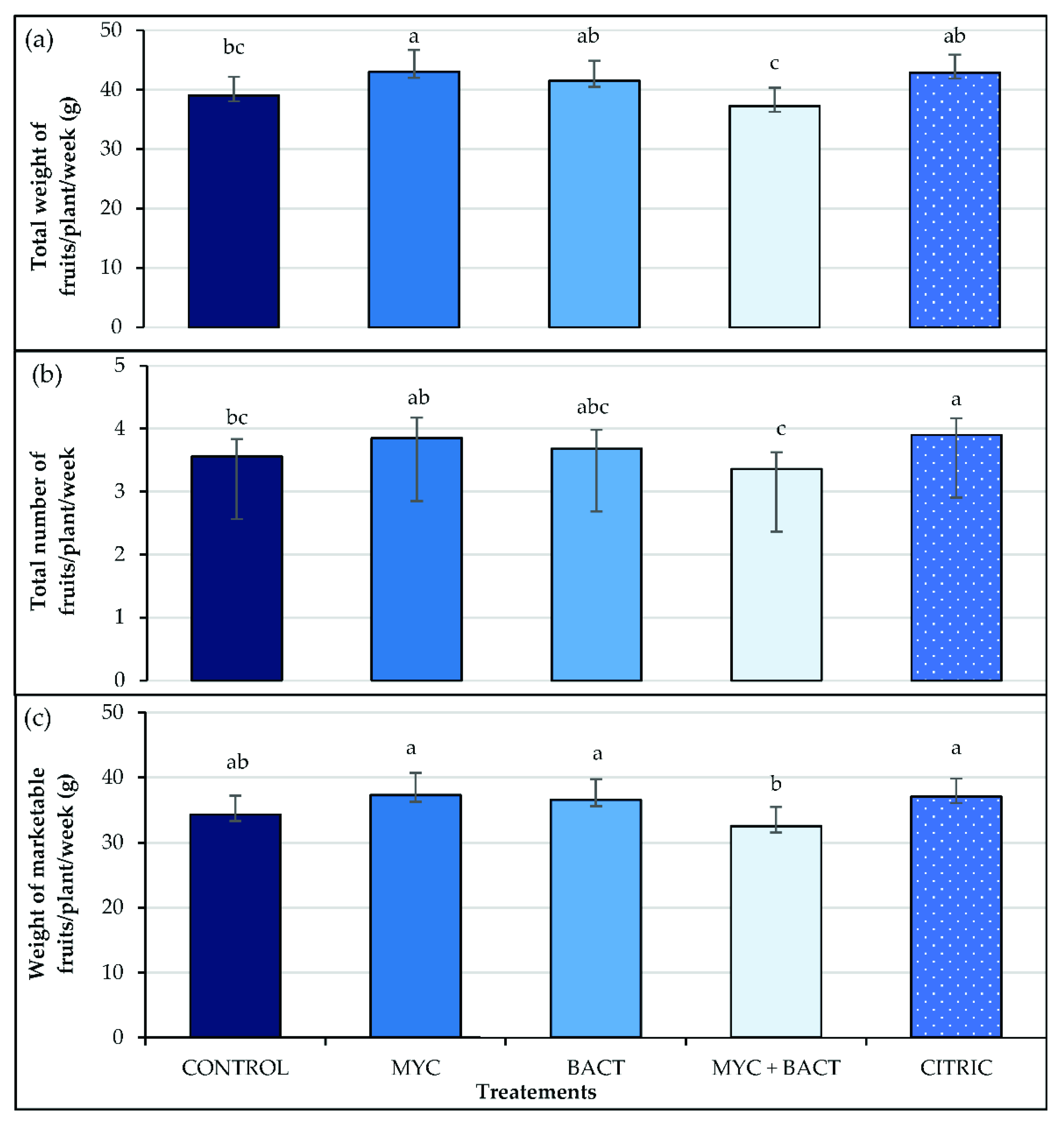
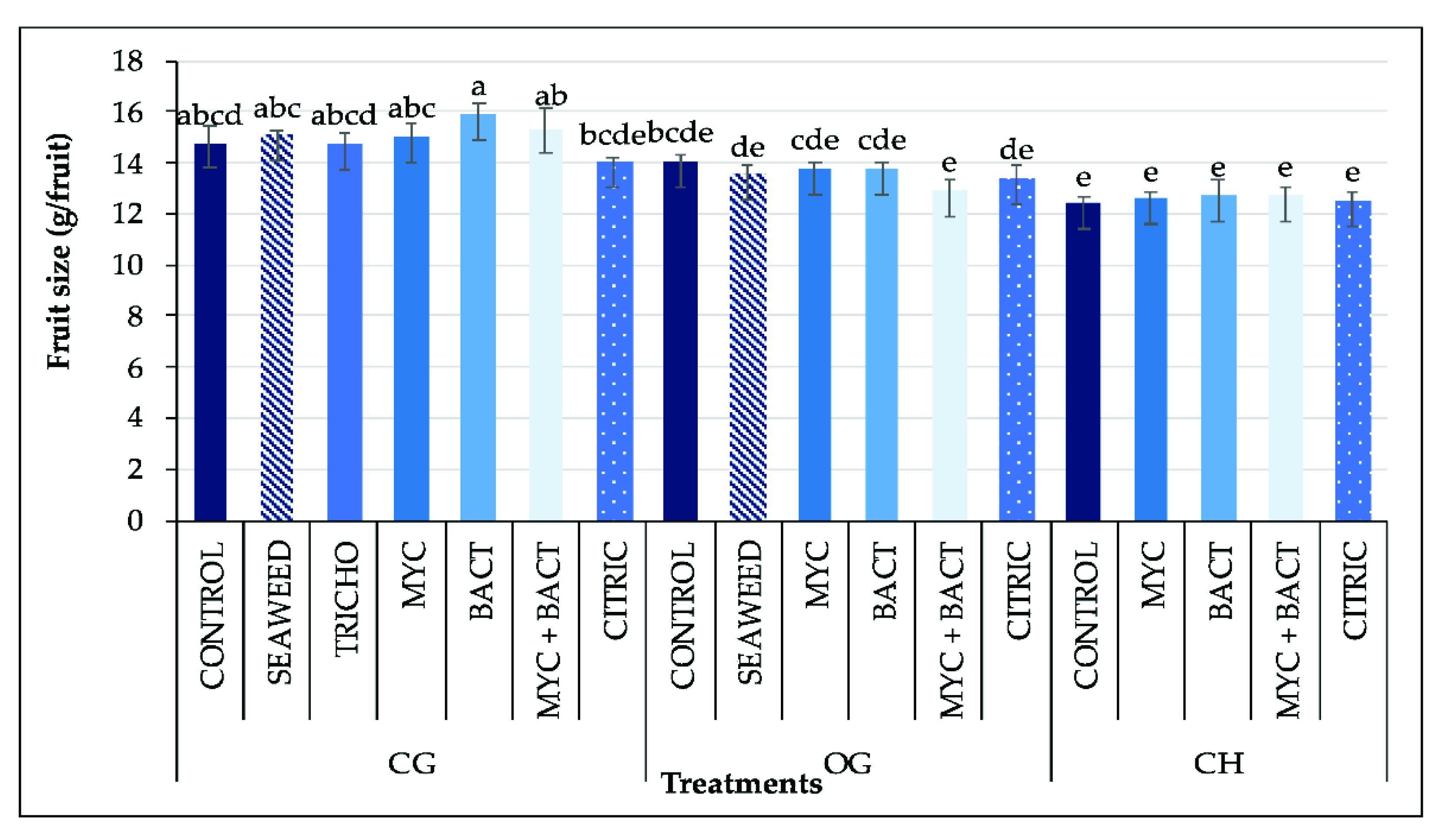

| Treatments | Fv/Fm | PI | SPAD | |
|---|---|---|---|---|
| CG z | CONTROL | 0.805 bcd x | 2.8 fgh | 37.3 def |
| SEAWEED | 0.803 bcde | 2.7 fgh | 37.0 ef | |
| TRICHO | 0.804 bcd | 3.1 defg | 37.1 ef | |
| MYC | 0.798 e * | 2.6 h | 36.7 f | |
| BACT | 0.802 cde | 2.6 gh | 37.2 def | |
| MYC + BACT | 0.801 de | 2.7 fgh | 37.4 def | |
| CITRIC | 0.801 de | 2.6 fgh | 36.8 ef | |
| OG | CONTROL | 0.803 bcde | 3.0 efgh | 38.1 cde |
| SEAWEED | 0.808 bc | 3.1 defg | 37.3 def | |
| MYC | 0.803 bcde | 2.9 efgh | 38.2 cde | |
| BACT | 0.802 bcde | 2.8 fgh | 37.7 cdef | |
| MYC + BACT | 0.803 bcde | 3.0 efgh | 37.1 ef | |
| CITRIC | 0.808 b | 3.1 cdef | 38.1 cde | |
| CH | CONTROL | 0.821 a | 3.5 abcd | 39.9 ab |
| MYC | 0.824 a | 3.9 a | 40.3 a | |
| BACT | 0.824 a | 3.7 ab | 39.1 abc | |
| MYC + BACT | 0.821 a | 3.5 abc | 40.6 a | |
| CITRIC | 0.820 a | 3.3 bcde | 38.7 bcd | |
| OG vs. CG | OG | 0.804 a | 3.0 a | 37.8 |
| CG | 0.802 b | 2.7 b | 37.1 | |
| CG vs. CH | CH | 0.822 a | 3.6 a | 39.7 a |
| CG | 0.802 b | 2.7 b | 37.1 b | |
| p values | ||||
| Biostimulant (B) | <0.001 | <0.001 | <0.001 | |
| OG vs. CG | 0.042 | 0.007 | 0.012 | |
| CG vs. CH | <0.001 | <0.001 | <0.001 | |
| Treatments | Number of Leaves | Number of Flowering Stalks | Number of Crowns | Diameter of Crowns (mm) | |
|---|---|---|---|---|---|
| CG z | CONTROL | 15.9 cd x | 4.9 efg | 3.5 cdefg | 38.1 defg |
| SEAWEED | 14.5 d | 4.7 efg | 3.2 fg | 36.5 defg | |
| TRICHO | 16.3 cd | 5.1 ef | 3.5 cdefg | 39.4 cde | |
| MYC | 15.4 cd | 4.8 efg | 3.3 efg | 36.9 defg | |
| BACT | 15.3 cd | 4.5 efg | 3.3 efg | 36.1 efg | |
| MYC + BACT | 14.1 d | 4.0 g | 3.2 efg | 34.4 fg | |
| CITRIC | 16.4 cd | 5.2 def | 3.7 bcde | 38.2 def | |
| OG | CONTROL | 15.9 cd | 4.8 efg | 3.4 defg | 40.2 cd |
| SEAWEED | 14.7 d | 4.3 fg | 3.4 efg | 34.2 g * | |
| MYC | 15.2 cd | 4.0 g | 3.2 g | 37.9 defg | |
| BACT | 15.2 cd | 5.1 ef | 3.3 efg | 37.0 defg | |
| MYC + BACT | 15.5 cd | 5.2 ef | 3.4 defg | 36.9 defg | |
| CITRIC | 17.3 bc | 5.5de | 3.6 bcdef | 39.4 cde | |
| CH | CONTROL | 21.1 a | 6.5 cd | 4.1 ab | 44.2 bc |
| MYC | 20.4 ab | 7.1 bc | 3.9 abcd | 48.1 ab | |
| BACT | 21.8a | 7.8 ab * | 4.3 a | 49.5 a * | |
| MYC + BACT | 20.6 ab | 8.1 ab * | 4.4 a | 44.5 bc | |
| CITRIC | 20.4 ab | 8.6 a * | 3.9 abc | 45.8 ab | |
| OG vs. CG | OG | 15.6 | 4.8 | 3.4 | 37.6 |
| CG | 15.4 | 4.7 | 3.4 | 37.1 | |
| CG vs. CH | CH | 20.8 a | 7.6 a | 4.1 a | 46.4 a |
| CG | 15.4 b | 4.7 b | 3.4 b | 37.1 b | |
| p values | |||||
| Biostimulant (B) | 0.040 | <0.001 | <0.001 | <0.001 | |
| OG vs. CG | 0.644 | 0.697 | 0.900 | 0.548 | |
| CG vs. CH | <0.001 | <0.001 | <0.001 | <0.001 | |
| Treatments | N (%) | P (%) | K (%) | Ca (%) | Mg (%) | |
|---|---|---|---|---|---|---|
| CG z | CONTROL | 1.83 fg x | 0.471 a | 1.41 | 0.634 cdef | 0.186 |
| SEAWEED | 1.93 efg | 0.466 a | 1.36 | 0.670 bcdef | 0.198 | |
| TRICHO | 1.98 bcdef | 0.494 a | 1.42 | 0.678 bcdef | 0.210 | |
| MYC | 2.02 abcdef | 0.486 a | 1.41 | 0.740 abcde | 0.209 | |
| BACT | 1.89 fg | 0.477 a | 1.32 | 0.685 bcdef | 0.199 | |
| MYC + BACT | 1.95 efg | 0.457 ab | 1.35 | 0.571 efg | 0.182 | |
| CITRIC | 1.86 fg | 0.460 ab | 1.42 | 0.809 ab * | 0.226 | |
| OG | CONTROL | 2.19 abc | 0.396 bc | 1.45 | 0.532 fg | 0.212 |
| SEAWEED | 2.15abcde | 0.389 bc | 1.47 | 0.434 g | 0.200 | |
| MYC | 2.00 abcdef | 0.363 cd | 1.38 | 0.520 fg | 0.206 | |
| BACT | 2.21 a | 0.382 cd | 1.36 | 0.612 cdef | 0.227 | |
| MYC + BACT | 2.18 abcd | 0.365 cd | 1.39 | 0.593 defg | 0.235 | |
| CITRIC | 1.96 defg * | 0.390 bc | 1.43 | 0.591 defg | 0.210 | |
| CH | CONTROL | 1.86 g | 0.276de | 1.38 | 0.903 a | 0.196 |
| MYC | 1.94 abcdef * | 0.242 e | 1.43 | 0.851 ab | 0.188 | |
| BACT | 1.99 abcdef * | 0.296 cde | 1.26 | 0.889 a | 0.220 | |
| MYC + BACT | 2.09 abcdef * | 0.236 e | 1.37 | 0.775 abcd | 0.189 | |
| CITRIC | 2.11 abcdef * | 0.233 e | 1.41 | 0.634 abc | 0.221 | |
| OG vs. CG | OG | 2.1 a | 0.381 b | 1.4 | 0.547 b | 0.215 |
| CG | 1.9 b | 0.473 a | 1.4 | 0.684 a | 0.201 | |
| CG vs. CH | CH | 2.0 | 0.257 b | 1.4 | 0.844 a | 0.203 |
| CG | 1.9 | 0.473 a | 1.4 | 0.684 b | 0.201 | |
| p values | ||||||
| Biostimulant (B) | 0.008 | <0.001 | <0.001 | <0.001 | 0.281 | |
| OG vs. CG | <0.001 | <0.001 | 0.285 | <0.001 | 0.076 | |
| CG vs. CH | 0.570 | <0.001 | 0.804 | <0.001 | 0.915 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soltaniband, V.; Brégard, A.; Gaudreau, L.; Dorais, M. Biostimulants Promote Plant Development, Crop Productivity, and Fruit Quality of Protected Strawberries. Agronomy 2022, 12, 1684. https://doi.org/10.3390/agronomy12071684
Soltaniband V, Brégard A, Gaudreau L, Dorais M. Biostimulants Promote Plant Development, Crop Productivity, and Fruit Quality of Protected Strawberries. Agronomy. 2022; 12(7):1684. https://doi.org/10.3390/agronomy12071684
Chicago/Turabian StyleSoltaniband, Veedaa, Annie Brégard, Linda Gaudreau, and Martine Dorais. 2022. "Biostimulants Promote Plant Development, Crop Productivity, and Fruit Quality of Protected Strawberries" Agronomy 12, no. 7: 1684. https://doi.org/10.3390/agronomy12071684
APA StyleSoltaniband, V., Brégard, A., Gaudreau, L., & Dorais, M. (2022). Biostimulants Promote Plant Development, Crop Productivity, and Fruit Quality of Protected Strawberries. Agronomy, 12(7), 1684. https://doi.org/10.3390/agronomy12071684






