Mode of Action of a Novel Synthetic Auxin Herbicide Halauxifen-Methyl
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Cultivation of Plants
2.2. Determination of Ethylene Production
2.3. Determination of ACC Content
2.4. Determination of ACC Synthase Activity
2.5. Determination of ACC Oxidase Activity
2.6. Determination of IAA and ABA
2.7. Molecular Cloning of the GaACS4, GaACS7, and GaNCED1 Fragments
2.8. Gene Expression Analysis Based on Real-Time Quantitative PCR
3. Results
3.1. Effect of Halauxifen-Methyl on Growth of Galium aparine
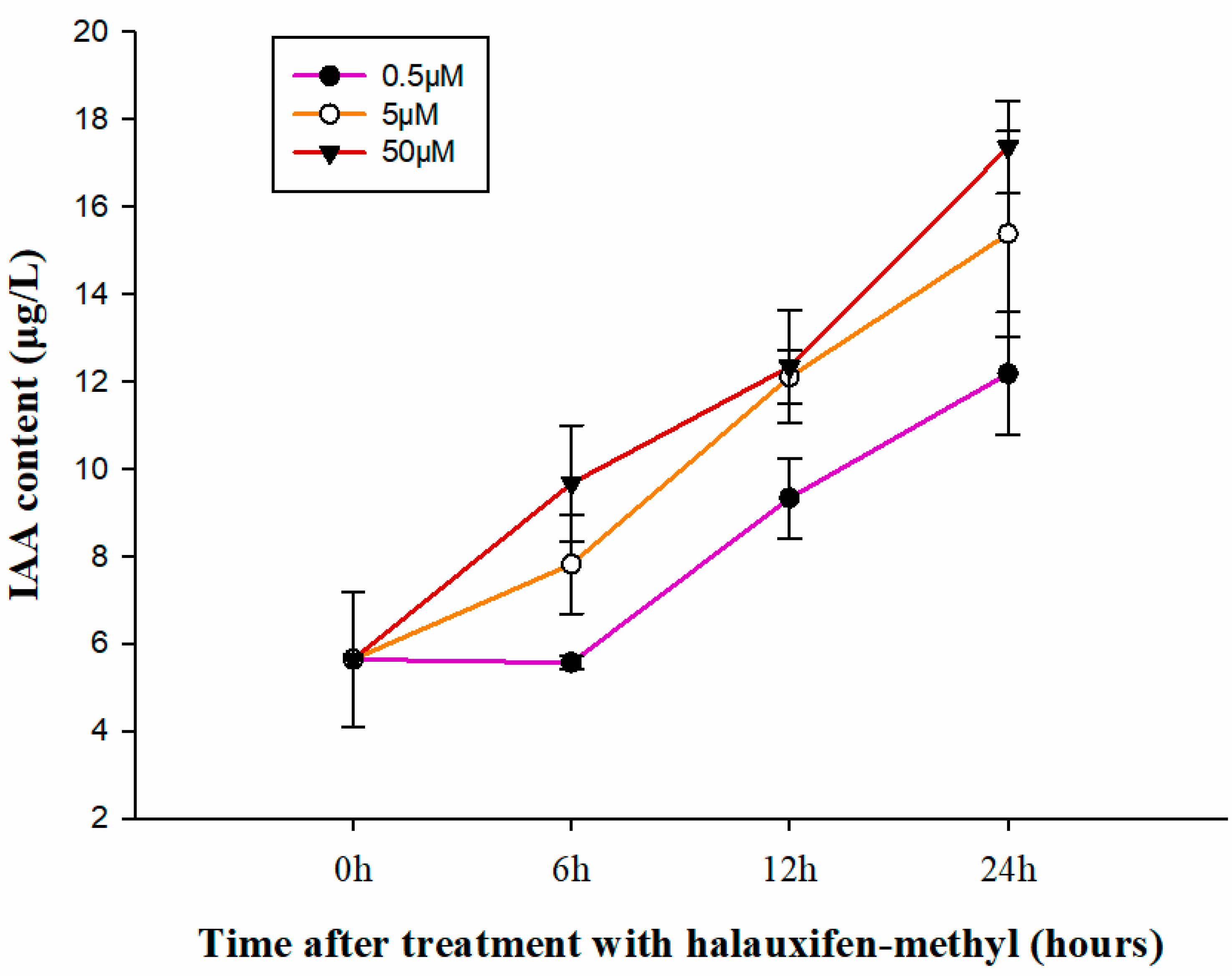
3.2. Effect of Halauxifen-Methyl on the Content of IAA in Galium aparine
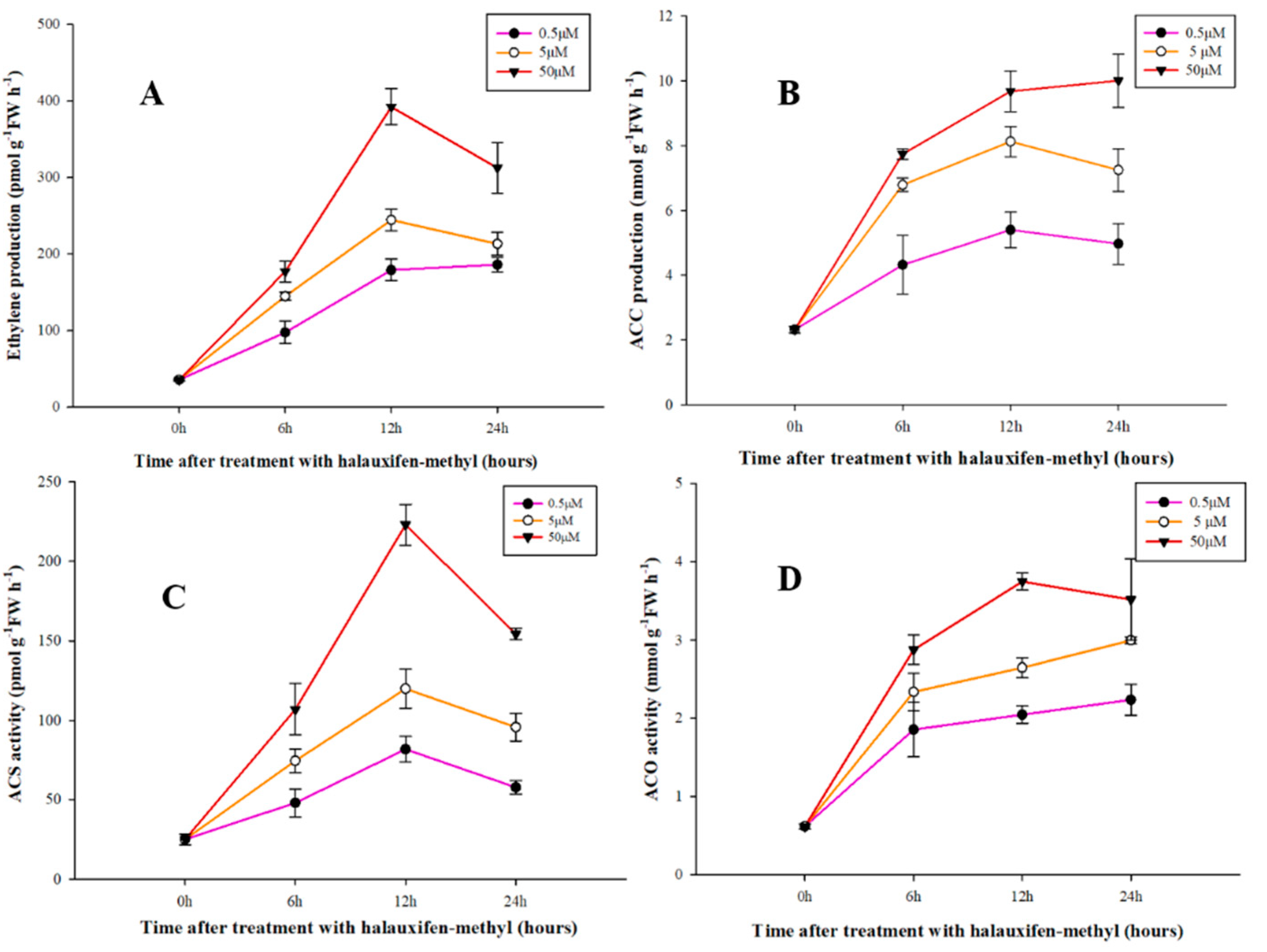
3.3. Effect of Halauxifen-Methyl on Ethylene Biosynthesis in Galium aparine
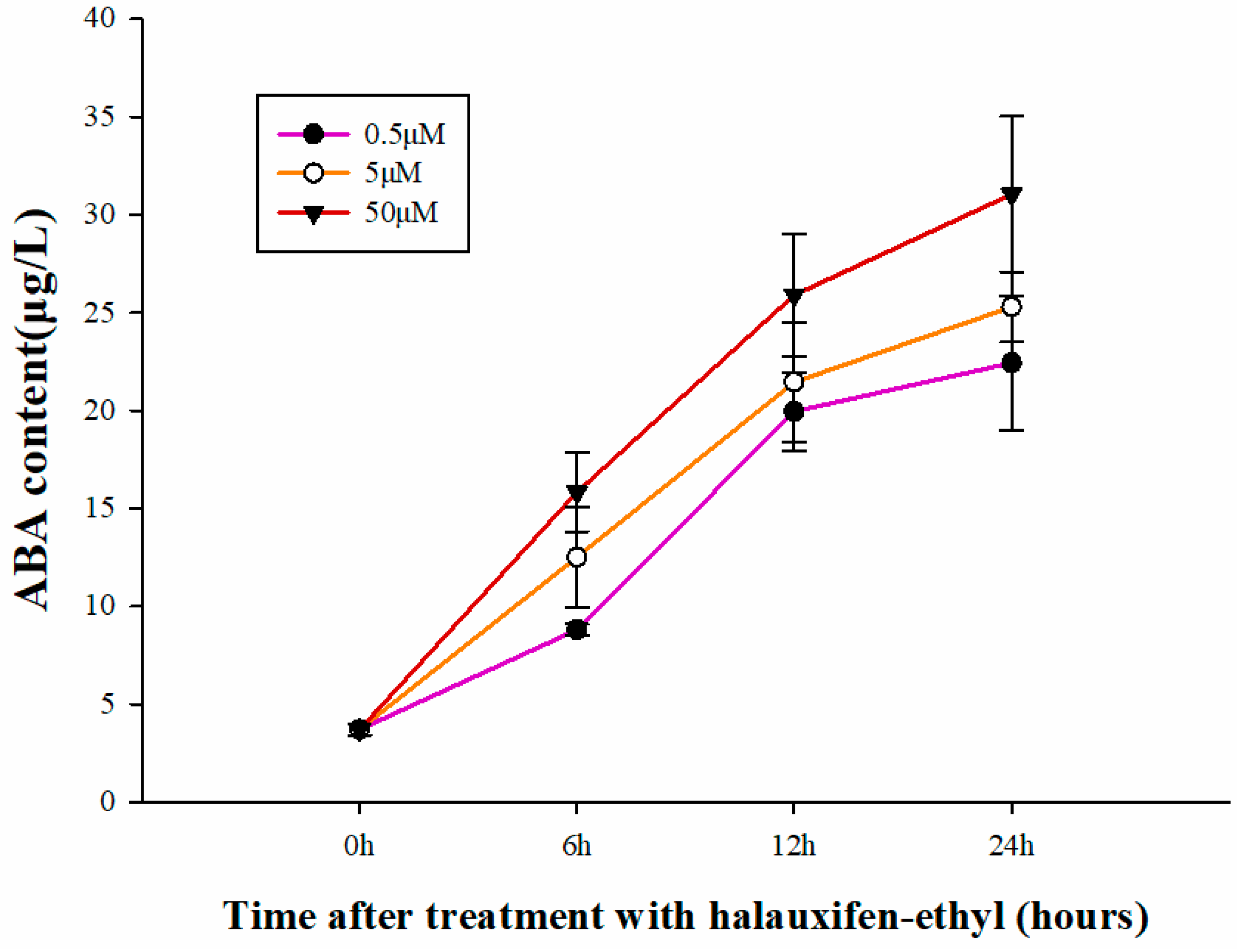
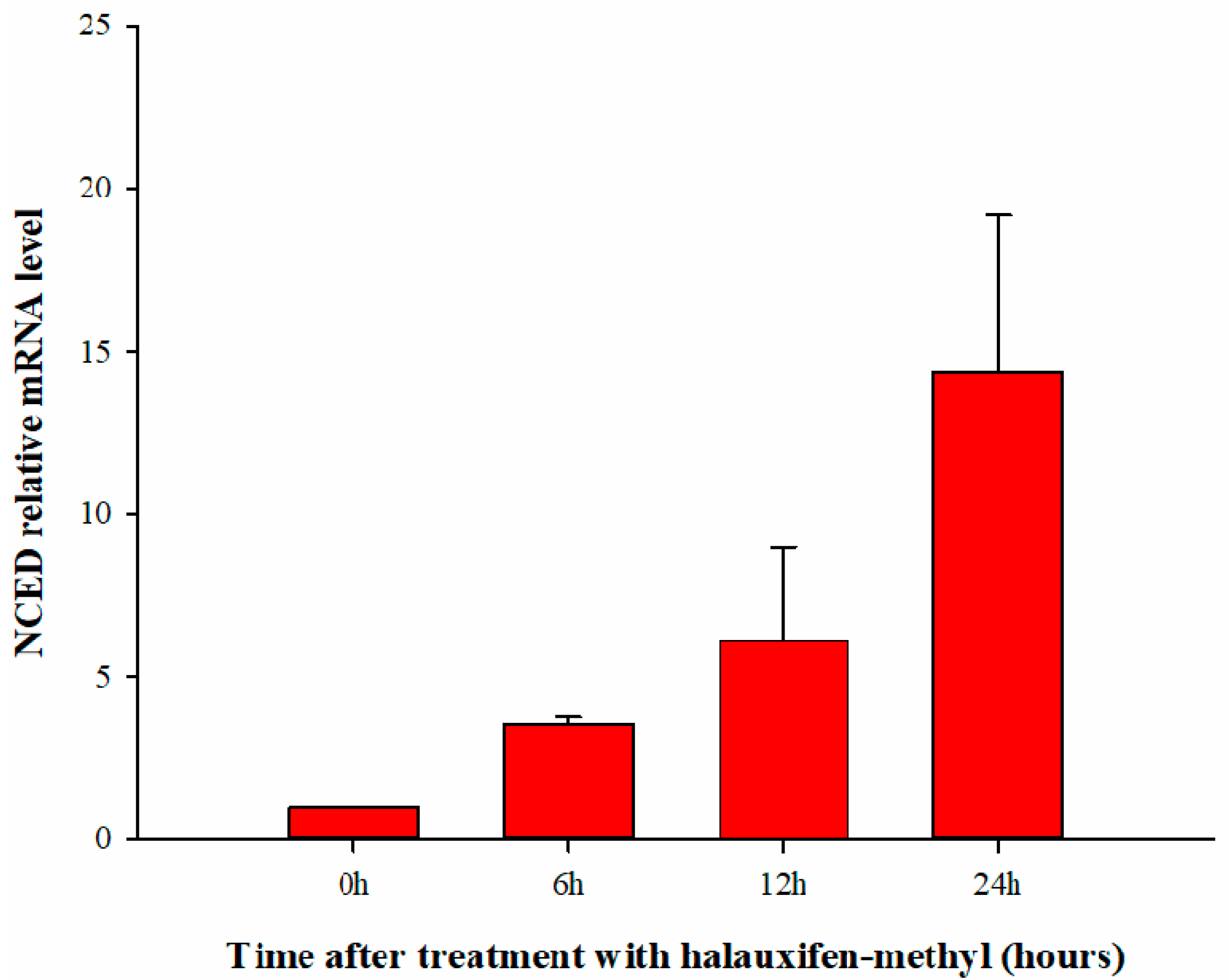
3.4. Effect of Halauxifen-Methyl on ABA Biosynthesis in Galium aparine
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeschke, P.; Witschel, M.; Kramer, W.; Schirmer, U. New Auxin Mimics and Herbicides. In Modern Crop Protection Compounds; Wiley: Hoboken, NJ, USA, 2019; pp. 303–350. [Google Scholar]
- Grossmann, K. Auxin herbicides: Current status of mechanism and mode of action. Pest Manag. Sci. 2009, 66, 113–120. [Google Scholar] [CrossRef]
- Tromas, A.; Perrot-Rechenmann, C. Recent progress in auxin biology. Comptes Rendus. Biol. 2010, 333, 297–306. [Google Scholar] [CrossRef]
- Hagen, G.; Guilfoyle, T. Auxin-responsive gene expression: Genes, promoters and regulatory factors. Plant Mol. Biol. 2002, 49, 373–385. [Google Scholar] [CrossRef]
- Kepinski, S.; Leyser, O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 2005, 435, 446–451. [Google Scholar] [CrossRef]
- Tan, X.; Villalobos, L.I.A.C.; Sharon, M.; Zheng, C.; Robinson, C.V.; Estelle, M.; Zheng, N. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 2007, 446, 640–645. [Google Scholar] [CrossRef]
- Peterson, M.A.; McMaster, S.A.; Riechers, D.E.; Skelton, J.; Stahlman, P.W. 2,4-D Past, Present, and Future: A Review. Weed Technol. 2016, 30, 303–345. [Google Scholar] [CrossRef]
- Soma, S.T.R. Characterization of a Novel RING-Type Ubiquitin E3 Ligase GhRING2 Involved in Cotton Fiber Development; Mississippi State University: Starkville, MS, USA, 2013. [Google Scholar]
- Dharmasiri, N.; Dharmasiri, S.; Estelle, M. The F-box protein TIR1 is an auxin receptor. Nature 2005, 435, 441–445. [Google Scholar] [CrossRef]
- Parry, G.; Calderon-Villalobos, L.I.; Prigge, M.; Peret, B.; Dharmasiri, S.; Itoh, H.; Lechner, E.; Gray, W.M.; Bennett, M.; Estelle, M. Complex regulation of the TIR1/AFB family of auxin receptors. Proc. Natl. Acad. Sci. USA 2009, 106, 22540–22545. [Google Scholar] [CrossRef] [Green Version]
- Prigge, M.J.; Greenham, K.; Zhang, Y.; Santner, A.; Castillejo, C.; Mutka, A.M.; O’Malley, R.C.; Ecker, J.R.; Kunkel, B.N.; Estelle, M. The Arabidopsis Auxin Receptor F-Box Proteins AFB4 and AFB5 Are Required for Response to the Synthetic Auxin Picloram. G3 Genes Genomes Genet. 2016, 6, 1383–1390. [Google Scholar] [CrossRef] [Green Version]
- Gleason, C.; Foley, R.C.; Singh, K.B. Mutant Analysis in Arabidopsis Provides Insight into the Molecular Mode of Action of the Auxinic Herbicide Dicamba. PLoS ONE 2011, 6, e17245. [Google Scholar]
- Lee, S.; Sundaram, S.; Armitage, L.; Evans, J.P.; Hawkes, T.; Kepinski, S.; Ferro, N.; Napier, R.M. Defining Binding Efficiency and Specificity of Auxins for SCFTIR1/AFB-Aux/IAA Co-receptor Complex Formation. Acs Chem. Biol. 2014, 9, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Kraft, M.; Kuglitsch, R.; Kwiatkowski, J.; Frank, M.; Grossmann, K. Indole-3-acetic acid and auxin herbicides up-regulate 9-cis-epoxycarotenoid dioxygenase gene expression and abscisic acid accumulation in cleavers (Galium aparine): Interaction with ethylene. J. Exp. Bot. 2007, 58, 1497–1503. [Google Scholar] [CrossRef] [Green Version]
- Grossmann, K. Mediation of Herbicide Effects by Hormone Interactions. J. Plant Growth Regul. 2003, 22, 109–122. [Google Scholar] [CrossRef]
- Schwartz, S.H.; Qin, X.; Zeevaart, J.A. Elucidation of the Indirect Pathway of Abscisic Acid Biosynthesis by Mutants, Genes, and Enzymes. Plant Physiol. 2003, 131, 1591–1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigo, M.-J.; Alquezar, B.; Zacarías, L. Cloning and characterization of two 9-cis-epoxycarotenoid dioxygenase genes, differentially regulated during fruit maturation and under stress conditions, from orange (Citrus sinensis L. Osbeck). J. Exp. Bot. 2006, 57, 633–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossmann, K.; Scheltrup, F.; Kwiatkowski, J.; Caspar, G. Induction of abscisic acid is a common effect of auxin herbicides in susceptible plants. J. Plant Physiol. 1996, 149, 475–478. [Google Scholar] [CrossRef]
- Grossmann, K. Auxin Herbicide Action Lifting the Veil Step by Step. Plant Signal. Behav. 2007, 2, 421–423. [Google Scholar] [CrossRef] [Green Version]
- Epp, J.B.; Schmitzer, P.R.; Crouse, G.D. Fifty years of herbicide research: Comparing the discovery of trifluralin and halauxifen-methyl. Pest Manag. Sci. 2018, 74, 9–16. [Google Scholar] [CrossRef]
- Epp, J.B.; Alexander, A.L.; Balko, T.W.; Buysse, A.M.; Brewster, W.K.; Bryan, K.; Daeuble, J.F.; Fields, S.C.; Gast, R.E.; Green, R.A.; et al. The discovery of Arylex (TM) active and Rinskor (TM) active: Two novel auxin herbicides. Bioorg. Med. Chem. 2016, 24, 362–371. [Google Scholar] [CrossRef]
- McCauley, C.L.; Johnson, W.G.; Young, B.G. Efficacy of Halauxifen-Methyl on Glyphosate-Resistant Horseweed (Erigeron canadensis). Weed Sci. 2018, 66, 758–763. [Google Scholar] [CrossRef]
- Ludwig-Müller, J.; Rattunde, R.; Rößler, S.; Liedel, K.; Benade, F.; Rost, A.; Becker, J. Two Auxinic Herbicides Affect Brassica napus Plant Hormone Levels and Induce Molecular Changes in Transcription. Biomolecules 2021, 11, 1153. [Google Scholar] [CrossRef]
- McCauley, C.L.; McAdam, S.A.M.; Bhide, K.; Thimmapuram, J.; Banks, J.A.; Young, B.G. Transcriptomics in Erigeron canadensis reveals rapid photosynthetic and hormonal responses to auxin herbicide application. J. Exp. Bot. 2020, 71, 3701–3709. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, K.; Retzlaff, G. Bioregulatory effects of the fungicidal strobilurin kresoxim-methyl in wheat (Triticum aestivum). Pestic. Sci. 1997, 50, 11–20. [Google Scholar] [CrossRef]
- Scheltrup, F.; Grossmann, K. Abscisic Acid is a Causative Factor in the Mode of Action of the Auxinic Herbicide Quinmerac in Cleaver (Galium aparine L.). J. Plant Physiol. 1995, 147, 118–126. [Google Scholar] [CrossRef]
- Concepcion, M.; Lizada, C.; Yang, S.F. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal. Biochem. 1979, 100, 140–145. [Google Scholar] [CrossRef]
- Dupille, E.; Zacarias, L. Extraction and biochemical characterization of wound-induced ACC oxidase from Citrus peel. Plant Sci. 1996, 114, 53–60. [Google Scholar] [CrossRef]
- Su, X.; Lu, L.; Li, Y.; Zhen, C.; Hu, G.; Jiang, K.; Yan, Y.; Xu, Y.; Wang, G.; Shi, M.; et al. Reference gene selection for quantitative real-time PCR (qRT-PCR) expression analysis in Galium aparine L. PLoS ONE 2020, 15, e0226668. [Google Scholar] [CrossRef]
- Tsuchisaka, A.; Theologis, A. Unique and Overlapping Expression Patterns among the Arabidopsis 1-Amino-Cyclopropane-1-Carboxylate Synthase Gene Family Members. Plant Physiol. 2004, 136, 2982–3000. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Chang, L.; Wu, S.; Li, P.; Liu, G.; Wang, N.N. Auto-regulation of the promoter activities of Arabidopsis 1-aminocyclopropane-1-carboxylate synthase genes AtACS4, AtACS5, and AtACS7 in response to different plant hormones. Plant Sci. 2008, 175, 161–167. [Google Scholar] [CrossRef]
- Raghavan, C.; Ong, E.K.; Dalling, M.J.; Stevenson, T.W. Regulation of genes associated with auxin, ethylene and ABA pathways by 2,4-dichlorophenoxyacetic acid in Arabidopsis. Funct. Integr. Genom. 2005, 6, 60–70. [Google Scholar] [CrossRef]
- Hansen, H.; Grossmann, K. Auxin-Induced Ethylene Triggers Abscisic Acid Biosynthesis and Growth Inhibition. Plant Physiol. 2000, 124, 1437–1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutler, A.J.; Krochko, E.J. Formation and breakdown of ABA. Trends Plant Sci. 1999, 4, 472–478. [Google Scholar] [CrossRef]
- Qin, X.; Zeevaart, J.A.D. The 9- cis -epoxycarotenoid cleavage reaction is the key regulatory step of abscisic acid biosynthesis in water-stressed bean. Proc. Natl. Acad. Sci. USA 1999, 96, 15354–15361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossmann, K. Mode of action of auxin herbicides: A new ending to a long, drawn out story. Trends Plant Sci. 2000, 5, 506–508. [Google Scholar] [CrossRef]
- Taylor, I.B.; Sonneveld, T.; Bugg, T.D.H.; Thompson, A.J. Regulation and Manipulation of the Biosynthesis of Abscisic Acid, Including the Supply of Xanthophyll Precursors. J. Plant Growth Regul. 2005, 24, 253–273. [Google Scholar] [CrossRef]
- Tan, B.-C.; Joseph, L.M.; Deng, W.-T.; Liu, L.; Li, Q.-B.; Cline, K.; McCarty, D.R. Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J. 2003, 35, 44–56. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Li, J.; Yu, J.; Lin, H.; Dong, L. Comparison of quintrione and quinclorac on mechanism of action. Pestic. Biochem. Physiol. 2022, 181, 105007. [Google Scholar] [CrossRef]
- Xu, J.; Lv, B.; Wang, Q.; Li, J.; Dong, L. A resistance mechanism dependent upon the inhibition of ethylene biosynthesis. Pest Manag. Sci. 2013, 69, 1407–1414. [Google Scholar] [CrossRef]
- Klaus, G.; Jacek, K.; Stefan, T. Auxin herbicides induce H2O2 overproduction and tissue damage in cleavers (Galium aparine L.). J. Exp. Bot. 2001, 52, 1811–1816. [Google Scholar]
- Gaines, A.T. The quick and the dead: A new model for the essential role of ABA accumulation in synthetic auxin herbicide mode of action. J. Exp. Bot. 2020, 71, 3383–3385. [Google Scholar] [CrossRef]
- Kholodar, A.V.; Sidorova, K.K.; Shumny, V. Effects of synthetic auxin (2,4-D) on the level of indolyl-3-acetic acid in cultivars and supernodulating mutants of pea (Pisum sativum L.). Dokl. Biol. Sci. 2002, 386, 460–461. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, C.; Ong, E.K.; Dalling, M.J.; Stevenson, T.W. Effect of herbicidal application of 2,4-dichlorophenoxyacetic acid in Arabidopsis. Funct. Integr. Genom. 2004, 5, 4–17. [Google Scholar] [CrossRef]
- Rey-Caballero, J.; Menéndez, J.; Giné-Bordonaba, J.; Salas, M.; Alcántara, R.; Torra, J. Unravelling the resistance mechanisms to 2,4-D (2,4-dichlorophenoxyacetic acid) in corn poppy (Papaver rhoeas). Pestic. Biochem. Physiol. 2016, 133, 67–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busi, R.; Goggin, D.E.; Heap, I.M.; Horak, M.J.; Jugulam, M.; Masters, R.A.; Napier, R.M.; Riar, D.S.; Satchivi, N.M.; Torra, J.; et al. Weed resistance to synthetic auxin herbicides. Pest Manag. Sci. 2018, 74, 2265–2276. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Liu, X.; Napier, R.; Dong, L.; Li, J. Mode of Action of a Novel Synthetic Auxin Herbicide Halauxifen-Methyl. Agronomy 2022, 12, 1659. https://doi.org/10.3390/agronomy12071659
Xu J, Liu X, Napier R, Dong L, Li J. Mode of Action of a Novel Synthetic Auxin Herbicide Halauxifen-Methyl. Agronomy. 2022; 12(7):1659. https://doi.org/10.3390/agronomy12071659
Chicago/Turabian StyleXu, Jiaqi, Xudong Liu, Richard Napier, Liyao Dong, and Jun Li. 2022. "Mode of Action of a Novel Synthetic Auxin Herbicide Halauxifen-Methyl" Agronomy 12, no. 7: 1659. https://doi.org/10.3390/agronomy12071659
APA StyleXu, J., Liu, X., Napier, R., Dong, L., & Li, J. (2022). Mode of Action of a Novel Synthetic Auxin Herbicide Halauxifen-Methyl. Agronomy, 12(7), 1659. https://doi.org/10.3390/agronomy12071659






